Abstract
Myeloid cells are capable of promoting or eradicating tumor cells and the nodal functions that contribute to their different roles are still obscure. Here, we show that mice with myeloid-specific genetic loss of the NF-κB pathway regulatory kinase IKKβ exhibit more rapid growth of cutaneous and lung melanoma tumors. In a BRAFV600E/PTEN−/− allograft model, IKKβ loss in macrophages reduced recruitment of myeloid cells into the tumor, lowered expression of MHC class II molecules, and enhanced production of the chemokine CCL11, thereby negatively regulating dendritic-cell maturation. Elevated serum and tissue levels of CCL11 mediated suppression of dendritic-cell differentiation/maturation within the tumor microenvironment, skewing it toward a Th2 immune response and impairing CD8+ T cell–mediated tumor cell lysis. Depleting macrophages or CD8+ T cells in mice with wild-type IKKβ myeloid cells enhanced tumor growth, where the myeloid cell response was used to mediate antitumor immunity against melanoma tumors (with less dependency on a CD8+ T-cell response). In contrast, myeloid cells deficient in IKKβ were compromised in tumor cell lysis, based on their reduced ability to phagocytize and digest tumor cells. Thus, mice with continuous IKKβ signaling in myeloid-lineage cells (IKKβCA) exhibited enhanced antitumor immunity and reduced melanoma outgrowth. Collectively, our results illuminate new mechanisms through which NF-κB signaling in myeloid cells promotes innate tumor surveillance.
Introduction
Malignant melanoma is a lethal disease due to its aggressive capacity for metastasis and resistance to therapy. For decades, considerable effort has gone toward development of immunotherapy for treatment of metastatic melanoma. Tumors can potentially be recognized as “altered self,” akin to allogeneic immunity, and leading to an antitumor immune response of potential value in the adjuvant setting. This motivated investigations of interactions between melanoma and immune cells and translation of this knowledge into effective clinical strategies. The majority of the early studies strove to increase T-cell responses to the tumor partly through manipulation of dendritic cells (DC), a key antigen-presenting cell (APC) type. However, macrophages and neutrophils were also found to be key mediators of inflammation and immunity in cancer. Their phenotypes depend on the physiologic or pathologic milieu in which they reside. Protumor macrophages (M2) and neutrophils (N2) can be contrasted with the classically activated macrophages (M1) and neutrophils (N1) that present antigen and/or produce reactive oxygen species (ROS) involved in the killing of foreign organisms and tumor cells (1, 2). Moreover, the cytokines and chemokines produced by myeloid cells can significantly affect DC and the Th1 (antitumor) versus Th2 (protumor) skew of the immune cells in the tumor microenvironment (TME).
Nuclear factor-kappa B (NF-κB) is a ubiquitous transcription factor that regulates expression of proinflammatory genes, playing a crucial role in immune response (3). NF-κB activation is regulated by the IκB kinase complex (IKKα, IKKβ, NEMO) that has become a major target for anti-inflammation and cancer therapy (4–6). Considering the importance of IKK, particularly IKKβ, in tumor immunity, a myriad of efforts have focused on the molecular mechanism for IKKβ regulation of the myeloid-mediated immune response during tumor development. Deletion of the Ikkβ gene in myeloid cells led to inhibition of colitis-induced colon cancer (7) and expression of an IκB-super repressor in resident macrophages (Kupffer cells) inhibited progression of hepatocellular carcinoma (8). Furthermore, introduction of NF-κB–deficient macrophages into mice with early ovarian cancer lesions slowed cancer progression (9). Despite these indications of a protumorigenic role of NF-κB in macrophages, other reports indicate that NF-κB is needed for the antitumorigenic function of macrophages in breast cancer metastasis and angiosarcoma (10, 11). Thus, the role of IKKβ/NF-κB signaling in macrophage pro- or antitumor responses remains controversial.
To address the role of IKKβ function in myeloid cells during melanoma tumorigenesis, we generated a C57Bl/6 mouse model with Cre-recombinase–mediated IKKβ deletion in myeloid cells (IKKβmyeΔ/Δ) and evaluated how loss of myeloid IKKβ affects melanoma tumor growth in allogenic and syngeneic melanoma models. In the allogenic model, melanoma cells were derived from a melanoma in a BRAFV600E/Pten−/− mouse that had been backcrossed from FVB to C57Bl/6 but retained FVB MHC. We evaluated the ability of these tumor cells to establish metastatic lesions in lung or liver in IKKβWT and IKKβmyeΔ/Δ C57Bl/6 mice. In the syngeneic model, growth of B16 melanoma tumors in mice with myeloid cells expressing IKKβ constitutively active (IKKβCA), IKKβWT or IKKβmyeΔ/Δ was evaluated. We now show that loss of Ikkβ in myeloid cells enhanced melanoma tumor growth in both the allograft and the syngeneic model, even though the mechanisms differed. In the allograft model, melanoma growth was enhanced in IKKβmyeΔ/Δ mice due to defects in myeloid cell MHCII expression and function, altered myeloid cytokine/chemokine expression, defects in DC maturation, and poor T-cell activation. In contrast, IKKβ and to a greater extent IKKβCA myeloid cells exhibited strong antitumor response to syngeneic B16 melanoma, compared with IKKβmyeΔ/Δ mice. These results indicate that IKKβ activity is important for the antitumorigenic function of myeloid cells, thus providing important therapeutic implications.
Materials and Methods
Myeloid Ikkβ knockout models and melanoma metastatic models
All animal experiments were approved by the Vanderbilt University Institutional Animal Care and Use Committee. To knockout Ikkβ in myeloid cells in either C57BL6 or FVB strain mice, LysM-Cre mice with Cre-recombinase expressed under the control of the murine lysozyme M gene regulatory region (1) were bred with Ikkβf/f mice (2). The C57Bl/6 mice IKKβf/f mice were backcrossed from FVB to C57BL/6 five generations. These mice were then bred to mice harboring the loxP-flanked tdTomato (mT) following the EGFP (mG) cassette, which was inserted into the Gt(ROSA)26Sor locus. These mT/mG mice served as a Cre-reporter strain and after Cre-mediated recombination, myeloid cells that are Ikkβ-null are green (3, 4). The mice with Ikkβ-null myeloid cells are designated here as “IkkβMyeΔ/Δ mice.” Littermates LysMCre::mT/mG mice without the Ikkβf/f alleles were used as IkkWT controls. IkkβCA mice with a genetic background of cfms-rtTA::TetOn-cIkkβ express a constitutively active form of Ikkβ in myeloid cells in response to doxycycline induction.
For generating metastasis models, melanoma cell lines expressing Gluc were derived from melanoma lesions (BrafV600E/Pten−/−) arising in the mixed strain of C57BL6/FVB (12), or B16F0 cells derived from C57Bl/6 mice were injected or implanted into IkkβMyeΔ/Δ or IkkβWT mice. To evaluate whether deletion or constitutive activation of Ikkβ in myeloid cells affected tumor growth in a syngeneic model of melanoma, IkkβMyeΔ/Δ mice, IkkβCA mice (10), or IkkβWT littermates were intravenously (i.v.) injected with 5 × 104 Gluc–expressing B16F0 melanoma cells to obtain lung metastases in 4 weeks. For both models, after 20 days tumor burden was determined by tumor-expressing Gluc reporter activity in 20 μg protein from lung tissue lysate or 5 μL of peripheral blood. N = 3 independent experiments with 6 mice per group/experiment. Detailed descriptions of breeding procedures and characterization of the mice and tumors are found in the Supplementary Methods.
Bone marrow transplant and inducible/spontaneous melanoma models
Recipient C57BL6/FVB mixed background mice carrying Braf+/−/Pten−/−/Tyr-Cre+ alleles were given 100 mg/L neomycin, 10 mg/L polymyxin B in pH2 water 1 week before transplant and continuously for 6 weeks after transplantation. Mice received one dose of 10-Gy irradiation (Cesium Gamma irradiator). Four hours later, the mice were injected via tail vein with bone marrow cells (1 × 106) from C57BL6 donor mice (myeloid IkkWT mice or myeloid IkkMyeΔ/Δ mice). The reconstitution of bone marrow in recipient mice was validated 3 weeks after transplant (Supplementary Fig. S1J–S1L) and proper function of recipient myeloid cells in response to tumor cells was verified and compared with that of donor mice (Supplementary Fig. S1J).
FACS analysis and antibodies
For FACS analyses, tissues were minced on a programmable dissociator and digested with an enzyme solution of collagenase, Dispase and DNase. A detailed list of antibodies used, staining, and FACS analyses protocols is found in Supplemental Methods.
Characterization of macrophage killing of tumor cells in the peritoneum
To study the role of myeloid Ikkβ activity on the ability to migrate into the peritoneum in response to tumor, BrafV600E/Pten−/− melanoma cells were injected into the peritoneum of mT/mG IkkWT mice. The infiltrating myeloid cells (GFP+, Tomato RFP−), lymphocytes (GFP−, Tomato RFP+), and melanoma cells were quantified by FACS analysis over an 8-hour time course (0, 2, 4, and 8 hours). To investigate the role of NF-κB in tumor cell phagocytosis, macrophages were isolated from IkkβMyeΔ/Δ mice or litter mate IkkβWt mice 18 hours after the mice had received a peritoneal injection of dead melanoma cells that had been fixed in 4% paraformaldehyde. The purity of isolated macrophages was more than 92% (Supplementary Fig. S3A).
Purification of mouse neutrophils and depletion of cellular subsets in vivo
Mouse blood was isolated as previously described (13). Cells were cultured in OptiMEM with 0.5% FBS. F4/80+ macrophages were depleted 90% or 97% in vivo by i.v. injections of 0.1 or 0.2 mL of clodronate (5 mg/mL), respectively, or liposome vehicle (without depletion of Gr1+ neutrophils or CD11c+ cells; Supplementary Fig. S2Bc and S2Bf). To evaluate the effect of macrophage depletion on tumor growth, clodronate or liposome vehicle were injected into mice 1 day before and every other day after implantation of 106 tumor cells, continuing throughout the experiment.
CD8+T cells or neutrophils were depleted using injections of 250 μg of anti-CD8 monoclonal antibody (mAb) YTS, or the Ly6G neutrophil marker mAb 1A8, or IgG2a mAb 2A3 (BioX-cell) as isotype control Gr1 for 3 days before implanting with melanoma cells, with 100-μg mAb injections every other day thereafter. Systemic depletion of these leukocytes in bone marrow was evaluated at the study endpoint using flow cytometry.
Immunocytochemistry, immunohistochemistry, cytokine array and ELISA
Immunostaining was performed according to the previously described protocol (5), using antibodies against S-100 and MART1. Inflammatory Cytokine Arrays and ELISA were performed as described previously (5).
Phagocytic latex bead assay ex vivo
Peritoneal cells were collected from mice 3 days after injection with 2 mL of 4% thioglycollate, then cultured in DMEM with 20% FBS 1 hour. The nonadherent cells were removed and adherent cells were cultured in fresh medium overnight, then cultured with 5 μL of fluorescent blue–labeled latex beads (size 2 μm; Sigma; #L0280) for 2 hours. Cells that phagocytized latex beads were analyzed by FACS and GFP+ macrophages that express blue fluorescence were counted.
Tetramer assay
A single-cell suspension was prepared from lung tumor tissues. H-2K(b) monomer loaded with SVYDFFVWL (TRP2) peptide was provided by the NIH Tetramer Core Facility and tetramerized using APC-labeled streptavidin. Cells were stained with PerCP-Cy5.5–conjugated anti-CD8 antibody and fluorescent TRP2 tetramer. The TRP2-tetramer–positive CD8+ T cells were enumerated by flow cytometry.
Statistical analysis
Data are expressed as mean ±SEM; the unpaired, two-tailed Student t test was used to determine P values. P < 0.05 were considered significant.
Results
Myeloid IKKβ is essential for antitumorigenic immunity
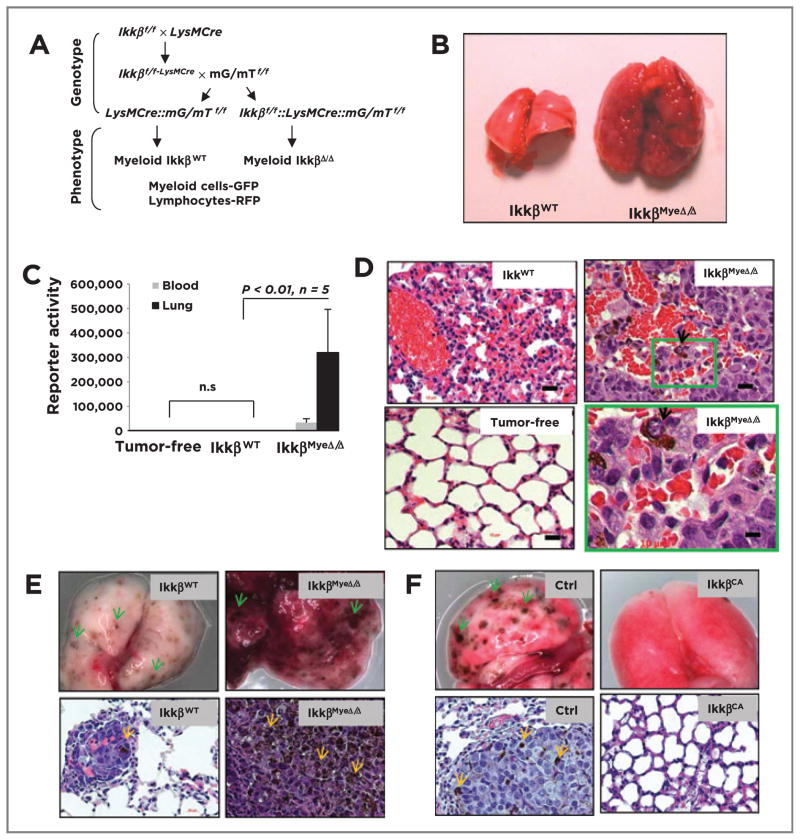
C57/BL6 and FVB mice with targeted deletion of Ikkβ in myeloid cells (IkkβMyeΔ/Δ) were generated by efficient Cre-loxP–mediated recombination in macrophages and neutrophils, but not in T cells or in the majority of B cells and DCs (Fig. 1A; refs. 11, 14). To test whether IKKβ in myeloid cells influences immunity against melanoma, 106 melanoma cells derived from BrafV600E/Pten−/− mice (on a mixed C57BL/6 × FVB background) after induction with 4-HT (Supplementary Fig. S1Aa; ref. 12), were i.v. injected into C57BL/6 IkkβMyeΔ/Δ mice (11) or into control IkkβWT C57BL/6 litter mates (20 mice/group). Three weeks later, all IkkβMyeΔ/Δ mice had difficulty breathing and had large abnormal lungs (0.99 ± 0.116 g) in contrast to the IkkβWT recipients (0.29 ± 0.013 g; Fig. 1B). To quantitate melanoma masses in the lung, melanoma cells were engineered to express Gaussian luciferase (Gluc). Four weeks after reporter animals (5 mice/group) received Gluc-expressing melanoma cells, Gluc activity was dramatically higher in lung tissue of IkkβMyeΔ/Δ mice (319,763 ± 176,717) compared with the IkkβWT mice (623 ± 182), or tumor-free controls (732 ± 117; Fig. 1C). Similar results were obtained when the BrafV600E/Pten−/− melanoma cells were injected into FVB mice (Supplementary Fig. S1B and S1C). H&E staining detected melanoma lesions in IkkβMyeΔ/Δ lungs, but not in IkkβWT controls (Fig. 1D), and lesions were verified as melanocytic by immunohistochemical staining with melanocyte and melanoma markers S-100 and MART-1, respectively (Supplementary Fig. S1D). When BrafV600E/Pten−/− melanoma cells (1 × 106) were injected intrasplenically into C57BL6 or FVB mice, similar results were obtained (Supplementary Fig. S1E–S1H), indicating that the metastatic potential in IKKβMyeΔ/Δ mice was not organ-specific. Moreover, when BrafV600E/Pten−/− melanoma cells were implanted subcutaneously, xenografts grew steadily in IkkβMyeΔ/Δ mice, while cells implanted to IkkβWT mice grew significantly slower for the first 2 weeks and subsequently regressed (Supplementary Fig. S1A and S1B; P < 0.01, n = 7).
Figure 1.
Deletion of Ikkβ in myeloid cells leads to protumorigenic immunity. A, generation of mice with myeloid Ikkβ deletion. Ikkβf/f mice (C57BL6) crossed with myeloid specific LysMCre mice (C57BL6) and mG/mT mice (C57BL6). Cre+ myeloid cells express GFP, whereas other Cre+ cells (e.g., lymphocytes) express Tomato red protein. B, BrafV600E/Pten−/− melanoma cells form melanoma lung lesions in IkkβMyeΔ/Δ mice, but not in IkkβWT mice. C, quantitation of lung melanoma lesions based on Gluc activity (mean ± SEM) in the blood or lung 4 weeks after G-luc-BrafV600E/Pten−/− cells were i.v. injected into IkkβΔ/Δ or IkkβWT C57B/6 mice or tumor-free IkkβWT mice. D, histology of melanoma lesions in lungs from C based on H&E staining. Arrows, melanoma cells in lungs of IkkβmyeΔ/Δ mice. E, myeloid IKKβ deletion enhances B16F0-Gluc melanoma growth in lung in syngeneic IkkβMyeΔ/Δ mice versus littermate IkkβWT mice. Twenty days after i.v. injection of tumor cells, lungs were perfused and photographed (top), or fixed and stained with H&E (bottom). F, B16F0 melanoma growth in C57BL6 myeloid IKKβCA mice and littermate control mice (single transgene cfms-rtTA or TetOn cIKKβ) received B16F0-Gluc melanoma cells i.v. and transgene was induced with doxycycline. After 20 days, lungs were collected for photography (top) and H&E staining (bottom). Arrows, the pigmented melanoma lesion. Scale bar, 50 μm.
To further explore the impact of IkkβMyeΔ/Δ myeloid cells on tumorigenesis in the inducible BrafV600E/Pten−/− mice, bone marrow cells from donor C57BL6 IkkβMyeΔ/Δ mice or littermate IkkβWT mice were transplanted into recipient C57BL6/FVB (Braff/f::Ptenf/f::Tyr-Cre) mice (Supplementary Fig. S1J–S1L). Recipient animals (20 mice/group) were treated with topical 4-HT to induce Tyr-Cre–mediated expression of BRAFV600E and deletion of PTEN in melanocytes, which then progress to melanoma (12). Five weeks after induction, typical pigmented melanomas occurred at the treatment site more frequently in irradiated mice transplanted with bone marrow from C57BL6 IkkβMyeΔ/Δ mice as compared mice transplanted with marrow from IkkβWT mice (Supplementary Fig. S1M and S1N). Because of the leakiness of the inducible Tyr-Cre system (15), spontaneous tumors appeared more frequently on mice transplanted with IkkβMyeΔ/Δ than IkkβWT marrow (Supplementary Fig. S1O and S1P).
When a syngeneic B16F0 melanoma model was examined using similar protocols, we observed that melanoma tumor burden was significantly enhanced in the lung of IkkβMyeΔ/Δ mice in comparison with that of IkkβWT mice (Gluc activity respectively: 30,489 ± 2,759 vs. 6,549 ± 3,457; P < 0.01; n =6; Fig. 1E). Also using this B16F0 model, mice expressing a constitutively active IKKβ (IKKβCA) showed only a few lung lesions based on gross visual and H&E-stained histologic analyses, in contrast to the numerous melanoma lesions in the lungs of IKKβWT littermates (Fig. 1F and Supplementary Fig. SIQ). Tumor reporter-Gluc activity was significantly reduced in the lungs of IKKβCA mice compared with controls (4,304 ± 1,479 vs. 31,396 ± 6,493; P < 0.01; n =6) with two experimental repeats. It should be noted that the B16F0 tumors grow faster in the pure C57Bl/6 vector control mice (control for the IkkβCA) as compared with the IkkβWT (control for the IkkβMyeΔ/Δ mice) that have been bred from FVB/129 background onto the C57Bl/6 background. Altogether, these data show deletion of IKKβ in myeloid cells results in a dramatic reduction of antitumor immunity in both syngeneic and allogenic models of melanoma, while enhanced IKKβ activity (IkkβCA) results in enhanced antitumor response.
The macrophage is a key mediator in antitumor immunity
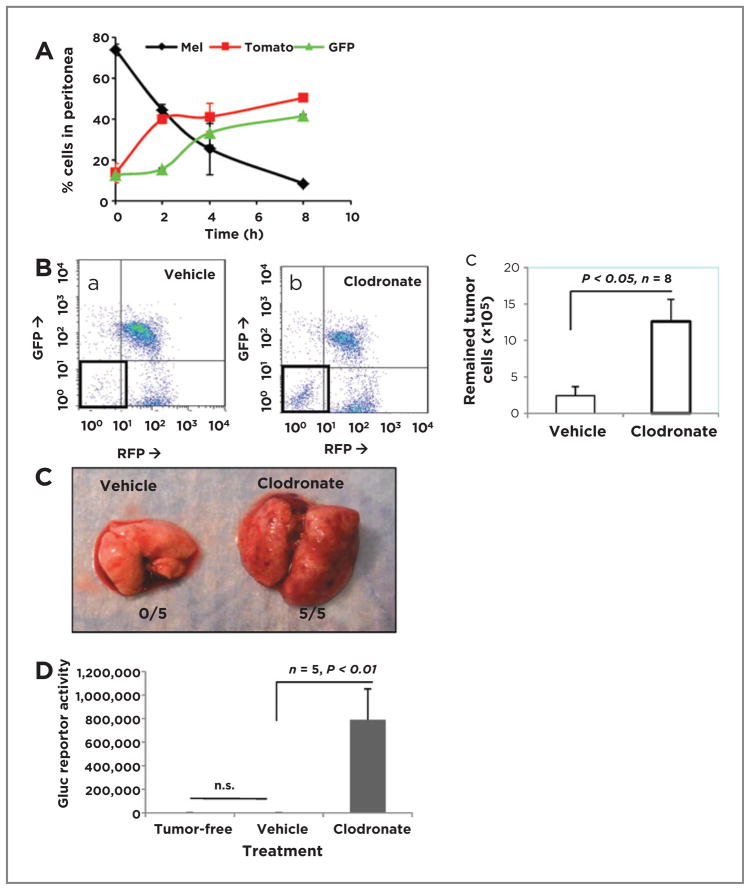
We sought first to explore whether macrophages might influence antimelanoma immunity using a peritoneal tumor cell recruitment assay. These analyses revealed that there were progressive increases in leukocytes and decreases in tumor cells in the peritoneum (Fig. 2A). As this finding suggested that recruited leukocytes may be eliminating the melanoma, macrophages were depleted by clodronate treatment, leading to a 5.25-fold increase in tumor cells (12.6 ± 3.0 × 105) compared with the liposome control group (2.4 ± 1.2 × 105; P < 0.05; n =8; Fig. 2Ba–c). Clodronate treatment had no effect on CD11c+ DCs within the time course of this assay (Supplementary Fig. S2Bf) and did not deplete Gr-1+ neutrophils or DCs (Supplementary Fig. S2Bc). Moreover, in vitro experiments demonstrated that effects of clodronate were similar to that of liposome vehicle controls on survival and growth of melanoma cells (Supplementary Fig. S2E).
Figure 2.
Macrophages play an essential role in melanoma innate immunity. A, BrafV600E/Pten−/− melanoma cells (1 × 107) were i.p. injected into mG/mT::lysM-Cre C57BL6 mice (n = 3). Infiltrating GFP myeloid cells, Tomato red lymphocytes, and tumor cells in the peritoneum were subsequently collected over 0 to 8 hours, analyzed by FACS, and graphed as percentage cells in peritoneum. B, BrafV600E/Pten−/− melanoma cells (107) were injected i.p. into each of 8 mice pretreated with clodronate or liposome vehicle to deplete macrophages. The next day, peritoneal cells were analyzed by FACS. C, Gluc-BrafV600E/Pten−/− cells delivered i.v. colonize lungs of mice with macrophages depleted 3 weeks of clodronate treatment. D, quantitation of Gluc activity in lungs of Gluc-BrafV600E/Pten−/− tumor-injected mice treated as in C (n = 5, P < 0.01).
To extend this finding, Gluc BrafV600E/Pten−/− melanoma cells were delivered i.v. to IKKβWT C57BL6 mice (5 per group). Melanoma lesions were identified in the lungs of clodronate-treated mice, but not in the liposome vehicle controls (Fig. 2C). Gluc activity was 439-fold higher in macrophage-depleted mice (788,198 ± 264,690) than in controls (1,793 ± 609; P < 0.01; n = 5). The lung Gluc activity was not different between control group and tumor-free mice (1,530 ± 191; P = 0.44; n = 5; Fig. 2D). In contrast, when neutrophils were depleted in IkkβWT mice using anti-Ly6G antibody (Supplementary Fig. S2C) there was only a 6-fold increase in Gluc reporter activity in lung, indicating enhanced melanoma growth in the lung of neutrophil-depleted mice (Supplementary Fig. S2D; 6,769 ± 4,085 vs. 1,131 ± 344; P < 0.05; n = 5). We conclude that both macrophages and neutrophils lead to inhibition of melanoma tumor growth, but macrophages have a much more striking effect (439- vs. 6-fold increases in tumor size after macrophage depletion vs. neutrophil depletion).
NF-κB modulates macrophage-mediated tumor cell death
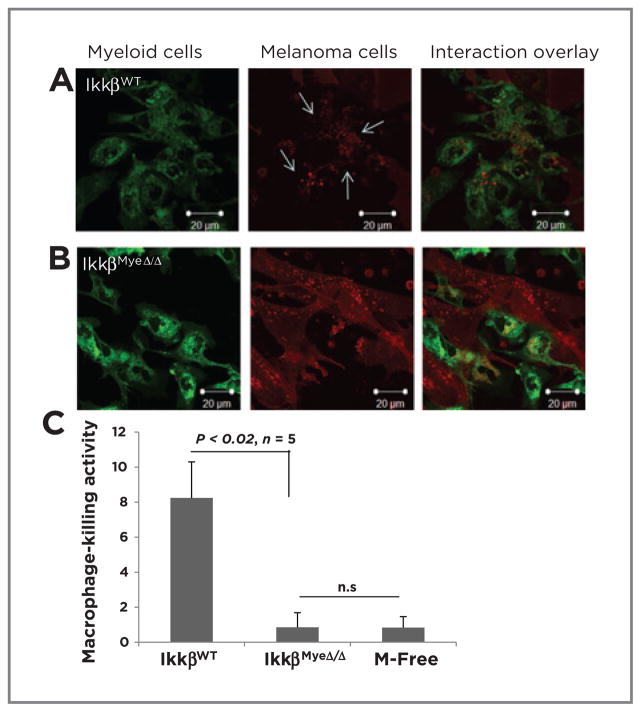
To determine whether defects in macrophage tumor cell killing and phagocytosis were responsible for the increased tumor growth in the IkkβMyeΔ/Δ mice, we cocultured macrophages isolated from the peritoneum (Supplementary Fig. S3A) with RFP-tagged tumor cells. In contrast to IkkβWT macrophages (Fig. 3A), IkkβMyeΔ/Δ macrophages exhibited poor phagocytic activity toward RFP-tagged BrafV600E/Pten−/− melanoma cells based on uptake of RFP (Fig. 3B). Moreover, the tumor cell kill after 5-hour coculture with macrophages was significantly lower with IkkβMyeΔ/Δ macrophages (0.9% ± 0.8%) than IkkβWT cells (8.2% ± 2.1%; P < 0.02; n = 5). Cell death in the macrophage-free cultured melanoma cells was comparable with that of cells incubated with IkkβMyeΔ/Δ macrophage (0.8% ± 0.6%; Fig. 3C). This result was confirmed by latex bead assays that found the efficiency of macrophage phagocytosis was significantly reduced upon loss of IKKβ (Supplementary Fig. S3C). However, when cell–cell interaction was observed after prolonged culture (3 days), IKKβΔ/Δ macrophages did eventually engulf tumor cells, though engulfed tumor cells appeared not to undergo digestion (Supplementary Fig. S3B). Thus, loss of macrophage IKKβ resulted in less efficient phagocytosis of tumor cells.
Figure 3.
IKKβ regulates macrophage phagocytosis. GFP-expressing macrophages isolated from IkkβWT (A) or IkkβMyeΔ/Δ (B) were cocultured with melanoma cells expressing RFP (3:1) for 5 hours and examined by confocal microscopy. Scale bars, magnification. C, macrophage killing activity of cells recovered after coculture in A and B were stained with 7-AAD and subjected to FACS analysis.
Myeloid IKKβ is important for macrophage and CD8 T cell–mediated cytotoxicity
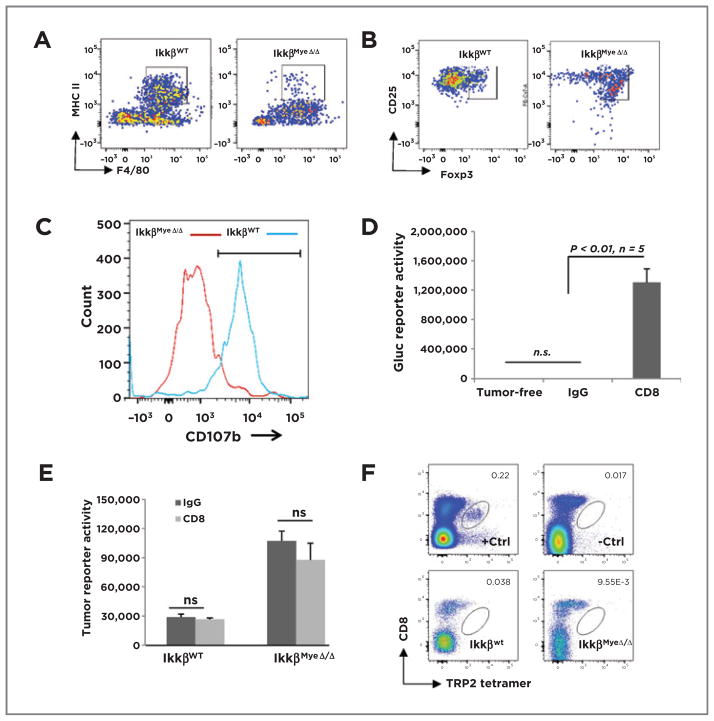
To determine the impact of IKKβ loss on the infiltrating lung macrophages and their expression of MHC II+, we performed FACS analysis of lungs from IkkβMyeΔ/Δ mice or IkkβWt littermates that had received BRAFV600E/PTEN−/−cells (1 × 106) i.v. The number of F4/80+ macrophages infiltrating into lungs of IkkβWt mice was nearly twice that of IkkβMyeΔ/Δ 24 hours after tumor cell injection (12,060 ± 1,660 vs. 6,943 ± 1,294, respectively). The IkkβMyeΔ/Δ mice exhibited greatly diminished numbers of F4/80+/MHC II+ double-positive cells in comparison with the IkkβWT mice (2.9% ± 1.0% vs. 50% ± 4.3%; P < 0.01; n = 6; Fig. 4A). These data suggest that loss of IKKβ reduces the number of F4/80+ cells in the lung in response to tumor by about 42%, but the number of F4/80+ macrophages expressing MHCII is reduced 16-fold (or >7-fold taking into consideration the reduction in total F4/80+macrophages in the lung).
Figure 4.
Myeloid IKKβ mediates macrophage and CD8+cytotoxicity. A, C57Bl/6 IkkβWT or IkkβMyeΔ/Δ mice were i.v. injected with BrafV600E/Pten−/− cells. After 24 hours, GFP+ lung macrophages were stained for F4/80 and MHCII and analyzed by FACS. B, BrafV600E/Pten−/− cells were injected i.v. into mice carrying IkkβWt or IkkβMyeΔ/Δ myeloid cells. Pulmonary Tomato-RFP CD4+ T cells double-positive for CD25 and Foxp3 were analyzed by FACS 3 days after injection. C, using the protocol described in B, lung Tomato-RFP lymphocytes positive for both CD8 and CD107b were evaluated by FACS. D, CD8+ cells were depleted and after 3 weeks, lung tumor burden was analyzed by Gluc activity. E, CD8+ T cells were depleted 3 days before subcutaneous implantation of 5 × 104 Gluc-B16F0 melanoma cells. CD8 or control antibody injections continued 16 days before tumor burden was assessed by Gluc activity assay. F, tetramer analysis of CD8+T cells infiltrating syngeneic melanoma tumor. IkkβWt or IkkβMyeΔ/Δ mice received B16F0 melanoma cells (5 × 104) i.v. After 16 days, cells from the lungs of these mice were stained with PerCP-Cy5.5–conjugated CD8 antibody and APC-labeled tetramer with monocyte-derived TRP2 (SVYDFFVWL) peptide and analyzed FACS. +Ctrl, positive control cells from splenocytes of TRP2-immunized mouse; −Ctrl, negative control cells from splenocytes of nonimmunized mouse.
To learn the impact of IKKβ expression in macrophages on CD4+ T-cell phenotype, IkkβMyeΔ/Δ mice or IkkβWT littermates received 106 BrafV600E/Pten−/− cells i.v.. Three days later, immune cells expressing Tomato-RFP were isolated from lung, F4/80+ macrophages were excluded, and expression of Th2 and Treg markers (CD25 and Foxp3) was analyzed by FACS. The number of CD25+/Foxp3+ CD4+ T cells (Tregs) from IKKβMyeΔ/Δ mice was significantly increased over IkkβWT mice (69% ± 7.8% vs. 23% ± 5.9%; P < 0.01; n = 4; Fig. 4B). Thus, an anti-inflammatory skewing occurs as a consequence of IKKβ loss in myeloid cells.
Activated CD8+T cells release perforin and granzymes from their lytic granules to kill targets by exocytic merging of the CD107a/b-containing granule membrane with the plasma membrane (16–19). To investigate the activation status of CD8+ T cells in lung of mice with melanoma tumors, lymphocytes expressing Tomato-RFP were sorted from lung tissues and CD8+T/CD107b+double-positive cells identified by FACS. The percentage of CD107b+ CD8+ cells from tumor-bearing IkkβMyeΔ/Δ mice declined by 40% in comparison with tumor-bearing IkkβWT mice (19% ± 6.0% vs. 60% ± 7.8%; P < 0.01; n = 4; Fig. 4C). Thus, loss of IKKβ activity in myeloid cells results in poor activation of CD8+ T cells in lung, additionally supported by Supplementary Fig. S4A. A similar immune response was observed in the cutaneous melanoma model. Together, the increase in Treg and decrease in activated CTL suggest that myeloid IKKβ activity is pivotal for driving tumor cytotoxicity of CD8+ T cells.
To test the role of CTL directly, CD8+ T cells were depleted using CD8-YTS antibody to achieve 98.4% CD8+ T-cell depletion (Supplementary Fig. S4D). In contrast to control mice that completely rejected melanoma formation after input of Gluc-BrafV600E/Pten−/− melanoma cells, mice with depleted CD8+ T cells exhibited 1,152-fold increased Gluc activity, indicating significant outgrowth of metastatic melanoma lesions (Fig. 4D; 1,303,308 ± 187,269 vs. 1,131 ± 344; P < 0.01). Thus, data suggest that CD8+ T cells are required for antitumor cytotoxicity and myeloid IKKβ is essential for activation of CD8+ T cells in response to melanoma cells in the tumor allograft model.
In the syngeneic model in which less immunogenic B16F0 melanoma cells were implanted into C57/BL6 mice, there was no influence on tumor progression compared with IgG-treated mice in either of IkkβMyeΔ/Δ mice or IkkβWT mice when CD8+T cells were depleted (Fig. 4E and Supplementary Fig. S5A–S5C). A similar result was observed in the IkkβCA mice (Supplementary Fig. S5D). Thus, immune cells other than CD8+ T cells play the major antitumor role in the syngeneic model. Also, TRP2 (SVYDFFVWL) tetramer staining did not reveal B16 melanoma-specific CD8+ T cells in the lung tumor milieu (Fig. 4F and Supplementary Fig. S5E), indicating that for B16 melanoma in the syngeneic melanoma tumor model, CD8+ T cells contributed little to the antitumor response. B16 cells are reported to be poor activators of an anti-melanoma CTL response due to a significant population of Treg cells (20, 21); our data are in agreement with those prior observations.
NF-κB is required for cytokine-mediated immunity
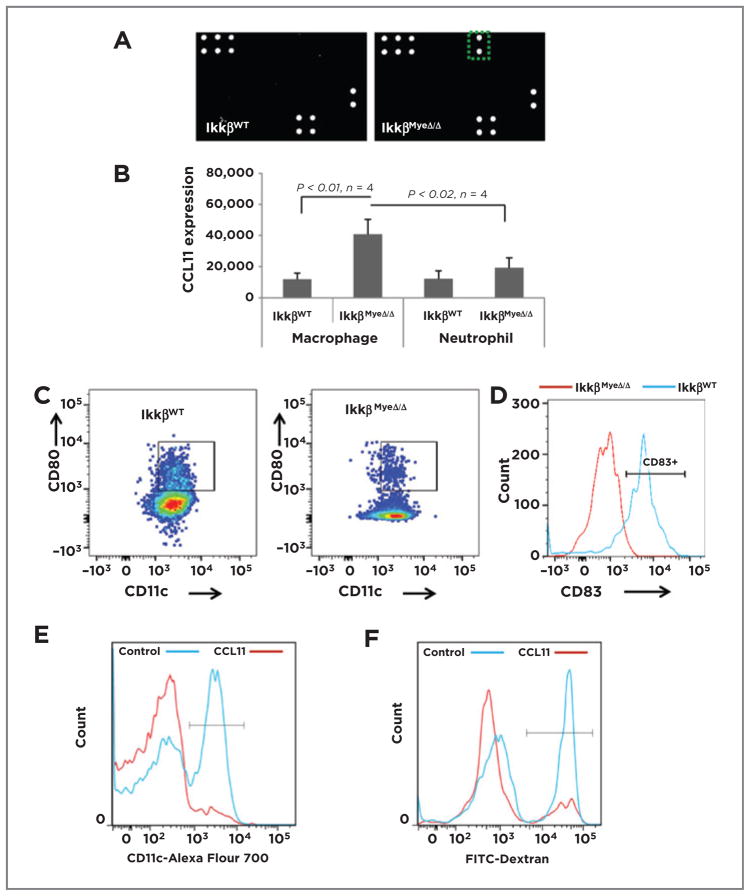
To learn whether myeloid IKKβ deletion leads to any alteration in cytokine profiles in vivo, which might affect the Th1- versus Th2-skew of immune cells, sera from non–tumor-bearing IkkβWT and IkkβMyeΔ/Δ mice were analyzed. CCL11 was elevated 19-fold in serum of IkkβMyeΔ/Δ mice compared with IkkβWT mice (Fig. 5A) and the CCL11 was expressed mainly in IKKβMyeΔ/Δ macrophages and to a lesser extent in the IKKβMyeΔ/Δ neutrophils (Fig. 5B). Because CCL11 can hinder DC differentiation (22) and affect a Th2 response (23), we hypothesized that CCL11 may link myeloid IKKβ loss with the TME. To examine the in vivo impact of CCL11 on the DC population, splenocytes isolated from IkkβWT and IkkβMyeΔ/Δ mice and stained for DCs (CD11c+, CD80+) were analyzed by FACS. IkkβWT mice had over 4-fold more DCs than mice whose myeloid lineage lacked IKKβ (9.5% ± 1.3% of DCs vs. 2.2% ± 0.12%, respectively; P < 0.01; n =5; Fig. 5C). To further study DC maturation, the cells were stained for CD83 (a marker for DC maturation) and subjected to FACS analysis. CD83 expression on the DCs of IkkβMyeΔ/Δ mice was very low (6.8% ± 3.8%), in contrast to the CD83 expression on the DCs of IkkβWT mice (82% ± 4.8%; P < 0.01; n = 4; Fig. 5D). To gain insight into the effect of CCL11 on DC generation, murine bone marrow cells from IkkβWT mice were cultured 7 days in medium with 20 ng/mL of GM-CSF and 20 ng/mL of IL4 ± 100 ng/mL of CCL11 or control PBS, collected on day 7, stained with CD11c-Alexa Fluor 700, and analyzed by FACS. CCL11 significantly reduced generation of DCs (7.7% ± 0.78% vs. 43.6% ± 1.72%; P < 0.01; n = 4; Fig. 5E). To test DC function, CD11c+ DCs were incubated with FITC-dextran 15 minutes and dextran endocytosis was analyzed by flow cytometry. CCL11-treated IKKβ-deficient DCs exhibited a 65% reduction in endocytosis compared with controls (Fig. 5F; 15.8% ± 0.84% vs. 44.7% ± 0.83%, respectively; P < 0.01; n = 4). Thus, IKKβ deletion in myeloid cells resulted in overexpression of CCL11, which contributed to reduced generation of DCs and reduced DC maturation. The loss of DC maturation in the IkkβMyeΔ/Δ mice could have significant consequences on the T-cell activation in the allograft tumor model.
Figure 5.
A, myeloid IKKβ restrains CCL11 expression. Results of cytokine arrays of sera isolated from IkkβWT and IkkβΔ/Δ mice; green rectangle (right) shows position of CCL11 signal of sera from IkkβmyeΔ/Δ mice. B, FACS analysis of intracellular CCL11 expression (mean ± SEM; n = 4) in macrophages or neutrophils isolated from spleen of the IkkβWT and IkkβΔ/Δ mice. C, splenic cells were prepared as in B and CD45+ cells were stained for CD11c and CD80 and analyzed by FACS (mean ± SEM). D, splenocytes isolated as in B were stained with CD11c and CD83 and analyzed by FACS. E, bone marrow cells from IkkβWT mice were cultured in medium containing GM-CSF and IL4 with or without 100 ng/mL of CCL11 for 7 days, then stained for CD11c by FACS. F, DCs generated from bone marrow as in E were incubated in 50 μL of FITC-dextran for 15 minutes, then cells were stained for CD11c and double-positive cells analyzed by FACS.
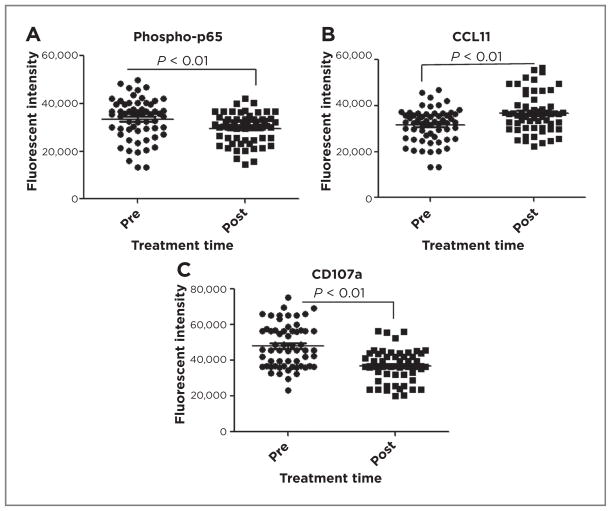
What might be clinical meanings of these findings in patients with melanoma? To explore the potential relevance of these findings in human melanoma, we evaluated CCL11 expression in macrophages (CD163+) of biopsy specimens from 6 patients with melanoma before and after treatment with the proteasome inhibitor, bortezomib (VELCADE), and temozolomide in a phase I/II clinical trial (24). VELCADE, an FDA-approved agent in some cancers, inhibits degradation of phosphorylated-IκB, thus reducing NF-κB activity by retaining RelA/p65 in the cytoplasm, but also affects a number of additional pathways (25–28). Tissues were stained for macrophages (CD163; ref. 29), phospho-RelA(p65), activated CD8+ T cells (CD107a), and CCL11, visualized by confocal microscopy, and quantitated using Metamorph (Supplementary Fig. S6A and S6B). Treatment resulted in a small but significant reduction in nuclear phospho-REL-A/p65 (29,586 ± 2,741 vs. 33,462 ± 3,460; P < 0.01; Fig. 6A), a 17% upregulation of CCL11 (36,772 ± 1,860 vs. 31,452 ± 2,224; P < 0.01; Fig. 6B), and a significant reduction in CD107a+ CD8+ T cells (36,924 ± 2,009 vs. 48,146 ± 4,641; P < 0.01; Fig. 6C) compared with pretreatment controls. The specificity of antibodies was verified on human peripheral lymphocytes (Supplementary Fig. S6C and S6D) and expression of CD107a on individual tumor-associated CD8+ T cells from VELCADE-treated patient tumors (Supplementary Fig. S6 E and S6F) was significantly reduced. Thus, our findings suggest that systemic therapy with VELCADE may act via NF-κB to change chemokine expression and CTL effector activity in patients with cancer, and that targeting NF-κB signaling in human melanoma risks negative effects on anti-tumor immunity.
Figure 6.
Association of NF-κB with myeloid CCL11 expression and T cell activity in human melanoma. Paraffin-embedded patient melanoma tumor samples, pre- or posttreatment with VELCADE and temozolomide, were stained for CD163, phosphor-p65 (A) and CCL11 (B) and fluorescent intensity of each was quantified. C, human melanoma samples were stained for CD8 and CD107a, expression was quantified and graphed (GraphPad Prism, mean ± SEM, t test, P < 0.01).
Discussion
Tumor-associated macrophages (TAM) exhibit both antitumor M1 and protumor M2 innate immunity phenotypes, indicating the highly complex milieu within the tumor (30–32). Loss-of-function studies indicate that various members of the NF-κB/Rel family of transcription factors regulate macrophage polarization (5, 10, 32, 33). Some work suggests that NF-κB activation confers an M2 phenotype to TAMs, based on observations that inhibition of NF-κB in myeloid cells elicits a switch from an M2 to M1 phenotype (33). However, Connelly and colleagues (10) showed that constitutive activation of NF-κB maintained the antitumor phenotype of macrophages, whereas NF-κB inhibition by expression of an IκBα “super-repressor” resulted in enhanced breast tumor promotion. Under certain chronic inflammatory conditions, lipopolysaccharide-tolerant macrophages accumulated p50 NF-κB homodimers that could act as negative regulators of the NF-κB signal pathway (34). Moreover, in an angiosarcoma model, loss of IKKβ in myeloid cells resulted in enhanced tumor growth (11), though in colon cancer and hepatocellular carcinoma, this myeloid loss of IKKβ had the opposite effect (7, 8). The controversy over the role of NF-κB in cells of the myeloid lineage in mediation of tumor immunity raises the intriguing question of whether one common NF-κB signal creates a diversity of transcriptional responses that are tailored to particular tissues and organs.
In this study, we advance understanding of how NF-κB affects innate immunity through the demonstration that deletion of IKKβ in myeloid cells results in macrophages with an M2-phenotype. Here, in allo- and syngeneic studies of melanoma as well as models in which melanoma is inducible in situ, loss of IKKβ in myeloid cells is associated with enhanced melanoma growth. In contrast, expression of a constitutively activated form of NF-κB in myeloid cells markedly inhibits tumor growth. Although neutrophils have been reported to have either tumor-inhibitory (N1) or tumor-enhancing (N2) properties (2), we observed that macrophages play a more dominant role in the innate immune response to melanoma.
Cytokines such as IL1 IL4, IL6, IL10, IL12, TNFα, IFNγ, and TGFβ, as well as chemokines, play an important role in the modulation of the pro- or antitumor properties of innate immune response (33). The chemokine CCL11 exhibits an inhibitory role on the differentiation of DCs and enhances subsequent Th2- polarization (22). CCL11 is upregulated by Th2 cytokines IL4 and IL13, whereas its expression is down-regulated by the Th1 cytokine IFNγ (35, 36). Here CCL11 was highly expressed in macrophages with IKKβ knockout and this blocked DC differentiation and enhanced the implied Th2 milieu, resulting in poor activation of CD8+ T cells. Although the number of clinical samples analyzed was small and not large enough to predict prognosis, our clinical data demonstrating that CCL11 was expressed by myeloid cells in melanoma tumors from a human trial using VELCADE (22) are of interest because they stress the importance of careful consideration of the immunologic effects of drugs that affect the NF-κB pathway.
A key advance of our studies is the definitive demonstration that the antitumor activity of TAMs requires NF-κB, because myeloid-targeted deletion of IKKβ resulted in macrophages with decreased ability to kill tumor cells in vitro. Moreover, we show that macrophages from IKKβmyeΔ/Δ mice exhibit marked reduction in expression of the MHC class II molecules needed to present antigens to CD4+ T cells to prime CD8+ T cells to become CTLs (37, 38). Moreover, our data suggest that in the absence of activation of the CD8+ T-cell response, the innate immune response is the major guardian in the host response to tumor.
Experiments described herein have important implications for therapeutic use of inhibitors of NF-κB in melanoma therapy. Although targeted deletion of Ikkβ in Ink4A/Arf-null melanocytes blocks mutant RAS-induced melanoma (5), systemic targeting of NF-κB with an IKKβ inhibitor is less effective in inhibiting the growth of RAS-transformed murine melanoma in immunocompetent mice, indicating a potential negative impact of the inhibitor on antitumor immunity (Hawkins, in preparation). On the basis of our data, IKKβ inhibitors will be most effective when delivered directly to tumor cells. Moreover, developing ways to heighten or retain IKKβ activity in myeloid cells, while blocking IKKβ in melanoma tumor cells, may prove to be effective for inhibition of melanoma tumor growth.
Supplementary Material
Figure S1. Myeloid Ikkβ is required for anti-tumorigenic immunity. A, Melanoma model. Mice harboring genotype of Braf CA::Ptenf/f::Tyr-CreER were maintained on a mixed C57BL6/FVB strain. These mice were treated with 1mg 4-HT. Skin tumours developed 1 month after treatment (a). H&E staining of the melanoma lesion (b) in contrast to the histology of mouse skin without lesion (c). 10× magnification. B&C, Lung metastatic melanoma. FVB mice (5/group) with a myeloid IkkβWt or IkkβMyeΔ/Δ were intravenously injected with BrafV600E/Pten−/− melanoma cells (1×106) expressing the Gluc reporter gene. Four weeks after injection, lung tissues were collected from myeloid IkkβWt mice or myeloid IkkβMyeΔ/Δ mice (B) and subjected to quantitation of live melanoma cells in the lung based on Gluc activity (C). Mock refers to negative controls of tumor-free mice. D, Immunohistochemistry. BrafV600E/Pten−/− melanoma cells (1×106) were intravenously injected into C57BL6 mice. Two weeks after injection, lung tissues were collected from IkkβWt mice (left panels) or IkkβMyeΔ/Δ mice (right panels). Melanoma cells were identified by immunohistochemical staining of S100 (upper-panels) and Mart1 (lower-panels). Bar, 50 μm. E&F, metastatic melanoma lesions in the liver. To evaluate the organ specific metastatic capacity of the mixed genetic background melanoma cells, (1×106) were intra-splenically injected into allogenic C57BL6 mice (5 animals/group). By four weeks post injection, liver metastasis were observed in IkkβMyeΔ/Δ mice but not in IkkβWt mice. Melanoma cells expressing the Gaussian luciferase (Gluc) reporter gene were injected into the spleen of C57BL/6 IkkβMyeΔ/Δ or IkkβWt mice. IkkβWt mice without tumor cell injection served as a tumor-free control. Histological analysis of H&E stained paraffin embedded sections of liver taken from mice four weeks after injection of tumor cells as described in E. Bar, 50 μm. Quantitation of the metastatic lesions based upon Gluc activity in in 10μg of liver tissue was determined to indicate the quantity of remaining live melanoma cells in IkkβWt and IkkβMyeΔ/Δ mice (F). Data demonstrated that myeloid C57BL6 IkkβMyeΔ/Δ mice were permissive for growth and metastasis of C57BL6/FVB melanoma cells, in contrast to C57BL6 IkkβWt mice that successfully rejected the allogeneic melanoma. Data are expressed as mean ± SEM and were statistically analyzed by the Student’s t-test. G&H, the phenotype exhibited in the C57BL6 strain mice was also observed in the allogeneic FVB mice. Mixed background BrafV600E/Pten−/− melanoma cells (1×106) expressing the Gluc reporter gene were intravenously injected into FVB mice (5/group) with myeloid IkkβWt or IkkβMyeΔ/Δ. Four weeks after injection, liver tissues were collected from myeloid IkkβWt mice or myeloid IkkβMyeΔ/Δ mice (G, Bar, 50 μm.) and subjected to Gluc activity analysis for a quantitative estimate of the live melanoma cells in the liver, (H). Mock refers to negative controls of tumor-free mice. Arrow indicates a metastatic lesion. I, melanoma xenografts. A melanoma cell line was derived from a 4-HT induced-BrafV600E/Pten−/− melanoma lesion growing on a mixed background C56BL6/FVB mouse and cultured in DMEM/F-12 medium containing 10% FBS. 1×107 of these melanoma cells were subcutaneously inoculated into C57BL6 mice with myeloid IkkβWt or IkkβMyeΔ/Δ background. Tumor size was measured on days 10, 15 and 27. Data were statistically analyzed by the Student’s t-test. **p<0.01, n=7. J, K, L, Bone Marrow Transplant (BMT). For evaluation of the reconstituted bone marrow, the mixed C57BL6/FVB recipient mice harboring BrafCA::Ptenf/f::Tyr-CreER alleles were irradiated and transplanted with 1×106 bone marrow cells of C57BL6 donor mice carrying LysM-Cre::mG/mT genes. Lymphocytes expressing tdTomato-RFP and appearing in the peripheral blood were monitored weekly by flow cytometry. The red-immune cells in peripheral blood were normalized to that of non-irradiated donor mice (LysM-Cre::mG/mT) and expressed as percentage of reconstituted bone marrow. However, to evaluate how soon bone marrow depletion occurs in recipient mice after irradiation, mice (LysM-Cre::mG/mT) were irradiated and transplanted with bone marrow cells not expressing fluorescent protein. The percentage of red immune cells in blood indicates the remaining bone marrow cells after irradiation (J). n=5
To examine the cell composition of the reconstituted bone marrow, bone marrow cells were isolated from recipient mice or donor mice as a control. Cells were stained with fluorescent conjugated specific antibodies to neutrophils (Gr1-APC-Cy7), macrophages (F4/80-Percp/Cy5.5), B cells (B220-Alexa Fluor 700), CD4+ T cells (CD4-pacific blue) and CD8+ T cells (CD8a-APC). Cells were sorted as myeloid expressing GFP or lymphocyte expressing tomato and analyzed by FACS (K). n=2.
To evaluate the immune response of recipient mice to tumor cells, recipient mice (n=5) or donor mice (n=5) were injected in the peritoneum with melanoma cells (1×107) or PBS as a vehicle control. Twenty-four hours after injection, the GFP+ myeloid cells in the peripheral blood stream were monitored by FACS analysis (L). M&N, inducible melanoma model. Mixed C57BL6/FVB recipient mice (20/group) harboring Braf CA::Ptenf/f::Tyr-CreER alleles received bone marrow cells (1×106) from C57BL6 strain IkkβMyeΔ/Δ mice (Ikkβf/f::LysM-Cre::mG/mT) or IkkβWt mice (LysM-Cre::mG/mT) as controls. Three weeks after BMT, 5 μl of 10 mmol/L 4-HT was topically applied daily to the shaven back of recipient mice for 3 days. Five weeks post induction, tumour size was photographed (M), measured and statistically analysed by the Student’s t-test (N). Data are expressed as mean ± SEM. O&P, spontaneous melanoma model. Mixed C57BL6/FVB recipient mice (20/group) harboring BrafCA::Ptenf/f::Tyr-CreER alleles received bone marrow cells (1×106) from C57BL6 IkkβMyeΔ/Δ mice (Ikkβf/f::LysM-Cre::mG/mT) or IkkβWt mice (LysM-Cre::mG/mT) as controls. Eight weeks after BMT, the pigmented neoplastic area was photographed (O), measured and statistically analysed by a Student’s t-test (P). Data are expressed as mean ±SEM. Q, diagram of inducible constitutively active IKKβ in myeloid cells of mice. C57BL6 mice harboring genetic background of cfms-rt-TA/TetOn-IkkβCA or littermates with cfms-rt-TA genes served as controls accessed to drinking water containing 2 mg/ml doxycycline between day 0 and day 9. Fifty thousand B16 melanoma cells were intravenously injected on day 7. On day 20, mice were sacrificed and lungs were perfused and collected for evaluation of both tumor growth and immune response.
Figure S2. Myeloid cell depletion alterstumor immunity. A, Depletion of macrophages in vivo. Mice were intravenously given (a–c) 0, 0.1 or 0.2 ml of clodronate, then 1 × 106 melanoma tumor cells were injected into the peritoneum. The next day cells in the peritoneum were collected, stained with fluorescence-conjugated APC-CD45, PerCP/Cy5.5-F4/80 antibody and analyzed by FACS. MΦ refers to macrophage. B, clodronate specifically depletes macrophages. Mice received intravenous injection of PBS (a, d), liposome vehicle (b, e) or 1 mg of clodronate (c, f) immediately followed by peritoneal injection of PBS (a, d), or melanoma cells (b, c, e, f). The next day after injection, peritoneal cells were collected and sorted for the Gr1+, F4/80+ and CD11c+ cell population. Neu, neutrophil; MΦ, macrophage; DC, dendritic cell. C, Depletion of neutrophils in vivo. Mice were intravenously injected with 250 μg of antibody to Ly6G or isotype IgG2a daily for three days prior to intravenous delivery of 1 × 106 melanoma cells. Then 100 μg antibody was delivered every other day. On day 5, mice were euthanized and the bone marrow cells were stained with PE-conjugated Ly6G and subjected to FACS analysis. D, depletion of neutrophils promotes lung metastasis. Mice (5/group) received intravenous treatment with ly6G antibody or IgG2a isotype control antibody and intravenously implanted with Gluc-melanoma cells as described in Methods. Three weeks later, mice were euthanized, lung tissue was sonically lysed, and 10 μg lung tissue protein was subjected to the Gluc activity assay. n=5, p<0.05. Ly6G antibody treatment vs IgG2a isotype control. Tumor-free, mice were neither treated with antibody nor given tumor cells. E, Clodronate toxicity on melanoma cells. The cultured melanoma cells engineered with Gluc reporter were treated with clodronate at increasing concentrations of 0, 125, 250, 500, 1000, 10000 ng/ml. The cells were treated with the same amount of vehicle liposome served as controls. After two days of culture, the remained cells were lysed and subjected to luciferase activity assay that reports activity that reflects the relative proportion of surviving cells. The experiment was repeated twice and data were expressed as mean ±SEM.
Figure S3. IKKβ loss alters macrophage phagocytosis capacity. Morphology of macrophage phagocytosis ex vivo. A, Isolation and purity of macrophages. Macrophages were isolated from the peritoneal cavity of either IkkβWt or IkkβMyeΔ/Δ mice 24h after injection with dead melanoma cells. The cell purity was assessed by staining with Percp-Cy5.5 conjugated F4/80 antibody and analyzed by FACS. Cells double positive for GFP and F4/80 are macrophages (MΦ). Macrophage purity was over 92%. B, visualization of phagocytosis of tumor cells. Macrophages (green) with either IkkβWt or IkkβMyeΔ/Δ genotype were allowed to interact with melanoma cells (red) using the co-cultured approach for three days. Tumor cell phagocytosis was visualized by Confocal microscopy (LSM 510META). C, Macrophages capacity assessed by phagocytic latex beads. Macrophages were isolated from the peritoneal cavity of either IkkβWt or IkkβMyeΔ/Δ mice 72h after injection with 2ml of 4% thioglycollate. Cells were co-cultured with fluorescent blue labeled-latex beads. After 2 hours culture, cells were subjected to flow cytometry analysis for the percent of cells expressing the fluorescent blue. IkkβMyeΔ/Δ macrophages showed significant reduction of taking up latex beads in compared with IkkβWt mice macrophages (1.6±0.6% vs. 8.5±2.7%, p<0.01, n=6).
Figure S4. Myeloid IKKβ deletion reduced the cytotoxicity of T cells. A, myeloid IKKβ deletion reduced the number of CD8+CD107b+ cells in the tumor tissue. Myeloid IkkβWt or IkkβMyeΔ/Δ mice were subcutaneously inoculated with melanoma cells (1×107). On days 1, 2, 3 post-implant, infiltrating RFP+ lymphocytes were sorted by FACS and the CD8 expressing CD107b were analyzed per 10,000 cells. B, Myeloid cell infiltration. IkkβWt or IkkβMyeΔ/Δ mice were intravenously injected with melanoma cells (1×106). The infiltration of myeloid cells (GFP positive) into lung tissues was analyzed by FACS over the time course as indicated. C, CD8 T cell infiltration. The infiltration of CD8+T cells in the above lung tissues were sorted using APC-conjugated antibody to CD8 and analyzed by FACS. Data are expressed as CD8+ T cells/10,000 lung cells and the statistical analysis utilized by the Student’s t-test. Wt refers to IkkβWt mice; Ko refers to IkkβMyeΔ/Δ mice. D, depletion of CD8+ T cells in vivo. Mice were intravenously injected with 250 μg of CD8 antibody or isotype IgG2a daily for three days prior to i.v. injection of 1 × 106 melanoma cells. Then 100 μg antibody was injected I.v. every other day. On day 5, the bone marrow cells were stained with APC-conjugated CD8 antibody and subjected to FACS analysis. E, Neutrophil purity and viability. Peripheral blood was collected from mice and the neutrophils were isolated from the Percoll gradient 1.077–1.119 interface. The percentage cell purity was determined by sorting cells expressing GFP and staining for Gr1-APC-Cy7 or F4/80-Percp Cy5.5. Cell viability was determined by staining with 7-AAD.
Figure S5. Cytokines play a major role in orchestrating the cellular make-up of the tumor microenvironment. A, Efficiency of CD8 cell depletion in peripheral blood. C57BL6 mice were intraperitoneally injected 200 μg CD8 antibody/mouse daily for three days and then continued injection twice a week for 16 days. Meanwhile, mice injected with the same amount of isotype IgG as a control. CD45+CD8+ cells were analyzed by flow cytometry in the peripheral blood (A) on day 5, in spleen (B) and lung (C) on day 16. D, elimination of CD8 T cells in the syngeneic reaction. CD8+ cells in C57BL6 mice carrying myeloid IkkβCA or IkkβWT were depleted by intraperitoneal injection CD8 antibody while mice injected with isotype IgG as a control for three days. Mice were intravenously injected with syngeneic B16 melanoma cells (5×104) expressing Gluc reporter. Antibody injection continued bi-weekly for 16 days until lung tumor burden was quantitatively determined by Gluc activity. E, TRP-2-specific CTL response in a syngeneic B16 melanoma model. C57BL6 mice carrying IkkβCA or IkkβWT myeloid cells intravenously received syngeneic B16 melanoma cells (5×104). After 16 days a single cell suspension was prepared from lung tumor tissues. Cells were stained with PerCP-Cy5.5-conjugated CD8 antibody and APC-labeled TRP2 (SVYDFFVWL) tetramer. The APC positive CD8 T cells were analyzed by flow cytometry. +Ctrl, positive control cells from the splenocytes of TRP2-immunized mouse; -Ctrl, negative control cells from the splenocytes of non-immunized mouse.
Figure S6. Immuno-fluorescent staining human melanoma tissues. A&B, Paraffin-embedded tissue samples from melanoma patients, pre- or post-treatment, were stained with fluorochrome-conjugated CD163, phospho-p65 and CCL11 antibodies. The stained tissues were visualized by confocal microscopy, photographed and quantified by linescan using the Metamorph program version 7.6.4. C, a representative photo of human peripheral lymphocytes stained with AlexaFlour448-conjugated CD8 antibody and AlexaFlour647-conjugated CD107a antibody is shown. D, Linescan quantitation of the CD8+ T cells expressing CD107a positive (a) or negative fluorescence (b). E-a, a representative photo of CD8 T cells in the pre-treated human melanoma or F-a, a representative photo of CD8 T cells in the post-treated human melanoma stained with AlexaFlour448-conjugated CD8 antibody and AlexaFlour647-conjugated CD107a antibody. Line Scan quantitation of the CD8+ T cells expressing CD107a in pre-treatment (E-b) or post-treatment group (F-b).
Acknowledgments
The authors thank Linda W. Horton, Melissa Downing, and Taylor Sherrill for technical assistance. The authors also thank Martin McMahon (University of California, San Francisco, San Francisco, CA) for the Tyr::CreER/BRAFCA::PTENf/f mice and Hal Moses for the LysM-Cre mice. The authors acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of TRP2 monomer. We also thank Jeff Sosman and Mark Kelley for their collaboration in obtaining the tissue blocks from the VELCADE/TMZ melanoma clinical trial.
Grant Support
Work was supported by grants from the Tennessee Valley Healthcare System, Department of Veterans Affairs: VA Merit (A Richmond, S. Joyce) and VA Research Career Scientist Awards (A. Richmond); and grants from the NIH: R01-CA116021 (A. Richmond) and 5P30CA068485 (J. Pietenpol) for Cancer Center Core Facility Support.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: M. Boothby, G.D. Ayers, F.E. Yull, A. Richmond
Development of methodology: J. Yang, P. Gilchuk, F.E. Yull
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): J. Yang, O.E. Hawkins, W. Barham, S. Joyce, M. Karin, F.E. Yull
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J. Yang, G.D. Ayers, S. Joyce
Writing, review, and/or revision of the manuscript: J. Yang, W. Barham, M. Boothby, F.E. Yull, A. Richmond
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): A. Richmond
Study supervision: M. Boothby, F.E. Yull, A. Richmond
Other (tetramers preparation, staining and flow cyotmetric analysis for enumeration of antigen-specific CD8+ T cells, data analysis, and figures preparation for tetramer staining experiments): P. Gilchuk, S. Joyce
References
- 1.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 2.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Ann Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–7. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Splittgerber R, Yull FE, Kantrow S, Ayers GD, Karin M, et al. Conditional ablation of Ikkb inhibits melanoma tumor development in mice. J Clin Invest. 2010;120:2563–74. doi: 10.1172/JCI42358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adli M, Baldwin AS. IKK-i/IKKepsilon controls constitutive, cancer cell–associated NF-kappaB activity via regulation of Ser-536 p65/RelA phosphorylation. J Biol Chem. 2006;281:26976–84. doi: 10.1074/jbc.M603133200. [DOI] [PubMed] [Google Scholar]
- 7.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–90. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–32. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 10.Connelly L, Barham W, Onishko HM, Chen L, Sherrill TP, Zabuawala T, et al. NF-kappaB activation within macrophages leads to an anti-tumor phenotype in a mammary tumor lung metastasis model. Breast Cancer Res. 2011;13:R83. doi: 10.1186/bcr2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Kantrow S, Sai J, Hawkins OE, Boothby M, Ayers GD, et al. Ikk4a/Arf inactivation with activation of the NF-kappaB/IL-6 pathway is sufficient to drive the development and growth of angiosarcoma. Cancer Res. 2012;72:4682–95. doi: 10.1158/0008-5472.CAN-12-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–14. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–77. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 15.Hooijkaas AI, Gadiot J, van der Valk M, Mooi WJ, Blank CU. Targeting BRAFV600E in an inducible murine model of melanoma. Am J Pathol. 2012;181:785–94. doi: 10.1016/j.ajpath.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. [PubMed] [Google Scholar]
- 17.Henkart PA. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994;1:343–6. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 18.Kannan K, Stewart RM, Bounds W, Carlsson SR, Fukuda M, Betzing KW, et al. Lysosome-associated membrane proteins h-LAMP1 (CD107a) and h-LAMP2 (CD107b) are activation-dependent cell surface glycoproteins in human peripheral blood mononuclear cells which mediate cell adhesion to vascular endothelium. Cell Immunol. 1996;171:10–9. doi: 10.1006/cimm.1996.0167. [DOI] [PubMed] [Google Scholar]
- 19.Peters PJ, Borst J, Oorschot V, Fukuda M, Krahenbuhl O, Tschopp J, et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173:1099–109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiger JD, Wagner PD, Cameron MJ, Shu S, Chang AE. Generation of T-cells reactive to the poorly immunogenic B16-BL6 melanoma with efficacy in the treatment of spontaneous metastases. J Immunother Emphasis Tumor Immunol. 1993;13:153–65. doi: 10.1097/00002371-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kline J, Brown IE, Zha YY, Blank C, Strickler J, Wouters H, et al. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14:3156–67. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson NJ, Addley MR, Ryan EJ, Boyd CR, Carroll HP, Paunovic V, et al. CCL11 blocks IL-4 and GM-CSF signaling in hematopoietic cells and hinders dendritic cell differentiation via suppressor of cytokine signaling expression. J Leuk Biol. 2009;85:289–97. doi: 10.1189/jlb.0708394. [DOI] [PubMed] [Google Scholar]
- 23.Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–54. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su Y, Amiri KI, Horton LW, Yu Y, Ayers GD, Koehler E, et al. A phase I trial of bortezomib with temozolomide in patients with advanced melanoma: toxicities, antitumor effects, and modulation of therapeutic targets. Clin Cancer Res. 2010;16:348–57. doi: 10.1158/1078-0432.CCR-09-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005;65:3828–36. doi: 10.1158/0008-5472.CAN-04-3684. [DOI] [PubMed] [Google Scholar]
- 26.Shahshahan MA, Beckley MN, Jazirehi AR. Potential usage of proteasome inhibitor bortezomib (Velcade, PS-341) in the treatment of metastatic melanoma: basic and clinical aspects. Am J Cancer Res. 2011;1:913–24. [PMC free article] [PubMed] [Google Scholar]
- 27.Fribley AM, Evenchik B, Zeng Q, Park BK, Guan JY, Zhang H, et al. Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem. 2006;281:31440–7. doi: 10.1074/jbc.M604356200. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez Y, Verhaegen M, Miller TP, Rush JL, Steiner P, Opipari AW, Jr, et al. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65:6294–304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- 29.Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004;122:794–801. doi: 10.1309/QHD6-YFN8-1KQX-UUH6. [DOI] [PubMed] [Google Scholar]
- 30.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–22. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 31.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A. 2009;106:14978–83. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–8. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe K, Jose PJ, Rankin SM. Eotaxin-2 generation is differentially regulated by lipopolysaccharide and IL-4 in monocytes and macrophages. J Immunol. 2002;168:1911–8. doi: 10.4049/jimmunol.168.4.1911. [DOI] [PubMed] [Google Scholar]
- 36.Miyamasu M, Yamaguchi M, Nakajima T, Misaki Y, Morita Y, Matsushima K, et al. Th1-derived cytokine IFN-gamma is a potent inhibitor of eotaxin synthesis in vitro. Int Immunol. 1999;11:1001–4. doi: 10.1093/intimm/11.6.1001. [DOI] [PubMed] [Google Scholar]
- 37.Fan ST, Edgington TS. Sufficiency of the CD8+ T cell lineage to mount an effective tumoricidal response to syngeneic tumor-bearing novel class I MHC antigens. J Immunol. 1989;143:4287–91. [PubMed] [Google Scholar]
- 38.Harding CV, Collins DS, Slot JW, Geuze HJ, Unanue ER. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991;64:393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Myeloid Ikkβ is required for anti-tumorigenic immunity. A, Melanoma model. Mice harboring genotype of Braf CA::Ptenf/f::Tyr-CreER were maintained on a mixed C57BL6/FVB strain. These mice were treated with 1mg 4-HT. Skin tumours developed 1 month after treatment (a). H&E staining of the melanoma lesion (b) in contrast to the histology of mouse skin without lesion (c). 10× magnification. B&C, Lung metastatic melanoma. FVB mice (5/group) with a myeloid IkkβWt or IkkβMyeΔ/Δ were intravenously injected with BrafV600E/Pten−/− melanoma cells (1×106) expressing the Gluc reporter gene. Four weeks after injection, lung tissues were collected from myeloid IkkβWt mice or myeloid IkkβMyeΔ/Δ mice (B) and subjected to quantitation of live melanoma cells in the lung based on Gluc activity (C). Mock refers to negative controls of tumor-free mice. D, Immunohistochemistry. BrafV600E/Pten−/− melanoma cells (1×106) were intravenously injected into C57BL6 mice. Two weeks after injection, lung tissues were collected from IkkβWt mice (left panels) or IkkβMyeΔ/Δ mice (right panels). Melanoma cells were identified by immunohistochemical staining of S100 (upper-panels) and Mart1 (lower-panels). Bar, 50 μm. E&F, metastatic melanoma lesions in the liver. To evaluate the organ specific metastatic capacity of the mixed genetic background melanoma cells, (1×106) were intra-splenically injected into allogenic C57BL6 mice (5 animals/group). By four weeks post injection, liver metastasis were observed in IkkβMyeΔ/Δ mice but not in IkkβWt mice. Melanoma cells expressing the Gaussian luciferase (Gluc) reporter gene were injected into the spleen of C57BL/6 IkkβMyeΔ/Δ or IkkβWt mice. IkkβWt mice without tumor cell injection served as a tumor-free control. Histological analysis of H&E stained paraffin embedded sections of liver taken from mice four weeks after injection of tumor cells as described in E. Bar, 50 μm. Quantitation of the metastatic lesions based upon Gluc activity in in 10μg of liver tissue was determined to indicate the quantity of remaining live melanoma cells in IkkβWt and IkkβMyeΔ/Δ mice (F). Data demonstrated that myeloid C57BL6 IkkβMyeΔ/Δ mice were permissive for growth and metastasis of C57BL6/FVB melanoma cells, in contrast to C57BL6 IkkβWt mice that successfully rejected the allogeneic melanoma. Data are expressed as mean ± SEM and were statistically analyzed by the Student’s t-test. G&H, the phenotype exhibited in the C57BL6 strain mice was also observed in the allogeneic FVB mice. Mixed background BrafV600E/Pten−/− melanoma cells (1×106) expressing the Gluc reporter gene were intravenously injected into FVB mice (5/group) with myeloid IkkβWt or IkkβMyeΔ/Δ. Four weeks after injection, liver tissues were collected from myeloid IkkβWt mice or myeloid IkkβMyeΔ/Δ mice (G, Bar, 50 μm.) and subjected to Gluc activity analysis for a quantitative estimate of the live melanoma cells in the liver, (H). Mock refers to negative controls of tumor-free mice. Arrow indicates a metastatic lesion. I, melanoma xenografts. A melanoma cell line was derived from a 4-HT induced-BrafV600E/Pten−/− melanoma lesion growing on a mixed background C56BL6/FVB mouse and cultured in DMEM/F-12 medium containing 10% FBS. 1×107 of these melanoma cells were subcutaneously inoculated into C57BL6 mice with myeloid IkkβWt or IkkβMyeΔ/Δ background. Tumor size was measured on days 10, 15 and 27. Data were statistically analyzed by the Student’s t-test. **p<0.01, n=7. J, K, L, Bone Marrow Transplant (BMT). For evaluation of the reconstituted bone marrow, the mixed C57BL6/FVB recipient mice harboring BrafCA::Ptenf/f::Tyr-CreER alleles were irradiated and transplanted with 1×106 bone marrow cells of C57BL6 donor mice carrying LysM-Cre::mG/mT genes. Lymphocytes expressing tdTomato-RFP and appearing in the peripheral blood were monitored weekly by flow cytometry. The red-immune cells in peripheral blood were normalized to that of non-irradiated donor mice (LysM-Cre::mG/mT) and expressed as percentage of reconstituted bone marrow. However, to evaluate how soon bone marrow depletion occurs in recipient mice after irradiation, mice (LysM-Cre::mG/mT) were irradiated and transplanted with bone marrow cells not expressing fluorescent protein. The percentage of red immune cells in blood indicates the remaining bone marrow cells after irradiation (J). n=5
To examine the cell composition of the reconstituted bone marrow, bone marrow cells were isolated from recipient mice or donor mice as a control. Cells were stained with fluorescent conjugated specific antibodies to neutrophils (Gr1-APC-Cy7), macrophages (F4/80-Percp/Cy5.5), B cells (B220-Alexa Fluor 700), CD4+ T cells (CD4-pacific blue) and CD8+ T cells (CD8a-APC). Cells were sorted as myeloid expressing GFP or lymphocyte expressing tomato and analyzed by FACS (K). n=2.
To evaluate the immune response of recipient mice to tumor cells, recipient mice (n=5) or donor mice (n=5) were injected in the peritoneum with melanoma cells (1×107) or PBS as a vehicle control. Twenty-four hours after injection, the GFP+ myeloid cells in the peripheral blood stream were monitored by FACS analysis (L). M&N, inducible melanoma model. Mixed C57BL6/FVB recipient mice (20/group) harboring Braf CA::Ptenf/f::Tyr-CreER alleles received bone marrow cells (1×106) from C57BL6 strain IkkβMyeΔ/Δ mice (Ikkβf/f::LysM-Cre::mG/mT) or IkkβWt mice (LysM-Cre::mG/mT) as controls. Three weeks after BMT, 5 μl of 10 mmol/L 4-HT was topically applied daily to the shaven back of recipient mice for 3 days. Five weeks post induction, tumour size was photographed (M), measured and statistically analysed by the Student’s t-test (N). Data are expressed as mean ± SEM. O&P, spontaneous melanoma model. Mixed C57BL6/FVB recipient mice (20/group) harboring BrafCA::Ptenf/f::Tyr-CreER alleles received bone marrow cells (1×106) from C57BL6 IkkβMyeΔ/Δ mice (Ikkβf/f::LysM-Cre::mG/mT) or IkkβWt mice (LysM-Cre::mG/mT) as controls. Eight weeks after BMT, the pigmented neoplastic area was photographed (O), measured and statistically analysed by a Student’s t-test (P). Data are expressed as mean ±SEM. Q, diagram of inducible constitutively active IKKβ in myeloid cells of mice. C57BL6 mice harboring genetic background of cfms-rt-TA/TetOn-IkkβCA or littermates with cfms-rt-TA genes served as controls accessed to drinking water containing 2 mg/ml doxycycline between day 0 and day 9. Fifty thousand B16 melanoma cells were intravenously injected on day 7. On day 20, mice were sacrificed and lungs were perfused and collected for evaluation of both tumor growth and immune response.
Figure S2. Myeloid cell depletion alterstumor immunity. A, Depletion of macrophages in vivo. Mice were intravenously given (a–c) 0, 0.1 or 0.2 ml of clodronate, then 1 × 106 melanoma tumor cells were injected into the peritoneum. The next day cells in the peritoneum were collected, stained with fluorescence-conjugated APC-CD45, PerCP/Cy5.5-F4/80 antibody and analyzed by FACS. MΦ refers to macrophage. B, clodronate specifically depletes macrophages. Mice received intravenous injection of PBS (a, d), liposome vehicle (b, e) or 1 mg of clodronate (c, f) immediately followed by peritoneal injection of PBS (a, d), or melanoma cells (b, c, e, f). The next day after injection, peritoneal cells were collected and sorted for the Gr1+, F4/80+ and CD11c+ cell population. Neu, neutrophil; MΦ, macrophage; DC, dendritic cell. C, Depletion of neutrophils in vivo. Mice were intravenously injected with 250 μg of antibody to Ly6G or isotype IgG2a daily for three days prior to intravenous delivery of 1 × 106 melanoma cells. Then 100 μg antibody was delivered every other day. On day 5, mice were euthanized and the bone marrow cells were stained with PE-conjugated Ly6G and subjected to FACS analysis. D, depletion of neutrophils promotes lung metastasis. Mice (5/group) received intravenous treatment with ly6G antibody or IgG2a isotype control antibody and intravenously implanted with Gluc-melanoma cells as described in Methods. Three weeks later, mice were euthanized, lung tissue was sonically lysed, and 10 μg lung tissue protein was subjected to the Gluc activity assay. n=5, p<0.05. Ly6G antibody treatment vs IgG2a isotype control. Tumor-free, mice were neither treated with antibody nor given tumor cells. E, Clodronate toxicity on melanoma cells. The cultured melanoma cells engineered with Gluc reporter were treated with clodronate at increasing concentrations of 0, 125, 250, 500, 1000, 10000 ng/ml. The cells were treated with the same amount of vehicle liposome served as controls. After two days of culture, the remained cells were lysed and subjected to luciferase activity assay that reports activity that reflects the relative proportion of surviving cells. The experiment was repeated twice and data were expressed as mean ±SEM.
Figure S3. IKKβ loss alters macrophage phagocytosis capacity. Morphology of macrophage phagocytosis ex vivo. A, Isolation and purity of macrophages. Macrophages were isolated from the peritoneal cavity of either IkkβWt or IkkβMyeΔ/Δ mice 24h after injection with dead melanoma cells. The cell purity was assessed by staining with Percp-Cy5.5 conjugated F4/80 antibody and analyzed by FACS. Cells double positive for GFP and F4/80 are macrophages (MΦ). Macrophage purity was over 92%. B, visualization of phagocytosis of tumor cells. Macrophages (green) with either IkkβWt or IkkβMyeΔ/Δ genotype were allowed to interact with melanoma cells (red) using the co-cultured approach for three days. Tumor cell phagocytosis was visualized by Confocal microscopy (LSM 510META). C, Macrophages capacity assessed by phagocytic latex beads. Macrophages were isolated from the peritoneal cavity of either IkkβWt or IkkβMyeΔ/Δ mice 72h after injection with 2ml of 4% thioglycollate. Cells were co-cultured with fluorescent blue labeled-latex beads. After 2 hours culture, cells were subjected to flow cytometry analysis for the percent of cells expressing the fluorescent blue. IkkβMyeΔ/Δ macrophages showed significant reduction of taking up latex beads in compared with IkkβWt mice macrophages (1.6±0.6% vs. 8.5±2.7%, p<0.01, n=6).
Figure S4. Myeloid IKKβ deletion reduced the cytotoxicity of T cells. A, myeloid IKKβ deletion reduced the number of CD8+CD107b+ cells in the tumor tissue. Myeloid IkkβWt or IkkβMyeΔ/Δ mice were subcutaneously inoculated with melanoma cells (1×107). On days 1, 2, 3 post-implant, infiltrating RFP+ lymphocytes were sorted by FACS and the CD8 expressing CD107b were analyzed per 10,000 cells. B, Myeloid cell infiltration. IkkβWt or IkkβMyeΔ/Δ mice were intravenously injected with melanoma cells (1×106). The infiltration of myeloid cells (GFP positive) into lung tissues was analyzed by FACS over the time course as indicated. C, CD8 T cell infiltration. The infiltration of CD8+T cells in the above lung tissues were sorted using APC-conjugated antibody to CD8 and analyzed by FACS. Data are expressed as CD8+ T cells/10,000 lung cells and the statistical analysis utilized by the Student’s t-test. Wt refers to IkkβWt mice; Ko refers to IkkβMyeΔ/Δ mice. D, depletion of CD8+ T cells in vivo. Mice were intravenously injected with 250 μg of CD8 antibody or isotype IgG2a daily for three days prior to i.v. injection of 1 × 106 melanoma cells. Then 100 μg antibody was injected I.v. every other day. On day 5, the bone marrow cells were stained with APC-conjugated CD8 antibody and subjected to FACS analysis. E, Neutrophil purity and viability. Peripheral blood was collected from mice and the neutrophils were isolated from the Percoll gradient 1.077–1.119 interface. The percentage cell purity was determined by sorting cells expressing GFP and staining for Gr1-APC-Cy7 or F4/80-Percp Cy5.5. Cell viability was determined by staining with 7-AAD.
Figure S5. Cytokines play a major role in orchestrating the cellular make-up of the tumor microenvironment. A, Efficiency of CD8 cell depletion in peripheral blood. C57BL6 mice were intraperitoneally injected 200 μg CD8 antibody/mouse daily for three days and then continued injection twice a week for 16 days. Meanwhile, mice injected with the same amount of isotype IgG as a control. CD45+CD8+ cells were analyzed by flow cytometry in the peripheral blood (A) on day 5, in spleen (B) and lung (C) on day 16. D, elimination of CD8 T cells in the syngeneic reaction. CD8+ cells in C57BL6 mice carrying myeloid IkkβCA or IkkβWT were depleted by intraperitoneal injection CD8 antibody while mice injected with isotype IgG as a control for three days. Mice were intravenously injected with syngeneic B16 melanoma cells (5×104) expressing Gluc reporter. Antibody injection continued bi-weekly for 16 days until lung tumor burden was quantitatively determined by Gluc activity. E, TRP-2-specific CTL response in a syngeneic B16 melanoma model. C57BL6 mice carrying IkkβCA or IkkβWT myeloid cells intravenously received syngeneic B16 melanoma cells (5×104). After 16 days a single cell suspension was prepared from lung tumor tissues. Cells were stained with PerCP-Cy5.5-conjugated CD8 antibody and APC-labeled TRP2 (SVYDFFVWL) tetramer. The APC positive CD8 T cells were analyzed by flow cytometry. +Ctrl, positive control cells from the splenocytes of TRP2-immunized mouse; -Ctrl, negative control cells from the splenocytes of non-immunized mouse.
Figure S6. Immuno-fluorescent staining human melanoma tissues. A&B, Paraffin-embedded tissue samples from melanoma patients, pre- or post-treatment, were stained with fluorochrome-conjugated CD163, phospho-p65 and CCL11 antibodies. The stained tissues were visualized by confocal microscopy, photographed and quantified by linescan using the Metamorph program version 7.6.4. C, a representative photo of human peripheral lymphocytes stained with AlexaFlour448-conjugated CD8 antibody and AlexaFlour647-conjugated CD107a antibody is shown. D, Linescan quantitation of the CD8+ T cells expressing CD107a positive (a) or negative fluorescence (b). E-a, a representative photo of CD8 T cells in the pre-treated human melanoma or F-a, a representative photo of CD8 T cells in the post-treated human melanoma stained with AlexaFlour448-conjugated CD8 antibody and AlexaFlour647-conjugated CD107a antibody. Line Scan quantitation of the CD8+ T cells expressing CD107a in pre-treatment (E-b) or post-treatment group (F-b).