Summary
Reducing the activity of the Insulin/IGF-1 Signaling pathway (IIS) modifies development, elevates stress resistance, protects from toxic protein aggregation (proteotoxicity) and extends lifespan of worms, flies and mice. In the nematode Caenorhabditis elegans (C. elegans), lifespan extension by IIS reduction is entirely dependent upon the activity of the transcription factors DAF-16 and the Heat Shock Factor-1 (HSF-1). While DAF-16 determines lifespan exclusively during early adulthood it is required for proteotoxicity protection also during late adulthood. In contrast, HSF-1 protects from proteotoxicity during larval development. Despite the critical requirement for HSF-1 for lifespan extension the temporal requirements for this transcription factor as a lifespan determinant are unknown. To establish the temporal requirements of HSF-1 for longevity assurance we conditionally knocked down hsf-1 during larval development and adulthood of C. elegans and found that unlike daf-16, hsf-1 is foremost required for lifespan determination during early larval development, required for a lesser extent during early adulthood and has small effect on longevity also during late adulthood. Our findings indicate that early developmental events affect lifespan and suggest that HSF-1 sets during development the conditions that enable DAF-16 to promote longevity during reproductive adulthood. This study proposes a novel link between HSF-1 and the longevity functions of the IIS.
Keywords: Aging, Insulin/IGF-1 signaling, DAF-16, Heat Shock Factor 1, Development
Introduction
The Insulin/IGF-1 Signaling pathway (IIS) is a key regulator of development, stress resistance, metabolism and longevity of worms (Kenyon et al, 1993), flies (Giannakou et al, 2007), mice (Bluher et al, 2003; Holzenberger et al, 2003; Selman et al, 2008; Taguchi et al, 2007) and presumably humans (Flachsbart et al, 2009; Suh et al, 2008; Willcox et al, 2008). In the nematode Caenorhabditis elegans (C. elegans) DAF-2, the sole insulin/IGF-1 receptor, initiates a cascade of events that promotes the phosphorylation of its downstream forkhead-like transcription factor, DAF-16 (Henderson & Johnson, 2001; Paradis & Ruvkun, 1998). Phosphorylated DAF-16 is prevented from entering the nucleus and from regulating the expression of its target gene networks (Lin et al, 2001). Thus, daf-2 knockdown by either mutation or RNA interference (RNAi) hyper-activates daf-16, creating long-lived, youthful and stress-resistant worms. These longevity and stress resistance effects of daf-2 knockdown are entirely dependent upon daf-16 (Lee et al, 2001; Ogg et al, 1997).
In C. elegans, the highly conserved (Liu et al, 1997), leucine zipper containing (Rabindran et al, 1993) transcription factor, Heat Shock Factor 1 (HSF-1) is an additional critical and specific player in the IIS longevity pathway. Accordingly, the knockdown of hsf-1 prevents IIS reduction from extending lifespan and hsf-1 over-expression extends lifespan in a daf-16 dependent manner (Hsu et al, 2003; Morley & Morimoto, 2004). Similarly to DAF-16, HSF-1 exhibits various biological functions in addition to lifespan regulation. It is critically required for heat stress response (Sarge et al, 1993), innate immunity (Singh & Aballay, 2006) and for proper development (Akerfelt et al, 2010; Walker et al, 2003).
In C. elegans, IIS reduction by daf-2 RNAi regulates lifespan solely during reproductive adulthood (days 1–6 of adulthood). Consistently, conditional daf-16 reduction during reproductive adulthood, but not larval development, has the reciprocal effect of shortening lifespan (Dillin et al, 2002a). Similarly, in the fruit fly Drosophila melanogaster, conditional gene expression of dFOXO (DAF-16 orthologue) during the reproductive phase of adulthood results in an increased longevity (Giannakou et al, 2007). Despite its critical roles in the IIS regulated longevity mechanism the temporal requirements for HSF-1 as a lifespan determinant are unknown.
IIS reduction provides protection from an additional aging-associated phenomenon, toxic protein aggregation (proteotoxicity) (David et al, 2010). This protection is entirely dependent upon both DAF-16 and HSF-1 (Cohen et al, 2006; Hsu et al, 2003; Morley & Morimoto, 2004; Zhang et al, 2011). In the case of the aggregation of the Alzheimer’s disease associated peptide Aβ, DAF-16 and HSF-1 were shown to promote opposing activities, DAF-16 mediates protective active aggregation while HSF-1 facilitates disaggregation (Cohen et al, 2006). The central role of HSF-1 in countering proteotoxicity was further established by the finding that it is critically needed for dietary restriction (DR) to promote protection from Aβ aggregation (Steinkraus et al, 2008). Recently we tested the timing requirements for daf-16 and hsf-1 in reduced IIS mediated protection from Aβ proteotoxicity and found that while daf-16 is required during both early and late adulthood but not during development, hsf-1 is predominantly required for protection from proteotoxicity during development (Cohen et al, 2010). These surprising findings raised the question of whether HSF-1 protects from proteotoxicity and mediates longevity during the same time window during the nematode lifecycle or whether it functions as a lifespan determinant concurrently with DAF-16. To address this issue we studied the temporal requirements for hsf-1 for longevity assurance using the nematode C. elegans and conditional RNAi knock down technique.
Results
We determined the natural expression levels of hsf-1 during the worm’s larval development, using two separate sets of specific hsf-1 primers and quantitative real-time PCR (qPCR). Our results indicate that hsf-1 expression steadily declines during development as hsf-1 mRNA transcripts were most abundant during embryonic development (in eggs), less in L2, even less in L4 larvae, while the least amount of hsf-1 mRNA was detected in young adults (Fig. 1A and S1). To establish these results we asked whether the relative HSF-1 protein levels in L2 larvae and L4 stages correlate with the mRNA levels. To directly compare the HSF-1 protein amounts we harvested and homogenized worms (strain CF512) that were grown on control bacteria carrying the empty RNAi plasmid (EV) up to either L2 or L4 larval stages. The homogenates were spun to separate the soluble (supernatant) and insoluble (pellet) fractions and equal amounts of total protein were subjected to Western Blot analysis (WB) using HSF-1 antibody. Our results (Fig. 1B) showed that HSF-1 is more abundant in L2 larvae than in their L4 counterparts in both supernatant and pellets, indicating that not only hsf-1 expression level is elevated in L2 larval stage but also the amounts of HSF-1protein. Therefore, if the expression pattern of hsf-1 was correlative with the requirement for hsf-1 to mediate longevity we would expect hsf-1 to be required primarily during embryogenesis, secondarily during larval development, and finally, weakly required during adulthood to mediate longevity.
Fig. 1.
hsf-1 is required foremost during development but also during adulthood to promote longevity A. Quantitative PCR revealed that hsf-1 expression is maximal during embryonic as well as early larval development and declines thereafter. B. The levels of HSF-1 protein in both soluble and insoluble fractions of L2 larvae are notably higher than in their L4 counterparts as detected by WB that was probed with an HSF-1 antibody. C. WB analysis revealed that eggs of wild-type worms grown on hsf-1 RNAi bacteria contained remarkably less HSF-1 than eggs of their EV-grown counterparts. D. Reduced HSF-1 levels only during embryogenesis had no effect on lifespan. Eggs obtained from hsf-1 RNAi grown worms placed on EV bacteria to enable hatching larvae to restore hsf-1 expression. The hatched worms (green line) and control worms (blue line) exhibited indistinguishable lifespans. E. daf-2(e1370) mutant worms were hatched on hsf-1 RNAi and were transferred onto dcr-1 RNAi bacteria at either L2 (green open circles) or L4 (purple open diamonds) stages. Unlike L2 transferred animals, those transferred at L4 had exceptionally short lifespans. For statistical data see table S1. F. Reciprocally to E; daf-2(e1370) mutant worms were hatched on EV bacteria and transferred onto hsf-1 RNAi bacteria at either larval stage L2 (blue open triangles) or L4 (green open circles). As in E, worms which had reduced hsf-1 levels during L2, but not L4, exhibited exceptionally short lifespans. For statistical data see table S2.
We tested whether the temporal expression pattern of hsf-1 was predictive of the temporal requirements of hsf-1 for longevity by determining whether embryonic expression of hsf-1 was needed for lifespan determination. Parental wild-type worms (strainN2) were cultured on hsf-1 RNAi bacteria to deplete HSF-1 in the F1 generation of embryos. Western blot analysis confirmed that HSF-1 quantities were greatly reduced in the eggs of hsf-1 RNAi treated worms (Fig. 1C). HSF-1 reduced eggs were either placed on hsf-1 RNAi bacteria to maintain compromised hsf-1 mRNA levels or on bacteria harboring an empty RNAi vector (EV) to enable the hatching larva to restore hsf-1 expression. The restoration of hsf-1 in worms that were hatched from hsf-1 reduced eggs and placed on EV bacteria was tested by functional analysis employing animals that express the Green Fluorescent Protein (GFP) under the regulation of the promoter of hsp-16.2, a well-established HSF-1 target gene (strain CL2070)(Link et al, 1999). Eggs of CL2070 worms were placed on hsf-1 RNAi bacteria, hatched, grown to reproductive adulthood and were bleached to obtain HSF-1 reduced eggs. These eggs were placed on EV bacteria and worms of the second generation were exposed to heat stress (33°C, 3h) at day 1 of adulthood to examine whether they restored the ability to induce the expression of hsp-16.2 in response to heat. Our results (Fig. S2) indicate that unlike worms that were fed hsf-1 RNAi bacteria for one or two generations (lanes 2 and 4), animals that were hatched from HSF-1reduced eggs and grown on EV bacteria have restored the ability to induce the expression of hsp-16.2 (lane 3). This observation indicates that these worms have restored HSF-1 to functional levels.
While worms that were grown for one generation on hsf-1 RNAi lived an extremely short life (Fig. 1D, red line) restoration of hsf-1 expression after hatching resulted in indistinguishable lifespans compared to those of animals grown continuously on the EV bacteria (Fig. 1D green and blue lines respectively and supplemental table S1). These results indicate that hsf-1 is not required for longevity assurance during embryogenesis.
Next we sought to test whether hsf-1 was required for longevity during larval development, a time in which the IIS pathway is dispensable for longevity regulation (Dillin et al, 2002a). To conditionally inactivate hsf-1 we used RNAi towards hsf-1 (to knockdown its expression) and RNAi towards dcr-1 to restore the expression of hsf-1. dcr-1 (dicer) encodes an RNase III enzyme that plays critical roles in RNA interference (Knight & Bass, 2001). Accordingly, the knockdown of dcr-1 reduces the endogenous RNAi response, enabling RNAi-inactivated genes to return to near normal expression levels (Bernstein et al, 2001; Dillin et al, 2002b). First we tested whether the knockdown of dcr-1 during development affects lifespan. To define the temporal requirements for dicer as a lifespan determinant we transferred temperature sensitive sterile worms (strain CF512) that were hatched and developed on control bacteria (EV) at 25°C (in this temperature HSF-1 does not induce the heat shock response (Fig S3, lane 2)), onto dcr-1 RNAi bacteria in three hours intervals during early development (up to 18 hours after hatching), or 36 hours after hatching (late development) and recording their lifespans. Our results (Fig. S4A) indicated that the knockdown of dcr-1 during larval development shortened lifespan but this effect was progressively less prominent in worms that had natural dicer levels for longer time from hatching. Similarly we tested whether the knockdown of dcr-1 during adulthood affects lifespans and found that dcr-1 RNAi treatment has no effect on lifespan when applied during adulthood (Fig S4B).
To assess the temporal requirement for hsf-1 in the longevity phenotype associated with reduced daf-2 activity we utilized the long-lived daf-2(e1370) mutant strain (Kenyon et al, 1993). To conditionally inactivated hsf-1 during specific times of larval development the worms were grown on hsf-1 RNAi bacteria and transferred onto plates spotted with dcr-1 RNAi bacteria at either larval stage L2 or L4 (20 hours or 36 hours after hatching, respectively). Quantitative real-time PCR indicated that in L2 larvae, hsf-1 expression was remarkably reduced within four hours after transferring the worms from control bacteria (EV) onto hsf-1 RNAi bacteria (Fig. S5A). A similar lag time was required for the worms to restore the expression of hsf-1 after transferring them from hsf-1 onto dcr-1 RNAi bacteria (Fig. S5C). L4 larvae exhibited reduced hsf-1 mRNA levels within 6 hours after transferring from EV bacteria onto hsf-1 RNAi (Fig. S5B). The restoration of hsf-1 expression occurred in L4 larvae within one and a half hours (Fig. S5D)(Possibly due to higher rate of food consumption of L4 larvae compared to their L2 counterparts). Therefore, in this set of experiments the animals had reduced hsf-1 expression from hatching up to the L2 or L4 larval stages, respectively, but restored expression quickly after transfer onto dcr-1 RNAi. Reduction of hsf-1 expression from hatching through the L4 larval stage resulted in a decreased longevity (Fig. 1E, mean lifespan (LS) 14.7±5.49 days), only slightly longer than that of control animals grown on hsf-1 RNAi throughout life (12.29±3.34 days). In a striking contrast, animals in which hsf-1 was inactivated from hatching until the L2 larval stage lived remarkably longer (mean LS 35.3±13.41 days). This mean lifespan was very similar to that of animals grown on dcr-1 RNAi throughout life (33.63±8.8 days, Fig. 1E and table S2). These results indicate that unlike daf-16, hsf-1 executes an essential longevity function during larval development, predominantly between the L2 and L4 larval stages.
To determine if the expression of hsf-1 during early larval development can set the rate of the animal’s aging for the rest of its life or whether hsf-1 expression also affects lifespan during later developmental stages we performed a reciprocal experiment to the one described above. Long-lived daf-2(e1370) mutant worms were grown on EV bacteria and transferred onto hsf-1 RNAi bacteria at either the L2 or L4 larval stages (Fig. 1F and table S3). Therefore, these animals had natural hsf-1 expression until the time of transfer to hsf-1 RNAi bacteria, either the L2 or L4 larval stages. Consistent with our prior results, worms transferred onto hsf-1 RNAi at the beginning of L2 larval stage had short lifespans (mean LS 12.97±5.74 days), while those transferred at L4 lived considerably longer (mean LS 19.85±6.37 days) but much shorter than their counterparts that had an active hsf-1 during adulthood (Fig. 1E). Together these results indicate that hsf-1 is foremost necessary between the L2 and L4 larval stages for the extended lifespan of daf-2 mutant animals. However, the observation that daf-2 mutant worms transferred onto hsf-1 RNAi at the L4 stage did not live as long as daf-2 mutant animals grown on control bacteria, indicates that hsf-1 is also needed during adulthood to promote the full longevity effect of reduced IIS.
To accurately determine the critical timing requirements of hsf-1 for longevity assurance during larval development we tested the lifespans of CF512 worm populations which were hatched and grown on control bacteria (EV) at 25°C and transferred onto hsf-1 RNAi at 3 hour intervals, beginning from 3 hours until 36 hours after hatching (12 time points in total). Worms that were transferred onto hsf-1 RNAi 24 hours after hatching or earlier had short mean lifespans almost identical to these of animals treated with hsf-1 RNAi bacteria throughout their lives (Fig. 2A,C and table S4). Distinctly, the lifespans of animals transferred onto hsf-1 RNAi bacteria at 27 hours after hatching or later lived progressively and significantly longer than their counterparts transferred earlier (Figs. 2B,C and table S4) but still much less than animals that had active hsf-1 up until adulthood. These results point to the late L2/early L3 developmental stages as the end of the time window in which hsf-1 is necessary during development for longevity.
Fig. 2.
Functions of hsf-1 during L2 developmental stage are critical to enable development and promote longevity. A–C. CF512 worms that were hatched on EV bacteria transferred onto hsf-1 RNAi bacteria at the indicated times. Transfer at 24h after hatching or earlier did not reverse the short lifespan phenotype associated with hsf-1 RNAi, whereas worms transferred 27h after hatching or later had longer lifespans. D–F. CF512 worms were hatched on hsf-1 RNAi bacteria and transferred onto dcr-1 RNAi at the indicated times. Transferring at either 3h or 6h after hatching resulted in short lifespans due to interference with the developmental functions of dicer. Transferring 9h after hatching had relatively long lifespan, comparable to that of EV-grown worms. Worms that were transferred onto dcr-1 RNAi 12h after hatching or later showed progressively shorter lifespans due to hsf-1 RNAi toxicity.
The last set of experiments defined the end of the time window in which hsf-1 exhibits its most prominent effect on lifespan during larval development. To define the beginning of this time window, we performed the reciprocal inactivation experiment. Animals were hatched on hsf-1 RNAi bacteria and transferred onto dcr-1 RNAi bacteria at the same 3 hour intervals 3 to 36 hours after hatching to enable the restoration of hsf-1 and rescue the worms from their extremely short lifespan phenotype. Functional dicer is required for appropriate worm development (Knight & Bass, 2001) thus, RNAi towards dicer is predicted to shorten lifespans of worms when applied during early development. Our results (Fig S4A) confirm that the knockdown of dcr-1 shortens lifespan only if it applied up to 12 hours after hatching. Indeed, worms transferred onto dcr-1 RNAi during the L1 developmental stage exhibited slightly reduced lifespans and this effect was weakened as the worms were transferred later in development (Figs. 2D, 2F, S6 and table S5). We found that the lifespan shortening effect of hsf-1 RNAi appeared in worms that were transferred 12 hours after hatching, a time point corresponding to the early L2 developmental stage. The ability of the dcr-1 RNAi to partially rescue the worms from the short lifespan phenotype associated with hsf-1 RNAi declined with time (Figs. 2E, F, S6 and table S5). Taken together, the role of hsf-1 in longevity determination during larval development appears to begin 12 hours after hatching (early L2 larval stage) and to extend for additional 12 hours into the late L2/early L3 larval stages. Interestingly, even worms that were transferred at either 30, 33 or 36 hours after hatching had longer lifespans compared to their counterparts that were grown on hsf-1 RNAi throughout life, supporting the idea that hsf-1 is also required, albeit for a lesser extent, during adulthood.
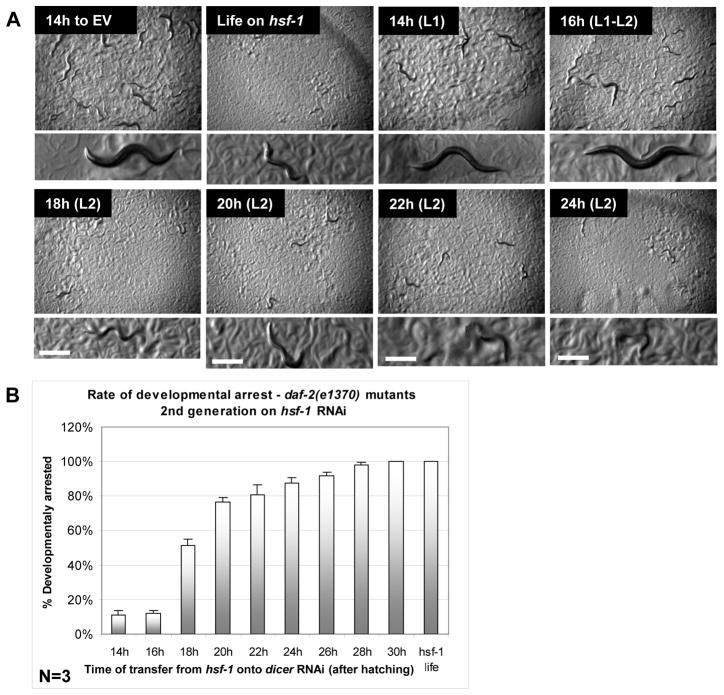
As an independent measure of the temporal requirement for hsf-1 during larval development, we utilized a unique phenotype of daf-2(e1370) mutant worms. When grown for two generations on hsf-1 RNAi, daf-2(e1370) mutant worms were developmentally arrested at the L2 larval stage (wild-type and weak daf-2(e1368) mutant worm strains completed development even if grown on hsf-1 RNAi for three generations (Fig. S7, A and B)). A similar observation of developmental arrest was observed in age-1 mutant worms (that also exhibit reduced IIS) following hsf-1 knockdown (Morley & Morimoto, 2004). Using this phenotype we pinpointed the time window in which the development of daf-2(e1370) mutant worms cultured for two generations on hsf-1 RNAi bacteria can be rescued from the L2 developmental arrest. Groups of daf-2(e1370) mutant worms of the second generation on hsf-1 RNAi were transferred onto dcr-1 RNAi bacteria every two hours, starting 14 hours after hatching, to reinstate hsf-1 expression and rescue development (Fig. S7C). The animals that were transferred from hsf-1 RNAi onto dcr-1 RNAi at the beginning of the L2 larval stage or later exhibited a dramatic increase in the percentage of larval arrest from about 10% (16 hours after hatching) to greater than 50% arrest a mere 2 hours later (18 hours after hatching) and nearly 78% after 20 hours (Fig. 3, A and B). Worms transferred at 30 hours or later after hatching were 100% arrested. Since hsf-1 expression is restored quickly after transferring the worms onto dcr-1 RNAi bacteria (Fig. S5 C and D), our results indicate that the time window in which hsf-1 is critical for the proper development and maturation of daf-2(e1370) mutant worms is also during the L2 larval stage, the time when hsf-1 is critically needed for longevity.
Fig 3.
hsf-1 is required during L2 larval stage for proper development of daf-2 mutant worms. A. Highly synchronized second generation on hsf-1 RNAi, daf-2(e1370) mutant worms were transferred onto dcr-1 RNAi bacteria at the indicated times after hatching to rescue the developmental arrest phenotype. Worms transferred 18h after hatching or later showed increasing developmental arrest. B. Bars represent the fraction of developmentally arrested worms at each time point in three independent experiments.
Two observations indicate that despite its relatively weak expression, hsf-1 is also needed during adulthood for longevity. First, initiating hsf-1 inactivation at the L4 larval stage still shortens the lifespan of daf-2(e1370) mutant animals (Fig. 1E). Secondly, worms transferred from hsf-1 RNAi onto dcr-1 RNAi later than 30 hours after hatching lived longer than their counterparts which were grown on hsf-1 RNAi throughout life (Fig. 2E and F). Thus, we sought to accurately define the secondary time window in which hsf-1 determines lifespan during adulthood. daf-2(e1370) mutant worms were cultured on hsf-1 RNAi throughout development and transferred onto dcr-1 RNAi at either day 1, 3, 5, 7 or 9 of adulthood (the efficiency of hsf-1 RNAi during adulthood was monitored and confirmed in CF512 worms. The worms were grown to either day 1, 4 or 8 of adulthood, fed hsf-1 RNAi bacteria for 24h, exposed to heat stress (33°C, 1h) and their abilities to induce the expression of hsp-70 were compared to the induction of this gene in their EV-fed counterparts of corresponding ages (Fig S8)). Inactivation of hsf-1 during larval development and early adulthood (up to day 5 or later) reduced longevity of daf-2(e1370) mutant worms to similar extent as inactivation throughout life (Fig. 4, A and B). However, when the worms were grown on hsf-1 RNAi throughout development and transferred onto dcr-1 RNAi earlier than day 5 of adulthood (day 1 or 3 of adulthood), their lifespans were significantly longer than animals grown on hsf-1 RNAi throughout life (Fig. 4A, B and table S1). Taken together, hsf-1 is prominently required during larval development and for a lesser extent up to day 5 of adulthood for lifespan determination. The role of hsf-1 as lifespan determinant during reproductive adulthood is of particular interest since daf-16 executes its lifespan functions at the same time window (Dillin et al, 2002a).
Fig 4.
hsf-1 determines lifespan also during early adulthood and required for full lifespan throughout life. A and B. daf-2(e1370) mutant worms that developed on hsf-1 RNAi were transferred onto dcr-1 RNAi bacteria at either day 1, 3, 5, 7 or 9 of adulthood. Worms transferred at either day 1 or 3, but not those transferred at day 5 or later, lived longer than their counterparts grown on hsf-1 RNAi throughout life. C and D. daf-2(e1370) mutant worms were let hatch and develop on EV bacteria and transferred onto hsf-1 RNAi at either day 1, 5, 9, 12 or 18 of adulthood. All worm groups exhibited similar average survival rates from the age of exposure to hsf-1 RNAi.
To test whether hsf-1 plays any role as a lifespan determinant during mid and late stages of life we performed the reciprocal experiment; daf-2(e1370) mutant worms were developed and grown on EV bacteria and then transferred onto hsf-1 RNAi bacteria at either day 1, 5, 9, 12 or 18 of adulthood. This experiment (Fig. 4C and D) revealed that feeding worms with bacteria expressing hsf-1 RNAi shortens life at any stage of life. This observation suggests that HSF-1 is required for the maintenance of health and to enable longevity even at late stages of life. Yet, the lifespan shortening effects of hsf-1 RNAi at late stages of life were relatively mild and worms that were transferred at different ages exhibited similar average lifespans from exposure to hsf-1 RNAi of 20 to 24 days (Fig. 4D). For instance, worms that were transferred to hsf-1 RNAi at day 9 of adulthood had a mean lifespan of 31.06±7.16 days (22.06 days from exposure to hsf-1 RNAi) while their counterparts that were transferred to hsf-1 RNAi at day 18 of adulthood exhibited mean lifespan of 42.55±8.22 days (24.55 days from exposure) (Supplemental table 3). These observations indicate that hsf-1 is required for the maintenance of health and promotion of full lifespan throughout life but its prominence declines over time.
Discussion
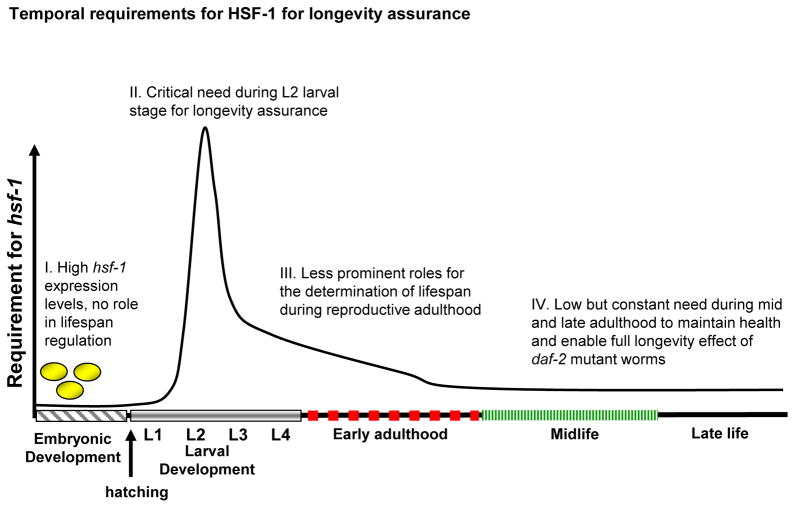
Because hsf-1 is foremost required for lifespan determination in two distinct times during the animal’s lifecycle, the L2 larval stage and days 1–5 of adulthood, we propose a model in which hsf-1 functions in a two-step mechanism for longevity assurance (Fig. 5). During early larval development it is critically required to set a competent state that enables IIS reduction to promote longevity later in life. Thus, hsf-1 knockdown during this stage prevents the creation of pivotal components of the longevity mechanism that enables IIS reduction to extend lifespan. Later, during early adulthood and for a lesser extent during late adulthood, it is required to maintain and perhaps strengthen these cellular mechanisms. What might be the nature of the developmental hsf-1 regulated mechanism? The well-established role of HSF-1 in various stress responses raises the prospect that during development it activates the expression of genes that encode molecular chaperones and other proteins which are required for the maintenance of proteostasis. Such developmental target genes might encode constitutive heat shock proteins such as HSP-1. The observation that treating worms with hsp-1 RNAi for two generations results in a developmental arrest (our unpublished data) supports this notion. The identification of developmental HSF-1 transcriptional targets that function as DAF-16 co-factors during adulthood will be critically required for the examination of this putative linkage between HSF-1 and the IIS.
Fig 5.
Schematic illustration of the temporal requirement for hsf-1 as a lifespan determinant during the worm lifecycle. I. There is no need for hsf-1 during embryogenesis for longevity assurance as hsf-1 reduction exclusively during embryogenesis has no effect on lifespan. II. hsf-1 is critically required during L2 developmental stage to enable longevity. hsf-1 reduction at this time has severe irreversible short life phenotype. III. hsf-1 functions as longevity determinant also during early adulthood yet, for a lesser extent compared to early development. IV. Although less prominently, hsf-1 is constantly required during midlife and late adulthood for daf-2 mutants to achieve their full longevity phenotype.
It is likely that the maintenance of proteostasis is a specific aspect of longevity. First, we have recently shown that HSF-1 is foremost required during development to protect from proteotoxicity (Cohen et al, 2010). Moreover, since even a single protein aggregation event can destabilize the proteome (Gidalevitz et al, 2006) the formation of an efficient proteostasis assurance mechanism appears to be required for IIS reduction to slow aging and promote longevity (Balch et al, 2008). The key role of HSF-1 in mediating disaggregation and subsequently aggregate detoxification (Cohen et al, 2006) strongly suggests that its activity during development is needed for the formation of tight protein integrity assurance machinery that supports longevity.
This study also provides an interesting insight into the temporal requirement for the worm’s microRNA mechanism for longevity. The experiments which determine the temporal need for dcr-1 as a lifespan determinant (Fig S4) show that the microRNA mechanism is important during early development for longevity. Since this mechanism was previously shown to be required for proper development of C. elegans (Knight & Bass, 2001) our finding might point at an additional link, perhaps ancillary, between developmental events and longevity. This hypothesized link might be supported by the report that components of the microRNA processing mechanism are required for daf-2 mutant worms to exhibit their full longevity phenotype (Boehm & Slack, 2005).
The requirement for hsf-1 during day 1–5 of adulthood correlates with the time window in which daf-16 is required to mediate the increased longevity of daf-2 mutant worms (Dillin et al, 2002a). This finding raises the prospect that HSF-1 is required for proper DAF-16 function. It is tempting to speculate that DAF-16 and HSF-1 acts in concert during reproductive adulthood to mediate the expression of specific genes. Accordingly, promoter regions of several small heat shock proteins were found to contain the canonical recognition sites of both DAF-16 and HSF-1 (Hsu et al, 2003) and daf-2 RNAi treatment was shown to increase the expression levels of these genes (Murphy et al, 2003).
This study provides the temporal insights that enable the definition of the roles played by HSF-1 in the mediation of longevity, the identification of its downstream gene networks and biological functions that regulate aging and the characterization of the links between this transcription factor and the IIS.
Experimental procedures
Worm and RNAi strains
N2, daf-2(e1370) mutant daf-2(e1368) mutant worm strains as well as hsp- 16.2p::GFP worms (strain CL2070) were obtained from the Caenorhabditis Genetics Center (Minneapolis, MN). The worms were grown at 20°C. CF512 (fer-15(b26)II; fem-1(hc17)IV) worms are heat-sensitive sterile that were routinely grown at 15°C. To avoid progeny, CF512 worm eggs were incubated at 20°C for 16h to enable efficient hatching, larvae transferred to 25°C for 48h and back to 20°C until harvested. To reduce gene expression we used previously described (Dillin et al, 2002a) bacterial strains expressing dsRNA: empty vector (pAD12), daf-2 (pAD48), daf-16 (pAD43) and dicer. hsf-1 dsRNA expressing bacterial strain was from genomic RNAi library (J. Ahringer). Each RNAi bacteria colony was grown at 37°C in LB with 100μg/ml carbenicillin, and then seeded onto NG-carbenicillin plates supplemented with 100mM IPTG.
Lifespan analysis
Synchronized worm eggs were placed on master NG-carbenicillin plates seeded with the indicated RNAi bacterial strain and supplemented with 100mM IPTG. The eggs were incubated at 20°C until transferred onto small NG- carbenicillin plates (10 animals per plate) at the indicated ages. Adult worms were transferred onto freshly seeded plates every four days. Worms that failed to move their noses when tapped twice with a platinum wire were scored as dead. Dead worms were scored daily. Lifespan analyses were conducted at 20°C.
Worm synchronization for developmental arrest experiment
Day 1 adult reproductive worms were transferred onto plates seeded with bacteria. The worms were removed after 30 minutes leaving highly synchronized eggs on the plates.
Antibodies
HSF-1 antibody (SPA-901) was from Stressgen. GFP antibody (mAb #2956) was purchased from Cell Signalling and anti γ-tubulin antibody clone GTU-88 (T-6557) was from Sigma. Secondary antibodies conjugated to HRP were purchased from Jackson Immuno-Research (West Grove, PA, USA).
Protein blotting
Worm eggs were purified by bleaching, boiled in loading buffer (10% glycerol, 125mM Tris base, 1% SDS), loaded on 12% Tris-Glycine PAA and proteins were separated and transferred onto PVDF membranes. Worms (either larvae or adults) were washed from plates with M9, homogenized using a dounce homogenizer and centrifuged for 3 minutes at 850g. Pellets were boiled in loading buffer (10% glycerol, 125 mM Tris-base, 1% SDS). Protein concentration of supernatants was determined using Bio-Rad Protein assay (#500-0006). 100 μg of the supernatant was boiled in loading buffer. Chemiluminescence was detected using a Luminescent Image Analyzer (Las-3000-Fujifilm). For reprobing, PVDF membranes were stripped by incubation in 300mM NaOH (5 min, RT with agitation), followed by neutralization by several rinses in TBST (10mM Tris-Cl pH 7.5, 150mM NaCl, 0.3% Tween-20).
RNA isolation and quantitative RT-PCR
Total RNA was isolated from synchronized worm populations at the indicated ages. Total RNA was extracted using Qiazol reagent (Qiagen #79306) and purified using RNeasy kit (QIAGEN #74104). cDNA was created using QuantiTect Probe RT-PCR Kit (QIAGEN #204443). Quantification was completed using SDS2.1 software (Applied biosystems), normalizing to control levels of act-1 cDNA. SybrGreen real-time qPCR experiments were performed as described in the manual using ABI Prism7900HT (Applied Biosystems). hsf-1 primer set 1:forward: TTGACGACGACAAGCTTCCAGT reverse: AAAGCTTGCACCAGAATCATCCC hsf-1 primer set 2: forward: GTCTCTGTCATGCAGCCAGG reverse: TTGGGTCCGGCAGTTCC. act-1 primers: forward: GAGCACGGTATCGTCACCAA reverse: TGTGATGCCAGATCTTCTCCAT. hsp-70 primers: forward: AGTGGATCCTCCGACAAGG reverse: CACCAAAGGCTACTGCTTCG
Supplementary Material
Fig S1. A and B. act-1 quantitative PCR calibration for hsf-1 mRNA quantification (corresponding figure 1A). C–F. Raw data of figure 1A.
Fig S2. Worms expressing GFP under the hsp-16.2 promoter were either grown on control bacteria (EV) or hsf-1 RNAi for one generation and bleached. Eggs obtained from EV-grown worms were placed on either EV bacteria (EV to EV, lane 1) or on hsf-1 RNAi bacteria (EV to hsf-1, lane 4). Similarly eggs of hsf-1 RNAi treated worms were placed on either EV (hsf-1 to EV, lane 3) or on hsf-1 RNAi (hsf-1 to hsf-1, lane 2). The worms were grown to day 1 of adulthood, exposed to heat stress (33°C, 3h) and subjected to WB analysis using GFP antibody. Worms that were hatched from hsf-1 reduced eggs and developed on EV bacteria (hsf-1 to EV, lane 3) restored their ability to induce the expression of GFP in response to heat.
Fig S3. To test whether exposure to 25°C induces the heat shock response, CL2070 worms that were grown to adulthood at 20°C were exposed for 3h to either 25°C (lane 2), 33°C (lane 3) or left at 20°C (lane 1). WB analysis using GFP antibody indicated that the hsp-16.2 promoter (a target of HSF-1) was not induced in worms that were exposed to 25°C.
Fig S4. A. To examine how dcr-1 RNAi treatment affects lifespan, CF512 animals were let hatched on EV bacteria and transferred onto dcr-1 RNAi in 3 hours intervals from 3 to 18 hours or 36 hours after hatching and their lifespans were recorded. Our results show that dcr-1 RNAi shortens lifespan when applied during larval development but this effect becomes less prominent when the worms had natural dicer levels for longer time from hatching. B. Similarly we tested whether the knockdown of dcr-1 during adulthood modifies lifespans of CF512 worms. No effects on lifespan were observed when dcr-1 RNAi was applied at either days 1, 5 or 9 of adulthood.
Fig S5. A and B. Quantitative PCR indicates that the hsf-1 expression levels are efficiently decreased (70–80% compared to control) in wild-type larvae within 4–6 hours after exposure to hsf-1 RNAi in L2 (A) and L4 (B) stages. C and D. The expression levels of hsf-1 are restored to nearly natural levels in L2 larvae ~6h after transfer onto dcr-1 RNAi (C) while in L4 larvae hsf-1 restoration is apparent 1.5h after exposure to dcr-1 RNAi (D).
Fig S6. Worms transferred from hsf-1 onto dcr-1 RNAi later than 12h after hatching exhibited progressively shorter lifespans (corresponding figure 2E, corresponding table S5)
Fig S7. A. daf-2(e1370) mutant, but neither wild-type nor daf-2(e1368) mutant worm are developmentally arrested when grown for two generation on hsf-1 RNAi (white arrows point to progeny). B. daf-2(e1368) grown for three generations on hsf-1 RNAi completed development. C. Experimental design of figures 3, A and B.
Fig S8. CF512 worms were grown on EV bacteria up to days 1, 4 or 8 of adulthood and either exposed for 24 hours to hsf-1 RNAi or left untreated prior to exposure to heat shock (33°C, 1h). qPCR analysis of hsp-70 levels revealed that hsf-1 RNAi efficiently reduces the worm ability to respond to heat in both; early adulthood and midlife.
Acknowledgments
This study was generously supported by the United states – Israel Binational Science Foundation (BSF 2009465)(EC and AD), Marie Curie Reintegration grant (279134)(EC), the Howard Hughes Medical Institute and NIA P01 AG031097 (AD).
Footnotes
Author Contribution
EC and AD designed and initiated this study. YV, MM, TD, MBS, DJ, EK and EC performed the experimental work. EC and AD wrote the manuscript.
References
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nature reviews. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science (New York, NY. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science (New York, NY. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Boehm M, Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science (New York, NY. 2005;310:1954–1957. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science (New York, NY. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- Cohen E, Du D, Joyce D, Kapernick EA, Volovik Y, Kelly JW, Dillin A. Temporal requirements of insulin/IGF-1 signaling for proteotoxicity protection. Aging Cell. 2010;9:126–134. doi: 10.1111/j.1474-9726.2009.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread Protein Aggregation as an Inherent Part of Aging in C. elegans. PLoS biology. 2010:8. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science (New York, NY. 2002a;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science (New York, NY. 2002b;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Jacobson J, Vinti G, Leevers SJ, Partridge L. Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell. 2007 doi: 10.1111/j.1474-9726.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–1474. doi: 10.1126/science.1124514. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science (New York, NY. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Knight SW, Bass BL. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science (New York, NY. 2001;293:2269–2271. doi: 10.1126/science.1062039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nature genetics. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Link CD, Cypser JR, Johnson CJ, Johnson TE. Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell stress & chaperones. 1999;4:235–242. doi: 10.1379/1466-1268(1999)004<0235:doosri>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XD, Liu PC, Santoro N, Thiele DJ. Conservation of a stress response: human heat shock transcription factors functionally substitute for yeast HSF. The EMBO journal. 1997;16:6466–6477. doi: 10.1093/emboj/16.21.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes & development. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabindran SK, Haroun RI, Clos J, Wisniewski J, Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science (New York, NY. 1993;259:230–234. doi: 10.1126/science.8421783. [DOI] [PubMed] [Google Scholar]
- Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Molecular and cellular biology. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. Faseb J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Singh V, Aballay A. Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc Natl Acad Sci U S A. 2006;103:13092–13097. doi: 10.1073/pnas.0604050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, Kaeberlein M. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008;7:394–404. doi: 10.1111/j.1474-9726.2008.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science (New York, NY. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Walker GA, Thompson FJ, Brawley A, Scanlon T, Devaney E. Heat shock factor functions at the convergence of the stress response and developmental pathways in Caenorhabditis elegans. Faseb J. 2003;17:1960–1962. doi: 10.1096/fj.03-0164fje. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Mullane PC, Periz G, Wang J. TDP-43 neurotoxicity and protein aggregation modulated by heat shock factor and insulin/IGF-1 signaling. Human molecular genetics. 2011 doi: 10.1093/hmg/ddr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. A and B. act-1 quantitative PCR calibration for hsf-1 mRNA quantification (corresponding figure 1A). C–F. Raw data of figure 1A.
Fig S2. Worms expressing GFP under the hsp-16.2 promoter were either grown on control bacteria (EV) or hsf-1 RNAi for one generation and bleached. Eggs obtained from EV-grown worms were placed on either EV bacteria (EV to EV, lane 1) or on hsf-1 RNAi bacteria (EV to hsf-1, lane 4). Similarly eggs of hsf-1 RNAi treated worms were placed on either EV (hsf-1 to EV, lane 3) or on hsf-1 RNAi (hsf-1 to hsf-1, lane 2). The worms were grown to day 1 of adulthood, exposed to heat stress (33°C, 3h) and subjected to WB analysis using GFP antibody. Worms that were hatched from hsf-1 reduced eggs and developed on EV bacteria (hsf-1 to EV, lane 3) restored their ability to induce the expression of GFP in response to heat.
Fig S3. To test whether exposure to 25°C induces the heat shock response, CL2070 worms that were grown to adulthood at 20°C were exposed for 3h to either 25°C (lane 2), 33°C (lane 3) or left at 20°C (lane 1). WB analysis using GFP antibody indicated that the hsp-16.2 promoter (a target of HSF-1) was not induced in worms that were exposed to 25°C.
Fig S4. A. To examine how dcr-1 RNAi treatment affects lifespan, CF512 animals were let hatched on EV bacteria and transferred onto dcr-1 RNAi in 3 hours intervals from 3 to 18 hours or 36 hours after hatching and their lifespans were recorded. Our results show that dcr-1 RNAi shortens lifespan when applied during larval development but this effect becomes less prominent when the worms had natural dicer levels for longer time from hatching. B. Similarly we tested whether the knockdown of dcr-1 during adulthood modifies lifespans of CF512 worms. No effects on lifespan were observed when dcr-1 RNAi was applied at either days 1, 5 or 9 of adulthood.
Fig S5. A and B. Quantitative PCR indicates that the hsf-1 expression levels are efficiently decreased (70–80% compared to control) in wild-type larvae within 4–6 hours after exposure to hsf-1 RNAi in L2 (A) and L4 (B) stages. C and D. The expression levels of hsf-1 are restored to nearly natural levels in L2 larvae ~6h after transfer onto dcr-1 RNAi (C) while in L4 larvae hsf-1 restoration is apparent 1.5h after exposure to dcr-1 RNAi (D).
Fig S6. Worms transferred from hsf-1 onto dcr-1 RNAi later than 12h after hatching exhibited progressively shorter lifespans (corresponding figure 2E, corresponding table S5)
Fig S7. A. daf-2(e1370) mutant, but neither wild-type nor daf-2(e1368) mutant worm are developmentally arrested when grown for two generation on hsf-1 RNAi (white arrows point to progeny). B. daf-2(e1368) grown for three generations on hsf-1 RNAi completed development. C. Experimental design of figures 3, A and B.
Fig S8. CF512 worms were grown on EV bacteria up to days 1, 4 or 8 of adulthood and either exposed for 24 hours to hsf-1 RNAi or left untreated prior to exposure to heat shock (33°C, 1h). qPCR analysis of hsp-70 levels revealed that hsf-1 RNAi efficiently reduces the worm ability to respond to heat in both; early adulthood and midlife.