SUMMARY
Decretins, hormones induced by fasting that suppress insulin production and secretion, have been postulated from classical human metabolic studies. From genetic screens, we identified Drosophila Limostatin (Lst), a peptide hormone that suppresses insulin secretion. Lst is induced by nutrient restriction in gut-associated endocrine cells. limostatin deficiency led to hyperinsulinemia, hypoglycemia and excess adiposity. A conserved 15-residue polypeptide encoded by limostatin suppressed secretion by insulin-producing cells. Targeted knockdown of CG9918, a Drosophila orthologue of Neuromedin U receptors (NMUR), in insulin-producing cells phenocopied limostatin deficiency, and attenuated insulin suppression by purified Lst, suggesting CG9918 encodes an Lst receptor. NMUR1 is expressed in islet β-cells, and purified NMU suppresses insulin secretion from human islets. A human mutant NMU variant that co-segregates with familial early-onset obesity and hyperinsulinemia fails to suppress insulin secretion. We propose Lst as an index member of an ancient hormone class called decretins, which suppress insulin output.
Keywords: Metabolism, Drosophila, Diabetes, Hormones, Starvation, Limostatin, Obesity, Incretin, Decretin, Insulin, Insulin-like peptides, CG9918, Neuromedin U, Pancreas, Beta cells, Corpora cardiaca, Insulin-producing cells, Ilp2HF ELISA
INTRODUCTION
The coupling of hormonal responses to nutrient availability is fundamental for metabolic control. In mammals, regulated secretion of insulin from pancreatic β cells is a principal hormonal response orchestrating metabolic homeostasis. Circulating insulin levels constitute a dynamic metabolic switch, signaling the fed state and nutrient storage (anabolic pathways) when elevated, or starvation and nutrient mobilization (catabolic pathways) when decreased (Cahill, 1971; Saltiel and Kahn, 2001). Thus, insulin secretion must be precisely tuned to the nutritional state of the animal. Increased circulating glucose stimulates β cell depolarization and insulin secretion (Rorsman and Braun, 2013). In concert with glucose, gut-derived incretin hormones amplify glucose-stimulated insulin secretion in response to ingested carbohydrates, thereby tuning insulin output to the feeding state of the host (La Barre, 1932; Campbell and Drucker, 2013; Creutzfeldt, 2005).
While the incretin effect on insulin secretion during feeding is well-documented, counter-regulatory mechanisms that suppress insulin secretion during or after starvation are incompletely understood (Campbell and Drucker, 2013; Longo and Mattson, 2014). Classical starvation experiments in humans and other mammals revealed that sustained fasting profoundly alters the dynamics of insulin production and secretion, resulting in impaired glucose tolerance, relative insulin deficits, and “starvation diabetes” (Cahill et al., 1966; Fery et al., 1990; Fink et al., 1974; Hofmeister, 1890; Lilavivathana et al., 1978; Unger et al., 1963). Remarkably, starvation-induced suppression of glucose-stimulated insulin secretion was not reverted by normalizing circulating glucose levels, suggesting that the dampening effect of starvation on insulin secretion perdures and is uncoupled from blood glucose and macronutrient concentrations (Lilavivathana et al., 1978). Based on these observations, it has been postulated that hormonal signals induced by fasting may actively attenuate insulin secretion (Lilavivathana et al., 1978; Unger et al., 1963). Ensinck et al (1997) suggested that enteroendocrine “decretin” hormones may constrain the release of insulin to prevent hypoglycemia. This concept is further supported by recent studies identifying a G-protein that suppresses insulin secretion from pancreatic beta cells (Wang et al., 2011). Thus, after nutrient restriction, decretin hormones could signal through G-protein coupled receptors to attenuate glucose-stimulated insulin secretion from beta cells.
The discovery of hormonal pathways regulating metabolism in mammals presents a formidable challenge. However, progress has revealed conserved mechanisms of metabolic regulation by insulin and glucagon-like peptides in Drosophila, providing a powerful genetic model to address unresolved questions relevant to mammalian metabolism (Baker and Thummel, 2007; Erion and Sehgal, 2013; Leopold and Perrimon, 2007). Similar to mammals, secretion of Drosophila insulin-like peptides (Ilps) from neuroendocrine cells in the brain regulates glucose homeostasis and nutrient stores in the fly (Broughton et al., 2005; Geminard et al., 2009; Ikeya et al., 2002; Rulifson et al., 2002). Ilp secretion from insulin-producing cells (IPCs) is responsive to circulating glucose and macronutrients, and is suppressed upon nutrient withdrawal (Geminard et al., 2009; Kréneisz et al., 2010). Notably, recent studies have identified hormonal and GPCR-linked mechanisms regulating the secretion of Ilps from IPCs in the fly, suggesting further conservation of pathways regulating insulin secretion in the fly (Geminard et al., 2009; Kwak et al., 2013; Owusu-Ansah et al., 2013; Rajan and Perrimon, 2012).
In mammals, the incretin hormones gastric inhibitory peptide (GIP) and glucagon-like peptide-1 (GLP-1) are secreted by enteroendocrine cells following a meal, and enhance glucose-stimulated insulin production and secretion from pancreatic beta cells (Campbell and Drucker, 2013; Creutzfeldt, 2005). Thus, we postulated that a decretin hormone would have the ‘opposite’ hallmarks of incretins. Specifically, a decretin (1) derives from an enteroendocrine source that is sensitive to nutrient availability, (2) is responsive to fasting or carbohydrate deficiency, and (3) suppresses insulin production and secretion from insulin producing cells. However, like incretins, the action of decretins on insulin secretion would be manifest during feeding, when a stimulus for secretion is present.
Here, we identify a secreted hormone, Limostatin (Lst) that suppresses insulin secretion following starvation in Drosophila. We show that lst is regulated by starvation and flies deficient for lst display phenotypes consistent with hyperinsulinemia. We localize Lst production to glucose-sensing, endocrine corpora cardiaca (CC) cells associated with the gut and show that lst is suppressed by carbohydrate feeding. Using calcium imaging and in vitro insulin secretion assays, we identify a 15-amino acid Lst peptide (Lst-15) sufficient to suppress activity of IPCs and Ilp secretion. We identify an orphan GPCR in IPCs as a candidate Lst receptor. Moreover, we show that Neuromedin U (NMU) is likely a functional mammalian orthologue of Lst that inhibits islet β-cell insulin secretion. These results establish a decretin signaling pathway that suppresses insulin output in Drosophila.
RESULTS
CG8317 Encodes Limostatin, a Polypeptide That Suppresses Insulin Production
To identify a hormone that may function as a decretin, we performed an ectopic misexpression screen of selected starvation-regulated Drosophila genes (Palanker et al., 2009; Zinke et al., 2002) predicted to encode peptide hormones. Expression of CG8317 in the fat body, a tissue capable of humoral secretion (Geminard et al., 2009; Rajan and Perrimon, 2012), induced phenotypes consistent with insulin deficiency in Drosophila (Rulifson et al., 2002), including hyperglycemia and developmental delay (Figure S1A, B). CG8317 encodes a predicted primary translation product of 139 amino acids with a signal peptide and four putative dibasic cleavage sites, characteristic features of metazoan pre-prohormones (Figure 1A, B). Quantitative RT-PCR (qPCR) in fasting adult flies showed induction of CG8317 by 16 hours with peak mRNA levels after 24–28 hours of nutrient deprivation (Figure 1C), confirming results from whole-genome expression analysis (Palanker et al., 2009; Zinke et al., 2002). CG8317 and its predicted products appear to be conserved in Drosophila species, including a 15 amino-acid region flanked by cleavage sites also conserved in mosquitoes and other Insecta (Figure S1D). Inactive pre-prohormones undergo post-translational processing, including cleavage at dibasic residues, prior to secretion as bioactive peptides (Duckert et al., 2004). To identify dibasic cleavage sites that are critical for CG8317 function, we generated misexpression lines with arginine/lysine to alanine substitutions at each of the four sites and screened for phenotypes (see Experimental Procedures). Substitution of arginines 101 and 102, immediately N-terminal to the most highly conserved region, eliminated CG8317 gain-of-function phenotypes, suggesting that this dibasic cleavage site is necessary for pro-hormone processing and function (Figure 1B, S1C–D). Thus, CG8317 encodes a starvation-regulated gene likely encoding a processed pre-propeptide that can inhibit insulin production and secretion (see below). We named CG8317 limostatin (lst) after Limos, the Greek goddess of starvation.
Figure 1. Loss of Lst, a Starvation-Regulated Prepropeptide, Causes Hyperinsulinemia.

(A) Genomic organization of lst locus (previously CG8317) with location of P{EP}G424 and breakpoints of lst1 deletion.
(B) Schematic of preprolimostatin with predicted signal peptide and dibasic cleavage sites (red, underline). The highly conserved region used to generate Lst-15 is indicated between cleavage sites #2 and #3 (magenta, bold). Lst antibodies and control peptide were generated using a 9-amino acid peptide as indicated (underline).
(C) Time-course of lst expression during starvation in wild type adult flies, normalized to fed condition.
(D) Glucose levels in control and lst mutant flies.
(E) Lifespan of yw; lstctrl (n=164) and yw; lst1 (n=173) male flies. Median survival times are 57 and 43 days for yw; lstctrl and yw; lst1 flies, respectively. P<0.0001 (Log-rank test).
(F) Ilp2 expression in adult yw; lst1 flies compared to isogenic controls (yw; lstctrl).
(G) Hemolymph levels of Ilp2HF in yw; lstctrl; Ilp2HF and yw; lst1; Ilp2HF flies (Ilp2HF homozygous). All data displayed as mean + s.d. * P<0.05, ** P<0.01, *** P<0.001 (n ≥ 5 for all conditions). See also Figure S1.
To investigate lst function, we mobilized a P-element (Bellen et al., 2004) near lst and identified an imprecise excision that deleted 2 kilobases (kb) encompassing the entire coding sequence (lst1 allele; Figure 1A). lst mRNA was undetectable in lst mutant flies by qPCR, indicating that lst1 is a null allele. Expression of an lst transgene rescued lst mutant phenotypes (see below). Insulin deficiency in Drosophila produces hyperglycemia, starvation resistance, and lifespan extension (Broughton et al., 2008, 2005; Rulifson et al., 2002). Thus, we hypothesized that lst deficient flies would display phenotypes consistent with insulin excess. As expected, lst mutants were hypoglycemic and short-lived compared to isogenic controls (Figure 1D, E). We next assessed insulin production in lst mutants. Drosophila Insulin-like peptides (Ilps) -2,-3, and -5 are produced by IPCs, median neurosecretory cells of the pars intercerebralis, and are essential regulators of growth and metabolism (Gronke et al., 2010; Ikeya et al., 2002; Rulifson et al., 2002). We measured transcript levels of Ilp2, Ilp3, and Ilp5 by qPCR and found elevated mRNAs encoding all brain-derived Ilps in lst1 flies during ad libitum feeding (Figure 1F, Figure S1E, F). While expression and IPC accumulation of Ilp protein have been used to assess insulin signaling in Drosophila (Buch et al., 2008; Geminard et al., 2009), we sought to directly measure circulating picomolar levels of Ilp2 in the hemolymph. To do this, we generated mutant flies and isogenic controls harboring a bioactive form of dual-epitope tagged Ilp2, Ilp2HF, in place of the endogenous locus (Park et al., 2014). If Lst functioned as a decretin, we hypothesized that circulating Ilp2 levels would be elevated in lst mutants. ELISA measurement of Ilp2HF revealed a significant increase in circulating Ilp2HF in lst1 flies compared to controls (Figure 1G). Collectively, these results demonstrate that lst is produced during fasting and is required to suppress insulin production by Drosophila IPCs.
Obesity in Hyperinsulinemic limostatin-deficient Flies
Elevated insulin signaling can stimulate obesity in flies by increasing both adipocyte number and lipid accumulation (DiAngelo and Birnbaum, 2009). Consistent with this precedent, increased IPC excitability by targeted expression of a bacterial sodium channel (NaChBAC) was sufficient to increase triglyceride stores in adult flies compared to age-matched controls (Figure 2A). Likewise, in hyperinsulinemic lst1 flies we found triglyceride content was elevated 150% compared to controls, using standard assays including colorimetry, nile-red staining and thin layer chromatography (Figure 2B, C, Figure S2). To assess whether IPC activity is necessary for obesity in lst mutants, we generated lines that permit electrical silencing of IPCs through targeted expression of the inward rectifying potassium channel Kir2.1. Upon silencing of IPCs, we detected no difference in triglyceride content of lst mutants and controls (Figure 2D). Thus, lst mutant flies are obese, and display phenotypes associated with insulin excess.
Figure 2. Obesity in lst Mutants.
(A) Triglyceride content of control Ilp2-GAL4 and Ilp2-GAL4>NaChBAC flies.
(B, C) Whole fly triglyceride content and nile red staining of abdominal lipid droplets in adult lst1 flies and controls.
(D) Triglyceride content after silencing of IPCs using Ilp2-GAL4 to drive UAS-Kir2.1 in yw; lstctrl and yw; lst1 background, normalized to yw; lstctrl; Ilp2-GAL4>UAS-Kir2.1.
(E) Triglyceride levels in yw; lst1 and controls in random fed, starved, and starved then overnight re-fed flies. Data are normalized to yw; lstctrl fed condition.
(F, G) Quantification of triglyceride depletion after starvation and triglyceride accumulation following refeeding after starvation, data from experiment in (E). Scale bar 15 μm in (C). Data displayed as mean + s.d. * P<0.05, ** P<0.01, and *** P<0.001, (n = 5–8 per condition). See also Figure S2.
To further evaluate the balance between catabolic and anabolic activity in lst mutants, we fasted flies for 24-hours to deplete lipid stores, then re-fed flies for 24-hours to promote lipid accumulation (Figure 2E). Starvation-induced lipid depletion remained fully intact in lst1 mutants, and was even slightly elevated (Figure 2F, G). Remarkably, lst mutants rapidly accumulated triglycerides upon re-feeding and displayed significant obesity after only 24-hours (Figure 2F, G). These results indicate that defects in catabolic defects are not the principal basis for obesity in lst mutants.
Lst is Regulated by Carbohydrate Feeding in Gut-Associated corpora cardiaca cells
To identify the tissue source(s) of Lst, we generated an lst reporter line (Lst-GAL4>mCD4::tdTomato) and a monoclonal antibody against the pre-propeptide (see Experimental Procedures). Lst-GAL4 mediated expression of mCD4::tdTomato co-localized with Adipokinetic hormone (AKH) in corpora cardiaca (CC) cells (Figure 3A). The CC cells comprise 14 gut-associated endocrine cells which send projections to the midgut and secrete hormones into the circulation from projections to the dorsal vessel (Cognigni et al., 2011; Kim and Rulifson, 2004; Park et al., 2011). CC cells secrete AKH, a hormone thought to be a functional ortholog of mammalian glucagon, indicating that CC cell have roles analogous to preproglucagon-expressing cells in the mammalian pancreas and gastrointestinal tract (Kim and Rulifson, 2004; Park et al., 2011). Lst protein co-localized with Akh, and with the dense-core vesicle marker ANF-EMD (Rao et al., 2001) in CC cell neurites ramifying on heart, consistent with the postulated role of Lst as a secreted hormone (Figure 3B, C).
Figure 3. Lst is Produced in Gut-Associated CC cells.
(A) Expression of Lst-GAL4>CD4::tdTomato and AKH immunoreactivity in CC cells. Labels here and below: cc, corpora cardiaca; ca, corpus allatum; he, heart/dorsal vessel.
(B) Dense-core vesicle marker preproANF::EMD (GFP) and Lst antibody staining of CC cells. Outline marks ring gland. Arrow indicates CC cell soma, arrowhead marks path of dorsal vessel.
(C) AKH and Lst immunoreactivity in ring gland of control and lst mutant flies. Labels as above, hatched denotes boundary of ring gland.
(D–F) Glucose, circulating Ilp2HF (heterozygous Ilp2HF flies) and triglyceride levels following knock-down of lst in CC cells using Akh-GAL4 (lstRNAi) compared to isogenic controls (VDRCctrl).
(G) Triglyceride content in controls (yw; lstctrl; Akh-GAL4), lst mutants (yw; lst1; Akh-GAL4) and following rescue with UAS-lst (yw; lst1; Akh-GAL4/UAS-lst). Scale bars 10 μm in (A–C). Data displayed as mean + s.d. ** P<0.01, and *** P<0.001. See also Figure S3.
Following specific knockdown of lst using RNAi (lstRNAi) in CC cells, Lst immunoreactivity was reduced or undetectable, as in homozygous lst mutants (Figure 3C, S3A). Lst knockdown in CC cells, but not the fat body (Figure S3B), recapitulated the hypoglycemia, elevated circulating Ilp2HF levels, and obesity observed in lst1 mutant flies (Figure 3D–F). Thus, CC cells are a crucial physiological source of Lst. To confirm that lst loss-of-function causes obesity in lst mutants, we expressed UAS-lst specifically in the CC cells of lst mutant flies. We observed reversion of lst1 obesity, with triglyceride levels indistinguishable from those in controls (Figure 3G). Thus, Lst is principally produced and secreted by CC cells, and phenotypes in lst mutants derive from Lst loss in CC cells.
A critical feature of incretin hormones is their regulation by carbohydrate feeding (Creutzfeldt, 2005). Thus, if Lst functioned as a decretin, we postulated that elevated lst expression after fasting should be reduced upon refeeding with carbohydrates. Refeeding fasted flies with carbohydrates, rapidly suppressed lst mRNA expression (Figure 4A). By contrast, refeeding with protein did not detectably affect lst expression (Figure 4B). Hence, lst expression is increased by dietary carbohydrate restriction. Carbohydrate refeeding after fasting led to significant increases of circulating Ilp2HF (Figure 4C). Consistent with our finding that lst is required to suppress insulin, we found that this post-prandial increase of circulating Ilp2HF was significantly greater in lst mutants compared to controls (Figure 4C). In summary, lst is regulated by dietary carbohydrate and is required to regulate insulin output in post-prandial settings.
Figure 4. Lst Regulates Insulin Secretion in Response to Dietary Sugar.

(A, B) qPCR analysis of lst expression in wild-type adult flies starved then refed for 30, 60, or 120 minutes with carbohydrate only (A) or protein only (B) food. 0 time point indicates flies starved and not refed. (C) Hemolymph Ilp2HF levels in lst mutants (purple bars) and controls (open bars) refed for 0, 30 or 60 minutes following starvation. 0 time point indicates starved. Flies here homozygous for Ilp2HF. Data displayed as mean + s.d. * P<0.05, ** P<0.01, and *** P<0.001
A Peptide Derived from Lst Inhibits IPC Activity and Insulin Secretion
Drosophila IPCs share electrophysiological properties with mammalian pancreatic β-cells, including coupling of electrical excitation to induction of calcium transients (Kréneisz et al., 2010). Incretins such as GLP-1 augment insulin secretion from β-cells by increasing the frequency and amplitude of intracellular calcium transients (MacDonald et al., 2002). If Lst functioned as a decretin, we hypothesized it should decrease the excitability of IPCs. To monitor IPC activity, we generated flies that produce the genetically-encoded calcium indicator GCaMP3 (Tian et al., 2009) specifically in IPCs. We quantified in vivo GCaMP3 fluorescence of IPCs by confocal microscopy in brains of immobilized live adult flies (see Experimental Procedures). GCaMP3 fluorescence was dose-dependently attenuated (Figure 5A) by exposure to a 15 amino acid peptide with carboxy-terminal amidation (Lst-15) corresponding to the highly conserved Lst region (Figure 1B, S1D). In contrast, exposure to a control peptide derived from an alternate domain in the pre-propeptide (Figure 1C) did not detectably affect GCaMP3 signal (Figure 5A). These results illustrate that a conserved region of the Lst peptide can regulate calcium signaling in IPCs.
Figure 5. Lst-15 Inhibits Electrical Activity and Insulin Secretion from IPCs.

(A) GCaMP3 fluorescence in head-fixed adult flies expressing GCaMP3 in IPCs under the control of the Ilp2 promoter. Baseline images from IPCs in standard AHL (3 mM glucose) before treatment. Treatment panel images were obtained 30s after treatment with control peptide or Lst-15 (125 nM and 1 μM) diluted in standard AHL and displayed with 16-color look-up table. IPC cell clusters are indicated by hatched outline and individual cells in the imaging plane are numbered around perimeter of the cluster. Average ΔF/F% from baseline for each condition are plotted (bottom, right).
(B) Normalized Ilp2HF protein secreted into supernatant from Drosophila heads incubated for 30 minutes in standard AHL with control peptide or Lst-15 peptide (1 μM) under basal and high-KCl conditions as indicated. Data are normalized to basal control condition. Scale bars 10 μm in (A). Data displayed as mean + s.d. *** P<0.001
To directly assess the effects of Limostatin on Ilp secretion, we developed an in vitro assay to measure Ilp2HF secretion from brain IPCs following exogenous application of purified Lst-15 peptide. Heads from Ilp21 gd2HF flies were cultured in artificial hemolymph-like (AHL) solution for 30 minutes and Ilp2HF concentration in supernatants was measured by ELISA (Park et al., 2014). Exposure to Lst-15 significantly depressed Ilp2HF secretion under basal conditions (Figure 5B). Secretion remained modestly depressed following addition of high-KCl AHL solution to depolarize IPCs (Figure 5B) and a control peptide had no effect on Ilp2HF secretion (Figure 5B). Taken together, these results further support classification of Lst as a peptide hormone, and suggest that Lst acutely regulates insulin secretion from IPCs (and see below). Furthermore, we have identified a minimal amidated peptide that is sufficient for the insulinostatic effect of Lst. Our demonstration that Lst is a hormone (1) produced by gut-associated endocrine cells, (2) regulated by carbohydrate restriction that (3) inhibits Ilp production and secretion from IPCs supports classification of Lst as a Drosophila decretin.
Knockdown of the GPCR CG9918 in IPCs Phenocopies lst Loss of Function
Many neuropeptides signal through G protein-coupled receptors (GPCRs) (Taghert and Nitabach, 2012), and receptor activity or expression is often modulated to balance signaling strength (Gardner and Nissenson, 2004). To identify a candidate receptor for Lst, we designed a qPCR-based screen to reveal GPCRs encoded by mRNAs that were both (1) reduced upon lst over-expression and (2) elevated in lst mutants. As proof of principle, ectopic expression of Akh in fat body reduced expression of the G-protein-coupled Akh receptor, AkhR (data not shown). We identified 3 candidate receptors with mRNA levels that appropriately and reciprocally varied in this manner upon lst gain- or loss-of-function (Figure 6A).
Figure 6. CG9918 is a Candidate Lst receptor.
(A) qPCR for expression of Drosophila GPCRs in lst over-expression (r4-G4>UAS-lst) or lst1 loss of function. Expression changes in comparison to control are indicated by gray (no change), green (decreased), purple (elevated), and black (not determined). Red arrowheads denote transcripts reciprocally regulated and assessed in (B).
(B) Triglyceride levels following IPC specific knockdown of receptors identified in (A) (red arrowheads). Receptors encoded by CG9918, CG4395, and CG7285 were knocked down in IPCs using Ilp2-GAL4; UAS-Dcr2. AKHR was included as a negative control.
(C, D) Ilp2 expression and hemolymph Ilp2HF levels in CG9918RNAi flies and controls (Ilp2HF heterozygous here).
(E) lst expression in CG9918RNAi flies. Data in (B,C, E) normalized to control condition.
(F) Fluorescent in situ hybridization (FISH) for CG9918 mRNA with immunohistochemistry (IHC) using antibodies against Ilp2 in control and CG9918RNAi flies.
(G) Normalized Ilp2HF levels in supernatant from CG9918RNAi and control heads incubated with 1 μM Lst-15 (red bars) or Lst control peptide (open bars).
(H) Summary model for Lst signaling. Ingested carbohydrates levels are monitored by CC-cells. Under carbohydrate poor conditions, secreted Lst hormonally suppresses activity and secretion of Ilps from Insulin-producing cells (IPCs). Scale bar 25 μm in (F). Data displayed as mean + s.d. * P<0.05, ** P<0.01, *** P<0.001. See also Figure S4.
Based on our findings that excitatory activity of the IPCs is crucial to lst loss of function phenotypes (Figure 2D), we reasoned that knockdown of a candidate Lst receptor specifically in IPCs should phenocopy the lst1 mutation. Only IPC-directed knockdown of the receptor encoded by CG9918 (CG9918RNAi) produced increased adiposity, accompanied by elevated Ilp2 mRNA levels (Figure 6B, C), phenotypes observed in lst1 flies. CG9918 has been called Pyrokinin 1 receptor (PK1r), based on its reported affinity for Drosophila pyrokinin Drm-PK-1 (Cazzamali et al., 2005), but other studies failed to activate CG9918 with Drm-PK-1 or pyrokinins (Park et al., 2002), a peptide class thought to regulate sex pheromone production (Choi and Vander Meer, 2012). Consistent with a role in lst signaling, CG9918RNAi in IPCs also increased hemolymph Ilp2HF levels, and endogenous lst expression (Figure 6D, E). To confirm expression of CG9918 in IPCs we performed fluorescent in situ hybridization (FISH) combined with immunohistochemistry (for Ilp2) in CG9918RNAi and controls. Hybridization signal for CG9918 co-localized with Ilp2 protein in IPCs, was reduced with CG9918RNAi and undetectable with sense probes (Figure 6F, S4). Thus, we have identified CG9918 as a GPCR expressed in IPCs that negatively regulates insulin expression and secretion in the adult fly.
To test further if CG9918 encodes a GPCR required for Lst signaling, we reasoned that CG9918RNAi in IPCs should alter effects of purified Lst-15 peptide on insulin secretion. Compared to controls, we observed that CG9918 knock-down prevented the effects of Lst-15 on attenuating Ilp2HF secretion (Figure 6G). These pharmacogenetic findings indicate that Lst regulates insulin secretion directly in IPCs, and support the view that CG9918 encodes an Lst receptor.
NMU is a functional orthologue of Lst that inhibits insulin secretion by human islets
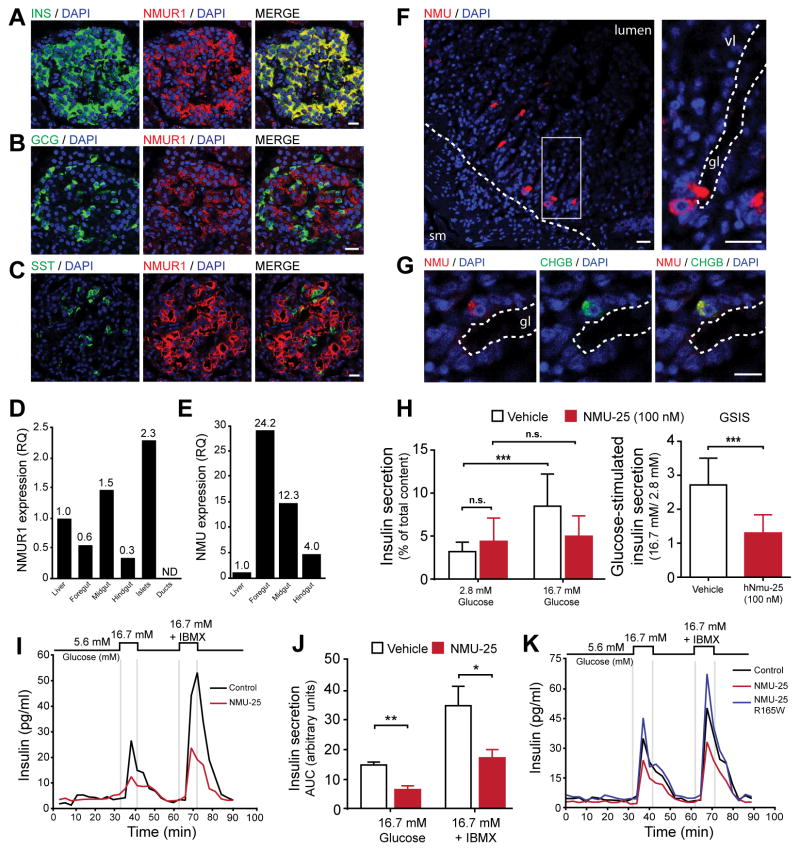
The GCPR encoded by CG9918 is conserved among a cluster of receptors within a phylogenetic group of peptide receptors that includes human Neuromedin U receptors (NMUR) (Metpally and Sowdhamini, 2005). Among these receptors, CG9918 is most closely related to human NMURs, displaying 32% conservation with NMUR1 and 30% conservation with NMUR2 (Figure S5A–B, Experimental Methods). In humans and other mammals, peripheral effects of NMU are mediated by NMUR1, while NMUR2 is primarily expressed in the CNS (Mitchell et al 2009). NMUR1 immunoreactivity and mRNA were detected in human pancreatic islets in insulin+ β cells (Figure 7A, S5C, S5F). By contrast, little to no protein or mRNA were detected in glucagon+ α cells, somatostatin+ δ cells, exocrine acinar cells or pancreatic ducts (Figure 7B, C, S5D–F). In addition, qPCR revealed expression of NMUR1 mRNA in human gastrointestinal tissues with enrichment in pancreatic islets (Figure 7D), consistent with prior reports (Howard et al., 2000). Lst is produced in CC cells that contact the Drosophila foregut. Thus, we assessed NMU expression in human gastrointestinal organs. NMU mRNA expression was enriched in human foregut organs (stomach, duodenum) (Figure 7E). Immunofluorescence localized NMU to ChgB+ duodenal cells with an ‘open-type’ morphology adjacent to the lumen of intestinal glands (Figure 7F, G). These results support the view that NMU from a gastrointestinal source might impact insulin output by pancreatic β-cells.
Figure 7. NMU signaling suppresses insulin secretion from human β-cells.
(A–C) Immunofluorescence for NMUR1 and Insulin (β-cells), Glucagon (α-cells), or Somatostatin (δ-cells) in adult human pancreas.
(D) qPCR analysis of NMUR1 expression in human gastrointestinal tissues, including purified human islets and pancreatic ductal cells. Data expressed as relative quantification (RQ) and normalized to liver sample. N.D., not detected.
(E) qPCR analysis of NMU expression in human gastrointestinal tissues. Data expressed as relative quantification (RQ) and normalized to liver sample.
(F) NMU immunoreactivity in villous mucosa of human duodenum. Mucosal villi (vl) oriented toward lumen at upper right of image. Hatched line in left panel denotes boundary of mucosal layer. Magnification of boxed region in right panel shows base of duodenal gland (gl), with lumen outlined by hatched line and open-type enteroendocrine cell immunoreactive for NMU.
(G) NMU and Chromogranin B immunoreactivity in open-type enteroendocrine cell. Lumenal edge of duodenal gland (gl) is marked by hatched line. Scale bars 10 μM in (A–C) and 20 μM in (D, E).
(H) Insulin secretion from human islets from 59 year-old male donor assayed in static batch assay with vehicle or 100 nM NMU-25. Data normalized to insulin content and expressed as percent of total content. Glucose-stimulated insulin secretion (GSIS) panel (right) displays ratio of stimulated (16.7 mM) to basal (2.8 mM) secretion.
(I) Insulin secretion from human islet perifusion assay using islets from 40 year-old male donor. NMU-25 (red trace) was applied at 100 nM and included in all incubation solutions. Top diagram depicts stimulation protocol. IBMX, 3-isobutyl-1-methylxanthine.
(J) Quantification of insulin secretion area under the curve (AUC) from independent perifusion experiments (in Figure 7I, 7K and Figure S5I) using islets from 3 human donors under stimulation conditions (16.7 mM glucose or 16.7 mM + IBMX). Islets were treated with vehicle (open bars) or 100 nM NMU-25 (red bars). Error bars SEM.
(K) Insulin secretion in human islet perifusion assay using islets from a 49 year-old male donor. NMU-25 (red trace) and mutant NMU-25 R165W (blue) were applied at 100 nM and included in all incubation solutions. * P < 0.05, ** P < 0.01, *** P < 0.001. See also Figure S5.
To test directly if NMU can suppress insulin secretion, we purified human islets and assessed glucose-stimulated insulin secretion (GSIS) at a concentration of NMU reported to elicit physiological responses (Kaczmarek et al., 2006). NMU-25 potently suppressed glucose-stimulated insulin secretion from human islets in static batch culture assays (Figure 7H, P < 0.001 for GSIS) and islet perifusion experiments (Figure 7I–K). An NMU R165W allele that encodes a mutant peptide was previously found to co-segregate in an autosomal dominant pattern with early-onset obesity (Hainerová et al 2006). In that study, a subset of carriers displayed elevated insulin C-peptide levels; based on these findings and mutation of the highly conserved NMU C-terminal pentapeptide in this family, we reasoned that suppression of insulin secretion might be impaired by the mutant NMU R165W variant. In human islet perifusion assays, the R165W NMU variant failed to suppress insulin secretion (Figure 7K, S5G) compared to wild-type NMU. These data suggest that the human NMU R165W mutation represents a hypomorphic loss-of-function allele, and that impaired regulation of insulin secretion by NMU could underlie metabolic changes in carriers of this allele.
DISCUSSION
Limostatin is a peptide hormone induced by carbohydrate restriction from endocrine cells associated with the gut that suppresses insulin production and release by insulin-producing cells. Thus, Drosophila Lst fulfills the functional criteria for a decretin and serves as an index member of this hormone class in metazoans. Results here also show that Lst signaling from corpora cardica cells may be mediated by the GPCR encoded by CG9918 in insulin-producing cells. In addition, our results reveal cellular and molecular features of a cell-cell signaling system in Drosophila with likely homologies to a mammalian entero-insular axis.
Reduction of nutrient-derived secretogogues, like glucose, is a primary mechanism for attenuating insulin output during starvation in humans (Cahill et al., 1966) and flies (Colombani et al., 2003; Geminard et al., 2009). Consistent with this, we found that circulating Ilp2HF levels were reduced to a similar degree in lst mutant or control flies during prolonged fasting (Figure 4C). Therefore, lst was dispensable for Ilp2 reduction during fasting. However, lst mutants upon re-feeding or during subsequent ad libitum feeding had enhanced circulating Ilp2HF levels compared to controls, findings that demonstrate a requirement for Lst to restrict insulin output in fed flies. Thus, while induced by nutrient restriction, Lst decretin function was revealed by nutrient challenge. This linkage of feeding to decretin regulation of insulin output is reminiscent of incretin regulation and action (Campbell and Drucker, 2013).
Recent studies have demonstrated functional conservation in Drosophila of fundamental hormonal systems for metabolic regulation in mammals, including insulin (Ikeya et al., 2002; Rulifson et al., 2002), glucagon (Kim and Rulifson, 2004; Lee and Park, 2004), and leptin (Rajan and Perrimon, 2012). Here we used Drosophila to identify a hormonal regulator of insulin output, glucose and lipid metabolism without an identified antecedent mammalian orthologue – emphasizing the possibility for work on flies to presage endocrine hormone discovery in mammals. Gain of Lst function in our studies led to reduced insulin signaling, and hyperglycemia, consistent with prior work by our group and others (Broughton et al., 2005; Kim and Rulifson, 2004). By contrast, loss of Lst function led to excessive insulin production and secretion, hypoglycemia and elevated triglycerides, phenotypes consistent with the recognized anabolic functions of insulin signaling in metazoans, and with the few prior metabolic studies of flies with insulin excess (Erion et al., 2012; Rajan and Perrimon, 2012).
Prior studies show that somatostatin and galanin are mammalian gastrointestinal hormones that can suppress insulin secretion. Somatostatin-28 (SST-28) is a peptide derivative of the pro-somatostatin gene that is expressed widely, including in gastrointestinal cells and pancreatic islet cells. Islet somatostatin signaling is thought to be principally paracrine, rather than endocrine, and serum SST-28 concentrations increase post-prandially (D’Alessio et al., 1989; Strowski and Blake, 2008). Galanin is an orexigenic neuropeptide produced throughout the CNS and in peripheral neurons, and has been reported to inhibit insulin secretion (Fehmann et al., 1995). Unlike enteroendocrine-derived hormones which act systemically, galanin is secreted from intrapancreatic autonomic nerve terminals and thought to exert local effects (Dunning et al., 1986; Dupré, 1988; Tang et al., 2012). In addition, Galanin synthesis and secretion are increased by feeding and dietary fat (Leibowitz et al., 2004; Wang and Leibowitz, 1997). Thus, like incretins, output of SST-28 and galanin are induced by feeding, but in contrast to incretins, these peptides suppress insulin secretion. Further studies are needed to assess the roles of these peptide regulators in the modulation of insulin secretion during fasting.
While sequence-based searches did not identify vertebrate orthologues of Lst, we found that the postulated Lst receptor in IPCs, encoded by CG9918, is most similar to the GPCRs Neuromedin U receptor 1 and 2 (NMUR1 and NMUR2). In rodents, NMU signaling may be a central regulator of satiety and feeding behavior (Hanada et al., 2004; Howard et al., 2000), and this role may be conserved in other organisms (Pang and Curran, 2014; Schoofs et al., 2014). In addition, NMU mutant mice have increased adiposity and hyperinsulinemia (Hanada et al 2004), but a direct role for NMU in regulating insulin secretion by insulin-producing cells was not identified. In rodents, the central effects of NMU on satiety are thought to be mediated by the receptor NMUR2; however, hyperphagia, hyperinsulinemia and obesity were not reported in NMUR2 mutant mice (Bechtold et al., 2009). Together, these studies suggest that a subset of phenotypes observed in NMU mutant mice may instead reflect the activity of NMU on peripheral tissues like pancreatic islets, but this has not been previously shown. Notably, humans harboring the NMU R165W allele displayed obesity and elevated insulin C-peptide levels, without evident hyperphagia – further suggesting that the central and peripheral effects of NMU reflect distinct pathways that may be uncoupled (Hainerová et al., 2006). Here we showed that NMU is produced abundantly in human foregut organs and suppresses insulin secretion from pancreatic β-cells, supporting the view that NMU has important functions outside the CNS in regulating metabolism. Thus, like the incretin GLP-1 (Drucker, 2006), NMU may have dual central and peripheral signaling functions in the regulating metabolism. Demonstration that NMU is a mammalian decretin will require further studies on NMU regulation and robust methods to measure circulating NMU levels in fasting and re-feeding. In summary, our findings should invigorate searches for mammalian decretins with possible roles in both physiological and pathological settings.
EXPERIMENTAL PROCEDURES
Drosophila methods
Experimental crosses were maintained at 25°C under 12 h: 12 h light/dark conditions and provided fresh food every 2–3 days. Unless otherwise indicated, standard molasses (6% molasses, 5% corn meal, 2.5% baker’s yeast, and 0.7% agar) food was used for all experiments. Adult flies were collected 2 days after eclosion and aged for 8–12 days on standard molasses food for all experiments. Carbohydrate-only food was comprised of 15% W/V dextrose or sucrose, 1% Agar. Protein only food was comprised of 10% W/V bacto-peptone (BD). For starvation experiments, flies were tipped to fresh vials or bottles containing 1% agar or wetted cotton plugs and fasted for 20–24 hours unless otherwise indicated. For re-feeding experiments, agar-starved flies were tipped to foods prepared with food coloring and feeding was verified by visualization of pigment in gut.
The lst1 allele was generated by imprecise excision of a P element upstream of the lst gene in the yw; P{EP}G424 line (Bellen et al., 2004) using standard methods. The extent of the deletion was assessed by PCR and sequencing. The deletion spans 1946 bp fragment (2R: 12462183…12464128 in the genome assembly release r5.52), only removing the lst gene, including the entire coding region. A control yw stock and the yw; lst1 line were then backcrossed into the original yw; P{EP}G424 line to generate isogenic yw; lstctrl and yw;lst1 stocks. To generate lines for epistasis experiments, yw; lstctrl and yw; lst1 (located on chromosome II) were combined with transgenes or deficiencies located on chromosome III by standard methods to generate isogenic flies. Thus, stocks were yw; lstctrl or yw; lst1 chromosomes I & II, and isogenic for indicated transgenic or mutant chromosome III.
Drosophila metabolic assays were performed using protocols described in detail (Tennessen et al., 2014). Insulin measurements in Drosophila were performed using flies homozygous or heterozygous for the Ilp2HF transgene, as indicated. Hemolymph Ilp2HF levels were quantified using custom made ELISA assays as described in (Park et al., 2014).
Human tissues
Institutional review board approval for research use of tissue was obtained from Stanford University School of Medicine and Vanderbilt University. Human pancreata and islets were obtained from previously healthy, non-diabetic organ donors by the Integrated Islet Distribution Program (IIDP). For histology studies, fresh human pancreata and gastro-intestinal organs were fixed and processed for sectioning by standard histology protocols. Pancreata from donors aged (3, 23, 30 years old) were used in immunofluorescence studies. Islets used in static batch incubation and perifusion assays were from donors aged (3, 40, 49, 51, 59 years old). Human gastrointestinal cDNA was obtained from Clontech (Human Digestive System MTC panel, cat: 636746) and derived from multiple donors. Human islet and pancreatic ductal cell RNA for qPCR was obtained as described previously (Lee et al., 2013). Adult human stomach slides used for histology were obtained from Abcam (cat: ab4371). Adult human pyloric stomach and duodenum specimens used in histology were procured by the National Disease Research Interchange (NDRI). Human pancreas sections used for RNAscope 2.0 assays were obtained from the Stanford Tissue Bank.
Peptides
Drosophila peptides used in this study were supplied by LifeTein (South Plainfield, NJ). Peptide sequences are as follows: Limostatin-control peptide (Lst-ctrl), AQPDSLRSKP; Limostatin-15 (Lst-15) AIVFRPLFVYKQQEI-amide; Akh, pyrQLTFSPDW-amide. Human NMU-25 was obtained from Sigma (N4284) and LifeTein, human NMU-R165W was obtained from LifeTein.
Supplementary Material
Acknowledgments
We thank the Bloomington Drosophila Stock Center, and TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing transgenic fly stocks used in this study; J. Mulholland and K. Lee at the Stanford Cell Sciences Imaging Facility (CSIF) for microscopy support; T. Anderson and W. Adams in the Stanford Fly Media Center. J. Leong for advice on calcium imaging experiments; S. Babur for assistance with the FISH protocol; Y. Liu and J. Wang for technical support; Stanford Tissue Bank and Dr. J. Lee for specimens; Drs. M. Scott, M. Goodman, and members of the Kim group for reading and improving the manuscript. R.W.A. is a student in the Stanford Medical Scientist Training Program and was also supported by the Paul and Daisy Soros Fellowships and a Stanford Bio-X Program fellowship. K.-R. S. was supported by a Stanford VPUE award. Stanford CSIF was supported by the NIH grant 1S10OD01058001A1. Work in the Powers lab was supported by grants from the Department of Veterans Affairs (Merit Review), the NIH (DK89572 and DK072473), the JDRF, and the Vanderbilt Diabetes Research and Training Center (DK20593). Work in the Kim group was supported by the Snyder Foundation and by the Howard Hughes Medical Institute (HHMI). S.K.K. is an Investigator of the HHMI.
Footnotes
Supplemental Information includes Extended Experimental Procedures, five figures, and one table, and can be found with this article online.
AUTHOR CONTRIBUTIONS
R.W.A. and S.K.K. designed experiments and wrote the manuscript. R.W.A., K-R.S., N.J., X.G., J.W. performed the experiments. R.W.A. and S.P. carried out the initial screen. S.P. and K-R.S. generated the limostatin null allele. S.P. generated transgenic lines and ELISA methods. L.K. performed experiments and assisted with experimental design. G.P. and A.C.P. designed and performed human islet perifusion experiments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Barre J. Sur les possibilites d’un traitement du diabète par l’incrétine. Bull Acad R Med Belg. 1932;12:620–634. [Google Scholar]
- Bechtold DA, Ivanov TR, Luckman SM. Appetite-modifying actions of pro-neuromedin U-derived peptides. Am J Physiol Endocrinol Metab. 2009;297:E545–51. doi: 10.1152/ajpendo.00255.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS One. 2008;3:e3721. doi: 10.1371/journal.pone.0003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton SJ, Piper MDW, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 2008;7:321–332. doi: 10.1016/j.cmet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Cahill GFJ. Physiology of Insulin In Man: The Banting Memorial Lecture 1971. Diabetes. 1971;20:785–799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- Cahill GF, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA, Kipnis DM. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966;45:1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Cazzamali G, Torp M, Hauser F, Williamson M, Grimmelikhuijzen CJ. The Drosophila gene CG9918 codes for a pyrokinin-1 receptor. Biochem Biophys Res Commun. 2005;335:14–19. doi: 10.1016/j.bbrc.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Choi M-Y, Vander Meer RK. Molecular Structure and Diversity of PBAN/pyrokinin Family Peptides in Ants. Front Endocrinol (Lausanne) 2012;3:32. doi: 10.3389/fendo.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Léopold P. A Nutrient Sensor Mechanism Controls Drosophila Growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W. The [pre-] history of the incretin concept. Regul Pept. 2005;128:87–91. doi: 10.1016/j.regpep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- D’Alessio DA, Sieber C, Beglinger C, Ensinck JW. A physiologic role for somatostatin 28 as a regulator of insulin secretion. J Clin Invest. 1989;84:857–862. doi: 10.1172/JCI114246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAngelo JR, Birnbaum MJ. Regulation of fat cell mass by insulin in Drosophila melanogaster. Mol Cell Biol. 2009;29:6341–6352. doi: 10.1128/MCB.00675-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Duckert P, Brunak S, Blom N. Prediction of proprotein convertase cleavage sites. Protein Eng Des Sel. 2004;17:107–112. doi: 10.1093/protein/gzh013. [DOI] [PubMed] [Google Scholar]
- Dunning BE, Ahren B, Veith RC, Böttcher G, Sundler F, Taborsky GJ. Galanin: a novel pancreatic neuropeptide. Am J Physiol. 1986;251:E127–33. doi: 10.1152/ajpendo.1986.251.1.E127. [DOI] [PubMed] [Google Scholar]
- Dupré J. Galanin: a selective inhibitor of insulin secretion? Pancreas. 1988;3:119–121. [PubMed] [Google Scholar]
- Erion R, Sehgal A. Regulation of insect behavior via the insulin-signaling pathway. Front Physiol. 2013;4:353. doi: 10.3389/fphys.2013.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erion R, DiAngelo JR, Crocker A, Sehgal A. Interaction between sleep and metabolism in Drosophila with altered octopamine signaling. J Biol Chem. 2012;287:32406–32414. doi: 10.1074/jbc.M112.360875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehmann HC, Janssen M, Göke B. Interaction of glucagon-like peptide-I (GLP-I) and galanin in insulin (beta TC-1)- and somatostatin (RIN T3)-secreting cells and evidence that both peptides have no receptors on glucagon (INR1G9)-secreting cells. Acta Diabetol. 1995;32:176–181. doi: 10.1007/BF00838488. [DOI] [PubMed] [Google Scholar]
- Fery F, d’Attellis NP, Balasse EO. Mechanisms of starvation diabetes: a study with double tracer and indirect calorimetry. Am J Physiol Endocrinol Metab. 1990;259:E770–777. doi: 10.1152/ajpendo.1990.259.6.E770. [DOI] [PubMed] [Google Scholar]
- Fink G, Gutman RA, Cresto JC, Selawry H, Lavine R, Recant L. Glucose-induced insulin release patterns: Effect of starvation. Diabetologia. 1974;10:421–425. doi: 10.1007/BF01221632. [DOI] [PubMed] [Google Scholar]
- Gardner DG, Nissenson RA. Mechanisms of Hormone Action. In: Gardner DG, Greenspan FS, editors. Basic and Clinical Endocrinology. 7. New York: Lange Meical Books/McGraw-Hill; 2004. pp. 61–84. [Google Scholar]
- Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Gronke S, Clarke DFF, Broughton S, Andrews TD, Partridge L, Grönke S. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainerová I, Torekov SS, Ek J, Finková M, Borch-Johnsen K, Jørgensen T, Madsen OD, Lebl J, Hansen T, Pedersen O. Association between neuromedin U gene variants and overweight and obesity. J Clin Endocrinol Metab. 2006;91:5057–5063. doi: 10.1210/jc.2006-1442. [DOI] [PubMed] [Google Scholar]
- Hanada R, Teranishi H, Pearson JT, Kurokawa M, Hosoda H, Fukushima N, Fukue Y, Serino R, Fujihara H, Ueta Y, et al. Neuromedin U has a novel anorexigenic effect independent of the leptin signaling pathway. Nat Med. 2004;10:1067–1073. doi: 10.1038/nm1106. [DOI] [PubMed] [Google Scholar]
- Hofmeister F. Ueber Resorption und Assimilation der Nährstoffe. Arch Für Exp Pathol Und Pharmakologie. 1890;26:355–370. [Google Scholar]
- Howard AD, Wang R, Pong SS, Mellin TN, Strack A, Guan XM, Zeng Z, Williams DL, Feighner SD, Nunes CN, et al. Identification of receptors for neuromedin U and its role in feeding. Nature. 2000;406:70–74. doi: 10.1038/35017610. [DOI] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Kaczmarek P, Malendowicz LK, Pruszynska-Oszmalek E, Wojciechowicz T, Szczepankiewicz D, Szkudelski T, Nowak KW. Neuromedin U receptor 1 expression in the rat endocrine pancreas and evidence suggesting neuromedin U suppressive effect on insulin secretion from isolated rat pancreatic islets. Int J Mol Med. 2006;18:951–955. [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Kréneisz O, Chen X, Fridell YWC, Mulkey DK. Glucose increases activity and Ca2+ in insulin-producing cells of adult Drosophila. Neuroreport. 2010;21:1116–1120. doi: 10.1097/WNR.0b013e3283409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak SJ, Hong SH, Bajracharya R, Yang SY, Lee KS, Yu K. Drosophila adiponectin receptor in insulin producing cells regulates glucose and lipid metabolism by controlling insulin secretion. PLoS One. 2013;8:e68641. doi: 10.1371/journal.pone.0068641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Sugiyama T, Liu Y, Wang J, Gu X, Lei J, Markmann JF, Miyazaki S, Miyazaki JI, Szot GL, et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife. 2013;2:e00940. doi: 10.7554/eLife.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF, Dourmashkin JT, Chang G-Q, Hill JO, Gayles EC, Fried SK, Wang J. Acute high-fat diet paradigms link galanin to triglycerides and their transport and metabolism in muscle. Brain Res. 2004;1008:168–178. doi: 10.1016/j.brainres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Leopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450:186–188. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- Lilavivathana U, Campbell RG, Brodows RG. Control of insulin secretion during fasting in man. Metabolism. 1978;27:815–821. doi: 10.1016/0026-0495(78)90216-0. [DOI] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PE, El-kholy W, Riedel MJ, Salapatek AMF, Light PE, Wheeler MB. The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes. 2002;51:S434–S442. doi: 10.2337/diabetes.51.2007.s434. [DOI] [PubMed] [Google Scholar]
- Metpally RPR, Sowdhamini R. Cross genome phylogenetic analysis of human and Drosophila G protein-coupled receptors: application to functional annotation of orphan receptors. BMC Genomics. 2005;6:106. doi: 10.1186/1471-2164-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JD, Maguire JJ, Davenport AP. Emerging pharmacology and physiology of neuromedin U and the structurally related peptide neuromedin S. Br J Pharmacol. 2009;158:87–103. doi: 10.1111/j.1476-5381.2009.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Song W, Perrimon N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell. 2013;155:699–712. doi: 10.1016/j.cell.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 2009;9:228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S, Curran SP. Adaptive capacity to bacterial diet modulates aging in C. elegans. Cell Metab. 2014;19:221–231. doi: 10.1016/j.cmet.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Bustamante EL, Antonova J, McLean GW, Kim SK. Specification of Drosophila corpora cardiaca neuroendocrine cells from mesoderm is regulated by Notch signaling. PLoS Genet. 2011;7:e1002241. doi: 10.1371/journal.pgen.1002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Alfa RW, Topper SM, Kim GES, Kockel L, Kim SK. A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion. PLoS Genet. 2014 doi: 10.1371/journal.pgen.1004555. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Kim YJ, Adams ME. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci U S A. 2002;99:11423–11428. doi: 10.1073/pnas.162276199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151:123–137. doi: 10.1016/j.cell.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Lang C, Levitan ES, Deitcher DL. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J Neurobiol. 2001;49:159–172. doi: 10.1002/neu.1072. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–179. doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science (80-) 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Schoofs A, Hückesfeld S, Schlegel P, Miroschnikow A, Peters M, Zeymer M, Spieβ R, Chiang A-S, Pankratz MJ. Selection of motor programs for suppressing food intake and inducing locomotion in the Drosophila brain. PLoS Biol. 2014;12:e1001893. doi: 10.1371/journal.pbio.1001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowski MZ, Blake AD. Function and expression of somatostatin receptors of the endocrine pancreas. Mol Cell Endocrinol. 2008;286:169–179. doi: 10.1016/j.mce.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Wang Y, Park S, Bajpayee NS, Vi D, Nagaoka Y, Birnbaumer L, Jiang M. Go2 G protein mediates galanin inhibitory effects on insulin release from pancreatic β cells. Proc Natl Acad Sci U S A. 2012;109:2636–2641. doi: 10.1073/pnas.1200100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennessen JM, Barry WE, Cox J, Thummel CS. Methods for studying metabolism in Drosophila. Methods. 2014 doi: 10.1016/j.ymeth.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Eisentraut A, Madison L, Upon S, Levels THE. The effects of total starvation upon the levels of circulating glucagon and insulin in man. J Clin Invest. 1963;42:1031–1039. doi: 10.1172/JCI104788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Leibowitz KL. Central insulin inhibits hypothalamic galanin and neuropeptide Y gene expression and peptide release in intact rats. Brain Res. 1997;777:231–236. doi: 10.1016/s0006-8993(97)00963-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Park S, Bajpayee NS, Nagaoka Y, Boulay G, Birnbaumer L, Jiang M. Augmented glucose-induced insulin release in mice lacking G(o2), but not G(o1) or G(i) proteins. Proc Natl Acad Sci U S A. 2011;108:1693–1698. doi: 10.1073/pnas.1018903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinke I, Schutz CS, Katzenberger JD, Bauer M, Pankratz MJ. Nutrient control of gene expression in Drosophila: microarray analysis of starvation and sugar-dependent response. EMBO J. 2002;21:6162–6173. doi: 10.1093/emboj/cdf600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.