Abstract
The correlation between diet and dental topography is of importance to paleontologists seeking to diagnose ecological adaptations in extinct taxa. Although the subject is well represented in the literature, few studies directly compare methods or evaluate dietary signals conveyed by both upper and lower molars. Here, we address this gap in our knowledge by comparing the efficacy of three measures of functional morphology for classifying an ecologically diverse sample of thirteen medium- to large-bodied platyrrhines by diet category (e.g., folivore, frugivore, hard object feeder). We used Shearing Quotient (SQ), an index derived from linear measurements of molar cutting edges and two indices of crown surface topography, Occlusal Relief (OR) and Relief Index (RFI). Using SQ, OR, and RFI, individuals were then classified by dietary category using Discriminate Function Analysis. Both upper and lower molar variables produce high classification rates in assigning individuals to diet categories, but lower molars are consistently more successful. SQs yield the highest classification rates. RFI and OR generally perform above chance. Upper molar RFI has a success rate below the level of chance. Adding molar length enhances the discriminatory power for all variables. We conclude that upper molar SQs are useful for dietary reconstruction, especially when combined with body size information. Additionally, we find that among our sample of platyrrhines, SQ remains the strongest predictor of diet, while RFI is less useful at signaling dietary differences in absence of body size information. The study demonstrates new ways for inferring the diets of extinct platyrrhine primates when both upper and lower molars are available, or, for taxa known only from upper molars. The techniques are useful in reconstructing diet in stem representatives of anthropoid clade, who share key aspects of molar morphology with extant platyrrhines.
Introduction
Numerous studies document a general relationship between primate molar occlusal morphology and diet, providing a useful way of inferring the diets of extinct species. To date, the bulk of this literature has focused on the use of lower molars in dietary reconstruction, with little consideration of the corresponding upper molar morphology. Among primates, measurements of the relative length of the lower molar shearing crests [1,2,3] and various measures of occlusal surface relief have been explored as correlates of diet in extant groups [4,5,6,7]. With the exception of a small data set of strepsirrhine upper molar crest lengths explored by Bajpai et al. [8], it has not yet been established whether a reliable diet signal may be captured from upper molar occlusal morphology as well. Furthermore, it is debated whether measures of occlusal relief and relative lengths of the shearing crests are comparably reliable indicators of diet category [5]. Here, we present a comparison of dietary signals present in occluding pairs of upper and lower first molars in medium- to large-bodied platyrrhines—a clade of primates for whom the phylogeny is well understood—using multiple measures of occlusal morphology (shear quotients and occlusal surface relief indices). Finding a generalizable method for inferring diets of extinct platyrrhines from upper as well as lower molars would introduce a previously unutilized source of information about platyrrhine paleobiology. Also, because Eocene-Oligocene anthropoids are similar to platyrrhines in body size and molar structure, this data set will provide an added source for inferring early anthropoid niche structure to that based on lower dental morphology [9,10].
In the introduction to their 1984 book “Adaptations for Foraging in Nonhuman Primates” Rodman and Cant [11] call attention to three sorts of adaptations: those for reproduction, avoidance of predation, and acquisition of food (foraging). Of the three, they note,
“… it is the ‘hunt’ and capture of food that seems to select most consistently for organismal design in primates…. Physiological potentials determine the suitability of any food to any specific forager, and physical structure of the food determines the potential of the forager to reduce the food to useful form” (page 2).
Consistent with Rodman and Cant’s observation, foraging and dietary specializations play a fundamental role in theories about the origin of the evolutionary novelties that characterize primate, anthropoid, and human origins [2,8,10,12,13,14,15,16,17,18,19]. Not surprisingly, the search for morphological signals of diet in fossils has produced an enormous literature emphasizing morphology, tooth wear, enamel structure, and the isotopic and physical properties of dental materials [20,21,22,23,24,25,26].
Most studies of diet-morphology correspondence emphasize how upper and lower cheek teeth interact to reduce food size during mastication [27,28]. It is assumed that tooth occlusal surfaces are subject to natural selection related to chewing efficiency [29] and to the ability of the teeth to remain functional in the face of chewing forces and attendant wear [30]. Increases in the surface area of masticated food as a consequence of chewing should increase the rate of digestion, thereby increasing the rate of energy return [31,32]. Thus, the structural properties of the food (hardness, ductility, etc.) select for the structural design of the teeth through adaptation for more efficient trituration and maintenance of functionality [33]. At the same time, tooth structure is selected to optimize the extraction of nutrients from food; foods like sugars and starches may not have to be chewed finely to achieve an optimal digestibility, whereas plant or animal structural carbohydrates require extensive oral processing to optimize digestibility [31]. Hard object consumers, for whom food processing and maintenance of dental function may be a balancing act, can often be differentiated from taxa consuming softer items through enamel specializations in combination the very low molar relief (e.g., [23]). Additionally, body size must also be taken into consideration owing to its dual association with metabolic needs and food passage time [31,34,35].
Some work has been done to examine the relationship between dental topography (molar crest lengths), chewing efficiency (chewed food particle size) and food physical properties. Sheine and Kay [29,32] report experiments showing that the relative lengths of molar shearing crests in six strepsirrhine species are related directly to efficiency in increasing food surface area. Yamashita [36] finds that lower molar crest lengths are correlated with leaf consumption in five Madagascar lemur species, such that animals consuming a higher proportion of leaves and materials with higher shear strength (measured as the stress at which the material tears, in kg/mm2) tend to have longer lower molar shear crests, relative to lower molar area. Likewise, research by Kay [1,2] has demonstrated that the degree of development of the cutting edges of the lower molars (measured in three dimensions with a binocular microscope, not as projected onto the occlusal plane) when corrected for tooth size and expressed as a Shearing Quotient (SQ) serves as a guide to the diet of extant species. A modification of this approach using Shearing Ratios (SR) used by Strait [3] yields similar results.
Recently, a number of techniques employ laser and μCT scanning to produce three-dimensional models of tooth crowns. A variety of different measures to capture the surface topography can then be collected. Several indices derived from surface topography have been demonstrated to correlate with dietary profile in select mammalian groups. These include measures of occlusal relief (e.g., Occlusal Relief, (OR; [4]), Relief Index (RFI; [7]), Orientation Patch Count (OPC; [37]), and Dirichlet Normal Surface Energy (DNSE; [6]). Relief indices (RFI and OR) represent the ratio of the three-dimensional surface area to the two-dimensional tooth cross-sectional area. The greater the occlusal relief, the higher the values of these indices [38]. Originally developed to study tooth wear, measures of molar relief (RFI and OR) have been applied to the study of surface topography of unworn teeth to search for diet-morphology correlates in euarchontan mammals [7], strepsirrhines [5], platyrrhines [5,39], and hominoids [4]. Unlike measurements of relative molar shear (SQ), no laboratory or field studies have examined whether mechanical food properties and/or food shape are correlated and functionally related to the three-dimensional dental topographic variables.
Simple proxies of tooth function and dental topographic methods each have strengths and weaknesses [6,7,39]. All techniques are sensitive to dental wear as would be expected from simple examination of the gross effects of tooth shape as teeth wear. Therefore, study samples usually include specimens that have little wear. Phylogenetic effects also must be considered in interspecific comparative analyses on a large number of species, to account for the likelihood that a part of the shared similarity between closely related species may be due to shared descent [7]. Inter-individual error in measurement has been considered a further potential drawback of shearing measurements because the technique is based on recognition of landmarks that often are difficult to identify precisely. Equally, however, surface topographic methods are not entirely landmark-free because the observer must choose how to ‘crop’ the tooth crown from the root and determine the type and degree of smoothing algorithms to apply to the surface prior to analysis.
Finally, we recognize an overarching problem of each of these current approaches is quantifying diet as a categorical variable to compare with molar structure. If we are evaluating the statistical ‘success’ of assigning a species to a particular diet category using a particular measure of molar morphology, we need to feel confident that the within-group variability is less than the between-group variability in the diet categories, for example that items within each dietary category have broadly similar structural properties [36]. The state of our current knowledge does not permit this except in a handful of cases.
Bearing in mind the above caveats and uncertainties we analyze occluding upper and lower molar structure in a sample of platyrrhines, with intent to evaluate the feasible use of upper molars in diet reconstruction, and to compare the efficacy of three methods of dietary inference—RFI, OR and SQ. Our dataset consists of thirteen species of medium to large-bodied New World monkeys. The phylogeny of the group is well known (see Materials) allowing us to measure and control for phylogenetic effects in the data. Large samples are used when calculating each index for the associated upper and lower molars of the same individual specimens. Previous evaluations of these measures focused on lower molars, whereas our new dataset includes measurements of both the lower teeth and the upper teeth with which they occlude.
We pose two questions: Controlling for sample size and phylogenetic effects, how efficiently do RFI, OR, and SQ of the lower and upper teeth correctly assign individuals and species to dietary categories? And, how do these measurements used together and in various combinations affect the accuracy of the assignments?
Materials and Methods
Data collection
The study sample consists of the upper and lower first molars of thirteen species of platyrrhine primates (an average of 7 specimens per species) covering a range of body sizes and dietary profiles (Table 1). All specimens used in this study were collected by mammalogists and are housed in systematic collections in the United States and Brazil. A full list of the specimens (with museum attribution) is found in S1 Table. No animals were sacrificed for the purposes of this study. Representatives from all three extant platyrrhine families were included; however, members of the subfamily Callitrichinae (marmosets and tamarins) were excluded. Due to the uniformly small size of callitrichines, the scanning tools we employed for surface analyses lack sufficient precision for these species to be accurately measured. Furthermore, early platyrrhine and catarrhine fossils—the target species for dietary reconstruction by these methods—demonstrate body sizes more consistent with extant non-callitrichine platyrrhines [19,40,41,42,43,44]. The sample was restricted to individuals with no more than minimal tooth wear. As a wear criterion, a specimen was rejected if the enamel was perforated to the dentine beyond the cusp tips (a simple circular exposure of dentine at cusp tips was acceptable but a ribbon of dentine exposure along a crest led to rejection). Specimens were also rejected when postmortem defects and breakage were encountered. Dietary information reported here is based on mean annual diet taken from the literature (see description in Analysis section) [45]. We calculated SQ and RFI for both the upper and lower first molars of each individual. These two dental measurements have previously been applied to lower molars and demonstrated to be useful for differentiating primates by diet. A third measure, (OR), was taken only for the lower first molar but not the first upper molar for reasons described in the methods section. Measurements of SQ were taken on high-precision epoxy casts while RFI and OR were calculated from laser scan models of the same casts.
Table 1. Platyrrhine sample with dietary profile.
| Species | N | Body Mass(grams) | Primary Dietary Components | Diet Category | Diet References |
|---|---|---|---|---|---|
| Alouatta palliata | 7 | 6250 | Leaves, fruit | Folivore | [46,47] |
| Alouatta seniculus | 5 | 5950 | Leaves, fruit | Folivore | [48,49,50] |
| Aotus vociferans | 9 | 703 | Fruit | Frugivore | [51,52] |
| Ateles geoffroyi | 9 | 7535 | Fruit | Frugivore | [53,54,55,56] |
| Brachyteles arachnoides | 9 | 8840 | Leaves, fruit | Folivore | [57,58,59] |
| Cacajao calvus | 10 | 3165 | Hard objects, fruit | Hard object feeder | [60] |
| Callicebus caligatus | 2 | 880 | Fruit | Frugivore | [61,62] |
| Callicebus cupreus | 8 | 1070 | Fruit | Frugivore | [61,62] |
| Cebus capucinus | 9 | 3160 | Fruit | Frugivore | [53,54] |
| Chiropotes santanas | 8 | 2740 | Hard objects, fruit | Hard object feeder | [63,64,65,66] |
| Lagothrix lagotricha | 9 | 7150 | Fruit | Frugivore | [62,67,68,69,70] |
| Pithecia irrorata | 9 | 2160 | Hard objects, fruit | Hard object feeder | [71] |
| Saimiri boliviensis | 9 | 811 | Fruit, insects | Frugivore | Genus-level information: [62,72,73] |
Shearing Quotient (SQ)
The lengths of shearing crests 1 through 6 and mesiodistal tooth length were measured for both upper and lower first molars (Fig. 1A,B) under a binocular microscope fitted with a reticle eyepiece at 12x or 25x magnification (depending on the size of the tooth). All measurements were taken in reticle units and converted to millimeters. ‘Total shear’ was calculated as the sum of shear crest lengths 1 through 6. Crest measurements were repeated by three observers (KLA, LAG, and RFK). Inter-observer error in the sum of shearing crests and tooth length was less than 5%.
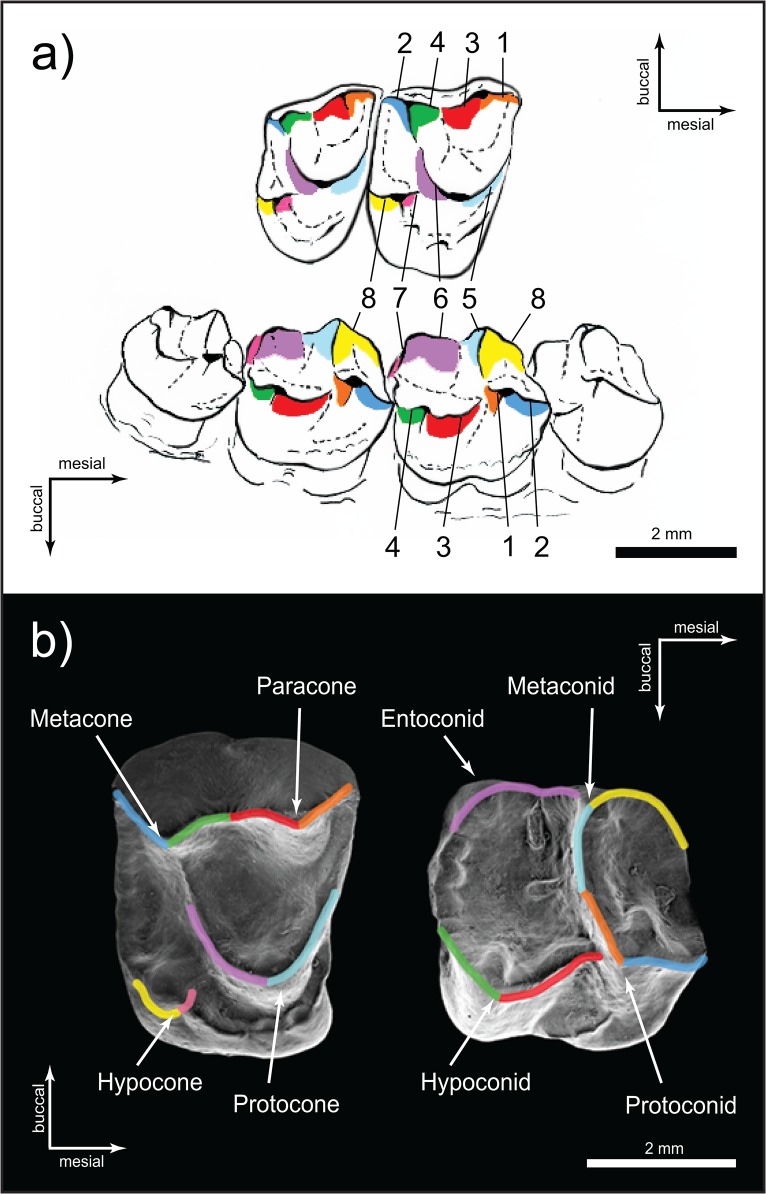
Fig 1. ) SEM image of the occlusal surface of the right first maxillary (on the left) and first mandibular (on the right) molars of Saimiri sciureus (USNM 546762, Para, Brazil).
a Crests 1–6 are shown. b) Saimiri sciureus (USNM 546762), oblique medial view (above) of right M1–2 and oblique lateral view (below) of P4-M3. The leading edges of eight functional crests are labeled, as are the wear surfaces distal to them. We measured crest lengths 1–6; crests 7 and 8 (associated with the margins of the hypocone and trigonid basin) were not measured. Numbering system of the crests follows Kay [102].
Three-dimensional dental relief: Relief Index (RFI) and Occlusal Relief (OR)
Entire maxillary and mandibular post-canine tooth rows were molded, cast, and laser surface scanned following a protocol outlined in Cooke [39].
Relief Index (RFI), a measure of the overall relief of a tooth crown, was calculated following Boyer [7] as ln(√TSA/√PSA) where TSA is the total surface area of the enamel crown cropped along the cemento-enamel junction (CEJ) and PSA (projected surface area) is the two-dimensional surface of the projection of the outline of the molar oriented in the occlusal view (Fig. 2). This procedure was completed for both upper and lower first molars. To examine the degree of intra-observer error that occurred during the cropping procedure one molar specimen was cropped ten times on several different days. Error was found to be less than 2% (S2 Table).
Fig 2. Laser-scan generated image of Saimiri boliviensis (AMNH 255858) first molars demonstrating calculations for surface relief measures.

See text for calculations. a) and b) oblique lateral view of lower first molar showing cropping at the talonid basin (a) and cemento-enamel junction (b, CEJ); c) upper (top row) and lower (bottom row) first molar in occlusal view with planometric surface area projection depicted to the right.
Occlusal Relief (OR) [4], was calculated as a ratio in which the numerator is the three-dimensional surface area of the occlusal surface tooth cropped from the lowest point in the talonid basin, and the denominator is the two dimensional planometric surface area of the occlusal table (Fig. 2). To determine the lowest point in the talonid basin, we passed a plane through the tooth parallel to the occlusal plane (x, z plane) using the “intersect a plane” function in Geomagic Studio 2012 (Geomagic, Inc.). The plane was lowered until it intersected only with the lowest point in the basin. The tooth was then cropped below this plane. OR was developed by M’Kirera and Ungar [4] for use on lower molars. The upper molars in our sample displayed considerable variation in cingulum development, which created difficulty in establishing a consistent cropping procedure. As such, we limited the use of OR in this study to the lower molars. RFI and OR were measured and calculated by SBC.
For both the upper and lower molars, Log10 TSA scales isometrically with Log10 PSA (i.e., the slope does not significantly differ from 1.0), so we followed the previous literature [4,7] in representing both as ratios rather than residuals. Lower molar TSA scales isometrically with molar length (slope = 2.0), whereas the upper molar TSAs scale with slight negative isometry (slope = 1.78, 95% CI for slope = 1.72 to 1.84).
Analysis
Closely related species may be expected to be more similar morphologically, owing to a greater degree of shared evolutionary history. A high degree of phylogenetic signal, or covariance between the species data and their phylogenetic relationships, may violate the assumption of independence of data points inherent in traditional correlation analyses [75,76]. We tested for the effects of phylogenetic signal in our variables by calculating Pagel’s lambda (λ) in the ‘geiger’ package for the statistical program ‘R’ [77]. Three fully resolved platyrrhine phylogenies with associated branch lengths were used, representing variable arrangements at the subfamily level: the maximum parsimony and maximum likelihood trees from Opazo [78] and the platyrrhine phylogeny from Perelman et al. [79].
Because the sum of the shear crests does not scale isometrically with molar length, the use of a ratio (sum shear/tooth length) fails to properly account for body size effects. Instead, SQs were calculated as the residual sum of the shear lengths using mesiodistal tooth length as a proxy for size. SQs are calculated in the following way: Using the ‘caper’ package for ‘R’ [80], a phylogenetically-corrected least-squares regression (PGLS) line was fitted to the Log10 species means of upper and lower first molar lengths (on the abscissa) and Log10 summed first molar shear (on the ordinate) of species means. The derived linear equation was used to generate an ‘expected’ shearing crest length from molar length of each included individual or taxon. First, expected and observed variables were converted to real space. The expected value was then subtracted from the observed value for each taxon, and divided by the expected value. The resultant (SQ value) is expressed here as a percentage.
Allen and Kay [46], synthesize the available behavioral data concerning platyrrhine diet composition, based on recorded feeding times and/or number of feeding bouts per food type [45]. In our analyses, taxa were categorized into feeding groups—frugivore, folivore, and hard object feeder—based on these feeding records. Species for which the mean annual percentage of the recorded feeding bouts/feeding time devoted to consuming leaves exceeded 50% were designated as “folivores”. Species in which fruit is the primary ingested component are categorized as “frugivores”. Species described in the literature as consuming a substantial amount of hard seeds and nuts in their diet are categorized as “hard object feeders”. Table 1 summarizes the primary diet components and assigned diet categories of each species. The SQ regression slopes described above were fit to frugivorous taxa alone. To visualize the distribution of values across different taxa and different dietary groups, SQ, RFI, and OR were plotted in standard box-and-whiskers plots in JMP Pro 10.0.2 for Mac, SAS Institute Inc. (Fig. 3A-E), and Kruskal-Wallis tests were computed to look for differences among species and diet categories. Because SQ values (residuals from the PGLS line) were found to contain a phylogenetic signal (λ = 1.0), a phylogenetic ANOVA was computed to test for differences among diet groups using the “phytools” package in “R” [81]. OR and RFI lambda values do not significantly differ (p>0.05) from 0 (OR: λ = 0.4, M1 RFI: λ = 0, M1 RFI: λ = 0), so traditional non-parametric statistical techniques were used. (The power to detect phylogenetic signal drops dramatically at small sample sizes (n < 20 species) [82], so there may, in fact be an undetected phylogenetic signal, a possibility that should be tested whenever samples are sufficiently large.) We applied post-hoc multiple pair-wise comparisons (Wilcoxon Rank Sum) to test for significant differences in SQ, RFI, and OR among diet categories. Significance criteria were determined using a Bonferroni correction [83].
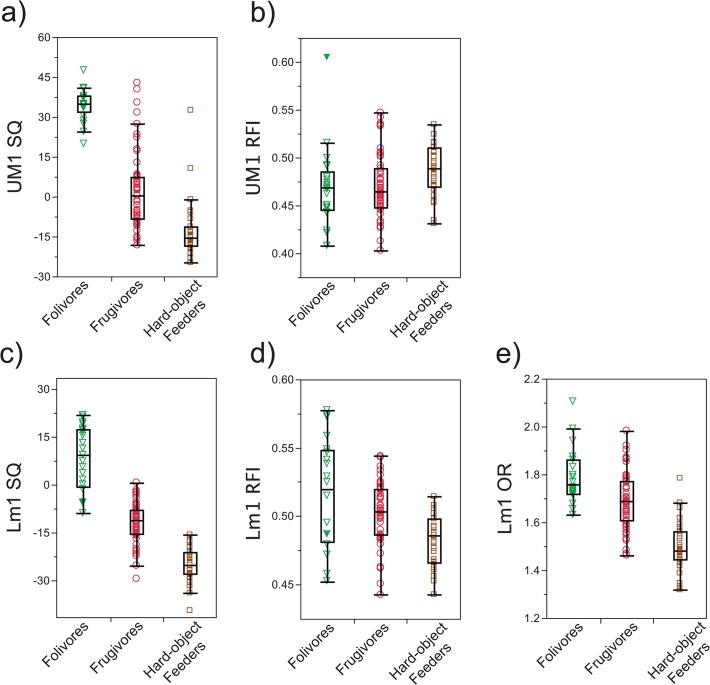
Fig 3. Box-and-whisker plots showing ranges of individual values for Diet-1 (3 group comparisons).
a) upper molar SQ, b) upper molar RFI, c) lower molar SQ, d) lower molar RFI, e) lower molar OR.
We employ discriminant function analyses (DFA) to examine the success rate of classifying individual platyrrhine specimens by diet from upper and lower first molar SQ and RFI, and lower molar OR. These analyses were conducted in SPSS Version 21.0 (SPSS, Inc.). M1 length and M1 length were included as proxies for size. DFAs were conducted by entering independent variables together with all prior probabilities equal to avoid sampling bias as a result of unequal number of individuals assigned to each dietary category. Results were cross validated using “leave one out classification”. Cross-validated classification rates are reported here. Additionally, the data are partitioned so that the classification success rates using all variables can be examined. The relationship among individual data points and group means are visualized in a plot of scores in canonical space, illustrating the axis of variation that provides the best differentiation among group means.
Results
Table 2 presents species means and standard deviations for all dental indexes. Box plots demonstrating distributions of molar indices by diet group are presented in Fig. 3.
Table 2. Species means and standard deviations (SD) for dental indexes.
| UM1 OR | UM1 RFI | Lm1 RFI | UM1 SQ | Lm1 SQ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Alouatta palliata | 1.72 | 0.06 | 0.46 | 0.07 | 0.5 | 0.02 | 35.72 | 2.1 | 12.54 | 8.6 |
| Alouatta seniculus | 1.82 | 0.12 | 0.47 | 0.03 | 0.55 | 0.04 | 34.71 | 6.19 | 25.87 | 7.73 |
| Aotus vociferans | 1.73 | 0.09 | 0.45 | 0.03 | 0.54 | 0.02 | 0.18 | 6.67 | 5.11 | 4.07 |
| Ateles geoffroyi | 1.7 | 0.07 | 0.52 | 0.02 | 0.53 | 0.02 | 18.46 | 12.24 | 0.17 | 3.25 |
| Brachyteles arachnoides | 1.84 | 0.13 | 0.48 | 0.02 | 0.57 | 0.02 | 33.52 | 8.04 | 22.83 | 8.65 |
| Cacajao calvus | 1.51 | 0.1 | 0.5 | 0.02 | 0.51 | 0.02 | -5.75 | 16.34 | -12.01 | 5.43 |
| Callicebus discolor | 1.61 | 0.06 | 0.45 | 0.03 | 0.54 | 0.02 | -9.25 | 4.7 | 0.307 | 2.6 |
| Callicebus moloch | 1.65 | 0.04 | 0.46 | 0.02 | 0.53 | 0.01 | -6.95 | 4.26 | -3.04 | 4.79 |
| Cebus capucinus | 1.59 | 0.11 | 0.45 | 0.03 | 0.51 | 0.03 | -11.74 | 3.98 | -6.86 | 6.82 |
| Chiropotes satanas | 1.46 | 0.08 | 0.48 | 0.02 | 0.49 | 0.01 | -15.4 | 7.37 | -13.8 | 4.3 |
| Lagothrix lagotricha | 1.69 | 0.1 | 0.47 | 0.02 | 0.53 | 0.02 | 11.45 | 19.12 | 4.53 | 4.7 |
| Pithecia irrorata | 1.53 | 0.13 | 0.48 | 0.03 | 0.51 | 0.02 | -16.67 | 3.28 | -14.15 | 6.6 |
| Saimiri boliviensis | 1.85 | 0.09 | 0.47 | 0.01 | 0.53 | 0.02 | 4.82 | 6.28 | -1.22 | -5.68 |
Sample sizes are listed in Table 1.
Shearing Quotient (SQ)
Results for SQ equations and residual distributions are consistent across all three phylogenies, indicating that the results are robust to deviations in family-level branching patterns. In the absence of differences we present only the results for the Perelman et al. [79] tree, which depicts Pitheciidae as the basal crown platyrrhine clade and Aotus as the first branch from the Cebidae. The family level branching patterns for this phylogeny are in concordance with data provided by Alu insertions, which are considered to have very low rates of homoplasy [84]. Among frugivores in our sample, the sum of the shear crests scale with negative allometry on tooth length for both the upper molars (log10 sum of M1 shearing crests = 0.44614 + 0.73751 * log10 M1 length; R-square = 0.72, p = 0.0006) and lower molars (log10 sum of M1 shearing crests = 0.29713 + 0.91435 * log10 M1 length; R-square = 0.97, p = 0.0003) in the PGLS regressions.
Frugivorous platyrrhine species are distributed around the grand mean for the total dataset, as expected from the method by which SQ is calculated (Table 2, Fig. 3A, C). For both the upper and lower first molars, SQ values for folivores occupy the upper end of the platyrrhine range, indicating a higher sum of shear crest lengths relative to tooth length than in frugivores, while hard object feeders occupy the low end of the sample distribution, indicating less molar shearing than in frugivores. The distributions of the upper molar SQ overlap considerably among the three diet categories, largely owing to the broad spread of values within the frugivore group. In particular, Ateles, has the highest M1 SQ values for the frugivores in our sample. Lower molar SQ values provide a greater degree of separation among diet groups owing to a more restricted frugivore range, although some specimens of Ateles still fall within the folivore range. For the upper and lower molars, folivore and hard object feeding categories occupy entirely separate ranges, with the exception of two individuals of Cacajao, whose upper molar SQ overlaps with frugivore SQs.
Distributions of indices by diet are summarized in Table 3. A phylogenetic ANOVA on species means indicates significant differences among groups (M1: p = 0.005, M1: p < 0.001). Post-hoc pair-wise comparisons on individual data points indicate highly significant differences among all diet categories (p = <0.0001; Bonferroni significance criterion: p ≤ 0.016) for both upper and lower molar SQ (Table 4).
Table 3. Sample parameters for M1 and M1 occlusal variables grouped by diet.
| Diet Category | M1 OR | M1 RFI | M1 RFI | M1 SQ | M1 SQ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% Conf. Int. | Mean | 95% Conf. Int. | Mean | 95% Conf. Int. | Mean | 95% Conf. Int. | Mean | 95% Conf. Int. | ||||||
| leaves | 1.8 | 1.85 | 1.74 | 0.47 | 0.49 | 0.45 | 0.54 | 0.56 | 0.52 | 34.54 | 37.26 | 31.81 | 20.04 | 24.58 | 15.67 |
| fruit | 1.7 | 1.73 | 1.67 | 0.47 | 0.48 | 0.46 | 0.53 | 0.53 | 0.52 | 2.27 | 6.17 | -1.62 | 0.09 | 1.75 | -1.56 |
| hard objects | 1.5 | 1.54 | 1.46 | 0.49 | 0.5 | 0.48 | 0.51 | 0.52 | 0.5 | -12.25 | -7.63 | -16.87 | -13.25 | -11.104 | -15.57 |
Values for 95% Conf. Int. indicate the upper and lower bounds of the 95% confidence interval for the diet grouping. See text for additional abbreviations.
Table 4. Results of post-hoc multiple pair-wise comparisons between tooth indices (Wilcoxon each pair) segregated by diet categories.
| Diet Category | p-value Wilcoxon each pair | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | M1 OR | M1 RFI | M1 RFI | M1 SQ | M1 SQ | |||||
| frugivore | folivore | 0.0034 | * | 0.8235 | 0.2453 | <0.0001 | * | <0.0001 | * | ||
| hard object | folivore | <0.0001 | * | 0.0126 | * | 0.0079 | * | <0.0001 | * | <0.0001 | * |
| hard object | frugivore | <0.0001 | * | 0.007 | * | <0.0001 | * | <0.0001 | * | <0.0001 | * |
* denotes statistical significance of p-value using Bonferroni significance criterion for 3 groups (p< 0.0167).
Relief Index (RFI)
Compared to SQ, both upper and lower molar RFIs show greater overlap of species means among the different dietary groups (species data in Table 2, group data in Table 3; Fig. 3B, D). Although means differ among groups, the ranges of individual specimens within each group overlap substantially. Upper molar RFI group ranges overlap completely. Statistically significant differences exist between the hard object feeders and both the frugivore and folivore distributions but not between frugivores and folivores (Kruskal-Wallis pairwise test with a Bonferroni correction criterion (p<0.017).
For lower molars, the folivore mean RFI and maximum individual values exceed those for frugivores, while hard object feeders have the lowest mean and maximum values (Table 3). All three groups have similar minimum values for each diet category (folivore = 0.48, frugivore = 0.47, hard object = 0.47). A Kruskal-Wallis test shows significant differences in hard object feeders versus frugivores and folivores but not between frugivores and folivores (Table 4).
Occlusal Relief (OR, lower molars only)
The results for M1 OR (Tables 3 and 4) mirror those of the lower molar SQs, with significant differences among means for diet groups (Kruskal-Wallis test: p < 0.0001, post-hoc pair-wise comparisons: p < 0.0167 for all groups) (Fig. 3E). Folivores have the highest OR values, hard object feeders have the lowest values, and frugivores are clustered around the grand mean of the total dataset. Despite the significant difference in means between frugivores and folivores, these groups show considerable overlap in distribution of individual values.
Discriminant function analyses
Summary of the results of the DFA analyses are given in Table 5 and S2 Table. Initially each variable was treated separately to determine its success rate in classifying individual specimens. Lower molar SQ is most effective with 82.5% of individuals correctly classified by dietary category, followed by lower molar length (60.2%), lower molar OR (57.3%), and lower molar RFI (44.7%). For all of the variables, classification rates increase substantially with the inclusion of body size information (M1 mesio-distal length). Highest classification rates occur when all lower molar variables—including molar length—are considered together (89.3%).
Table 5. Summary of correct classification rates for discriminant function analyses using upper molar (M1), lower molar (M1), and upper and lower molar occlusal variables combined.
| Variables | M1 | M1 | M1 + M1 |
|---|---|---|---|
| molar length | 60.2 | 60.2 | |
| SQ | 68.9 | 82.5 | 88.3 |
| SQ + molar length | 69.9 | 87.4 | 90.3 |
| OR | 57.3 | ||
| OR + molar length | 79.6 | ||
| RFI | 47.6 | 44.7 | 51.5 |
| RFI + molar length | 69.9 | 69.9 | 81.6 |
| All variables | 82.6 | 89.3 | 93.2 |
Values represent percentage of correctly classified specimens.
Of the dietary groups, frugivores had the lowest classification rates. For lower molar SQ, errors occur predominantly with Cebus capucinus specimens classified as hard object feeders. In fact, if species group means are used, only Cebus capucinus is misclassified. This is unsurprising as some species of this genus do consume hard objects (e.g., C. apella), though C. capucinus does not make these food types a major component of its diet. Additionally, two Ateles geoffroyi and two Lagothrix lagotricha are misclassified as folivores. For the two indices of topography, errors are less patterned and in the case of RFI, correct classification rates are only slightly higher than random (in a classification scheme with three categories, an individual would have a random chance of 33.3% of being correctly classified vs. our classification rate of 44.7%).
Upper molar analyses largely mirror those of lower molars, but have lower classification rates. SQ classifies 68.9% of individuals correctly by diet and RFI classifies 47.6% correctly. The addition of upper molar length improves both rates to 69.9%; when SQ, RFI, and molar length are used together the classification rate is 82.5%. For upper molar SQ, major errors in classification again occur most frequently within the frugivores. All specimens of C. capucinus and all but one specimen of Callicebus cupreus are classified as hard object feeders. A. geoffroyi and L. lagotricha individuals are both frequently misclassified as folivores. While these errors mirror results for lower molars they occur more frequently.
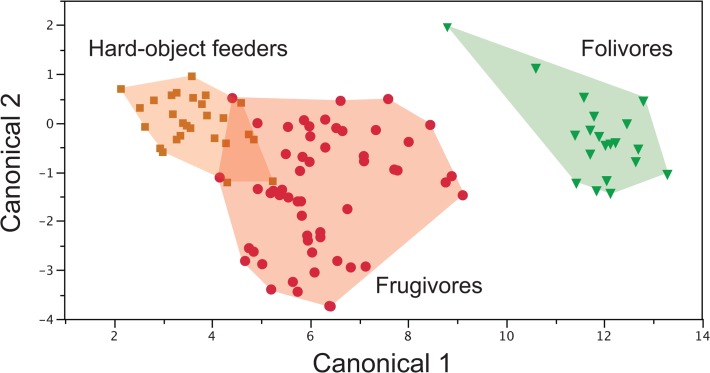
Classification rates are the highest when all upper and lower molar variables are considered together (93.2%) (Fig. 4). Three of 54 frugivorous individuals are misclassified as hard object feeders and one specimen of hard object feeder (of 27) as a frugivore; of the twenty specimens of folivores, one Alouatta palliata is misclassified as a frugivore. In our sample, the combination of upper and lower SQ with molar length returns a classification rate of 90.3%. Some L. lagotricha individuals classify as folivores, and a few C. capucinus specimens misclassify as hard object feeders.
Fig 4. Plot of a discriminant function analysis including M1 length, RFI, OR, and phylogenetically corrected SQ and M1 RFI and phylogenetically corrected SQ.
93.6% of variance is accounted for by discriminant function 1 and 6.4% by function 2. Polygons are drawn to include all individual specimens.
Upper and lower molar RFI together with molar lengths return a classification rate of 81.6% with major errors occurring when several different individual frugivore specimens were misclassified as hard object feeders. This occurs most often for A. geoffroyi and Saimiri boliviensis. Without the inclusion of molar lengths, classification rates remain high using SQs of upper and lower molars (88.3%), but classification rates using RFI alone decrease to 51.5%. Indeed, comparison of RFI success rates on upper and lower molars with or without molar length shows that the correct classification rates for RFI and molar length are largely being driven by molar length. Ultimately, as long as molar length is included as one of the variables, most combinations of upper and lower molar variables achieve high rates of classification.
Using species means rather than individual specimens, the three lower tooth indices taken together correctly classify all but one of our 13 species to a diet group (93%), the single exception being frugivorous Cebus capucinus, classified as a hard object feeder. The same overall classification rate is achieved using SQ alone (93%), whereas, the success rate for OR alone misclassifies four of 13 species with most of the errors being misclassification of frugivores as either folivores or hard object feeders. RFI performs less well than either of the other lower molar indices, missing 5 of 13 (almost 40%).
Again, when using species means rather than individual specimens, the two upper tooth indices taken together correctly classify all 13 species (100%). Using upper SQ alone, the success rate is 85%, with two frugivorous taxa misclassified as hard object feeders: Cebus capucinus and Callicebus cupreus. The success rate for upper molar RFI performs less well than either of the other lower molar indices, successfully assigning 10 of 13 taxa (76.2%).
Discussion
Understanding the relationship between dental morphology and behavior in extant primate species has been a key component for reconstructing the dietary niche of extinct species. Our study examines the occlusal morphology of platyrrhine upper and lower molars, separately and together, in relation to diet, controlling for phylogenetic effects. Several indices designed to capture occlusal morphology (SQ, RFI, and OR) are applied to upper and lower molars of the same individuals among taxa in a restricted taxonomic clade with a highly resolved phylogeny.
Early efforts to infer diet from molar morphology concentrated on allocation of an extinct species into one of several dietary categories [1,16]. But if only one or two specimens of the extinct species are measurable, we cannot establish that the individual values reasonably approximate species means. In such a case, the most conservative approach is to compare the single value with the ranges of extant values. Here, we use DFA to assess the success rate for assigning a single specimen to a particular diet group [5,6,7,39] and to look at success rates for species means of the indices.
An important outcome of this study is the demonstrated significance of using both upper and lower molar data to ‘predict’ dietary behavior in extant species. When training the predictive model using all variables for upper and lower molar concurrently, the predictive power exceeds 90%, even for individual specimens. When possible, we recommend a combination of both upper and lower molar measurements for ‘retrodicting’ the behavior of extinct platyrrhine species. However, when only the upper or the lower first molar is available, predictive success rates remain at ∼85% for individuals and even higher for samples that are sufficient to calculate the species mean with reasonable accuracy. Moreover, the ‘success rate’ exceeds 85% using linear measurements alone. Thus, the more expensive and technologically labor-intensive surface methods do not provide greater accuracy than the simple linear methods.
The findings of this study open the way for more precise interpretation of early platyrrhine evolution. The list of extinct platyrrhine taxa that have both lower and upper molars available, but for which only lower molars have been used in dietary assements, includes Late Oligocene Branisella [85,86], Early Miocene Dolichocebus [87,88], Mazzonicebus [89], Carlocebus, Soriacebus, and middle Miocene Lagonimico [90], Cebupithecia, Neosaimiri [91], Stirtonia [92], and Mohanamico [93,94]. Added to this are taxa for which there are only upper teeth and consequently, have been excluded from efforts to make dietary reconstructions: Early Miocene Chilicebus [40] and “Kilikaike” [95].
Upper versus lower molar dietary signal
Considered either as individual specimens or as species means, indices of lower molar occlusal morphology generally outperform the same measures from the associated upper molars in assigning species and individuals to a commonly used scheme of diet categories. The lower success rate of the upper molar in diet discrimination is largely due to the greater variability of upper molar indices, compared to those of the associated lower molars. Upper molar shear quotient and topographic variables show greater overlap in values among diet categories than do those for the lower molars, resulting in a somewhat greater degree of uncertainty in diet reconstruction. For example, the coefficients of variation for the upper molar SQs are roughly twice those of the lower molars for the frugivore and hard object feeding categories (SI: DFA classification errors). This variation may indicate a relaxed constraint on upper molar shearing in these species, and/or reflect a great deal of variability in the secondary source of nutrition for these species. For example, the frugivorous Cebus capucinus may include hard objects (seeds, nuts) in its diet, while Ateles supplements its frugivory with young leaves [45].
It is also possible that the greater variability in upper molar topography may reflect phylogenetic effects. For instance, the frugivorous Ateles has a lower molar shear quotient in the middle of the frugivore range, but a high upper molar shear quotient more in line with that of its close atelid relatives, the more folivorous Brachyteles and Alouatta. Nevertheless, upper molar shear quotients do show significant differences among all diet pairings, and a discriminant function analysis of individual specimens for all upper molar measures returns high classification rates. These results indicate that upper molar occlusal morphology is useful for dietary reconstruction, especially when combined with size information (i.e., molar length).
Topographic versus shear crest measures
Topographic surface measurements of molar relief (e.g., RFI, OR) have been argued to be a three-dimensional version of shearing quotient [38], however, our results show that the incorporation of additional information about surface anatomy does not necessarily replicate SQ results, nor does it dramatically improve the predictive signal for dietary reconstruction. In our study sample, RFI underperformed both SQ and OR for lower molars and SQ for upper molars. RFI was initially designed to quantify morphological differences among dental relief of markedly different morphologies—primates versus other eurachontans [7]. While it has proved useful for that purpose, RFI is not successful here in capturing minor variations in functionally relevant occlusal relief (e.g., shear) within this more phylogenetically circumscribed dataset. Several reasons may account for this discrepancy. RFI describes the relative occlusal surface area of the tooth, a measurement that will be affected by several factors in addition to molar shear, including hypsodonty [5], the presence of accessory cusps and associated crests, as well as other non-occulsal parts of the tooth—sidewall curvature, increasing molar breadth as one nears the CEJ [96], and the presence or absence of a cingulum [39]. Many of these features could in themselves be understood as functionally relevant, compensating for the mechanical demands of food processing, such as resisting dental attrition, or arresting crack propagation. However, feature distribution related to dietary or even non-dietary factors (grit ingested with food, for example) is as yet unclear and may cloud the signal captured by traditional two-dimensional shear measurements.
Many aspects of molar tooth structure picked up by topographic methods may be unrelated to chewing efficiency, as defined by how finely a food is chewed before swallowing with attendant benefits for digestibility. For example, the presence of cingula (low shelves on the crowns of many primate teeth) may be protective, deflecting food particles away from the gingiva [97], or they may reinforce the crown enamel against crack propagation and structural failure [22]. In either event, cingula are not functionally important for trituration, at least until wear is extreme. Another signal that would be picked up by dental topographic studies is enamel crenulation. The crenulated occlusal surface of Pongo or Chiropotes molars may be a functional design for the routine consumption of relatively tough and hard foods [23,98,99], but relates more to resistance against crack propagation than to masticatory efficiency. Thus, many surface features picked up by dental topographic analyses may well be unrelated to the primary function of reduction of food particle size before swallowing, and in some cases may be correlated effects of enamel structure or thickness. Although these features may convey information on dietary evolution, surface relief measures such as RFI and OPC fail to distinguish surface features like enamel crenulation or cingula from those provided by shearing blades used in trituration. As a result, a surface relief index of a flat tooth containing many crenulations may resemble that of a smoothly curved tooth with multiple shearing blades. Reducing topographic complexity down to a single index would in such a case fail to distinguish two very different adaptive strategies for coping with mastication of food particles requiring fundamentally dissimilar mechanical demands.
In this study, OR yields a somewhat clearer signal than RFI, possibly owing to the elimination of some of the variability in sidewall morphologies, as the tooth is cropped at the lowest point in the basin rather than at the CEJ. This cropping procedure, while feasible and perhaps more appropriate for studies of platyrrhines, is not always possible across a broad range of taxa given the very deep basins seen in some species [7]. Additionally, this method was developed for lower molars; given the greater morphological variability in upper molars, developing a repeatable cropping procedure for these teeth has eluded us. For example, in Saimiri, cropping at the low portion of the basin may include the some cingulum in some specimens and not in others.
A recent study by Winchester et al. [5] analyzed lower molar shape of platyrrhines. For each individual specimen they calculated a set of relief indices (e. g., RFI, OPC [37]) and linear variables equivalent to SQ. Winchester et al. reported a higher classification success rate using dental relief indices than using SQs for discrimination among most of their broad dietary categories. Unlike in many previous studies and the data for lower molars reported here, their version of SQ, which used M2 and sampled different but overlapping set of crests, failed to separate platyrrhine folivores from frugivores. In a recently published commentary, Boyer et al. [100] re-analyzed the shearing crest data of Winchester et al., concluding that that the most salient reason for differences between their results and those of prior studies (and also this study) are attributable principally to the tooth position examined: they used the lower second molar whereas ours and all previous studies or platyrrhines used the lower first molar. We speculate that the different findings for the two teeth have to do with the overall biomechanics of the platyrrhine masticatory system. Platyrrhines have a tendency to reduce the size of the molar battery from back to front. Callitrichines have lost the M3/3 in most cases but even large bodied 3-molared platyrrhines show an allometric trend toward M3/3 reduction that extends mesially to encompass the M2/2. Thus, M2/2 size is negatively allometric relative to M1/1. Bearing this in mind, we used M1/1 anticipating future research on smaller-bodied platyrrhines like callithrichines with third molar loss and extreme second molar reduction.
Body size and diet
Body size has a relatively predictable association with broad diet categories due to metabolic demands of food acquisition and processing [101]. Larger-bodied mammals require less caloric input per gram of body mass than do much smaller mammals. Owing to the absolute quantity of resources required for sustenance, a larger-bodied mammal is unlikely to be able to meet its nutritional demands for protein by eating non-social insects, while a small-bodied mammal (generally, below 500g) may be able to do so. The dataset used herein does not include the smallest bodied and most insectivorous primate species, nevertheless, as expected, we find that molar length on its own goes a long way towards predicting the broad dietary regime of a platyrrhine primate. We found that the addition of body size information (molar length) improves the DFA success rate for assigning individual specimens to diet categories for all variables; however, the classification rates for RFI by itself, in particular, are quite low without associated body size information. This is not surprising given the broad degree of overlap in RFI values among diet categories for both the upper and lower molars. Thus, for our broadly based sample of middle- to large-bodied platyrrhines, we do not find RFI to be particularly useful for assigning species to dietary categories, as they do not provide much information above that demonstrated by molar length alone. We do, however, recommend the consideration of body size as an informative variable in dietary reconstructions.
Conclusions
We explored the ability of dental indices on the lower and upper first molars in medium to large-bodied platyrrhines to differentiate among diet classes. Three diet categories were proposed: frugivore”, “folivore”, and “hard object feeder”.
When controlling for sample size and phylogenetic effects, M1 SQ most accurately allocates individual specimens and species to the dietary categories “frugivore”, “folivore”, and “hard object feeder”. First molar RFI and OR, are less successful in DFA analyses. We interpret this result as stemming from the fact that RFI and OR incorporate added functional elements of tooth design, such as those that prolong functional lifespan in the face of enamel wear (e.g., crown height), or protect the gingival and reduce the likelihood that teeth will fracture (cingulum development). These added elements might not correspond to the immediate functional aspects of mechanical preparation—breaking up food so as to increase its surface area and speed digestive processes by enzyme and microbial action.
Upper first molar indices, particularly SQ, also contain significant dietary information. However, the accuracy of the assignment of individuals or species to diet categories is poorer in general than accuracy achieved by corresponding measures on the lower molars.
When both upper and lower indices are considered together with tooth length, we achieve a 93% success rate in allocation of individuals to three-diet category scheme and 92.2% to the four-diet scheme. Species are correctly allocated to diet groups 100% of the time.
The results of this study open the way for improved accuracy in inferring the diets of extinct platyrrhines for which both upper and lower molars are available, or, for taxa known only from upper molars. As well, it offers a new window into the dietary behavior of Eocene and Oligocene stem anthropoids and early catarrhines.
Supporting Information
Sheet 1 for lower molars. Sheet 2 for upper molars. Sheet 3 for combined upper and lower molars.
(XLSX)
Data for individual specimens measured in this study.
(XLSX)
Acknowledgments
We thank Doug Boyer for reading and commenting on our results. We thank the curators at The American Museum of Natural History and Smithsonian Institution for their assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by NSF grants NSF BNS-0851272 and NSF EAR 1338694 to RFK and NSF DDIG 40761-0001 to SBC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kay RF (1975) The functional adaptations of primate molar teeth. American Journal of Physical Anthropology 43: 195–216. [DOI] [PubMed] [Google Scholar]
- 2. Kay RF, Covert HH (1984) Anatomy and behaviour of extinct primates In: Chivers DJ, Wood BA, Bilsborough A, editors. Food Acquisition and Processing in Primates. New York: Plenum Press; pp. 467–508. [Google Scholar]
- 3. Strait SG (2001) Dietary reconstruction of small-bodied omomyoid primates. Journal of Vertebrate Paleontology 21: 322–334. [Google Scholar]
- 4. M'Kirera F, Ungar PS (2003) Occlusal relief changes with molar wear in Pan troglodytes troglodytes and Gorilla gorilla gorilla . American Journal of Primatology 60: 31–41. [DOI] [PubMed] [Google Scholar]
- 5. Winchester JM, Boyer DM, St. Clair EM, Gosselin-Ildari AD, Cooke SB, et al. (2014) Dental topography of platyrrhines and prosimians: convergence and contrasts. American Journal of Physical Anthropology 153: 29–44. 10.1002/ajpa.22398 [DOI] [PubMed] [Google Scholar]
- 6. Bunn JM, Boyer DM, Lipman Y, St. Clair EM, Jernvall J, et al. (2011) Comparing Dirichlet normal surface energy of tooth crowns, a new technique of molar shape quantification for dietary inference, with previous methods in isolation and in combination. American Journal of Physical Anthropology 145: 247–261. 10.1002/ajpa.21489 [DOI] [PubMed] [Google Scholar]
- 7. Boyer DM (2008) Relief Index of second mandibular molars is a correlate of diet among prosimian primates and other euarchontan mammals. Journal of Human Evolution 55: 1118–1137. 10.1016/j.jhevol.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 8. Bajpai S, Kay RF, Williams BA, Das DP, Kapur VV, et al. (2008) The oldest Asian record of Anthropoidea. Proceedings of the National Academy of Sciences of the United States of America 105: 11093–11098. 10.1073/pnas.0804159105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kay RF, Simons EL (1980) The ecology of Oligocene African Anthropoidea. International Journal of Primatology 1: 21–37. [Google Scholar]
- 10. Kirk EC, Simons EL (2000) Diet of fossil primates from the Fayum Depression of Egypt: A quantitative analysis of molar shearing. Journal of Human Evolution 40: 203–229. [DOI] [PubMed] [Google Scholar]
- 11. Rodman PS, Cant JGH (1984) Introduction: From comparative Morphology and socioecology to an organismal biology of primates In: Rodman PS, Cant JGH, editors. Adaptations for Foraging in Nonhuman Primates. New Yory: Columbia University Press; pp. 1–20. [Google Scholar]
- 12. Beard KC, Wang J (2004) The eosimiid primates (Anthropoidea) of the Heti Formation, Yuanqu Basin, Shanxi and Henan Provinces, People’s Republic of China. Journal of Human Evolution 46: 401–432. [DOI] [PubMed] [Google Scholar]
- 13. Beard KC (2002) Basal anthropoids In: Hartwig WC, editor. The Primate Fossil Record. Cambridge: Cambridge University Press; pp. 133–150. [Google Scholar]
- 14. Cartmill M (1992) New views on primate origins. Evolutionary Anthropology 2: 105–111. [DOI] [PubMed] [Google Scholar]
- 15. Cartmill M (1972) Arboreal adaptations and the origin of the order Primates In: Tuttle RH, editor. The Functional and Evolutionary Biology of Primates. Chicago: Aldine; pp. 97–122. [Google Scholar]
- 16. Kay RF (1977) Diets of early Miocene African hominoids. Nature 268: 628–630. [Google Scholar]
- 17. Ravosa MJ (1999) Anthropoid origins and the modern symphysis. Folia primatologica 70: 65–78. [DOI] [PubMed] [Google Scholar]
- 18. Sussman RW (1991) Primate origins and the evolution of angiosperms. American Journal of Primatology 23: 209–223. [DOI] [PubMed] [Google Scholar]
- 19. Williams BA, Kay RF, Kirk EC (2010) New perspectives on anthropoid origins. Proceedings of the National Academy (USA) 107: 4797–4804. 10.1073/pnas.0908320107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anthony MRL (1994) Molar wear and molar function in primates Durham: Duke University. [Google Scholar]
- 21. Lee-Thorp JA, Sponheimer M, Passeyy BH, de Ruiter DJ, Cerling TE (2010) Stable isotopes in fossil hominin tooth enamel suggest a fundamental dietary shift in the Pliocene. Philosophical Transactions, Royal Society B 365: 3389–3396. 10.1098/rstb.2010.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lucas P, Constantion P, Wood B, Lawn B (2008) Dental enamel as a dietary indicator in mammals. BioEssays 30: 374–385. 10.1002/bies.20729 [DOI] [PubMed] [Google Scholar]
- 23. Martin LB, Olejniczak AJ, Maas MC (2003) Enamel thickness and microstructure in pitheciin primates, with comments on dietary adaptations of the middle Miocene hominoid Kenyapithecus. Journal of Human Evolution 45: 351–367. [DOI] [PubMed] [Google Scholar]
- 24. Rosenberger AL, Kinzey WG (1976) Functional patterns of molar occlusion in platyrrhine primates. American Journal of Physical Anthropology 45: 261–298. [DOI] [PubMed] [Google Scholar]
- 25. Ungar P (1998) Paleobiological inference and dental evidence for diet in primates Evolutionary Anthropology in press. [Google Scholar]
- 26. Ungar PS, Scott RS, Grine FE, Teaford MF (2010) Molar microwear textures and the diets of Australopithecus anamensis and Australopithecus afarensis . Philosophical Transactions, Royal Society B 365: 3345–3354. 10.1098/rstb.2010.0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Butler PM (1972) Some functional aspects of molar evolution. Evolution 26: 474–483. [DOI] [PubMed] [Google Scholar]
- 28. Kay RF, Hiiemae KM (1974) Jaw movement and tooth use in recent and fossil primates. American Journal of Physical Anthropology 40: 227–256. [DOI] [PubMed] [Google Scholar]
- 29. Sheine W, Kay RF (1982) A model for comparison of masticatory effectiveness in primates. Journal of Morphology 172: 139–149. [DOI] [PubMed] [Google Scholar]
- 30. Kaiser TM, Müller DWH, Fortelius M, Schulz E, Codron D, et al. (2011) Hypsodonty and tooth facet development in relation to diet and habitat in herbivorous ungulates: implications for understanding tooth wear. Mammal Review 2011: 1–20. [Google Scholar]
- 31. Kay RF, Sheine WS (1979) On the relationship between chitin particle size and digestibility in the primate Galago senegalensis . American Journal of Physical Anthropology 50: 301–308. [Google Scholar]
- 32. Sheine WS, Kay RF (1977) An analysis of chewed food particle size and its relationship to molar structure in the primates Cheirogaleus medius and Galago senegalensis, and the insectivoran Tupaia glis . American Journal of Physical Anthropology 47: 15–20. [Google Scholar]
- 33. Lucas PW (2004) Dental Functional Morphology: How Teeth Work Cambridge, UK: Cambridge University Press. 355 p. [Google Scholar]
- 34. Remis MJ (2000) Initial studies on the contributions of body size and gastrointestinal passage rates to dietary flexibility among gorillas. American Journal of Physical Anthropology 112: 171–180. [DOI] [PubMed] [Google Scholar]
- 35. Demment MW, Van Soest PJ (1985) A Nutritional Explanation for Body-Size Patterns of Ruminant and Nonruminant Herbivores. The American Naturalist 125: 641–672. [Google Scholar]
- 36. Yamashita N (1998) Functional dental correlates of food properties in five Malagasy lemur species. American Journal of Physical Anthropology 106: 169–188. [DOI] [PubMed] [Google Scholar]
- 37. Evans AR, Wilson GP, Fortelius M, Jernvall J (2007) High-level similarity of dentitions in carnivorans and rodents. Nature 445: 78–81. [DOI] [PubMed] [Google Scholar]
- 38. Ungar PS, Williamson M (2000) Exploring the effects of tooth wear on functional morphology: a preliminary study using dental topographic analysis. Palaeontologia Electronica 3: 1–18. [Google Scholar]
- 39. Cooke SB (2011) Paleodiet of extinct platyrrhines with emphasis on the Caribbean forms: three-dimensional geometric morphometrics of mandibular second molars. Anatomical Record 294: 2073–2091. 10.1002/ar.21502 [DOI] [PubMed] [Google Scholar]
- 40.Sears KE, Finarelli JA, Flynn JJ, Wyss A (2008) Estimating body mass in New World "monkeys" (Platyrrhini, Primates), with a consideration of the Miocene platyrrhine, Chilecebus carrascoensis. American Museum Novitates: 1–29.
- 41. Simons EL, Seiffert ER, Ryan TM, Attia Y (2007) A remarkable female cranium of the early Oligocene anthropoid Aegyptopithecus zeuxis (Catarrhini, Propliopithecidae). Proceedings of the National Academy of Sciences of the United States of America 104: 8731–8736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conroy GC (1987) Problems of body-weight estimation in fossil primates. International Journal of Primatology 8: 115–137. [Google Scholar]
- 43. Bush EC, Simons EL, Allman J (2004) High-resolution computed tomography study of the cranium of a fossil anthropoid primate, Parapithecus grangeri: New insights into the evolutionary history of primate sensory systems. Anatomical Record Part A 281A: 1083–1087. [DOI] [PubMed] [Google Scholar]
- 44. Kay RF, Meldrum DJ, Takai M (2013) Pitheciidae and other platyrrhine seed predators In: Veiga L, Barnett A, Ferrari S, Norconk M, editors. Evolutionary Biology and Conservation of Titis, Sakis and Uacaris. Cambridge, UK: Cambridge University Press; pp. 3–12. [Google Scholar]
- 45. Allen KL, Kay RF (2012) Dietary quality and encephalization in platyrrhine primates. Proceedings of the Royal Society, B, Biological Sciences 279: 715–721. 10.1098/rspb.2011.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Teaford MF, Glander KE (1996) Dental Microwear and Diet in a Wild Population of Mantled Howling Monkeys (Alouatta palliata) In: Norconk MA, Rosenberger AL, Garber PA, editors. Adaptive Radiations of Neotropical Primates: Springer; US: pp. 433–449. [Google Scholar]
- 47. Milton K (1980) The Foraging Strategy of Howler Monkeys: A study in Primate Economics New York: Columbia University Press. [Google Scholar]
- 48. Gaulin SJ, Gaulin CK (1982) Behavioral ecology of Alouatta seniculus in Andean cloud forest. International Journal of Primatology 3: 1–32. [Google Scholar]
- 49. Julliot C, Sabatier D (1993) Diet of the red howler monkey (Alouatta seniculus) in French Guiana. International Journal of Primatology 14: 527–550. [Google Scholar]
- 50. Simmen B, Sabatier D (1996) Diets of some French Guianan primates: food composition and food choices. International Journal of Primatology 17: 661–693. [Google Scholar]
- 51. Hladik A, Hladik CM (1969) Rapports trophiques entre vegetation et primates dans la foret de Barro Colorado (Panama). La terre et la vie 23: 25–117. [Google Scholar]
- 52. Wright PC (1981) The night monkeys, genus Aotus . Ecology and behavior of neotropical primates 1: 211–240. [Google Scholar]
- 53. Chapman C (1987) Flexibility in Diets of Three Species of Costa Rican Primates. Folia Primatologica 49: 90–105. [Google Scholar]
- 54. Hladik CM, Hladik A, Bousset J, Valdebouze P, Viroben G, et al. (1971) Le régime alimentaire des Primates de l’île de Barro-Colorado (Panama). Folia primatologica 16: 85–122. [DOI] [PubMed] [Google Scholar]
- 55. Cant J (1990) Feeding ecology of spider monkeys (Ateles geoffroyi) at Tikal, Guatemala. Human Evolution 5: 269–281. [Google Scholar]
- 56. Russo SE, Campbell CJ, Dew JL, Stevenson PR, Suarez SA (2005) A multi-forest comparison of dietary preferences and seed dispersal by Ateles spp. International Journal of Primatology 26: 1017–1037. [Google Scholar]
- 57. Milton K (1984) Habitat, diet, and activity patterns of free-ranging woolly spider monkeys (Brachyteles arachnoides E. Geoffroy 1806). International Journal of Primatology 5: 491–514. [Google Scholar]
- 58. Strier KB (1991) Diet in one group of woolly spider monkeys, or muriquis (Brachyteles arachnoides). American Journal of Primatology 23: 113–126. [DOI] [PubMed] [Google Scholar]
- 59. de Carvalho O Jr, Ferrari SF, Strier KB (2004) Diet of a muriqui group (Brachyteles arachnoides) in continuous primary forest. Primates 45: 201–204. [DOI] [PubMed] [Google Scholar]
- 60.Ayres JM (1986) Uakaries and Amazonian flooded forest [Ph. D.]: Cambridge University.
- 61. Nadjafzadeh MN, Heymann EW (2008) Prey foraging of red titi monkeys, Callicebus cupreus, in comparison to sympatric tamarins, Saguinus mystax and Saguinus fuscicollis . American Journal of Physical Anthropology 135: 56–63. [DOI] [PubMed] [Google Scholar]
- 62. Youlatos D (2004) Multivariate analysis of organismal and habitat parameters in two neotropical primate communities. American Journal of Physical Anthropology 123: 181–194. [DOI] [PubMed] [Google Scholar]
- 63. Ayres J (1989) Comparative feeding ecology of the Uakari and Bearded Saki, Cacajao and Chiropotes . Journal of Human Evolution 18: 697–716. [Google Scholar]
- 64. Van Roosmalen GM, Mittermeier RA, Fleagle JG (1988) Diet of the Northern Bearded Saki (Chiropotes satanas chiropotes): A neotropical seed predator. American Journal of Primatology 14: 11–35. [DOI] [PubMed] [Google Scholar]
- 65. Kinzey WG (1992) Dietary and dental adaptations in the Pitheciinae. American Journal Physical Anthropology 88: 499–514. [DOI] [PubMed] [Google Scholar]
- 66. Kinzey WG, Norconk MA (1993) Physical and chemical properties of fruit and seeds eaten by Pithecia and Chiropotes in Surinam and Venezuela. International Journal of Primatology 14: 207–227. [Google Scholar]
- 67. Peres CA (1994) Diet and feeding ecology of gray woolly monkeys (Lagothrix lagotricha cana) in central Amazonia: comparisons with other atelines. International Journal of Primatology 15: 333–372. [Google Scholar]
- 68. Stevenson PR, Quiñones MJ, Ahumada JA (1994) Ecological strategies of woolly monkeys (Lagothrix lagotricha) at Tinigua National Park, Colombia. American Journal of Primatology 32: 123–140. [DOI] [PubMed] [Google Scholar]
- 69. Defler TR, Defler SB (1996) Diet of a group of Lagothrix lagotricha lagotricha in southeastern Colombia. International Journal of Primatology 17: 161–190. [Google Scholar]
- 70. Dew JL (2005) Foraging, food choice, and food processing by sympatric ripe-fruit specialists: Lagothrix lagotricha poeppigii and Ateles belzebuth belzebuth . International Journal of Primatology 26: 1107–1135. [Google Scholar]
- 71. Palminteri S, Powell G, Adamek K, Tupayachi R (2013) Competition between two specialist seed-eaters: The diets of Pitheciines and large Ara macaws In: Veiga LM, Barnett AA, Ferrari SF, Norconk MA, editors. Evolutionary Biology and Conservation of Titis, Sakis and Uacaris. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 72. Terborgh J (1983) Five New World Primates: A Study of Comparative Ecology Princeton: Princeton University Press. [Google Scholar]
- 73. Lima EM, Ferrari SF (2003) Diet of a free-ranging group of squirrel monkeys (Saimiri sciureus) in eastern Brazilian Amazonia. Folia Primatologica 74: 150–158. [DOI] [PubMed] [Google Scholar]
- 74. Smith RJ, Jungers WL (1997) Body mass in comparative primatology. Journal of Human Evolution 32: 523–559. [DOI] [PubMed] [Google Scholar]
- 75. Felsenstein J (1985) Phylogenies and the comparative method. American Naturalist 125: 1–15. [DOI] [PubMed] [Google Scholar]
- 76. Nunn CL, Barton RA (2001) Comparative methods for studying primate adaptation and allometry. Evolutionary Anthropology 10: 81–98. [Google Scholar]
- 77.Harmon L, Weir J, Brock C, Glor R, Challenger W, et al. (2009) geiger: Analysis of evolutionary diversification. R package version 1.3–1.
- 78. Opazo JC, Wildman DE, Prychitko T, Johnson RM, Goodman M (2006) Phylogenetic relationships and divergence times among New World monkeys (Platyrrhini, Primates). Molecular Phylogenetics and Evolution 40: 274–280. [DOI] [PubMed] [Google Scholar]
- 79. Perelman P, Johnson WE, Roos C, Seuánez HN, Horvath JE, et al. (2011) A Molecular Phylogeny of Living Primates. PLoS Genetics 3: e1001342 10.1371/journal.pgen.1001342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, et al. (2010) caper: Comparative Analyses of Phylogenetics and Evolution in R. R package version 0.4/r71.
- 81. Revell LJ (2012) phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- 82. Rezende EL, Diniz-Filho JAF (2012) Phylogenetic analyses: Comparing species to infer adaptations and physiological mechanisms. Comprehensive Physiology 2: 639–674. 10.1002/cphy.c100079 [DOI] [PubMed] [Google Scholar]
- 83. Sokal RR, Rohlf FJ (1995) Biometry (3rd Edition). New York, NY: W.H. Freeman and Co. [Google Scholar]
- 84. Ray DA, Xing J, Hedges DJ, Hall MA, Laborde ME, et al. (2005) Alu insertion loci and platyrrhine primate phylogeny. Molecular Phylogenetics and Evolution 35: 117–126. [DOI] [PubMed] [Google Scholar]
- 85. Takai M, Anaya F (1996) New specimens of the oldest fossil platyrrhine, Branisella boliviana from Salla, Bolivia. American Journal of Physical Anthropology 99: 301–318. [DOI] [PubMed] [Google Scholar]
- 86. Kay RF, Williams BA, Anaya F (2002) The adaptations of Branisella boliviana, the earliest South American monkey In: Plavcan JM, van Schaik C, Kay RF, Jungers WL, editors. Reconstructing Behavior in the Primate Fossil Record. New York: Kluwer Academic/Plenum Publishers; pp. 339–370. [Google Scholar]
- 87. Fleagle JG, Kay RF (1989) The dental morphology of Dolichocebus gaimanensis, a fossil monkey from Argentina. American Journal of Physical Anthropology 78: 221. [Google Scholar]
- 88. Kay RF, Fleagle JG, Mitchell TRT, Colbert MW, Bown TM, et al. (2008) The anatomy of Dolichocebus gaimanensis, a primitive platyrrhine monkey from Argentina. Journal of Human Evolution 54: 323–382. [DOI] [PubMed] [Google Scholar]
- 89. Kay RF (2010) A New Primate from the Early Miocene of Gran Barranca, Chubut Province, Argentina: Paleoecological Implications In: Madden RH, Vucetich G, Carlini AA, Kay RF, editors. The Paleontology of Gran Barranca: Evolution and Environmental Change through the Middle Cenozoic of Patagonia Cambridge: Cambridge University Press; pp. 220–239. [Google Scholar]
- 90. Kay RF (1990) A possible "giant" tamarin from the Miocene of Colombia. American Journal of Physical Anthropology 81: 248. [DOI] [PubMed] [Google Scholar]
- 91. Takai M (1994) New specimens of Neosaimiri fieldsi, a middle Miocene ancestor of the squirrel monkeys from La Venta, Colombia. Journal of Human Evolution 27: 329–360. [Google Scholar]
- 92. Kay RF, Madden RH, Plavcan JM, Cifelli RL, Guerrero-Diaz J (1987) Stirtonia victoriae, a new species of Miocene Colombian primate. Journal of Human Evolution 16: 173–196. [Google Scholar]
- 93. Kay RF, Johnson DJ, Meldrum DJ (1999) Proteropithecia, new name for Propithecia Kay, Johnson and Meldrum, 1998 non Vojnits 1985. American Journal of Primatology 47: 347. [Google Scholar]
- 94. Luchterhand K, Kay RF, Madden RH (1986) Mohanamico hershkovitzi, gen et sp. nov., un primate du Miocène moyen d'Amérique du Sud. Comptes Rendus de l'Académie de Sciences, Paris Ser. II, 303: 1753–1758. 16053911 [Google Scholar]
- 95. Kay RF, Perry JMG, Malinzak MD, Allen KL, Kirk EC, et al. (2012) The paleobiology of Santacrucian primates In: Vizcaíno S, Kay RF, Bargo M, editors. Early Miocene Paleobiology in Patagonia: High-latitude paleocommunities of the Santa Cruz Formation. Cambridge, UK: Cambridge University Press; pp. 306–330. [Google Scholar]
- 96. Kay RF (1978) Molar structure and diet in extant Cercopithecoidea In: Butler PM, Joysey K, editors. Development, Function and Evolution of Teeth. London: Academic Press; pp. 309–339. [Google Scholar]
- 97. Crompton AW, Hiiemae K (1969) Functional occlusion in tribosphenic molars. Nature 222: 678–679. [DOI] [PubMed] [Google Scholar]
- 98. Ledogar JA, Winchester JM, St. Clair EM, Boyer DM Diet and dental topography in pitheciine seed predators. American Journal of Physical Anthropology 150: 107–121. 10.1002/ajpa.22181 [DOI] [PubMed] [Google Scholar]
- 99. Vogel ER, van Woerden JT, Lucas PW, Utami Atmoko SS, van Schaik CP, et al. (2008) Functional ecology and evolution of hominoid molar enamel thickness:Pan troglodytes schweinfurthii and Pongo pygmaeus wurmbii . Journal of Human Evolution 55: 60–74. 10.1016/j.jhevol.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 100. Boyer DM, Winchester J, Kay RF (2014) The effect of differences in methodology among some recent applications of shearing quotients. American Journal of Physical Anthropology 156: 166–178. 10.1002/ajpa.22619 [DOI] [PubMed] [Google Scholar]
- 101. Kay RF (1984) On the use of anatomical features to infer foraging behavior in extinct primates In: Cant J, Rodman P, editors. Adaptations for Foraging in Nonhuman Primates. New York: Columbia University Press; pp. 21–53. [Google Scholar]
- 102. Kay RF (1977) The evolution of molar occlusion in Cercopithecidae and early catarrhines. American Journal of Physical Anthropology 46: 327–352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sheet 1 for lower molars. Sheet 2 for upper molars. Sheet 3 for combined upper and lower molars.
(XLSX)
Data for individual specimens measured in this study.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.