Abstract
The objective of the current study was to characterize luteal function in vervet monkeys. Urine from 12 adult female vervets housed at an academic research center was collected for 10 weeks from single-caged monkeys in order to assess evidence of luteal activity (ELA) as determined by urinary excretion of pregnanediol glucuronide (Pdg) and estrone conjugates (E1c). Dual energy X-ray absorptiometry (DXA) was performed on the monkeys to assess body composition, bone density, and fat mass. Menstrual cyclicity was determined using records of vaginal bleeding. ELA was observed in 9 monkeys and was characterized by a late follicular rise in E1c followed by a progressive increase in Pdg excretion. Mean menstrual cycle length was 26.7 ± 3.8 days and the average day of luteal transition was 14 ± 1.8. Three monkeys without ELA had a clearly defined E1c rise (mean 12-fold from nadir) followed by an E1c drop that was not accompanied by Pdg rise and coincided with vaginal bleeding. Among the 9 ELA monkeys, excretion of E1c tended to negatively associate with fat mass, although this finding did not reach statistical significance (r = −0.61, p = 0.08). Similar to women, vervet monkeys experience an increase in E1c late in the follicular phase of the menstrual cycle which is followed by a subsequent luteal Pdg peak. Assessment of urinary reproductive hormones allows for identification of cardinal menstrual cycle events; thus, the similarity of vervet cycles to human menstrual cycles makes them a useful model for obesity-related human reproductive impairment.
Keywords: Chlorocebus aethiops, corpus luteum, non-human primates, obesity, ovary, vervet
Introduction
The vervet monkey (Chlorocebus aethiops) is an Old World non-human primate (NHP) that belongs to the same subfamily as macaques and baboons. Based upon the observation of a fully pedigreed and genotyped colony of more than 400 animals from the Vervet Research Colony (VRC) [Newman et al. 2002], adult vervets develop obesity and its associated metabolic profile in a manner very similar to humans. Heritability analyses of this colony demonstrated that patterns of obesity in vervets are highly heritable, as they are among humans [Kavanagh et al. 2007]. Thus, the VRC represents a source of a potentially translatable animal model to elucidate the impact of obesity on various target organs, including the reproductive axis. Adult female vervets exhibit similarities to women in hormonal regulation of menstruation [Carroll et al. 2007]. The average cycle length is about 30 days and the serum levels of pituitary gonadotropins follow a pattern very similar to humans [Molskness et al. 2007]. Urine collected from wild African vervets has been shown to be useful for assessment of progesterone excretion to predict conception [Andelman et al. 1985]. Additionally, an evaluation of urinary sex steroids excreted by a single vervet across one menstrual cycle and pregnancy has been reported [Setchell et al., 1980]. A comprehensive assessment of luteal function in the vervets has not been performed. The objective of the current study was to perform a quantitative assessment of luteal function in vervet monkeys using urine excretion of pregnanediol glucuronide (Pdg) and estrone conjugates (E1c) across a menstrual cycle. Our overarching goal for this project is three-fold: 1) demonstrate that extant evidence on the detrimental impact of human female obesity on reproduction is abundant; 2) highlight the glaring absence of a menstrual cycle model for obesity-related reproductive impairment; and 3) establish an inherent advantage of a reliable model for this purpose that would necessitate long-term, convenient, and minimally invasive monitoring of luteal function. The latter requirement would not be satisfied by daily blood draws over an extended period of time as we aim to demonstrate feasibility of obtaining urine for 10 weeks which is clearly less invasive than daily serum for a comparable time period.
Results
Evidence of luteal activity (ELA)
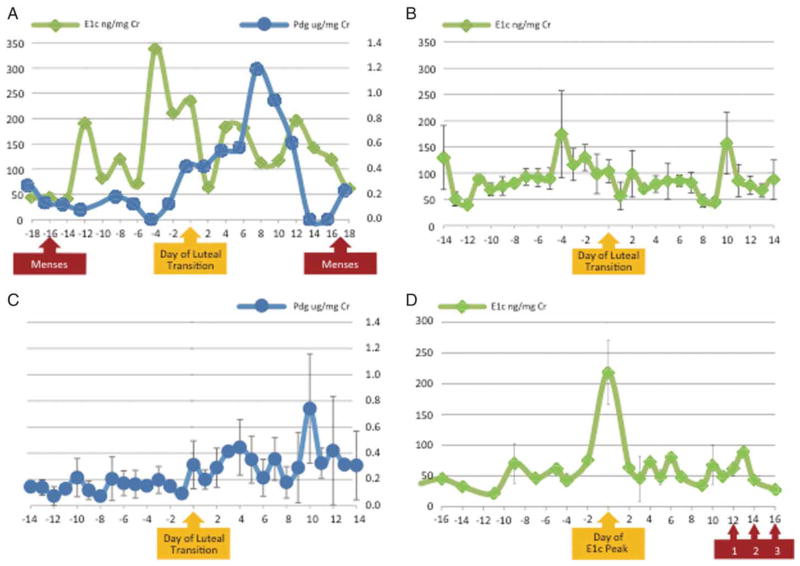
Nine monkeys exhibited ELA as determined by the algorithm established prospectively [Kassam et al. 1996; Santoro et al. 2003] to identify ovulatory cycles using urinary hormone assays. In this method, evidence of luteal activity is established as three times the baseline of Pdg. Cycles with sustained observation of at least three consecutive values above the threshold are classified as ELA. Absolute measures of urinary hormones were consistent with ELA status by the pre-specified algorithm, with the peak Pdg being almost twice as high in the ELA vs. non-ELA monkeys. A representative urinary hormone pattern in an ELA monkey (Fig. 1A) demonstrates a late follicular rise in E1c followed by a progressive increase in daily Pdg excretion. Composite delineation of E1c excretion in all ELA monkeys (Fig. 1B) demonstrated a bimodal pattern for E1c during the menstrual cycle, with well-defined pre-ovulatory and post-ovulatory peaks. The mean day of luteal transition (DLT) was 14 (SD 1.8 days). A composite graph of Pdg excretion demonstrated that the Pdg peak occurred approximately 10 days after the day of luteal transition (Fig. 1C). The three monkeys without ELA all had a rise in E1c (12 fold from the nadir, on average), followed by a drop that coincided with vaginal bleeding 12–16 days later (Fig. 1D). Eight monkeys had data for more than 1 complete cycle that was available for analysis during the study period. Among the monkeys with multiple cycles, 10 out of 16 cycles (62.5 %) had ELA, which was comparable to the overall prevalence observed in the analytic sample.
Figure 1.
Determination of evidence of luteal activity (ELA) in vervet monkeys with urinary hormones, Pdg (pregnanediol glucuronide, A and C) and E1c (estrone conjugates, A, B and D). Error bars indicate standard error of the mean for composite whole cycle data. Data was centered on the day of luteal transition (A, B, and C) or the day of E1c peak (D). A) Representative hormone pattern in one ELA monkey; B) Composite whole cycle E1c for nine ELA monkeys; C) Composite whole cycle Pdg for nine ELA monkeys; D) Composite E1c for three monkeys without ELA for at least 30 days preceding a vaginal bleeding episode. Data centered on the day of E1c peak. Vaginal bleeding following E1c peak is depicted by the red arrow for three individual vervets as 1, 2, and 3, respectively.
Comparisons of monkeys with and without ELA
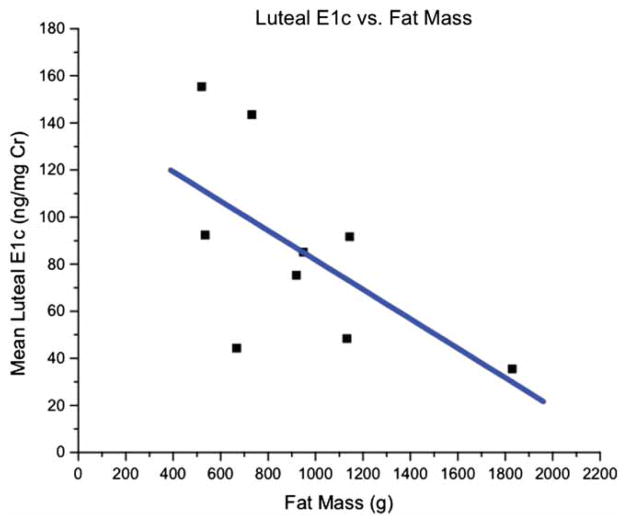
Morphometric and menstrual cycle characteristics by evidence of luteal activity are depicted in Table 1. ELA animals did not differ significantly from monkeys without ELA in menstrual cycle characteristics. Hormonal and menstrual characteristics were examined as a function of fat mass determined by DXA (Fig. 2). Among the nine ELA monkeys, lower excretion of luteal E1c was associated with greater fat mass, although the finding did not reach statistical significance (r = −0.61, p = 0.08) (Fig. 3). Other findings included a significant (p < 0.02) negative association between cycle length and percent body fat, a trend (p < 0.08) for a negative association between cycle length and body fat mass, and a trend (p < 0.09) for reduced body weight in females with no evidence of luteal activity (acyclic). Reduced body weight can lead to the absence of ovulatory cycles, a possible factor in this case. Furthermore, as the significant association between percent body fat and cycle length is negative for all females combined, i.e., lower percent body fat associates with longer cycles, it would suggest that lower proportions of body fat linked with a trend for less body weight may contribute to infrequent ovulatory cycles in these monkeys.
Table 1.
Morphometric and menstrual cycle characteristics by evidence of luteal activity.1
| Determinant | Evidence of Luteal Activity (n = 9) | No Evidence of Luteal Activity (n = 3) | P Value2 | Correlation with % Fat
|
Correlation with Fat Mass
|
||
|---|---|---|---|---|---|---|---|
| r | p | r | P | ||||
| Age, years | 6.82 (2.16) | 8.05 (2.28) | 0.41 | 0.37 | 0.24 | 0.45 | 0.14 |
| Urinary Pdg, ug/mg Cr | |||||||
| Peak | 0.56 (0.29) | 0.35 (0.15) | 0.27 | 0.03 | 0.92 | 0.01 | 0.99 |
| Mean, luteal | 0.35 (0.17) | n/a | −0.08 | 0.83 | −0.05 | 0.90 | |
| Urinary E1c, ng/mg Cr | |||||||
| Peak | 181.5 (90.3) | 168.0 (95.3) | 0.83 | −0.23 | 0.47 | −0.31 | 0.32 |
| Mean, total cycle | 90.0 (35.9) | 61.5 (12.3) | 0.22 | −0.23 | 0.48 | −0.29 | 0.35 |
| Mean, follicular | 95.4 (30.7) | n/a | −0.44 | 0.23 | −0.46 | 0.21 | |
| Mean, luteal | 85.7 (41.8) | n/a | −0.58 | 0.10 | −0.61 | 0.08 | |
| Menstrual Cycle Parameters | |||||||
| Cycle length, days | 29.6 (2.9) | 31.3 (7.5) | 0.56 | −0.64 | 0.02 | −0.53 | 0.08 |
| Duration of menses, days | 2.6 (0.7) | 2.5 (0.8) | 0.84 | −0.24 | 0.45 | −0.11 | 0.73 |
| Bleeding score 3 | 1.84 (0.26) | 1.64 (0.32) | 0.30 | 0.44 | 0.15 | 0.50 | 0.10 |
| Luteal length | 12.7 (3.3) | n/a | |||||
| Day of luteal transition | 14 (1.9) | n/a | |||||
| Body Weight, kg | 5.0 (0.6) | 4.3 (0.3) | 0.09 | 0.72 | 0.01 | 0.77 | 0.003 |
| Bone Mineral Density, g/cm3 | 0.32 (0.03) | 0.31 (0.03) | 0.55 | 0.64 | 0.02 | 0.79 | 0.002 |
| Fat Mass, g | 893.4(309.6) | 567.2 (99.2) | 0.12 | 0.96 | 0.0001 | ||
| Percent Fat | 17.5 (4.0) | 13.2 (2.6) | 0.12 | 0.96 | 0.0001 | ||
values presented as mean (standard deviation)
comparison by evidence of luteal activity, t test
0 (no bleeding), 1 (light), 2 (moderate), or 3 (heavy)
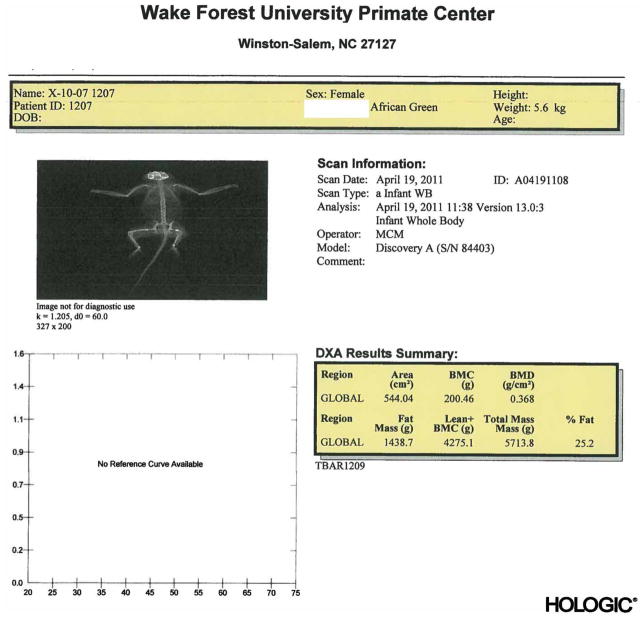
Figure 2.
Sample DXA scan of a female vervet monkey.
Figure 3.
Examination of luteal E1c excretion (ng/mg Cr) in 9 ELA monkeys as a function of fat mass (g) determined by DXA.
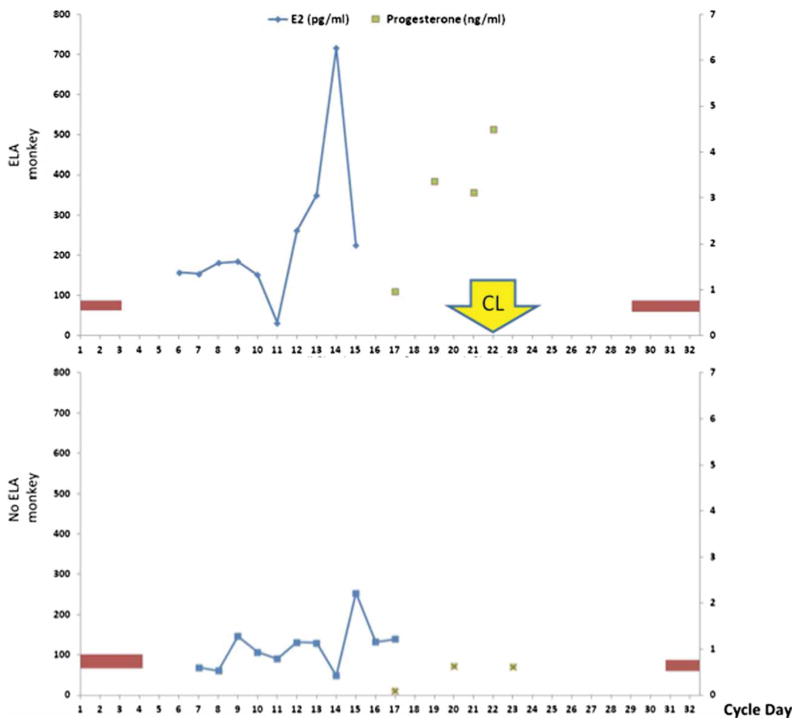
Urinary hormonal data were confirmed by measuring serial serum estradiol and progesterone for one monkey with ELA and one monkey without ELA (Fig. 4). This was obtained in a subsequent cycle along with a corpus luteum biopsy to minimize the impact of stress related to serial phlebotomy and surgery on urinary assays.
Figure 4.
Representative serum hormone pattern in one ELA monkey and one no ELA monkey. Vaginal bleeding is depicted by the red bars for corresponding cycle days. Removal of the corpus luteum was performed on cycle day #21 for the ELA monkey.
Discussion
In this analysis of urinary reproductive hormone metabolites, we found that the onset of vervet menses could be predicted by E1c and Pdg patterns, in a manner identical to ovulatory human menstrual cycles. In cycles without ELA, a rise in E1c followed by a drop in E1c levels predicted bleeding. Presence of an E1c peak that preceded vaginal bleeding in the animals without ELA suggests a failed ovulatory event rather than failed follicular development. Pathophysiology of incident sporadic anovulation among eumenorrheic females is not well understood [Mumford et al. 2011] and deserves to be further explored. Urine collection in non-human primates minimizes the concerns of stress-related endocrine changes (i.e., LH inhibition) that have been reported to be associated with frequent blood collection [Golub et al. 2003]. While the reproducibility of assaying urinary reproductive hormones has been well established in other non-human primate species [Shideler et al. 2001; Shideler et al. 2003], the current report is the first comprehensive evaluation of the vervet corpus luteum based upon serial assessment of excreted sex steroid metabolites. Our data are in agreement with evaluation of the vervet ovary by serum markers [Molskness et al. 2007] and expand this paradigm to establish the feasibility of long-term non-invasive analysis of urinary hormones in this species. The overarching goal of this vervet monkey project is to model the impact of obesity on reproductive hormones with intentional experimental weight acquisition. The data presented herein describe a cross-sectional assessment at baseline of normal weight monkeys and establish feasibility for subsequent studies.
The vervet monkeys used in this study represent a particularly attractive translational model due to the known parentage and lineage in the VRC, thus allowing investigations of potential genetic and environmental modifiers of chronic disease. The VRC was established in 1975, with 57 founders who originated in the wild and were captured in St. Kitts, West Indies. Today, the colony consists of over 400 vervets that are fully genotyped and pedigreed [Newman et al. 2002]. Obesity was shown to be a heritable disease among the vervets in the VRC, accounting for 20% of the population, who were all consuming a low-fat chow diet [Kavanagh et al. 2007]. These findings make the vervet a promising model to study the relationships between obesity and other organ systems, including reproduction. In this communication, we present data on the feasibility of quantitative non-invasive analysis of luteal function that is well suited for longitudinal studies specifically designed to evaluate the impact of intentional weight fluctuations on female reproductive hormones.
Assessment of luteal function in non-human primates enables the study of ovarian function in response to body mass fluctuations. Intentional obesity is not ethical and likely impossible to accomplish experimentally in humans. Thus, measurement of vervet urinary reproductive hormones allows us to quantify corpus luteum function and permits the use of vervets as a model of adiposity-related human reproductive impairment in studies of experimental obesity acquisition. The vervet model also permits subsequent weight loss or manipulation, in a controlled environment, not tenable in patients.
We have observed a relatively high prevalence of regular menstrual cycles without evidence of luteal activity (3 out of 12). While prevalence of eumenorrheic anovulation among women had been classically reported to be very low or non-existent [Malcolm and Cumming 2003], the original data proposing this phenomenon relied on a single measurement of serum progesterone in midluteal phase. In contrast, recently published data from the BioCycle Study characterized cycles from regularly menstruating women with eight hormonal measurements per cycle [Mumford et al. 2011]. Prevalence of sporadic anovulation in that cohort was 13.5% which was consistent with a 10% prevalence among certain groups of eumenorrheic women studied with daily urinary collection [De Souza et al. 1998]. Thus, the preserved menstrual cycle length in our vervets without ELA is consistent with human studies. We intend to follow some of the reported markers of sporadic anovulation in eumenorrheic women (such as decreased sex hormone binding globulin [Mumford et al. 2011]) longitudinally to gain insight into the mechanism underlying this phenomenon. A potential alternative explanation to the observed results is that the apparent luteal defects may represent diminished ovarian reserve (DOR). While permanent non-pathologic cessation of ovulation has been described in many NHP species, the literature suggests that the onset of female reproductive aging in NHP typically occurs much later than in humans [Walker and Herndon 2008]. To the best of our knowledge, there are no published reports of spontaneous premature ovarian failure in vervet monkeys. While AMH testing in vervets is not yet available, FSH assays were performed in the VRC approximately two years prior to this study. The average FSH value in the VRC was 1.0 ± 1.1 ng/ml. We herein report that all of the animals from the current study without ELA had FSH levels that were well within one standard deviation from the rest of the colony. These additional data suggest that spontaneous DOR is an unlikely explanation in this setting. We did not have the ability to provide a measure of visceral fat as the available DXA modality did not differentiate between visceral and subcutaneous fat tissue (Fig. 2). In the future, we plan to take advantage of the newly developed software that provides an estimate of visceral fat with DXA technology [Micklesfield et al. 2012].
A suggestion of decreased luteal E1c excretion in association with greater fat mass of the examined animals is intriguing. Reduced E1c excretion in obese women as compared with their normal weight counterparts has been described [De Pergola et al. 2006; Rochester et al. 2009]. De Pergola et al. [2006] reported that 22 overweight or obese fertile women demonstrated lower FSH, LH, inhibin B, and estradiol levels in the early follicular phase, independent of age and insulin levels. Although the mechanism for this trend is unclear, a further decrease in daily E1c after bariatric surgery in the morbidly obese has been attributed to loss of adipose tissue [Rochester et al. 2009]. Generalizability of this hormonal pattern across ethnically diverse human populations was reported by Randolph et al. [2003] whereas higher BMI was associated with decreasing serum estradiol, FSH, dehydroepiandrosterone sulphate, and serum hormone binding globulin levels and increasing serum testosterone levels in each of five sampled ethnic groups. Alternatively, a random chance finding is another consideration, which will be verified in a future longitudinal follow-up. Prospective follow-up of non-human primates with experimentally designed weight fluctuations promises to uncover mechanistic clues for this and other stigmata of reproductive hormone alterations that are associated with obesity.
A limitation to this study is the possibility that the cycles that did not exhibit ELA were in fact ovulatory cycles with a very low Pdg. The original luteal algorithm [Kassam et al. 1996] was formulated to accurately confirm ovulation, rather than anovulation. Luteal function is possibile even if there is ‘no luteal function’ as defined by Pdg. In future studies, we plan to alter the protocol and dilute the urine samples less to assess whether we can detect and quantify low, sustained levels of Pdg. Nonetheless, the modified algorithm we employed [Santoro et al. 1996] has been successfully used in clinical studies for longitudinal follow up of women and is a validated measure of human corpus luteum function. While the current study did not include serum measurements of the reported trends in urinary hormones, the observed associations are consistent with human studies and other HNP reports. Another limitation is considerable variability between animals with respect to the magnitude of the E1c peak, possibly owing to the gaps in specimen acquisition (collected three to five days per week, therefore allowing the possibility of missing the true peak for some animals. We plan to collect daily urine for subsequent studies to address this issue). Finally, the absence of measured FSH and LH levels corresponding to the urinary hormone metabolites is a limitation. However, our data are consistent with other studies that included pituitary assessments [Molskness et al. 2007].
Materials and Methods
Animal housing, selection, and study design
Twelve adult female vervet monkeys (Chlorocebus aethiops) were selected from the VRC at the Wake Forest University Primate Center (Winston-Salem, NC) for this study. All selected monkeys were within the normal weight range for this species (mean weight of 4.9 ± 0.76 kg) and were sexually mature with a mean age of 6.9 ± 2.2 y (range 4.6 to 10.6 y) at study entry. Animals were pair-housed in cages constructed of mesh flooring with a pan underneath to collect excrement and other waste. For environmental enrichment, all cages were equipped with perches and hanging mirrors. The monkeys were fed a commercial non-human primate diet (Purina Monkey Chow®) once daily in the afternoon. They were provided water ad libitum and received enrichment feedings and foraging opportunities 3–4 times per w. All policies and procedures were done in compliance with state and federal laws, and regulations and guidelines established by the Wake Forest University Animal Care and Use Committee. Wake Forest University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), which provided approval for this study.
Urinary collection
Urine was collected 3 – 5 d per w for 10 w. At 8 am every morning a mesh panel was inserted into each cage to separate pairs of monkeys and allow the collection of individual urine samples. Once the monkeys were separated, the metal pans underneath the mesh flooring were cleaned of all shavings and waste. Within 4 h after cage preparation, a sterile 10-mL syringe was used to withdraw at least 5-mL of urine from the pan, avoiding collection of samples contaminated by water or feces. The urine was transferred to a conical tube for centrifugation at 10,000 rpm for 30 min and the supernatant was transferred into polypropylene tubes for storage at −20°C.
Body composition
Monkeys were sedated with Ketamine HCL (15 mg/kg) intramuscularly and transported to a dual energy X-ray absorptiometry (DXA, Hologic, Bedford, MA, USA) scanner for measurements of body composition. For each scan, monkeys were placed in the supine position and the tail was secured to the animal’s thigh to ensure that it was included in the scanning region. A whole body scan was performed to determine lean and fat mass as well as bone density. Scans were analyzed using the Hologic Software, APEX 3.3 (Fig. 2).
Menstrual cycles
Due to light vaginal bleeding characteristic for this species, monkeys were vaginally swabbed daily to document menstrual bleeding, a technique that has been shown to be highly reproducible over extended follow-up [Carroll et al. 2007]. Swabbing consisted of using a ‘squeeze’ mechanism located on the housing racks, which allowed the technician to bring the monkey to the front of the cage. A cotton tipped swab was then placed in the vaginal opening until it reached the cervix. The cotton swab was then retracted and the presence and the amount of blood was recorded using a scoring system of 0 (no bleeding), 1 (light), 2 (moderate), or 3 (heavy). The monkey was then released from the squeeze mechanism and positively reinforced with fruit or vegetables.
Assays
Urine was assayed for estrone conjugates (E1c) and pregnanediol glucuronide (Pdg) using previously described methods. The E1c and Pdg levels were measured in duplicate by ELISA using antibodies and conjugate tracers provided by the laboratory of two coauthors (BL, NG). Quality controls reading close to the assay ED50 were used for calculation of coefficients of variation (CV). Interassay CV for E1c was 16.8% and intraassay CV was 6.2%. Corresponding CVs for Pdg were 18.5% and 11.1%, respectively. The lowest detectable limit was 10 ng/ml for both Pdg and E1c. Hormone concentrations were adjusted for glycerol and normalized to creatinine (Cr) [Taussky 1954].
Data analysis
A previously published algorithm validated in human studies [Santoro et al. 1996] was modified and luteal function was assessed by a three-step process. First, Pdg nadir during the first 5 samples after menses was determined. Second, a sustained 3-fold rise from the nadir was defined as evidence of luteal activity (ELA). Third, the day of a 60% drop in the E1c/Pdg ratio in proximity to the Pdg peak was set as the day of luteal transition (DLT). Comparisons between groups by ELA status were performed using unpaired t tests, and the Pearson coefficient was used for correlation of body composition data with menstrual and hormonal parameters. To avoid a scenario whereby some animals contributed more cycles to the analysis than others, we selected one menstrual cycle per monkey for analysis (the first cycle with ELA if more than one was observed during the course of the study per animal). All statistical tests used a two-tailed alpha of 0.05. Analyses were performed using STATA 9.2 (StataCorp LP, College Station, TX, USA) and OriginPro 7.5 (Northampton, MA, USA).
Abbreviations
- DXA
dual energy X-ray absorptiometry
- ELA
evidence of luteal activity
- Pdg
pregnanediol glucuronide
- E1c
estrone conjugates
- LH
luteinizing hormone
- VRC
Vervet Research Colony
- Cr
creatinine
- DLT
day of luteal transition
- FSH
follicle stimulating hormone
- NHP
non-human primate
- DOR
diminished ovarian reserve
Footnotes
Author contributions: Conceived and designed the experiments: SEA, AJP; Performed the experiments: MCK, MCM, JC, APB; Analyzed the data: MCK, MCM, JC, APB, BL, NG, NS, SEA, AJP; Contributed reagents/materials/analysis tools: BL, NG, NS; Wrote the manuscript: MCK, SEA, AJP.
Declaration of interests: This work was supported by: NIH R21 1R21HD060944 (AJP and SEA), NIH U54 HD058155 Center for the Study of Reproductive Biology (NS and AJP) and the VRC grant RR019963 (currently OD010965). The authors report no conflicts of interests. The authors alone are responsible for the content and writing of this paper.
References
- Andelman SJ, Else JG, Hearn JP, Hodges JK. The non-invasive monitoring of reproductive events in wild Vervet monkeys (Cercopithecus aethiops) using urinary pregnanediol-3α-glucuronide and its correlation with behavioural observations. J Zool. 1985;205:467–477. [Google Scholar]
- Carroll RL, Mah K, Fanton JW, Maginnis GN, Brenner RM, Slayden OD. Assessment of menstruation in the vervet (Cercopithecus aethiops) Am J Primatol. 2007;69:901–916. doi: 10.1002/ajp.20396. [DOI] [PubMed] [Google Scholar]
- De Pergola G, Maldera S, Tartagni M, Pannacciulli N, Loverro G, Giorgino R. Inhibitory effect of obesity on gonadotropin, estradiol, and inhibin B levels in fertile women. Obesity (Silver Spring) 2006;14:1954–1960. doi: 10.1038/oby.2006.228. [DOI] [PubMed] [Google Scholar]
- De Souza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG, et al. High Frequency of Luteal Phase Deficiency and Anovulation in Recreational Women Runners: Blunted Elevation in Follicle-Stimulating Hormone Observed during Luteal-Follicular Transition. J Clin Endocrinol Metab. 1998;83:4220–4232. doi: 10.1210/jcem.83.12.5334. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Lasley BL, Natarajan K, Tarantal AF. Effects of exogenous estrogenic agents on pubertal growth and reproductive system maturation in female rhesus monkeys. Toxicol Sci. 2003;74:103–113. doi: 10.1093/toxsci/kfg090. [DOI] [PubMed] [Google Scholar]
- Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect. 1996;104:408–413. doi: 10.1289/ehp.96104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, et al. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity. 2007;15:1666–1674. doi: 10.1038/oby.2007.199. [DOI] [PubMed] [Google Scholar]
- Malcolm CE, Cumming DC. Does anovulation exist in eumenorrheic women? Obstet Gynecol. 2003;102:317–318. doi: 10.1016/s0029-7844(03)00527-1. [DOI] [PubMed] [Google Scholar]
- Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 2012;20:1109–1114. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molskness TA, Hess DL, Maginnis GM, Wright JW, Fanton JW, Stouffer RL. Characteristics and regulation of the ovarian cycle in vervet monkeys. Am J Primatol. 2007;69:890–900. doi: 10.1002/ajp.20395. [DOI] [PubMed] [Google Scholar]
- Mumford SL, Schisterman EF, Siega-Riz AM, Gaskins AJ, Steiner AZ, Daniels JL, et al. Cholesterol, endocrine and metabolic disturbances in sporadic anovulatory women with regular menstruation. Hum Reprod. 2011;26:423–430. doi: 10.1093/humrep/deq322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TK, Fairbanks LA, Pollack D, Rogers J. Effectiveness of human microsatellite loci for assessing paternity in a captive colony of vervets (Chlorocebus aethiops sabaeus) Am J Primatol. 2002;56:237–243. doi: 10.1002/ajp.1078. [DOI] [PubMed] [Google Scholar]
- Randolph JF, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516–1522. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- Rochester D, Jain A, Polotsky AJ, Polotsky H, Gibbs K, Isaac B, et al. Partial recovery of luteal function after bariatric surgery in obese women. Fertil Steril. 2009;92:1410–1415. doi: 10.1016/j.fertnstert.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- Santoro N, Crawford SL, Allsworth JE, Gold EB, Greendale GA, Korenman S, et al. Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrinol Metab. 2003;284:E521–530. doi: 10.1152/ajpendo.00381.2002. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Bull R, Adlercreutz H. Steroid excretion during the reproductive cycle and in pregnancy of the vervet monkey (Cercopithecus aethiopus pygerythrus) J Steroid Biochem. 1980;12:375–384. doi: 10.1016/0022-4731(80)90296-4. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biol Reprod. 2001;65:1718–1725. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Laughlin LS, Rapp PR, Morrison JH, et al. Contribution of ovarian steroid production to urinary estrone conjugate concentrations in Macaca mulatta. Am J Primatol. 2003;61:111–121. doi: 10.1002/ajp.10114. [DOI] [PubMed] [Google Scholar]
- Taussky HH. A microcolorimetric determination of creatine in urine by the Jaffe reaction. J Biol Chem. 1954;208:853–861. [PubMed] [Google Scholar]
- Walker ML, Herndon JG. Menopause in Nonhuman Primates? Biol Reprod. 2008;79:398–406. doi: 10.1095/biolreprod.108.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]