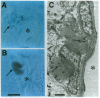
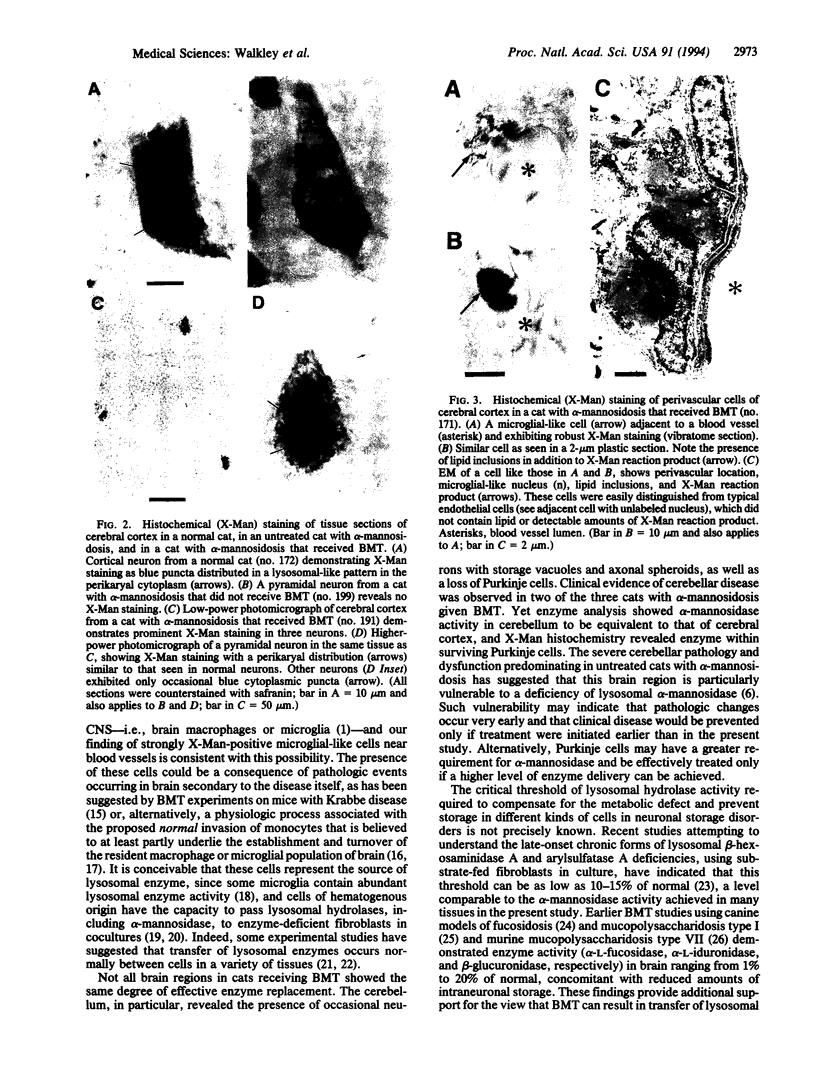
Abstract
Neuronal storage disorders are fatal neurodegenerative diseases of humans and animals that are caused by inherited deficiencies of lysosomal hydrolase activity. Affected individuals often appear normal at birth but eventually develop progressive neurologic symptoms including sensory and motor deficits, mental retardation, and seizures. We have examined efficacy of bone marrow transplantation as a means of enzyme replacement, using cats with the lysosomal storage disease alpha-mannosidosis. Treated animals showed little or no progression of neurologic signs 1-2 years after transplant, whereas untreated cats became severely impaired and reached endstage disease by 6 months of age. Increased lysosomal alpha-mannosidase activity was found in brain tissue of the treated animals, and electron microscopy revealed no evidence of lysosomal storage within most neurons. Histochemical localization of acidic alpha-D-mannoside mannohydrolase (EC 3.2. 1.24), using 5-bromo-4-chloro-3-indolyl alpha-D-mannopyranoside, showed that functional enzyme was present in neurons, glial cells, and cells associated with blood vessels. This study provides direct evidence that bone marrow transplantation as treatment for a neuronal storage disease can lead to significant levels of a missing lysosomal hydrolase within neurons of the central nervous system and to compensation for the genetic metabolic defect.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham D., Muir H., Winchester B., Olsen I. Lymphocytes transfer only the lysosomal form of alpha-D-mannosidase during cell-to-cell contact. Exp Cell Res. 1988 Mar;175(1):158–168. doi: 10.1016/0014-4827(88)90263-7. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Barker J. E., Vogler C. A., Kyle J. W., Sly W. S., Gwynn B., Levy B., Pegors C. Increased life span and correction of metabolic defects in murine mucopolysaccharidosis type VII after syngeneic bone marrow transplantation. Blood. 1991 Dec 1;78(11):3081–3092. [PubMed] [Google Scholar]
- Cummings J. F., Wood P. A., de Lahunta A., Walkley S. U., Le Boeuf L. The clinical and pathologic heterogeneity of feline alpha-mannosidosis. J Vet Intern Med. 1988 Oct-Dec;2(4):163–170. doi: 10.1111/j.1939-1676.1988.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Dobrenis K., Joseph A., Rattazzi M. C. Neuronal lysosomal enzyme replacement using fragment C of tetanus toxin. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2297–2301. doi: 10.1073/pnas.89.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder N. Solitary cells and enzyme exchange in tetraparental mice. Nature. 1976 Sep 2;263(5572):67–69. doi: 10.1038/263067a0. [DOI] [PubMed] [Google Scholar]
- Gasper P. W., Thrall M. A., Wenger D. A., Macy D. W., Ham L., Dornsife R. E., McBiles K., Quackenbush S. L., Kesel M. L., Gillette E. L. Correction of feline arylsulphatase B deficiency (mucopolysaccharidosis VI) by bone marrow transplantation. 1984 Nov 29-Dec 5Nature. 312(5993):467–469. doi: 10.1038/312467a0. [DOI] [PubMed] [Google Scholar]
- Goodman L. A., Livingston P. O., Walkley S. U. Ectopic dendrites occur only on cortical pyramidal cells containing elevated GM2 ganglioside in alpha-mannosidosis. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11330–11334. doi: 10.1073/pnas.88.24.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K., Mullen R. J. Intercellular transfer of beta-glucuronidase in chimeric mice. J Cell Sci. 1979 Dec;40:21–31. doi: 10.1242/jcs.40.1.21. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hoogerbrugge P. M., Suzuki K., Suzuki K., Poorthuis B. J., Kobayashi T., Wagemaker G., van Bekkum D. W. Donor-derived cells in the central nervous system of twitcher mice after bone marrow transplantation. Science. 1988 Feb 26;239(4843):1035–1038. doi: 10.1126/science.3278379. [DOI] [PubMed] [Google Scholar]
- Jolly R. D., Thompson K. G., Murphy C. E., Manktelow B. W., Bruere A. N., Winchester B. G. Enzyme replacement therapy--an experiment of nature in a chimeric mannosidosis calf. Pediatr Res. 1976 Apr;10(4):219–224. doi: 10.1203/00006450-197604000-00003. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leinekugel P., Michel S., Conzelmann E., Sandhoff K. Quantitative correlation between the residual activity of beta-hexosaminidase A and arylsulfatase A and the severity of the resulting lysosomal storage disease. Hum Genet. 1992 Mar;88(5):513–523. doi: 10.1007/BF00219337. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Light and electron microscopic demonstration of some lysosomal enzymes in the amoeboid microglia in neonatal rat brain. J Anat. 1977 Jul;123(Pt 3):637–648. [PMC free article] [PubMed] [Google Scholar]
- Olsen I., Dean M. F., Harris G., Muir H. Direct transfer of a lysosomal enzyme from lymphoid cells to deficient fibroblasts. Nature. 1981 May 21;291(5812):244–247. doi: 10.1038/291244a0. [DOI] [PubMed] [Google Scholar]
- Parkman R. The application of bone marrow transplantation to the treatment of genetic diseases. Science. 1986 Jun 13;232(4756):1373–1378. doi: 10.1126/science.3520819. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Hume D. A., Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985 Jun;15(2):313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Shull R. M., Hastings N. E., Selcer R. R., Jones J. B., Smith J. R., Cullen W. C., Constantopoulos G. Bone marrow transplantation in canine mucopolysaccharidosis I. Effects within the central nervous system. J Clin Invest. 1987 Feb;79(2):435–443. doi: 10.1172/JCI112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Kreutzberg G. W. Lectin binding by resting and reactive microglia. J Neurocytol. 1987 Apr;16(2):249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Taylor R. M., Stewart G. J., Farrow B. R., Byrne J., Healy P. J. Histological improvement and enzyme replacement in the brains of fucosidosis dogs after bone marrow engraftment. Transplant Proc. 1989 Feb;21(1 Pt 3):3074–3075. [PubMed] [Google Scholar]
- Vandevelde M., Fankhauser R., Bichsel P., Wiesmann U., Herschkowitz N. Hereditary neurovisceral mannosidosis associated with alpha-mannosidase deficiency in a family of Persian cats. Acta Neuropathol. 1982;58(1):64–68. doi: 10.1007/BF00692699. [DOI] [PubMed] [Google Scholar]
- Walkley S. U., Baker H. J., Rattazzi M. C., Haskins M. E., Wu J. Y. Neuroaxonal dystrophy in neuronal storage disorders: evidence for major GABAergic neuron involvement. J Neurol Sci. 1991 Jul;104(1):1–8. doi: 10.1016/0022-510x(91)90208-o. [DOI] [PubMed] [Google Scholar]
- Will A., Cooper A., Hatton C., Sardharwalla I. B., Evans D. I., Stevens R. F. Bone marrow transplantation in the treatment of alpha-mannosidosis. Arch Dis Child. 1987 Oct;62(10):1044–1049. doi: 10.1136/adc.62.10.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]