Abstract
Background
Emergent studies show that similar to other substances of abuse, cue-reactivity to cannabis is also associated with neural response in the brain’s reward pathway (Filbey et al., 2009). However, the inter-relatedness of brain regions during cue-reactivity in cannabis users remains unknown.
Methods
In this study, we conducted a series of investigations to determine functional connectivity during cue-reactivity in 71 cannabis users. First, we used psychophysiological interaction (PPI) analysis to examine coherent neural response to cannabis cues. Second, we evaluated whether these patterns of network functional connectivity differentiated dependent and non-dependent users. Finally, as an exploratory analysis, we determined the directionality of these connections via Granger connectivity analyses.
Results
PPI analyses showed reward network functional connectivity with the nucleus accumbens (NAc) seed region during cue exposure. Between-group contrasts found differential effects of dependence status. Dependent users (N = 31) had greater functional connectivity with amygdala and anterior cingulate gyrus (ACG) seeds while the non-dependent users (N = 24) had greater functional connectivity with the NAc, orbitofrontal cortex (OFC) and hippocampus seeds. Granger analyses showed that hippocampal and ACG activation preceded neural response in reward areas.
Conclusions
Both PPI and Granger analyses demonstrated strong functional coherence in reward regions during exposure to cannabis cues in current cannabis users. Functional connectivity (but not regional activation) in the reward network differentiated dependent from non-dependent cannabis users. Our findings suggest that repeated cannabis exposure causes observable changes in functional connectivity in the reward network and should be considered in intervention strategies.
Keywords: Craving, Ventral striatum, Marijuana, PPI, Granger, Cue-reactivity
1. Introduction
The mesocorticolimbic reward system is important in evaluating salient and rewarding stimuli and regulating appetitive behavior, and, as such, is an intense area of focus in studies of substance use disorders (SUDs). The reward system has primary dopaminergic projections from the ventral tegmental area (VTA) that innervate limbic (amygdala, hippocampus), dorsal and ventral striatum and prefrontal regions (orbitofrontal cortex, anterior cingulate gyrus; O’Connell and Hofmann, 2011). Functional imaging studies combined with cue exposure paradigms have provided strong evidence for the role of the reward system during craving (Hommer, 1999; Volkow et al., 2002), one of the primary behavioral symptoms of SUDs. Enhanced response in the reward system during cue-elicited craving has been reported in the common substances of abuse such as alcohol (Filbey et al., 2007), nicotine (Claus et al., 2013), and cocaine (Wilcox et al., 2011). To date, two studies have reported concordant findings of enhanced neural response to cues for the most widely used illicit drug in the world – cannabis – as in other drugs of abuse. For example, in response to tactile and visual cannabis cues (relative to neutral cues), Filbey et al. (2009) reported greater neural response in the ventral tegmental area (VTA), thalamus, anterior cingulate gyrus (ACG), insula, and amygdala in heavy cannabis users (Filbey et al., 2012). Relative to non-using controls, Cousijn et al. (2012) showed that in response to images of cannabis cues, cannabis users had greater neural response in the VTA. Among the cannabis users, those with higher cannabis related problems had greater activity in the orbitofrontal cortex (OFC), ACG and striatum compared to those with fewer cannabis-related problems, which also overlaps with findings from Filbey et al. (2009). Taken together, the existing literature suggests that alterations in reward system function underlie response to cannabis cues and may drive drug-seeking behavior in cannabis users (Cousijn et al., 2012; Filbey and DeWitt, 2012; Filbey et al., 2009). Notably, response in these areas was found to be associated with pathology related to cannabis use (e.g., craving, problem severity) but not with measures of cannabis use.
A framework proposed by Filbey et al. (2012) suggests that cannabis cues trigger activation in several areas including (1) the ACG that detects salience of the cue, (2) the amygdala that evaluates the emotional content, (3) the insula that engages interoceptive processes, and, (4) the hippocampus that incorporates memory information. These events trigger dopamine release from the VTA to the striatum and OFC, which is necessary for the encoding of learned association of the drug with its relevant cues. While this model proposes associations (and directionality) between these regions, how these regions are functionally organized has not yet been directly examined. Moreover, existing regional activation findings through traditional general linear modeling (GLM)-based analyses may fail to completely characterize dysfunction in the reward system.
In other SUDs, altered functional connectivity has been reported in the reward system. For example, tobacco smokers (Claus et al., 2013) have been shown to have greater functional connectivity with two seed regions (OFC, insula) across several areas (somatosensory areas and parietal lobe, striatum) during tobacco cues compared to food cues. Notably, this connectivity was positively correlated with severity of nicotine dependence. Similarly enhanced functional connectivity between OFC and striatum was also reported in other populations characterized with heightened reward-sensitivity or increased ability to derive pleasure from rein-forcers, such as in obese individuals (Stoeckel et al., 2009). These findings suggest that the interaction between reward regions may be accountable for the increased reward salience/motivation in substance abusing individuals. Inversely, in a study that looked at the opposing process of hypo-responsivity to reward, namely, anhedonia or the inability to experience pleasure, it was found that attenuated functional connectivity within reward areas (NAc, paralimbic areas) was associated with trait anhedonia (Keller et al., 2013). In summary, sensitivity to rewards appears to be associated with greater functional coherence or integration of the reward network. To date, however, functional connectivity in the reward network in cannabis users has yet to be determined.
In this report, we expand the growing literature on reward network functioning in cannabis users by examining functional connectivity or the temporal correlation of activity within this network in response to cannabis cues. To that end, we carried out three series of analyses: (1) psychophysiological interaction (PPI) analysis to examine functional connectivity, (2) t-tests to determine functional connectivity differences between severity of cannabis use disorder (CUD), and, (3) exploratory Granger connectivity analyses to evaluate the effective functional connectivity or the influential relationship between reward network areas.
2. Methods
This study was approved by the University of New Mexico and University of Texas at Dallas Institutional Review Boards.
2.1. Participants
We scanned 99 regular cannabis users with some having been previously described (see Table 1; Filbey et al., 2009, 2010; Schacht et al., 2012). The study’s inclusion criteria were: (1) self-reported regular use of cannabis defined as cannabis use for a minimum of six months prior to study participation at a rate of at least four occasions per week (presence of THC metabolites was verified via urinalysis at study entry to confirm cannabis use), (2) right-handedness, and, (3) English as the primary language. The participants were excluded from the study if: (1) they had a positive urinalysis for other drugs of abuse other than cannabis at study entry, (2) reported current or history of psychosis, traumatic brain injury, or MRI contraindications (e.g., pregnancy, non-removal metallic implants, claustrophobia) and (3) had a current diagnosis of non-cannabis abuse/dependence (past diagnosis was acceptable). Of note, any other Axis I disorder besides psychosis was not an exclusion criterion. Further, there was no IQ requirement for inclusion in the study.
Table 1.
Demographic characteristics of cannabis dependent and non-dependent groups.
| Dependent Mean (SD) | Non-dependent Mean (SD) | Statistical test for dependent vs. non-dependent | |
|---|---|---|---|
| N | 37 | 34 | – |
| Age | 24.46 (6.91) | 24.47 (8.06) | t(69) = −0.006; p = .995 |
| % Males | 75.7% | 73.5% | χ2(1) = 0.043; p = .835 |
| Years of education | 13.77 (3.24) | 13.35 (2.23) | t((69) = 0.626; p = .533 |
| WASI verbal IQ | 54.35 (10.91) | 55.18 (9.45) | t(69) = −0.34; p = .735 |
| Freq. of cannabis use (# days use from past 90) | 80.81 (14.28) | 82.59 (14.77) | t(69) = −0.515; p = .608 |
| Cigarettes per day (N = 17, N = 16) | 6.26 (6.73) | 11.48 (6.96) | t(31) = −2.19; p = .036 |
| # drinking days/90 days | 20.76 (19.48) | 24.44 (28.22) | t(69) = −0.645; p = .521 |
| Alcohol drinks/occasion (N = 32, N = 28) | 4.91 (2.44) | 6.43 (10.70) | t(29.46*) = −0.735; p = .468 |
| Self-reported craving (N = 36, N = 29) | 1.86 (2.55) | 2.19 (2.50) | t(63) = −0.52; p = .605 |
| MPS (N = 31, N = 28) | 4.35 (5.05) | 1.86 (2.21) | t(41.94*) = 2.50; p = .016 |
| MCQ | 212.8 (133.1) | 142.1 (120.6) | t(69) = 2.34; p = .022 |
| MWC (N = 37, N = 34) | 6.19 (4.92) | 5.15 (3.56) | t((69) = 0.835; p = .406 |
| Duration of regular use (N = 36, N = 34) | 5.81 (5.82) | 7.59 (7.75) | t(68) = −1.09; p = .279 |
| Age of regular use onset (N = 36, N = 34) | 18.17 (3.62) | 17.04 (2.60) | t((68) = 1.48; p = .143 |
WASI = Wechsler Abbreviated Scale of Intelligence; MPS = Marijuana Problem Scale; MCQ = Marijuana Craving Questionnaire; MWC = Marijuana Withdrawal Checklist.
Corrected for inequality of variances.
Of the 99 participants who met our eligibility criteria, 28 had motion exceeding 3mm (in translation) or 3 degrees (in rotation) between TRs during the fMRI task (described below) and were subsequently excluded from further analyses, leaving a total of 71 participants.
2.2. Procedure
Those who met the study’s inclusion criteria were scheduled for two separate study visits. The first visit consisted of obtaining informed consent as well as completing behavioral measures. Recent use of marijuana and other substances was assessed with a Time Line Follow Back interview (TLFB; Sobell et al., 1979), drug use questionnaire, marijuana use questionnaire, smoking history questionnaire, cannabis history questionnaire, and the Marijuana Problem Survey (MPS; Stephens et al., 2002). Lifetime and current symptoms of drug dependence were assessed with the SCID for DSM IV Research Version (First et al., 1997).
The participants were then instructed to abstain from cannabis use until their second visit, which consisted of an MRI scan. This was scheduled ~72 h after the first visit. Similar to our previous studies (Filbey et al., 2009, 2010; Schacht et al., 2012), we followed a bogus pipeline whereby participants were informed that a urinalysis would be performed for verification of their compliance to the abstinence instructions. Although THC urinalysis for short abstinence periods is unreliable, use of this bogus pipeline has been shown to improve compliance (Roese and Jamieson, 1993). The participants were also instructed to refrain from alcohol for 24 h, and, from caffeine and cigarettes for the preceding 2 h prior to their scan. Compliance with these instructions was confirmed by self-report (cannabis, alcohol, caffeine and cigarettes) and by breath alcohol level of 0.000 (alcohol) at the start of their MRI session. Participants with positive self-reported cannabis, alcohol, caffeine and cigarette, and/or breath alcohol levels > 0.000 were excluded from the MRI session. Immediately prior to their scan, participants completed a Marijuana Craving Questionnaire (MCQ; Heishman et al., 2001) as well as the Marijuana Withdrawal Checklist (MWC; Budney and Moore, 2002; Budney et al., 2003).
MRI images were collected using a 3T Siemens Trio whole body scanner equipped with Sonata gradient subsystem (40 mT/m amplitude, 200 µs rise time, 100% duty cycle) with a 12-channel coil combined with body coil transmission to achieve greater sensitivity in cortical areas. A high resolution whole brain anatomical MRI scan was also collected with a T1-weighted multi-echo Magnetization Prepared Rapid Gradient Echo or MPRAGE (MEMPR) sequence with the following parameters: TR/TE/TI = 2300/2.74/900 ms, flip angle = 8°, 192 sagittal slices, FOV = 256 × 256 mm, Slab thickness = 176 mm, Matrix = 256 × 256 × 176, Voxel size = 1 × 1 × 1 mm, Number of echos = 4, Pixel bandwidth = 650 Hz. Whole brain fMRI scans were collected using a gradient echo, echoplanar (EPI) sequence with ramp sampling correction using the intercomissural line (AC-PC) as a reference (TR: 2.0 s, TE: 27ms (39ms for 1.5 T), : 70o, matrix size: 64 64, 32 slices, voxel size: 3 3 4 mm3) ventral to the surface of the OFC. A tilting acquisition previously described in Filbey et al. (2008) was applied in order to increase the signal-to-noise ratio in the OFC.
During the fMRI session, the participants underwent a cannabis cue-exposure task previously described by Filbey et al. (2009). During the task, participants were pseudorandomly presented 12 tactile cannabis cues and 12 neutral cues across 2 separate runs. We presented a marijuana pipe for the cannabis cue and a pencil for the neutral cue. Cue presentations were both visual and tactile whereby the participants held the cue objects in their left hand, which was visible in the mirror above their eyes. The task was presented using a front projection to a mirror system mounted on the head coil. Individual trials consisted of a 20s cue presentation, followed by a 5s urge-to-use rating on a 0–10 Likert scale and a 20s washout period. Responses were recorded using a fiber-optic pad. Stimulus presentations were delivered using E-Prime (Schneider et al., 2002). The timing of the stimulus presentation was synchronized with trigger pulses from the magnet in order to ensure precise temporal integration of stimulus presentation and fMRI data acquisition. The task lasted for 288 TRs across two runs for a total task time of 19min and 12 s.
2.3. fMRI analyses
Pre-processing
The functional imaging time series was preprocessed using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). Before starting analyses, the first seven volumes of each EPI run were discarded to allow the MR signal to reach steady state. Pre-processing of these volumes started with motion correction using SPM’s realignment module (Friston et al., 1995). This was followed by slice timing correction, which corrected for temporal differences in acquisition time of the BOLD signal across slices within each volume. The functional data were then normalized into the Montreal Neurological Institute (MNI) standard space using the template provided in SPM (Ashburner and Friston, 1999). The resultant time series was then smoothed using a 10 mm (FWHM) Gaussian kernel.
Functional connectivity analysis
To determine functional connectivity, we performed a whole brain psychophysiological interactions analysis (PPI; Friston et al., 1997; O’Reilly et al., 2012) in FSL using seed regions in seven areas of the reward network based on a review by Filbey and Dewitt (2012): (1) nucleus accumbens (NAc), (2) orbitofrontal cortex (OFC), (3) ventral tegmental area (VTA), (4) insula, (5) hippocampus, (6) amygdala, and (7) anterior cingulate gyrus (ACG). PPI finds areas whose activity is modulated by both task and the ongoing activity in a seed region (i.e., the interaction effect of the two). In this study, a physiological regressor was generated for each subject based on the mean activation timecourse of an anatomical mask defined for each seed ROI. Anatomical masks for the seed ROIs were defined according to the atlas of Nielsen and Hansen (2002) as used in Filbey et al. (2008), except for the OFC, which used Desikan et al. (2006) and ACG, which used a mask centered around 5, +19, +28 (grown to 8 mm in diameter) described in Margulies et al. (2007) (see ROI masks in Supplemental materials, Fig. S11). Task regressors for cannabis cue ON, neutral cue ON, cannabis cue RATING, and neutral cue RATING were convolved with a double gamma hemodynamic response function provided by FEAT. Psychophysiological interaction (PPI) regressors were defined by the interaction of the physiological regressor with the cannabis cue ON and neutral cue ON regressors. Regression analysis was performed in FEAT using the cannabis cue ON, neutral cue ON, cannabis cue RATING, and neutral cue RATING regressors, a physiological seed regressor, and PPI regressors for cannabis cue ON and neutral cue ON. The multiple regression analysis was run separately for each seed, and, the final alpha level was Bonferroni corrected for the 7 comparisons. Parameter estimates for the two interaction regressors were used to compute a contrast between the cannabis and neutral cue ON interactions with the seed, expressed as a t-statistic. For each seed, second level analyses using FLAME generated t-maps of the contrast between cannabis and neutral cues. The final statistical images were thresholded at z > 2.3 and cluster-corrected to p < 0.007 using Gaussian Random Field (GRF) theory-based maximum height thresholding (Worsley et al., 1996).
Between-group comparisons
Additional analyses were performed to compare differences in the PPI contrasts between the dependent (N = 37) and non-dependent (N = 34) groups of subjects. Because of the potential confounding effects of tobacco use on reward network activation, participants who smoked tobacco >10 cigarettes per day were removed from between-group comparisons, leaving N = 31 dependent and N = 24 non-dependent users. Between-group analyses generated t-maps of between-group differences in the modulation induced by the task (see O’Reilly et al., 2012) for a tutorial view of between-group PPI analysis). In addition to standard cluster-level corrections for multiple comparisons using GRF, the significance for second level analyses was also Bonferroni-corrected for the seven seeds (p < 0.007).
Effective connectivity analysis
Because PPI does not indicate the direction of modulation, only its presence, the direction of effect in the extended reward network was investigated using Granger causality analysis (Goebel et al., 2003; Roebroeck et al., 2005). The same seven anatomical seed ROIs defined for each subject in the PPI analysis were used in the Granger analysis (NAc, insula, hippocampus, amygdala, ACG, OFC, and, VTA). Mean time series were determined for each seed ROI and for each subject during the cannabis cue ON segments using FSL. We began with a fully connected model, where each ROI acted as both a source and a target region. For each pair of target and source ROIs, Granger analysis was performed in Matlab using the ARFit package (Neumaier and Schneider, 2001; Schneider and Neumaier, 2001). Granger causality between ROIs was expressed as a log ratio of reduction in residual variance, comparing the variance accounted for by fitting a degree 1 autoregressive (AR) model for the target ROI alone, to the variance accounted for by adding the source ROI. In addition to a better model fit, a larger log ratio suggests that activity in the source ROI precedes activity in the target ROI. The significance of the connections between ROIs was tested using a nonparametric bootstrap by resampling the timecourses of the individual cue trials with replacement (10,000 samples), generating a bootstrap distribution of effect sizes for each possible connection. A critical value of p < 0.01 was used to determine significance for within-group analysis. Between-group analysis was performed by testing for overlap between bootstrap-based 99% confidence intervals. Between-group significance values (p-values) were estimated by determining the minimum separating confidence interval between groups for each connection.
3. Results
The 71 participants who had minimal movement in the scanner (i.e., <3 degrees rotation and 3mm translation) and subsequently used in these analyses had a mean duration of marijuana use of 6.67 years (SD = 6.84), mean marijuana use occasions of 3.38 times per day (SD = 2.16) (based on the Cannabis History Questionnaire (CHQ)), and mean marijuana withdrawal checklist (MWC) score of 5.75 (SD = 4.68). Thirty seven users had a DSM-IV diagnosis of current cannabis dependence based on SCID-IV interview (First et al., 1997), leaving 34 participants in the non-dependent users group.
Regarding other drugs of abuse, there were no reports of daily use (one participant reported using crystal meth once a month, eight participants reported using cocaine once a month, one participant reported using cocaine once a week and one person reported using ecstasy once a month). Participants reported a mean frequency of alcohol use of 22.5 days of the past 90 days (SD = 23.96) and among those who drank, reported a mean of 5.6 drinks on a drinking day (SD = 7.49). Of the 33 participants who reported some tobacco use, the average number of cigarettes on smoking days was 8.79 (SD = 7.23).
Pre-scan craving ratings, as measured by MCQ, showed differences between the groups, t(69) = 2.34, p = 0.022 where dependent users had greater MCQ scores than the non-dependent users. During the fMRI task, urge ratings were higher following the cannabis cue trials compared to neutral cue trials, t(64) = 6.43, p < 0.001 (M = 2.01; 95% CI 1.38–2.63). Dependent and non-dependent groups, however, did not show differences in urge ratings during the fMRI task, t(63) = −0.52, p = 0.605. Of note, although withdrawal symptoms are a feature of substance dependence, the acute withdrawal symptoms reported after 3 days of abstinence did not differ between the two groups (p = ns). We speculate that problems related to cannabis use distinguished the groups more whereby the dependent group had greater self-reported problems as assessed by the MPS compared to the non-dependent group (t(41.94) = 2.50; p = 0.016) (Table 1).
3.1. Functional connectivity in response to cues: PPI results
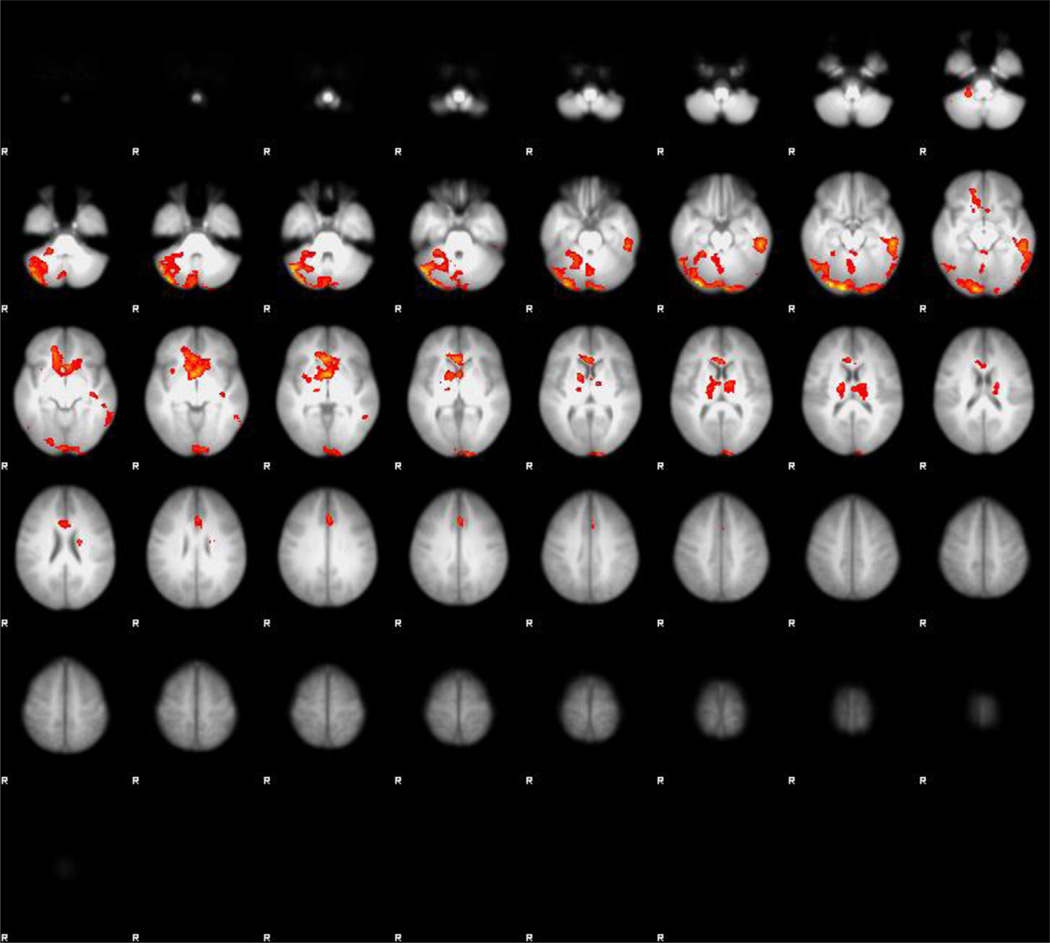
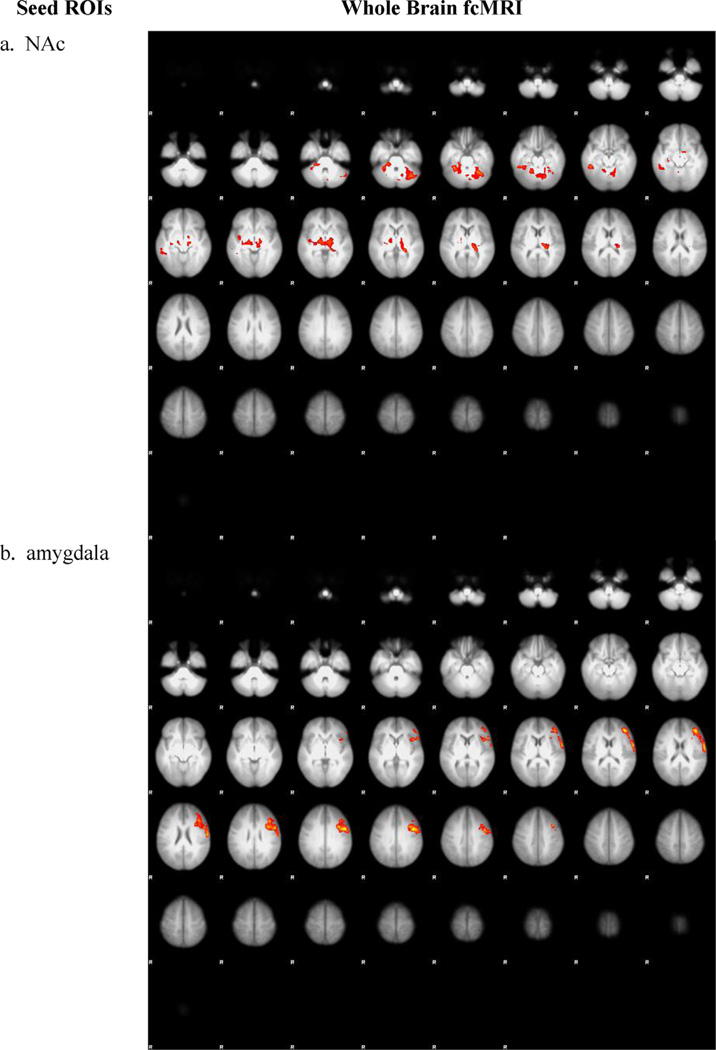
As expected, there was increased functional connectivity between NAc and areas within the reward network during exposure to cannabis cue ON vs. neutral cue ON. These clusters included the ACG, striatum, and the cerebellum (peaks are listed in Table 2, Fig. 1) (cluster-corrected p < 0.007, z = 2.3).
Table 2.
Loci of significant connectivity between nucleus accumbens (NAc) seed and other brain regions in all users (N = 71) during exposure to cannabis cue (vs. neutral cue) (cluster-corrected, z = 2.3, p < .007). Peak Z-scores are listed.
| 1. NAC | ||||||
|---|---|---|---|---|---|---|
| Cluster 1 | ||||||
| # voxels | Z | x | y | z | Localization | BA |
| 6238 | 4.37 | 16 | −90 | −16 | R cerebellum | – |
| 4.24 | 36 | −86 | −24 | R cerebellum | – | |
| 4.23 | 50 | −66 | −30 | R cerebellum | – | |
| 4.15 | 46 | −72 | −28 | R cerebellum | – | |
| 4.12 | 36 | −84 | −28 | R cerebellum | – | |
| Cluster 2 | ||||||
| # voxels | Z | x | y | z | Localization | BA |
| 3048 | 4.05 | −2 | 6 | 0 | L caudate head | – |
| 3.78 | 4 | 28 | −2 | R anterior cingulate | 24 | |
| 3.76 | 14 | 32 | −6 | R anterior cingulate | 24 | |
| 3.7 | 4 | 26 | 4 | R caudate head | – | |
| 3.48 | −2 | 18 | −6 | L anterior cingulate | 25 |
L = left, R = right, BA = Brodmann’s Area.
Fig. 1.
Whole brain functional connectivity between the nucleus accumbens (NAc) and other brain areas in response to cannabis cues (vs. neutral cues) in all participants (cluster-corrected z = 2.3, p < 007).
No functional connectivity differences were found with PPI between the cannabis cue ON and neutral cue ON conditions at the group level for the amygdala, insula, hippocampus, VTA, OFC, or ACG seeds.
3.2. Functional connectivity and severity of cannabis dependence
We examined how severity of cannabis use disorders (CUDs) modulated reward network functional connectivity by examining differences between dependent and non-dependent users. Because smoking rates differed between dependent and non-dependent users, smokers whose mean usage was at least 10 cigarettes per day (half a pack) were excluded from group comparisons. Although the individual group maps appear qualitatively different between the two groups (See Supplement, Fig. S22), direct contrasts between dependent (N= 31) and non-dependent users (N= 24) were not significant at the chosen threshold and significance level (n.s., i.e., z = 2.3).
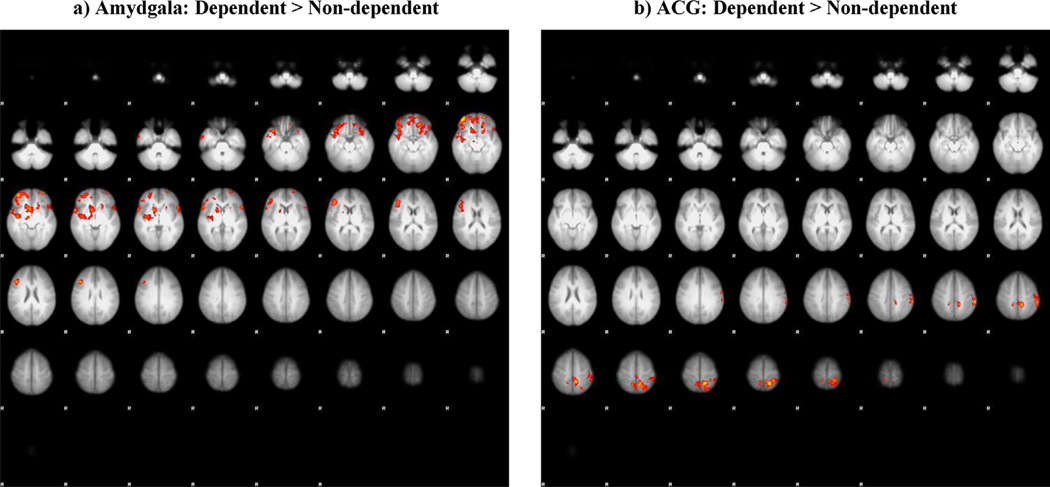
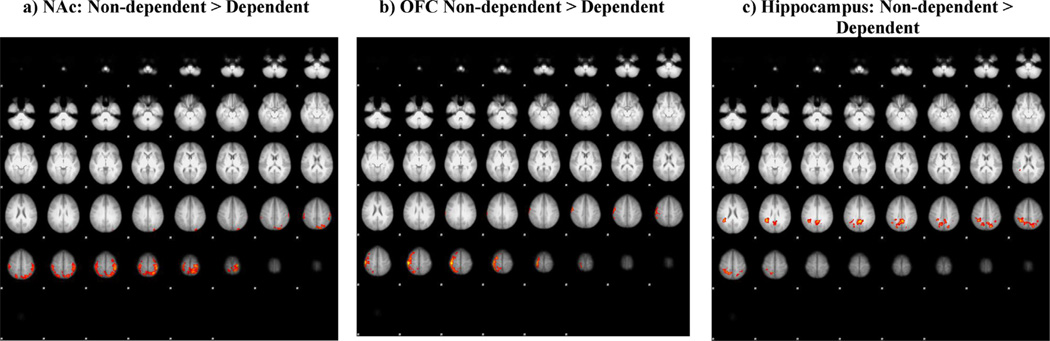
Because of these observed qualitative differences between groups, we examined whether these group differences would survive a less conservative height threshold of z = 1.96 but keeping the cluster and Bonferroni correction of p < 0.07. Interestingly, we found differential effects of CUD severity. The results showed greater functional connectivity for dependent cannabis users (vs. non-dependent cannabis users) during cannabis cue ON (vs. neutral cue ON) between the amygdala seed and middle and inferior frontal gyri as well as superior temporal gryus (cluster-corrected p < 0.007, z = 1.96), while the ACG seed showed greater connectivity to superior and inferior parietal cortex, precuneus and postcentral gyrus (cluster-corrected p < 0.007, z = 1.96) (see Fig. 2, Table 4). Non-dependent cannabis users had greater functional connectivity during cannabis cue ON (vs. neutral cue ON) compared to dependent cannabis users between the NAc seed and postcentral gyrus, superior parietal, and inferior parietal (cluster-corrected p < 0.007, z = 1.96), between the OFC seed and pre/postcentral gyri and superior frontal gyrus (cluster-corrected p < 0.007, z = 1.96), and between the hippocampus seed and the precuneus (cluster-corrected p < 0.007, z = 1.96) (see Fig. 3, Table 4). No group difference was found for the VTA or insula seeds at this lower threshold.
Fig. 2.
Connectivity differences between participants who did and did not meet SCID IV cannabis dependence criteria for the cannabis cue ON > neutral cue ON contrast psychophysiological interaction (PPI) analysis for the a) amygdala and b) anterior cingulate gyrus (ACG) seeds (cluster-corrected, z = 1.96, p < 0.007).
Table 4.
Loci of significant contrast between participants who did and did not meet SCID IV cannabis dependence criteria for the cannabis cue ON > neutral cue ON contrast psychophysiological interaction (PPI) for the (a) amygdala, (b) anterior cingulate gyrus (ACG), (c) nucleus accumbens (NAc), (d) orbitofrontal cortex (OFC), and (e) hippocampus seeds (cluster-corrected, z = 1.96, p < .007). Peak Z-scores are listed.
| (a) Amygdala: cannabis dependent > cannabis non-dependent | ||||||
| # voxels | Z | x | y | z | Localization | BA |
| 5926 | 3.40 | 38 | 56 | −12 | R middle frontal gyrus | 10 |
| 3.39 | 44 | 52 | −12 | R middle frontal gyrus | 10 | |
| 3.31 | 44 | 24 | 26 | R middle frontal gyrus | 9 | |
| 3.07 | 60 | 12 | −2 | R superior temporal gyrus | 22 | |
| 3.04 | −58 | 20 | 0 | L inferior frontal gyrus | 45 | |
| 3.01 | 40 | 20 | −22 | R inferior frontal gyrus | 47 | |
| (b) Anterior cingulate: cannabis dependent > cannabis non-dependent | ||||||
| # voxels | Z | x | y | z | Localization | BA |
| 2425 | 3.2 | −18 | −50 | 68 | L superior parietal | 7 |
| 3.12 | −8 | −44 | 56 | L precuneus | 7 | |
| 3.03 | −14 | −48 | 64 | L superior parietal | 7 | |
| 2.92 | −62 | −32 | 46 | L inferior parietal | 40 | |
| 2.88 | 16 | −38 | 74 | R postcentral gyrus | 5 | |
| 2.86 | −28 | −38 | 66 | L postcentral gyrus | 5 | |
| (c) NAc: cannabis non-dependent > cannabis dependent | ||||||
| # voxels | Z | X | y | z | Localization | BA |
| 4789 | 3.48 | −22 | −24 | 74 | L postcentral gyrus | 3 |
| 3.31 | −38 | −36 | 62 | L inferior parietal | 40 | |
| 3.3 | −40 | −36 | 66 | L postcentral gyrus | 2 | |
| 3.16 | −24 | −54 | 70 | L superior parietal | 7 | |
| 3.1 | −8 | −46 | 74 | L postcentral gyrus | 7 | |
| 3.09 | −12 | −46 | 74 | L postcentral gyrus | 7 | |
| (d) OFC: cannabis non-dependent > cannabis dependent | ||||||
| # voxels | Z | x | y | z | Localization | BA |
| 2590 | 3.41 | 42 | −28 | 64 | R postcentral gyrus | 3 |
| 3.31 | 46 | −24 | 60 | R postcentral gyrus | 3 | |
| 3.05 | 54 | −24 | 58 | R postcentral gyrus | 2 | |
| 3.01 | 40 | −18 | 66 | R precentral gyrus | 4 | |
| 2.99 | 24 | −6 | 70 | R superior frontal gyrus | 6 | |
| 2.98 | 42 | −36 | 66 | R postcentral gyrus | 5 | |
| (e) Hippocampus: cannabis non-dependent > cannabis dependent | ||||||
| # voxels | Z | x | y | z | Localization | BA |
| 3228 | 3.27 | −2 | −48 | 36 | L precuneus | 31 |
| 3.08 | 34 | −46 | 50 | R precuneus | 7 | |
| 3.02 | −20 | −70 | 48 | L precuneus | 7 | |
| 2.81 | 26 | −46 | 52 | R precuneus | 7 | |
| 2.79 | 14 | −54 | 58 | R precuneus | 7 | |
L = left, R = right, BA = Brodmann’s Area.
Fig. 3.
Connectivity differences between participants who did and did not meet SCID IV cannabis dependence criteria for the cannabis cue ON > neutral cue ON contrast psychophysiological interaction (PPI) analysis for the (a) nucleus accumbens (NAc), (b) orbitofrontal cortex (OFC), and (c) hippocampus seeds (cluster-corrected, z = 1.96, p < 0.007).
3.3. Directionality of functional connectivity in response to cues: Granger causality results
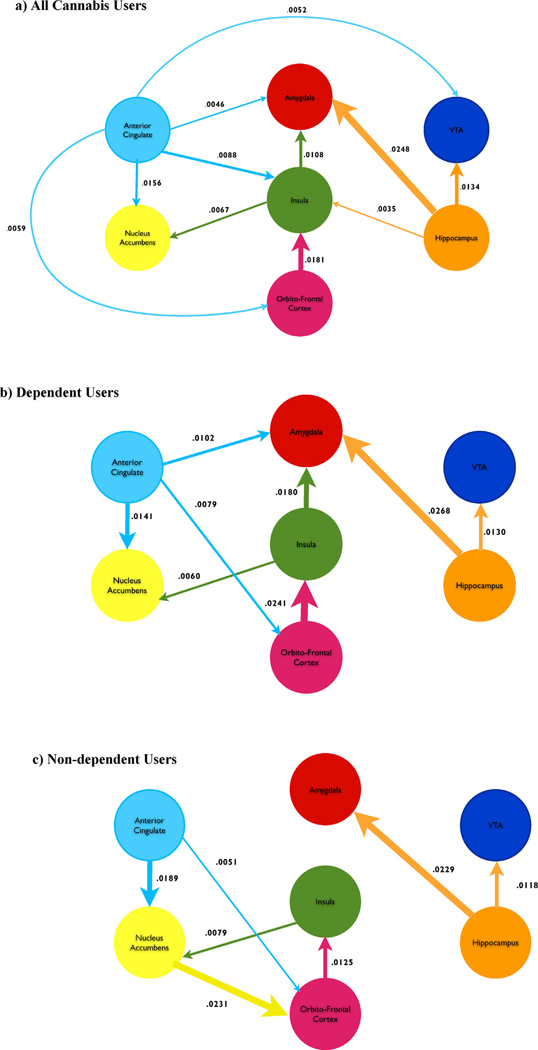
Granger causality analysis suggested a putative organization of the network of areas involved during exposure to the cannabis cue (cannabis cue ON). A strong connection from hippocampus-to-amygdala was the most reliable projection, measured as a log ratio reduction in error 0.0248 (99% CI 0.0132–0.0426). The amygdala, NAc and VTA appear to be receptive (mostly preceded), whereas hippocampus and ACG appear to be projective (mostly preceding). The remaining areas of the OFC, and insula had a mix of incoming and outgoing connections. The insula and ACG were the areas with the largest number of significant connections (highest degree).
The differences in Granger causality between the dependent and non-dependent groups as measured by reduction in variance did not reach significance when comparing bootstrap confidence limits. However, there were qualitative differences. For example, in the non-dependent group, a connection was present between NAc and OFC, 0.0251 (99% CI 0.0013–0.0603), and absent in the dependent group, 0.0065 (99% CI 0–0.0122); the difference was nearly significant with p = 0.06, inferred from a minimally separating CI. The non-dependent group did not show significant precedence from ACG-to-amygdala (dependent: 0.0242 [99% CI 0.0005–0.031], non-dependent: 0.0006 [99% CI 0–0.0084]), hippocampus-to-insula (dependent: 0.0156 [99% CI 0.0001–0.0381], non-dependent: 0.0006 [99% CI 0–0.0093]) and insula-to-amygdala (dependent: 0.0321 [99% CI 0.0029–0.0393], non-dependent: 0.0066 [99% CI 0–0.0291]). These differences approached significance for the ACG-to-amygdala connection (p = 0.11), but not for hippocampus-to-insula (p = 0.25) or insula-to-amygdala (p = 0.24). These qualitative differences suggest greater NAc connectivity in the non-dependent group with less ACG connectivity and greater ACG, insula, and amygdala connectivity in the dependent group (see Fig. 5).
Fig. 5.
Granger error reduction (log ratio of residual improvement) for: (a) all cannabis users, (b) dependent users, and, (c) non-dependent users. Only connections whose bootstrap confidence intervals exceeded threshold at p < 0.01 are included. Color and arrow indicate the temporal precedence of the connection and the size is proportional to the magnitude of error reduction (strength of connection).
3.4. Post-hoc analyses
To further examine the clinical significance of network connectivity during exposure to cannabis cues, we correlated PPI indexes during response to cannabis cue ON vs. neutral cue ON with MPS and MCQ in the entire sample (N = 71). These analyses showed that functional connectivity positively correlated with subjective craving. Specifically, the greater the total score from the MCQ, the greater the functional connectivity between the NAc and clusters encompassing the thalamus, pulvinar, and cerebellum (peaks listed in Table 3, Fig. 4a) (cluster-corrected p < 0.007, z = 2.3). In addition, greater MCQ scores were associated with greater functional connectivity between amygdala and left precentral and middle frontal gyri (cluster-corrected p < 0.007, z = 2.3; see Table 3, Fig. 4b). No other correlations were found between functional connectivity and behavioral measures.
Table 3.
Loci of significant positive correlation between (a) nucleus accumbens (NAc) and (b) amygdala seed connectivity and marijuana craving questionnaire total scores (cluster-corrected, z = 2.3, p < .007). Peak Z-scores are listed.
| (a) NAc | ||||||
| Cluster 1 | ||||||
| # voxels | Z | x | y | z | Localization | BA |
| 1785 | 3.47 | 34 | −40 | −24 | R cerebellum (Culmen) | – |
| 3.44 | −40 | −64 | −28 | L cerebellum (Tuber) | – | |
| 3.42 | 48 | −38 | −16 | R fusiform gyrus | 20 | |
| 3.31 | 22 | −32 | −30 | R cerebellum (Anterior, not labeled) | – | |
| 3.21 | 26 | −34 | −30 | R cerebellum (Culmen) | – | |
| Cluster 2 | ||||||
| # voxels | Z | x | y | z | Localization | BA |
| 1333 | 3.24 | −16 | −14 | −4 | L thalamus | – |
| 3.23 | −18 | −34 | 2 | L thalamus: pulvinar | – | |
| 3.19 | 16 | −12 | −2 | R thalamus | – | |
| 3.16 | 8 | −18 | −2 | R thalamus | – | |
| 3.16 | −22 | −28 | 12 | L thalamus: pulvinar | – | |
| (b) Amygdala | ||||||
| # voxels | Z | x | y | z | Localization | BA |
| 2752 | 3.61 | −44 | 4 | 36 | L precentral gyrus | 6 |
| 3.61 | −40 | 40 | 18 | L middle frontal gyrus | 46 | |
| 3.4 | −62 | −2 | 20 | L precentral gyrus | 4 | |
| 3.39 | −40 | 24 | 26 | L middle frontal gyrus | 9 | |
| 3.32 | −32 | 10 | 32 | L precentral gyrus | 6 | |
L = left, R = right, BA = Brodmann’s Area.
Fig. 4.
Correlation between subjective craving scores (MCQ) and cannabis cue ON > neutral cue ON contrast PPI for the (a) nucleus accumbens (NAc) seed and (b) amygdala seed (cluster-corrected, z = 2.3, p < 0.007). ROIs = regions of interest, fcMRI = functional connectivity MRI.
Typical of substance abusing populations, our sample included participants with other drug use co-morbidities. This effect was especially large for nicotine, where participants who did and did not meet criteria for cannabis dependence reported some smoking in similar numbers (roughly 40%), but the smokers in the dependent group on average reported a greater volume of usage. We eliminated potential confounds by excluding heavy smokers from both groups in the group comparisons (see Section 2). However, their inclusion in the overall sample may have influenced the strength of the reported finding. Thus, in order to determine the contributing effects of other drugs, we also performed a post-hoc analysis whereby participants who reported other drug use, including alcohol and tobacco, were excluded (N = 28 excluded, remainingN = 43). Significant connectivity remained for the NAc and ACG seeds (see Supplement Fig. S33).
4. Discussion
These results demonstrate widespread altered integration of distributed nodes that mediate various processes related to drug-seeking behavior in cannabis users. Similarly enhanced reward network coherence has been reported in other substance abusing populations such as nicotine users (Claus et al., 2013), alcohol users (Filbey et al., 2007), cocaine users (Wilcox et al., 2011), and obese individuals (Stoeckel et al., 2009). Moreover, increased connectivity in inhibitory control networks has been reported in cannabis users during successful response inhibition (via Stop Signal Task; Filbey and Yezhuvath, 2013). The current findings suggest that greater integration of regions within the reward network may reflect a greater bias towards reward salience (NAc), emotion (amygdala), and, internal bodily sensations (insula) during exposure to cannabis cues.
Notably, in other substances of abuse, such as cocaine, variability in the direction of alteration in functional connectivity (e.g., during resting state) has been noted, which have been attributed to the region of effect (Cisler et al., 2013). For example, while greater PFC-insula connectivity was suggested to lead to heightened awareness of internal bodily states that may then disrupt decision making, decreased anterior cingulate-insula functional connectivity may reduce one’s ability to divert attention from drug cues. Similarly differential patterns of connectivity were observed to be associated with severity of cannabis use in the current study. Specifically, PPI showed that dependent users had greater coupling between both amygdala and ACG seeds and other areas compared to the non-dependent users. Both the amygdala and ACG play important roles in affective responsiveness (Kapina et al., 2010). Some postulate that these regions form a network involved in detecting and discriminating subconscious and emotionally-laden stimuli (Lassen et al., 2007). Thus, the current findings of greater integration between the amygdala and inferior frontal gyrus, as well as ACG and parietal lobe during cue exposure suggest that cannabis cues trigger an enhanced detection and discrimination of the emotional content of the cues in dependent cannabis users relative to non-dependent users.
On the other hand, the non-dependent users had greater coherence in areas underlying hedonic and motivational aspects of reward (both NAc, OFC) compared to dependent users. Because of the role of this circuitry in reinforcement learning, we speculate that greater integration of NAc-parietal lobe and OFC-superior frontal gyrus in the non-dependent users may reflect enhanced conditioned response to cannabis cues due to greater self-awareness. Non-dependent users also showed greater coherence with the hippocampus seed compared to the dependent users. The hippocampus provides memory information particularly related to learned contextual stimuli. Because studies have shown that blockage of the hippocampus results in the extinction of drug seeking by contextual stimuli, greater integration of this region with the precuneus in response to cannabis cues, suggests greater influence of contextual memory and self-awareness in non-dependent users compared to dependent users. Overall, this differential response in connectivity patterns across cannabis severity suggests that nuanced processes are associated with different stages of cannabis use disorder. Specifically, based on these findings, those who are non-dependent have greater integration of reward-motivation and memory processes with self-awareness in response to cues, whereas those who are dependent on cannabis have greater reward network response related to the emotional content/processing of the cues.
We also found that the degree of connectivity between NAc-pulvinar as well as amygdala-IFG/PFC was positively associated with self-reported craving for cannabis. As our exploratory Granger connectivity suggests that both NAc and amygdala are mostly receptive, it is possible that the greater the visual attention to the cues (pulvinar) and discrimination of the cues (IFG/PFC) that may be influencing the emotional and incentive salience of the cues, the greater the subjective craving for cannabis. This positive association between functional connectivity in response to cues and self-reported craving (via MCQ) suggest that these effects in connectivity can indeed be attributed to increases in craving. In short, the connectivity patterns within the reward network may reflect the consequences of craving as a result of exposure to cannabis cues. This is consistent with a recent report in treatment seeking cannabis users, therefore, providing strong support for a link between reward network response to cannabis cues and craving (Goldman et al., 2013; Wetherill et al., 2014).
Finally, our exploratory analysis using Granger connectivity showed no quantitative group difference in terms of directionality of functional connectivity, which may be due to limitations of the method. However, some of the sub-threshold differences in patterns of effective connectivity are worth discussing. For instance, the broad effective connectivity from the ACG to other areas of the network suggests a modulatory role of the ACG on the reward network in response to cues. The effective connectivity from the hippocampus to amygdala suggests the guiding role of memory in determining emotional valence. Consistent with the observed group differences observed in PPI, the amygdala shows a more diverse pattern of connectivity in the dependent users than in the non-dependent users, including connections from the insula and ACG.
Are these alterations pre-existing? Studies in those who are at risk have suggested that these altered network connectivity may predate the clinical presentations of SUDs (Houck et al., 2013). For example, Weiland et al. (2013) found greater NAc connectivity with paracentral lobule/precuneus and sensorimotor areas in youth with a family history of alcohol use disorders during incentive anticipation, which correlated positively with sensation-seeking. Thus, differences in NAc connectivity with attention/motor/default networks, rather than control systems, may influence the reward system’s role in vulnerability for substance abuse. Moreover, Granger analyses showed that hippocampal and ACG activation preceded neural response in the reward neurocircuitry. These findings support the notion that dependence to cannabis is associated with greater integration within the reward network underlies dependence to cannabis.
4.1. Limitations
Some consideration must be taken in the interpretation of these findings. First, this study characterized the connectivity patterns in the cue reactivity task without respect to a group of non-using controls. We acknowledge that a comparison against a non-using group could provide an indicator of how cannabis may alter function in an otherwise healthy, non-cannabis affected reward network. However, based on the literature, we expect that this difference may be minimal. For example, Cousijn et al. (2012) found regional activation differences only in the VTA between cannabis users and non-using controls. Differences in reward area response were more pronounced between different cannabis users broken down by severity of use. Comparisons between using and non-using groups bring unique challenges. For instance, differences between a control group and the substance using group are likely to be very difficult to interpret, given that the task has a substantially different craving component for the controls (i.e., the cannabis cue is unlikely to produce craving in non-cannabis using subjects). Future studies should consider more effective approaches to disentangle these issues.
4.2. Conclusions
Building upon growing evidence of changes in regional activation in brain reward areas in cannabis users, we sought to characterize changes in the functional organization of the reward circuitry in cannabis users. We found that the reward circuitry is altered in cannabis users such that there is greater integration within the network. Using Granger analysis, we also noted that the response in key areas within the reward network is driven by ‘auxiliary’ areas involved in memory (hippocampus) and attention (ACG). More importantly, while no regional activation difference was found between the two groups, the degree of functional connectivity with the reward network regions distinguished dependent from non-dependent cannabis users, albeit at a lower threshold. These findings suggest a differential effect of CUD severity on degree of functional connectivity within the reward network. The importance of this finding is in demonstrating a neurobiological basis for the progression of or the risk for CUDs that can inform growing studies of functional connectivity changes as a result of treatment (e.g., methylphenidate; Janes et al., 2010; Ramaekers et al., 2013) and morbidity for substance dependence (i.e., family history; Weiland et al., 2013).
4.3. Future directions
The whole brain PPI and ROI-based Granger analysis allowed us to explore the patterns of connectivity in the reward network that participate in cue-reactivity in cannabis abuse and dependence. These findings would need to be replicated by direct tests of changes in the network with increased power. Subsequent analyses may make use of Dynamic Causal Modeling or Multivariate Granger analyses to directly test competing models.
Supplementary Material
Acknowledgments
Role of funding source
Funding for this study was provided by NIDA Grant K01 DA021632.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
FF designed the study and wrote the protocol. FF and JD managed the literature searches and summaries of previous related work. JD undertook the statistical analysis, and FF wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest statement
The authors do not have any conflict of interest to disclose.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2014.04.002.
References
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum. Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Moore BA. Development and consequences of cannabis dependence. J. Clin. Pharmacol. 2002;42:28S–33S. doi: 10.1002/j.1552-4604.2002.tb06000.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J. Abnorm. Psychol. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, Andrew James G, Kilts CD. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res. 2013;213:39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38:2363–2372. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Neural responses associated with cue-reactivity in frequent cannabis users. Addict. Biol. 2012;18:570–580. doi: 10.1111/j.1369-1600.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Filbey F, Yezhuvath U. Functional connectivity in inhibitory control networks and severity of cannabis use disorder. Am. J. Drug Alcohol Abuse. 2013;39:382–391. doi: 10.3109/00952990.2013.841710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2007;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus ED, Morgan M, Forester GR, Hutchison K. Dopaminergic genes modulate response inhibition in alcohol abusing adults. Addict. Biol. 2012;17:1046–1056. doi: 10.1111/j.1369-1600.2011.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, DeWitt SJ. Cannabis cue-elicited craving and the reward neurocircuitry. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;38:30–35. doi: 10.1016/j.pnpbp.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol Clin. Exp. Res. 2008;32:1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht J, Myers U, Chavez R, Hutchison K. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc. Nat. Acad. Sci. U.S.A. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams JBW. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders—SCID. Washington, D.C.: American Psychiatric Press; 1997. [Google Scholar]
- Friston K, Ashburner J, Frith CD, Poline JP, Heather JD, Frackowiak RS. Spatial registration and normalization of images. Hum. Brain Mapp. 1995;2:165–189. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Goebel R, Roebroeck A, Kim DS, Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn. Reson. Imaging. 2003;21:1251–1261. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Goldman M, Szucs-Reed RP, Jagannathan K, Ehrman RN, Wang Z, Li Y, Suh JJ, Kampman K, O’Brien CP, Childress AR, Franklin TR. Reward-related brain response and craving correlates of marijuana cue exposure: a preliminary study in treatment-seeking marijuana dependent subjects. J. Addict. Med. 2013;7:8–16. doi: 10.1097/ADM.0b013e318273863a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Hommer DW. Functional imaging of craving. Alcohol Res. Health. 1999;23:187–196. [PMC free article] [PubMed] [Google Scholar]
- Houck JM, Bryan AD, Feldstein Ewing SW. Functional connectivity and cannabis use in high-risk adolescents. Am. J. Drug Alcohol Abuse. 2013;39:414–423. doi: 10.3109/00952990.2013.837914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol. Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapina V, Vargas MI, Vulliemoz S, Landis T, Picard F, Lalive PH. VGKC antibody-associated encephalitis, microbleeds and progressive brain atrophy. J. Neurol. 2010;257:466–468. doi: 10.1007/s00415-009-5370-5. [DOI] [PubMed] [Google Scholar]
- Keller J, Young CB, Kelley E, Prater K, Levitin DJ, Menon V. Trait anhedonia is associated with reduced reactivity and connectivity of mesolimbic and paralimbic reward pathways. J. Psychiatr. Res. 2013;47:1319–1328. doi: 10.1016/j.jpsychires.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Lassen MB, Brown JE, Stobbs SH, Gunderson SH, Maes L, Valenzuela CF, Ray AP, Henriksen SJ, Steffensen SC. Brain stimulation reward is integrated by a network of electrically coupled GABA neurons. Brain Res. 2007;1156:46–58. doi: 10.1016/j.brainres.2007.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Neumaier A, Schneider T. Estimation of parameters and eigenmodes of multivariate autoregressive models. ACM Trans. Math. Softw. 2001;27:27–57. [Google Scholar]
- Nielsen FA, Hansen LK. Automatic anatomical labeling of Talairach coordinates and generation of volumes of interest via the BrainMap database. Eighth International Conferences on Functional Mapping of the Human Brain; Senai, Japan. 2002. [Google Scholar]
- O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Soc. Cogn. Affect. Neurosci. 2012;7:604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Evers EA, Theunissen EL, Kuypers KP, Goulas A, Stiers P. Methylphenidate reduces functional connectivity of nucleus accumbens in brain reward circuit. Psychopharmacology (Berlin) 2013;229:219–226. doi: 10.1007/s00213-013-3105-x. [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. NeuroImage. 2005;25:230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Roese NJ, Jamieson DW. Twenty years of bogus pipeline research: a critical review and meta-analysis. Psychol. Bull. 1993;114:363–375. [Google Scholar]
- Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology. 2012;37:2368–2376. doi: 10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Neumaier A. Algorithm 808: ARfit—a matlab package for the estimation of parameters and eigenmodes of multivariate autoregressive models. ACM Trans. Math. Softw. 2001;27:58–65. [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User’s Guide. Pittsburgh, PA: Psychology Software Tools, Inc.; 2002. [Google Scholar]
- Sobell MB, Sobell LC, VanderSpek R. Relationships among clinical judgment, self-report, and breath-analysis measures of intoxication in alcoholics. J. Consult. Clin. Psychol. 1979;47:204–206. doi: 10.1037//0022-006x.47.1.204. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Babor TF, Kadden R, Miller M. The Marijuana Treatment Project: rationale, design and participant characteristics. Addiction. 2002;97(Suppl. 1):109–124. doi: 10.1046/j.1360-0443.97.s01.6.x. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, 3rd, Horwitz B. Effective connectivity of a reward network in obese women. Brain Res. Bull. 2009;79:388–395. doi: 10.1016/j.brainresbull.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav. Pharmacol. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Weiland BJ, Welsh RC, Yau WY, Zucker RA, Zubieta JK, Heitzeg MM. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend. 2013;128:130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Childress AR, Jagannathan K, Bender J, Young KA, Suh JJ, O’Brien CP, Franklin TR. Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology (Berlin) 2014;231:1397–1407. doi: 10.1007/s00213-013-3342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K, Marrett S, Neelin P, Vandal A, Friston K, Evans A. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.