Abstract
The effective treatment of coronary artery disease targets two distinct goals, controlling symptomatic angina and decreasing the adverse events associated with ischemia. Traditional anti-anginal and anti-ischemic drugs function by altering the determinants of myocardial oxygen supply or demand, usually by altering loading conditions, changing the heart rate, or impacting contractility. Blockade of the late inward sodium current, INa, offers another target for the treatment of ischemia. Blockade of INa reduces the sodium and calcium overload that follows ischemia. This improves myocardial relaxation and reduces left ventricular diastolic stiffness, in turn enhancing myocardial contractility and perfusion. Ranolazine, a selective INa inhibitor, has been shown to provide both anti-anginal and anti-ischemic benefits without significant alterations in the heart rate and blood pressure in patients with stable coronary artery disease. When evaluated in patients with acute coronary syndrome, ranolazine has been shown to decrease recurrent ischemia, but not significantly reduce the risk of death or myocardial infarction.
Keywords: ischemia, angina, sodium current, ranolazine
Introduction
Coronary artery disease (CAD) is the leading cause of death in the United States, and due to the epidemic of obesity and diabetes, ischemic heart disease is overtaking infectious disease as the leading cause of death worldwide.[1] There are two goals in the optimal treatment of CAD. The first is to diminish the progression of disease, thereby reducing the risk of future myocardial infarction (MI) or cardiovascular death. The second goal is the relief of symptoms by reducing angina and improving functional status and quality of life.[2] This review examines treatment of CAD by modifying the late sodium current in the heart with the drug ranolazine.
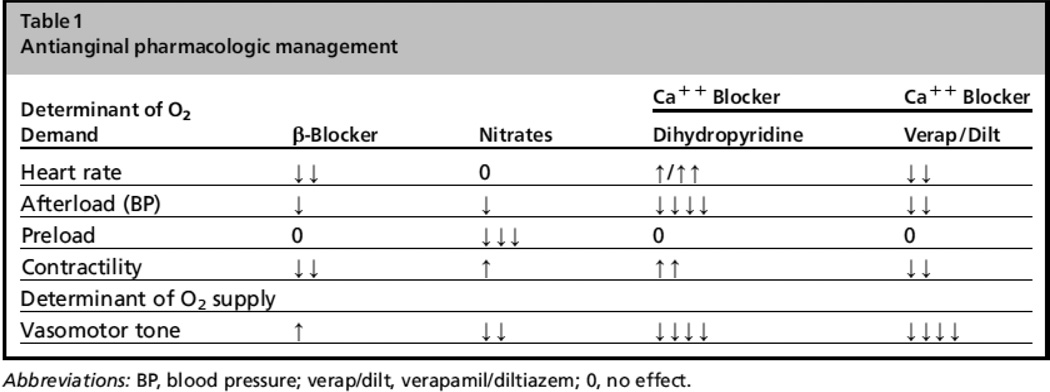
Anti-ischemia medications have previously focused on optimizing the determinants of the myocardial oxygen supply to demand balance. As myocardial oxygen extraction is maximal even at rest, the only method to improve the balance pharmacologically has been to reduce myocardial oxygen demand. This can be achieved by several mechanisms including lowering heart rate, decreasing afterload or decreasing blood pressure, decreasing myocardial contractility, or decreasing preload. Traditional anti-anginal medications, including beta-blockers, nitrates, and calcium channel blockers, work by one of these mechanisms as described in Figure 1. Despite improving ischemic symptoms, none of these classes of medications have demonstrated superiority over placebo for preventing death or MI in patients with chronic angina without previous MI or reduced left ventricular function.[3]
Figure 1.
Traditional anti-anginal agents (With permission from Stone PH, Cardiol Clin (2008) 26:603).
Many patients with angina require multiple medications to sufficiently control their symptoms. However, dose titration and the use of additional agents is often limited by side effects, namely hypotension or bradycardia.[4] Thus, additional and alternative therapeutic approaches for the treatment of CAD symptoms are necessary.
This review will address the rationale that inhibition of the late sodium current is beneficial to reduce the cardiac dysfunction during ischemia and will discuss the clinical studies supporting the use of ranolazine for its anti-anginal and anti-ischemic effects.
The role of the late sodium current in ischemia
A hallmark of myocardial ischemia is the imbalance of oxygen supply and demand leading to the dysregulation of ionic homeostasis in the cardiomyocyte. Available cellular energy production, in the form of adenosine triphosphate (ATP), rapidly decreases during ischemia, leading to a reduced energy supply for several important proteins involved in excitation-contraction coupling. Among these proteins is the Na+/K+ ATPase which is crucial in maintaining the normal resting membrane potential. If ischemia is prolonged and severe, intracellular ATP levels will decrease and the Na+/K+ ATPase will be unable to maintain the normal resting membrane potential. Eventually, these events will lead to marked cellular depolarization, and cell death.[5] An early event during ischemia is the rise in the intracellular sodium concentration and a decrease in intracellular pH.[6] There are several mechanisms by which this occurs.
In normal myocytes, initial electrical activation triggers entry of sodium through the membrane sodium channel generating the fast upstroke of the action potential. This early sodium rush causes further membrane depolarization leading to activation of voltage gated L-type calcium channels and influx of calcium into the cytosol. During normal conditions, sodium channels are rapidly inactivated followed by a fast recovery, allowing them to be activated again by a subsequent action potential. Following electrical activation, other ion channels also open, including calcium channels, and allow calcium ions to enter the cell during the plateau phase of the action potential triggering the release of large stores of calcium from the sarcoplasmic reticulum. The increased concentration of cytoplasmic calcium initiates the interaction between the contractile filaments, actin and myosin, leading to cellular contraction. After contraction, calcium ions are actively pumped back into the sarcoplasmic reticulum and cellular relaxation occurs. This mainly occurs through the sarcolemmal Na+/Ca2+ exchanger that normally exchanges 1 calcium ion for 3 sodium ions per cycle.[7] This exchanger can function in two different directions. In its forward mode, it eliminates calcium outside the cell to accomplish diastolic relaxation and calcium reuptake into the sarcoplasmic reticulum. During its reverse mode, it transports calcium into the cell in exchange for transsarcolemmal elimination of sodium. The activity and direction of transport depend on the pattern of protein expression, the membrane potential, and the intracellular sodium and calcium concentration.[7]
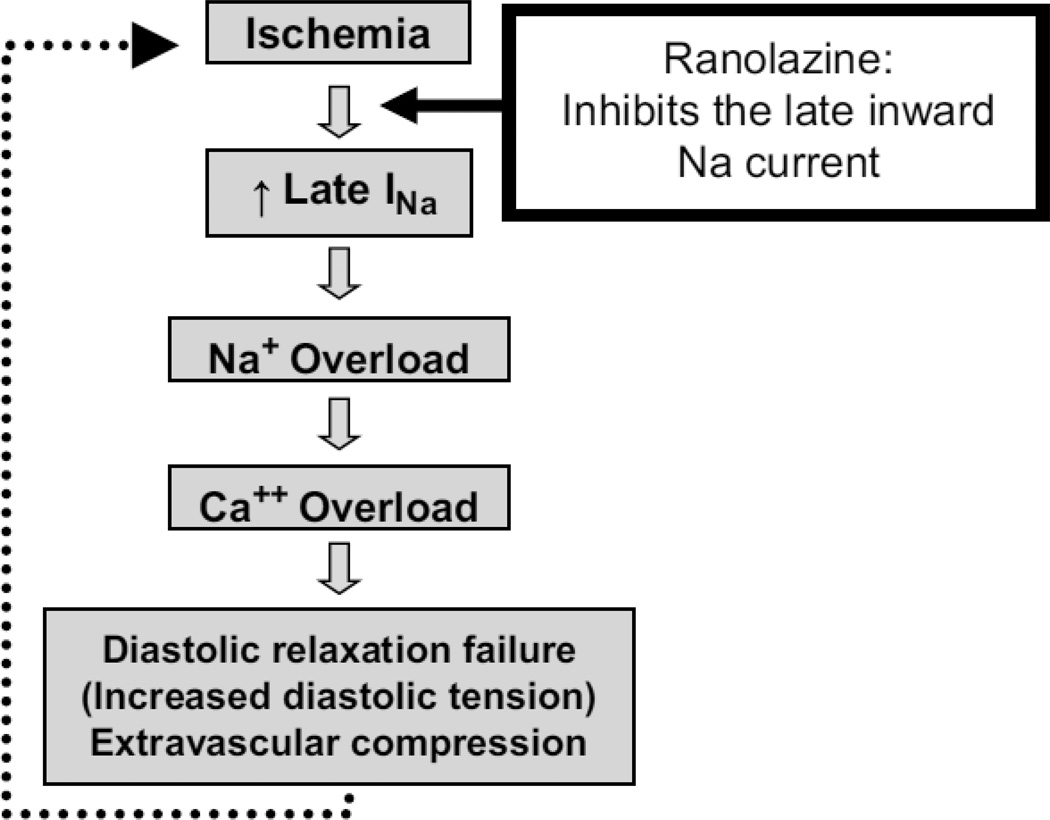
The initial inward sodium current can be altered in several conditions such as hypoxia, heart failure, or when exposed to metabolites such as reactive oxygen species.[8] Under these conditions, there is late opening, or possibly re-opening, of the sodium channels after inactivation leading to late influx of sodium ions. [9–11] This is referred to as the late inward sodium current (late INa). Although changes in the intracellular sodium concentration do not directly alter myofilament contraction, the increase in intracellular sodium leads to increased exchange of intracellular sodium for calcium through the sodium/calcium exchanger mechanism. When calcium concentrations increase, there can be diastolic activation of contractile proteins causing tonic contraction and increased energy consumption in an already energy-depleted myoctye.[12] Then, with increased diastolic tone, there can also be increased resistance to blood flow in the microcirculation resulting in worsening oxygen delivery. Eventually, a vicious cycle is set up where diastolic dysfunction after ischemia increases energy consumption and aggravates oxygen delivery to already hypoxic tissues. (Figure 2) While traditional anti-anginals act by mitigating the initial development of ischemia, ranolazine has a different mechanism of action, as described below.
Figure 2.
Scheme of the pathophysiology of myocardial ischemia and the role of ranolazine. (With permission from Stone PH, Cardiol Clin (2008) 26:603).
Ranolazine: mechanism of action
Ranolazine is a potent inhibitor of the late sodium current. It interrupts an important step in the pathophysiology of ischemia by protecting against intracellular ion dysregulation. Ranolazine was first believed to be effective as an anti-anginal drug by inhibiting metabolism of free fatty acids, but it has become clear that this effect only takes place at serum concentrations higher than those achieved in clinical use.[13, 14] A more important mechanism of action is the prevention of both calcium overload and the subsequent increase in diastolic tension due to inhibition of the late inward sodium channel.[15, 16] In cardiomyocytes from guinea pigs and dogs, ranolazine has been shown to cause a concentration-dependent, voltage-dependent, and frequency-dependent inhibition of the late INa‥[17] Given the normal rapid inactivation of the inward sodium channel in normal cardiac myocytes, the drug does not exert a significant effect on normal myocardium at the usual anti-anginal doses of up to 1,000 mg twice daily. Its effectiveness is heightened, however, under conditions of ischemia, as will be discussed further below.
Ranolazine: Clinical Use
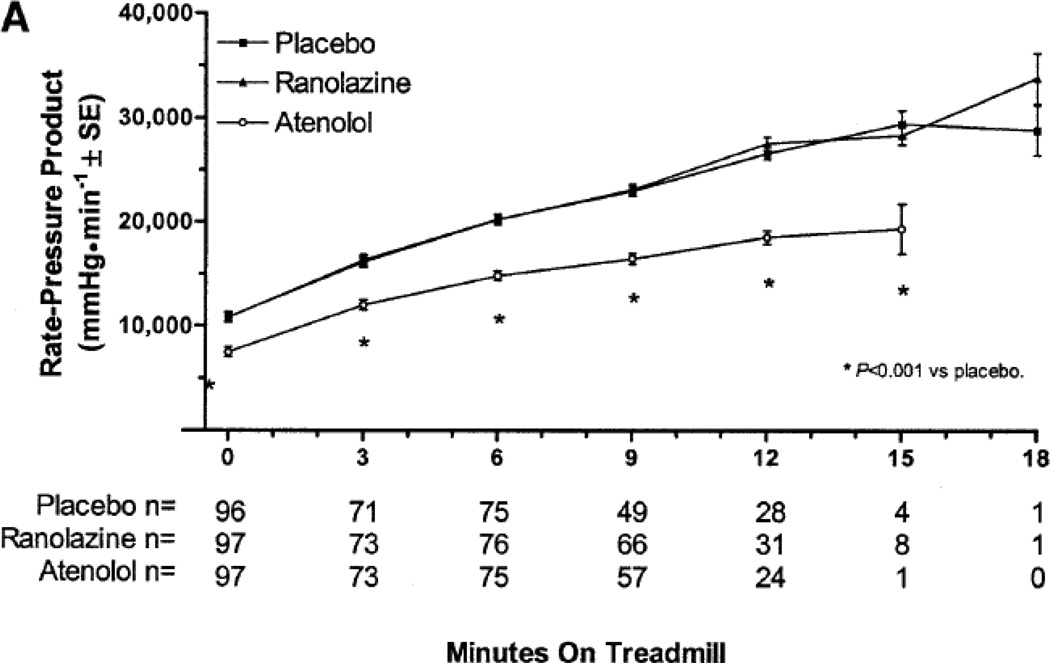
Ranolazine provides an alternative mechanism of action to treat ischemia. There is clinical evidence in humans that ranolazine functions as an anti-anginal in humans without impacting the traditional targets of myocardial oxygen demand, heart rate and blood pressure. As part of the Monotherapy Assessment of Ranolazine in Stable Angina (MARISA) trial (described in more detail below), patients were exercised at four different exercise levels on and off ranolazine. In the study, ranolazine produced a dose-dependent reduction in ST-segment depression that became more marked as exercise-induced ischemia became more pronounced, with only minor decreases in heart rate or blood pressure. At 12 minutes of exercise, for instance, the amount of ST-segment depression compared with placebo, and controlled for the rate-pressure product, was reduced by 22.3% on ranolazine 500 mg twice daily, by 35.4% on 1,000 mg twice daily, and by 45.8% on 1,500 mg twice daily. This progressive reduction in ischemia was more substantial than the minor changes in heart rate of blood pressure, leading to the conclusion that ranolazine acts via a mechanism independent from its effect on heart rate or blood pressure.[18] (Figure 3)
Figure 3.
Anti-ischemic effect of ranolazine without affecting heart rate of blood pressure during exercise. (With permission from Rousseau MF, et al. Am J Cardiol (2005) 95:314).
Mechanistic Evidence of the Role of Ranolazine
While improvement in diastolic function and reduction in diastolic wall tension leading to decreased oxygen consumption and ATP utilization are thought to be the main mechanism of action underlying the beneficial effects of ranolazine, there is only limited clinical data supporting this specific mechanism in humans. Figueredo, et al. have demonstrated improvement in the left ventricular myocardial performance index as measured by Doppler echocardiography in 22 study subjects treated with ranolazine for a mean of 2 months.[19] More recently, Venkataraman, et al. described improvement in both systolic and diastolic left ventricular synchrony in patients treated with ranolazine as measured by single photon emission computed tomographic myocardial perfusion imaging.[20]
Ranolazine in Clinical Use
Ranolazine, or N-(2,6-dimethylphenyl)-4(2-hydroxy-3-[2-methoxyphenoxyl]propyl)-1-piperazineacetamidedihidrochloride, was patented in 1986 and was approved by the US Food and Drug Administration in January 2006 for patients who remain symptomatic while on standard anti-anginal therapy. It is also available in the European Union. Ranolazine is available as film-coated sustained release tablets. The half-life for the extended release tablets is approximately 7 hours. Steady state concentrations are generally achieved within 3 days of twice daily dosing.[21] The drug is metabolized mostly by the cytochrome P450 enzyme CYP3A4 and to a lesser degree by CYP2D6, with approximately 5% excreted unchanged by the kidney. Drug clearance is reduced in patients with moderate to severe renal insufficiency. Given the CYP3A enzymatic system’s role in the metabolism of a number of other important drugs, ranolazine should be used with caution with diltiazem, ketoconazole, verapamil, macrolide antibiotics, HIV protease inhibitors, and others. Ranolazine also is a substrate and inhibitor of P-glycoprotein. Verapamil, which inhibits P-glycoprotein, inhibits absorption of ranolazine, with a consequent increase in plasma levels. Ranolazine also increases plasma digoxin levels.
Efficacy and Clinical Trials in Stable Angina
Early studies of ranolazine in patients with stable angina were performed using the immediate-release formulation of the drug, whereas more recent studies have used the sustained release formulation.
Immediate Release Formulation in Stable Angina
An early study examined the immediate release form of ranolazine at reasonably low doses.[22] Utilizing a randomized, double-blind, placebo-controlled, crossover design, 312 patients with chronic stable angina were withdrawn from other anti-anginal medications, and were treated with ranolazine 400 mg twice daily, 400 mg three times daily, or 267 mg three times daily, or placebo. Patients exhibited an improved total duration of exercise on ranolazine compared to placebo. However, the immediate release formulation did not provide continuous protection, and only provided short-term improvements in exercise duration, time to the onset of angina during exercise, and time to 1-mm ST segment depression during exercise.
Another study of the immediate release formulation of ranolazine was performed in nine centers across the US and Canada comparing the impact of the drug against atenolol (100 mg daily) and against placebo in patients with chronic stable angina and documented ST-segment depression with exercise testing.[23] In this study, ranolazine significantly reduced anginal episodes and nitroglycerin use, as well as significantly improving time to exercise-induced ischemia. Again this occurred without a significant change in rate-pressure product compared to placebo, in contrast to what occurred with atenolol. In the atenolol group, subjects had significantly decreased blood pressure, heart rate, and rate-pressure product at rest and during exercise compared with placebo or ranolazine.
Sustained Release Formulation in Stable Angina
Three randomized controlled trials have evaluated the extended release form of ranolazine in patients with chronic, stable angina. One trial, the Monotherapy Assessment of Ranolazine in Stable Angina (MARISA) evaluated ranolazine as an anti-anginal alone, while the Combination of Ranolazine in Stable Angina (CARISA) and Efficacy of Ranolazine in Chronic Angina (ERICA) trials evaluated the impact of ranolazine on a background of other anti-anginal agents.
Monotherapy - MARISA
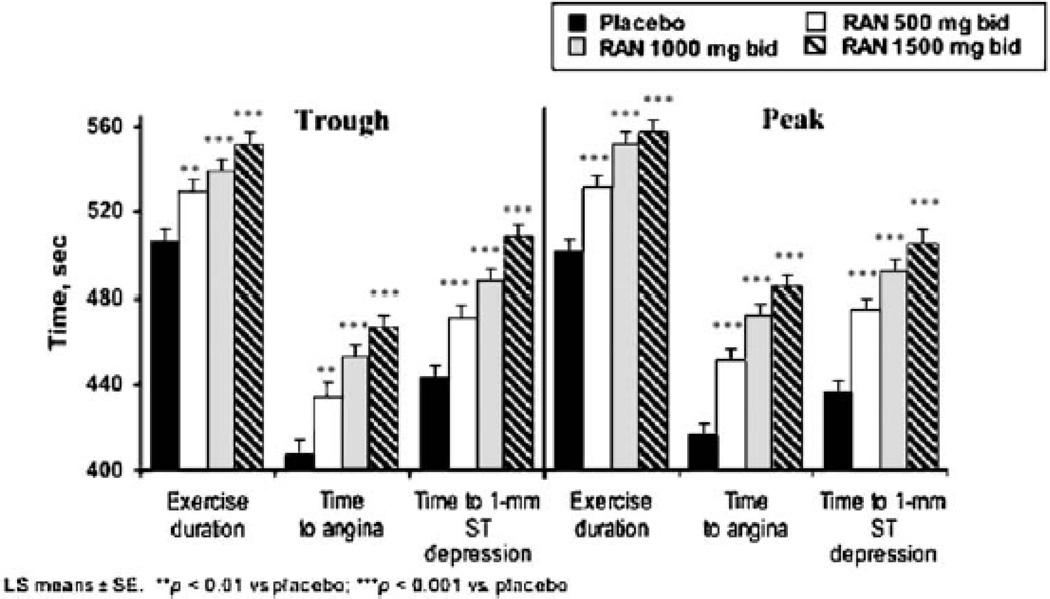
The MARISA trial investigated the effect of the sustained-release formulation of ranolazine as single agent therapy for angina.[24] The trial enrolled 191 patients with chronic exertional angina and reproducible ischemia on exercise testing in a 4 week, double-blind, placebo-controlled, cross-over trial at doses of 500 mg twice daily, 1,000 mg twice daily, or 1,500 mg twice daily. All three dosing regimens resulted in significant increases in exercise duration, exercise time to angina, and exercise time to 1-mm ST segment depression. The results were dose dependent, although the benefit was somewhat attenuated at 1,500 mg twice daily dosing. (Figure 4)
Figure 4.
Monotherapy with ranolazine increases exercise performance: the MARISA trial. (With permission from Chaitman et al. J Am Coll Cardiol (2004) 43:1378).
Combination regimens - CARISA, ERICA
In the CARISA trial, 823 patients receiving background anti-anginal therapy with either a calcium channel blocker or atenolol were randomly assigned to placebo or one of two doses of ranolazine (750 or 1,000 mg twice daily).[25] After 12 weeks of therapy, both doses of ranolazine significantly increased the total exercise time, the time to onset of angina during exercise, and (at peak ranolazine blood level) the time to ST-segment depression. Angina frequency was also reduced by 0.8 and 1.2 episodes per week (at the 750 mg and 1,000 mg doses, respectively) compared to placebo. There was no evidence of differential treatment effects based on the different background anti-anginal therapy a patient was receiving. In a recent substudy of those subjects on maximally tolerated beta-blocker or calcium channel blockers at the time of enrollment, a similar reduction in the number of angina attacks per week was seen.[26]
Adverse events were reported in 26% of patients in the placebo group, 31% of patients in the 750 mg group, and 33% of patients in the 1,000 mg group. The most common dose-related effects were constipation, dizziness, nausea, and asthenia. There was a small, dose-related increase in the QTc interval of 6.1 and 9.2 milliseconds in the 750 mg and 1,000 mg groups, but no clinically evident arrhythmias.
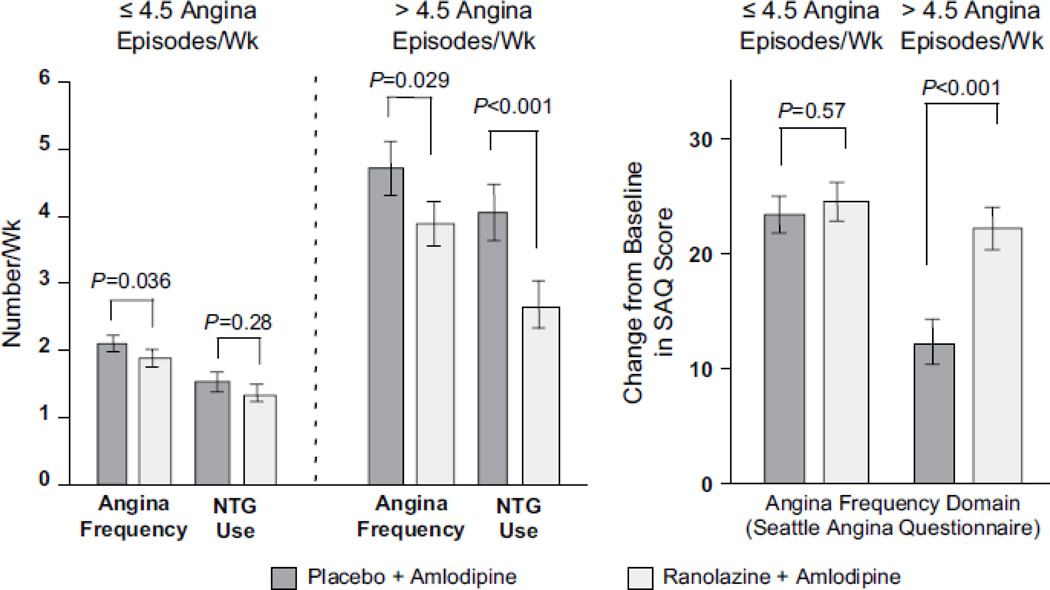
The ERICA is a more recent trial evaluating the effects of ranolazine in 565 patients who had persistent angina on a background of maximum dose amlodipine (10 mg daily).[27] In the trial, patients were also allowed to continue on long acting nitrates, but not beta-blockers. At baseline, the patients had 5.6 episodes of angina per week. Ranolazine was administered at 1,000 mg twice daily, and this regimen was compared to administration of placebo. Compared to placebo, ranolazine significantly reduced the number of weekly angina episodes (from 3.31 episodes per week on placebo to 2.88 episodes per week on ranolazine) as assessed using the Seattle Angina Questionnaire. (Figure 5)
Figure 5.
Effect of ranolazine in patients who have refractory angina despite maximum amlodipine therapy. (With permission from Stone et al. J Am Coll Cardiol (2006) 48:572).
The effect of ranolazine was consistent across the subgroups analyzed, including those on long-acting nitrates compared to those who were not, men compared to women, and patients older than 65 years compared to those younger than 65. Interestingly, patients with more than 4.5 episodes of angina weekly at baseline (the median) saw a more marked improvement with the drug than those who began the study with less frequent angina. This finding supports the idea that ranolazine is more effective in blunting the effects of ischemia when ischemia is actually present, rather than as a preventive measure.
Unstable Angina / NSTEMI – MERLIN-TIMI 36
The MERLIN-TIMI 36 trial has addressed the value of using ranolazine in the setting of non-ST segment elevation acute coronary syndrome (ACS) instead of stable angina.[28] The trial enrolled 6,560 patients within 48 hours of ischemic symptoms who were treated with ranolazine or placebo both during the acute phase of management of their presenting ACS, as well as during the chronic phase of secondary prevention (median duration approximately 1 year). Patients were initially treated with an intravenous formulation of ranolazine followed by 1,000 mg of the oral, extended-release formulation twice daily. The primary analysis of the trial showed no significant difference in the rate of the primary endpoint of cardiovascular death, MI, or recurrent ischemia between ranolazine and placebo (26.3% vs. 30.1%, hazard ration 0.92, 95% CI 0.83 to 1.02; p=0.11).
Analysis of the individual components of the primary endpoint revealed that ranolazine had no impact on the rate of death or recurrent MI (12.9% vs. 13.7%, p=0.87). However, ranolazine did reduce recurrent ischemia (17.3% vs. 20.0%, HR 0.87, 95% CI 0.76–0.99). Assessment of the other endpoints reflecting the anti-anginal properties of the drug demonstrated a 23% reduction in the rate of worsening angina and a 19% reduction in the need for any additional or increase in anti-anginal therapy in the ranolazine group compared to placebo.
While the overall results of the MERLIN-TIMI 36 trial did not alter the standard of care for patients with ACS, the study does provide some evidence for the anti-ischemic properties of ranolazine in a larger and less selected population of individuals with coronary artery disease than did the MARISA, CARISA, or ERICA trial described above.
In a substudy analysis that stratified patients within the MERLIN-TIMI 36 study based on the brain natriuretic peptide (BNP) measurements obtained at the time of randomization, [29] patients who had elevated BNP over 80 pg/mL experienced a significantly higher incidence of cardiovascular death, MI, or recurrent ischemia compared to those with BNP less than 80 pg/mL. Interestingly, those with higher BNP seemed to benefit more from exposure to ranolazine. In patients with BNP >80 pg/ml, ranolazine reduced the composite endpoint of cardiovascular death, MI, and recurrent ischemia (hazard ratio [HR]: 0.79; 95% confidence interval [CI]: 0.66 to 0.94, p = 0.009). Initial elevated BNP levels on presentation likely reflect diastolic stiffness resulting from more severe or prolonged ischemia. Thus, this substudy provides further evidence of enhanced efficacy in more severely ischemic patients.
In the course of ACS, ranolazine also had a significant effect in preventing ventricular arrhythmias and atrial arrhythmias in the study population of high-risk patients with non-ST segment elevation ACS.[30] In patients treated with ranolazine, there was a 3% lower frequency of ventricular tachycardia, a 10.3% lower incidence of supraventricular tachycardia, and a 0.7% reduction in new onset atrial fibrillation. While some of this anti-arrhythmic effect may be related to direct effects on the late INa, minimizing ischemia may also be one of the anti-arrhythmic mechanisms of the drug.
Due to the size of the MERLIN-TIMI 36 trial, the safety of ranolazine in ischemic patients was confirmed during this clinical trial. There was no difference in mortality between patients treated with ranolazine versus placebo. There was also no difference in sudden death. Discontinuation of the drug due to an adverse event also occurred more frequently in the ranolazine group compared to the placebo group (8.8% vs. 4.7%, p<0.001). The most frequent adverse events were dizziness (13% with ranolazine vs. 7% with placebo), nausea (9% vs. 6%), and constipation (9% vs. 3%). Syncope did occur more frequently in the ranolazine group than the placebo group (3.3% vs. 2.3%, p=0.01). Most of these were thought to be due to vasovagal episodes. There were only two cases of torsades de pointes noted in the study, one case in each group despite the expected QTc prolongation.
Post-percutaneous coronary intervention (PCI)
Recently, a pilot randomized clinical trial has evaluated ranolazine in the setting of PCI in order to attempt to reduce peri-procedural ischemia. Seventy patients undergoing elective PCI were randomized to treatment with ranolazine or placebo for 7 days prior to the PCI procedure. The group pretreated with ranolazine was found to have fewer peri-procedural MIs (6% vs. 22%, p=0.041) and less release of troponin I. These data are the first to suggest that ranolazine is effective in decreasing the incidence of myocardial injury during PCI.
Ranolazine in women with ischemia but without obstructive CAD
There is some suggestion in the literature that ranolazine may be particularly effective in the prevention of ischemia for women, particularly those without documented obstructive CAD. In a substudy of the MERLIN-TIMI 36 trial, women were noted to have less significant epicardial CAD disease than men. However, women were more likely to have an elevated B-type natriuretic peptide, more likely to have worse median angina frequency scores, and more likely to have had an ischemic episode on continuous ECG during the first seven days of the study compared to men. Despite these differences, ranolazine treatment in women was still associated with a significant reduction in recurrent ischemia (13.0% vs. 18.2%, p=0.002).
More recent data supports the possible use for ranolazine in women with evidence of myocardial ischemia in the absence of obstructive CAD. A pilot, randomized controlled trial was performed where 20 women with ≥ 10% ischemic myocardium on adenosine stress cardiac magnetic resonance imaging (MRI), but no evidence of obstructive CAD on angiography were randomized to ranolazine or placebo. Patients treated with ranolazine had significantly better scores on the Seattle Angina Questionnaire in areas of physical functioning, angina stability, and quality of life. There was also a suggestion of improvement in the quantitative myocardial perfusion reserve index in those women with coronary flow reserve ≤3 who were treated with ranolazine compared to placebo.[31]
QTc Prolongation
Ranolazine can increase the duration of the action potential and the QT interval.[32] Although prolongation of the QT interval is apparent on electronic analysis of population-wide ECGs, the average prolongation in a given patient might be difficult to pick up in view of the normal intra-patient variability. The mean prolongation on ranolazine is up to 10 msec in patients treated with 500 to 1,000 mg twice daily. While QTc prolongation has been observed, an increase in significant arrhythmias has not been seen across the MARISA, CARISA, ERICA and MERLIN-TIMI 36 studies.[25, 24, 27, 28] Interestingly, in the MERLIN-TIMI 36 trial, ranolazine significantly reduced the rate of a pre-specified set of arrhythmias during the first 7 days of treatment compared to placebo (73.7% vs. 83.1%, p<0.001).[30] With regard to ventricular tachycardia, fewer patients on ranolazine had an episode of ventricular tachycardia lasting ≥ 8 beats (5.3% vs. 8.3%, p<0.001) compared to placebo. There were also significant decreases in the rates of new-onset atrial fibrillation, supraventricular tachycardia, or pauses ≥ 3 seconds.
The prolongation of the QT interval with ranolazine results from modest inhibition of the IKr that is partially counterbalanced by the effect of ranolazine on late INa. A study in isolated guinea pig hearts measured the effects of ranolazine alone and in the presence of anemone toxin, whose action mimics the sodium channelopathy associated with long QT syndrome. Although ranolazine prolonged the duration of the action potential, it reduced ventricular arrhythmias caused by agents that increased the late sodium current.[33, 34]
Summary
Ranolazine is an anti-anginal agent that is effective via its effect on the late INa, independent of its effect on heart rate and loading conditions. Evidence of its clinical efficacy has been demonstrated in trials in chronic stable angina when used as a sole anti-anginal or in combination with other, more traditional agents. Ranolazine has also been studied in the setting of ACS, where it has not been shown to be effective in modifying the natural history of CAD, but its anti-ischemic properties and safety have been confirmed. At present, ranolazine is approved for use as first line therapy for chronic stable angina and is suitable for use as first line therapy when low heart rate or blood pressure limits the use of the other, more conventional, anti-anginal agents. Ranolazine is also particularly effective as an adjunctive therapy in patients without adequate symptom relief from conventional anti-anginal therapies, particularly beta blockers, nitrates, and calcium channel blockers. In non-ST-segment elevation ACS, it reduces ischemia, but has no effect on mortality thus limiting its use compared to other agents, particularly beta blockers. There is emerging evidence that ranolazine may be useful in the future for myocardial protection during PCI or in the treatment of the subset of patients with documented ischemia on functional cardiac testing, but no obstructive CAD on angiography. At present, there is insufficient data in these areas to recommend routine use.
Overall, ranolazine has been shown to be effective in improving symptoms, quality of life, and multiple objective measures of ischemia in patients with stable coronary artery disease. Overall, we recommend its use as an adjunctive therapy when limited by low heart rate or hypotension with the use of other anti-anginals including beta-blockers, nitrates, or calcium channel blockers. We do not recommend its routine use during ACS at this time. More studies are needed to definitively address other possible indications with regard to the prevention of ischemia.
Acknowledgments
Funding: Dr. Wimmer receives research support from NIH T32HL007604.
Footnotes
Disclosures: Dr. Wimmer has no disclosures. Dr. Stone has no disclosures.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics-2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. doi:CIRCULATIONAHA.106.179918 [pii] 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina) Circulation. 2003;107(1):149–158. doi: 10.1161/01.cir.0000047041.66447.29. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, McDonald KM, Hastie T, Fadel B, Hagan V, Lee BK, et al. Meta-analysis of trials comparing beta-blockers, calcium antagonists, and nitrates for stable angina. JAMA. 1999;281(20):1927–1936. doi: 10.1001/jama.281.20.1927. doi:jma80047 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Abrams J. Clinical practice. Chronic stable angina. N Engl J Med. 2005;352(24):2524–2533. doi: 10.1056/NEJMcp042317. doi:352/24/2524 [pii] 10.1056/NEJMcp042317. [DOI] [PubMed] [Google Scholar]
- 5.Silverman HS, Stern MD. Ionic basis of ischaemic cardiac injury: insights from cellular studies. Cardiovasc Res. 1994;28(5):581–597. doi: 10.1093/cvr/28.5.581. doi-0008-6363(94)90159-7 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Imahashi K, Kusuoka H, Hashimoto K, Yoshioka J, Yamaguchi H, Nishimura T. Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ Res. 1999;84(12):1401–1406. doi: 10.1161/01.res.84.12.1401. [DOI] [PubMed] [Google Scholar]
- 7.Bers DM. Excitation-contraction coupling and cardiac contractile force. 2nd ed. Developments in cardiovascular medicine. Vol. 237. Dordrecht; Boston: Kluwer Academic Publishers; 2001. [Google Scholar]
- 8.Hale SL, Shryock JC, Belardinelli L, Sweeney M, Kloner RA. Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardiol. 2008;44(6):954–967. doi: 10.1016/j.yjmcc.2008.03.019. doi:S0022-2828(08)00363-5 [pii] 10.1016/j.yjmcc.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol. 1996;497(Pt 2):337–347. doi: 10.1113/jphysiol.1996.sp021772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Undrovinas AI, Fleidervish IA, Makielski JC. Inward sodium current at resting potentials in single cardiac myocytes induced by the ischemic metabolite lysophosphatidylcholine. Circ Res. 1992;71(5):1231–1241. doi: 10.1161/01.res.71.5.1231. [DOI] [PubMed] [Google Scholar]
- 11.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997;500(Pt 3):631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandes R, Maier LS, Bers DM. Regulation of mitochondrial [NADH] by cytosolic [Ca2+] and work in trabeculae from hypertrophic and normal rat hearts. Circ Res. 1998;82(11):1189–1198. doi: 10.1161/01.res.82.11.1189. [DOI] [PubMed] [Google Scholar]
- 13.McCormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation. 1996;93(1):135–142. doi: 10.1161/01.cir.93.1.135. [DOI] [PubMed] [Google Scholar]
- 14.MacInnes A, Fairman DA, Binding P, Rhodes J, Wyatt MJ, Phelan A, et al. The antianginal agent trimetazidine does not exert its functional benefit via inhibition of mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2003;93(3):e26–e32. doi: 10.1161/01.RES.0000086943.72932.71. doi:10.1161/01.RES.0000086943.72932.71 01.RES.0000086943.72932.71 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113(20):2462–2472. doi: 10.1161/CIRCULATIONAHA.105.597500. doi:113/20/2462 [pii] 10.1161/CIRCULATIONAHA.105.597500. [DOI] [PubMed] [Google Scholar]
- 16.Maier LS. A novel mechanism for the treatment of angina, arrhythmias, and diastolic dysfunction: inhibition of late I(Na) using ranolazine. J Cardiovasc Pharmacol. 2009;54(4):279–286. doi: 10.1097/FJC.0b013e3181a1b9e7. doi:10.1097/FJC.0b013e3181a1b9e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, et al. Electrophysiologic properties and antiarrhythmic actions of a novel antianginal agent. J Cardiovasc Pharmacol Ther. 2004;9(Suppl 1):S65–S83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 18.Stone PH, Chaitman BR, Stocke K, Sano J, DeVault A, Koch GG. The anti-ischemic mechanism of action of ranolazine in stable ischemic heart disease. J Am Coll Cardiol. 2010;56(12):934–942. doi: 10.1016/j.jacc.2010.04.042. doi:S0735-1097(10)02463-0 [pii] 10.1016/j.jacc.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 19.Figueredo VM, Pressman GS, Romero-Corral A, Murdock E, Holderbach P, Morris DL. Improvement in left ventricular systolic and diastolic performance during ranolazine treatment in patients with stable angina. Journal of cardiovascular pharmacology and therapeutics. 2011;16(2):168–172. doi: 10.1177/1074248410382105. doi:10.1177/1074248410382105. [DOI] [PubMed] [Google Scholar]
- 20.Venkataraman R, Chen J, Garcia EV, Belardinelli L, Hage FG, Heo J, et al. Effect of Ranolazine on Left Ventricular Dyssynchrony in Patients With Coronary Artery Disease. The American journal of cardiology. 2012 doi: 10.1016/j.amjcard.2012.06.055. doi:10.1016/j.amjcard.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerling M. Clinical pharmacokinetics of ranolazine. Clin Pharmacokinet. 2006;45(5):469–491. doi: 10.2165/00003088-200645050-00003. doi:4553 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Pepine CJ, Wolff AA. A controlled trial with a novel anti-ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional antianginal agents. Ranolazine Study Group. Am J Cardiol. 1999;84(1):46–50. doi: 10.1016/s0002-9149(99)00190-3. doi:S0002914999001903 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol. 2005;95(3):311–316. doi: 10.1016/j.amjcard.2004.09.025. doi:S0002-9149(04)01593-0 [pii] 10.1016/j.amjcard.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Chaitman BR, Skettino SL, Parker JO, Hanley P, Meluzin J, Kuch J, et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004;43(8):1375–1382. doi: 10.1016/j.jacc.2003.11.045. doi:10.1016/j.jacc.2003.11.045 S0735109704001925 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Chaitman BR, Pepine CJ, Parker JO, Skopal J, Chumakova G, Kuch J, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291(3):309–316. doi: 10.1001/jama.291.3.309. doi:10.1001/jama.291.3.309 291/3/309 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Sendon JL, Lee S, Cheng ML, Ben-Yehuda O. Effects of ranolazine on exercise tolerance and angina frequency in patients with severe chronic angina receiving maximally-tolerated background therapy: analysis from the Combination Assessment of Ranolazine In Stable Angina (CARISA) randomized trial. Eur J Prev Cardiol. 2012;19(5):952–959. doi: 10.1177/2047487312450133. doi:10.1177/2047487312450133. [DOI] [PubMed] [Google Scholar]
- 27.Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol. 2006;48(3):566–575. doi: 10.1016/j.jacc.2006.05.044. doi:S0735-1097(06)01532-4 [pii] 10.1016/j.jacc.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 28.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297(16):1775–1783. doi: 10.1001/jama.297.16.1775. doi:297/16/1775 [pii] 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 29.Morrow DA, Scirica BM, Sabatine MS, de Lemos JA, Murphy SA, Jarolim P, et al. B-type natriuretic peptide and the effect of ranolazine in patients with non-ST-segment elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST Elevation Acute Coronary-Thrombolysis In Myocardial Infarction 36) trial. J Am Coll Cardiol. 2010;55(12):1189–1196. doi: 10.1016/j.jacc.2009.09.068. doi:S0735-1097(10)00235-4 [pii] 10.1016/j.jacc.2009.09.068. [DOI] [PubMed] [Google Scholar]
- 30.Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116(15):1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. doi:CIRCULATIONAHA.107.724880 [pii] 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 31.Mehta PK, Goykhman P, Thomson LE, Shufelt C, Wei J, Yang Y, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging. 2011;4(5):514–522. doi: 10.1016/j.jcmg.2011.03.007. doi:10.1016/j.jcmg.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belardinelli L, Shryock JC, Wu L, Song Y. Use of preclinical assays to predict risk of drug-induced torsades de pointes. Heart Rhythm. 2005;2(2 Suppl):S16–S22. doi: 10.1016/j.hrthm.2004.10.032. doi:10.1016/j.hrthm.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004;310(2):599–605. doi: 10.1124/jpet.104.066100. doi:10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 34.Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. Journal of cardiovascular pharmacology. 2004;44(2):192–199. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]