Abstract
Phthalates provide one of the most documented example evidencing how much we must be cautious when using the traditional paradigm based on extrapolation of experimental data from rodent studies for human health risk assessment of endocrine disruptors (EDs). Since foetal testis is known as one of the most sensitive targets of EDs, phthalate risk assessment is routinely based on the capacity of such compounds to decrease testosterone production by the testis or to impair masculinization in the rat during foetal life. In this paper, the well-established inhibiting effects of phthalates of the foetal Leydig cells function in the rat are briefly reviewed. Then, data obtained in humans and other species are carefully analysed. Already in January 2009, using the organotypic culture system named Fetal Testis Assay (FeTA) that we developed, we reported that phthalates might not affect testosterone production in human foetal testes. Several recent experimental studies using xenografts confirm the absence of detectable anti-androgenic effect of phthalates in the human foetal testes. Epidemiological studies led to contradictory results. Altogether, these findings suggest that phthalates effects on foetal Leydig cells are largely species-specific. Consequently, the phthalate threshold doses that disturb foetal steroidogenesis in rat testes and that are presently used to define the acceptable daily intake levels for human health protection must be questioned. This does not mean that phthalates are safe because these compounds have many deleterious effects upon germ cell development that may be common to the different studied species including human. More generally, the identification of common molecular, cellular or/and phenotypic targets in rat and human testes should precede the choice of the toxicological endpoint in rat to accurately assess the safety threshold of any ED in humans.
Keywords: Endocrine disruptors, Phthalates, Leydig cells, Masculinization, Human health, Risk assessment, Toxicity test, Development, Reproduction, Foetus, Testis, Testosterone
Résumé
En toxicologie réglementaire, l’évaluation du risque sanitaire d’un perturbateur endocrinien (PE) est basée sur le paradigme traditionnel qui consiste à extrapoler à l’espèce humaine les données obtenues chez l’animal. Les phtalates fournissent un des exemples les mieux documentés montrant combien nous devons être prudents dans cette démarche. Le testicule fœtal est une cible privilégiée des PE et l’évaluation du risque sanitaire des phtalates a été construite sur la capacité de ces produits à inhiber la production testiculaire de testostérone ou à réduire la masculinisation pendant la vie fœtale chez le rat. Dans cet article, nous présentons brièvement les effets inhibiteurs bien connus des phtalates sur les fonctions des cellules de Leydig fœtales chez le rat. Puis nous détaillons les études effectuées chez l’homme et les autres espèces. Dès janvier 2009, en utilisant un système de culture organotypique original que nous avions mis au point et nommé hFeTA pour human Fetal Testis Assay, nous avons montré que les phtalates ne réduisent pas la production de testostérone par le testicule fœtal humain. En utilisant des modèles de xénogreffes, plusieurs études ont confirmé récemment l’absence d’effet antiandrogénique détectable des phtalates sur le testicule fœtal humain. Les études épidémiologiques ont conduits à des conclusions contradictoires. En définitive, l’effet des phtalates sur les cellules de Leydig fœtales est largement dépendant de l’espèce. En conséquence, on doit s’interroger sur le bien-fondé de l’utilisation actuelle de la dose minimale induisant un déficit de la stéroïdogenèse dans le testicule fœtal de rat pour définir les normes réglementaires d’exposition aux phtalates en santé humaine. Il faut noter que, bien que les phtalates semblent dépourvus d’effet antiandrogénique sur le testicule fœtal humain, ils ne sont pas sans danger puiqu’ils altèrent le développement de la lignée germinale chez l’Homme comme chez toutes les espèces étudiées. De façon plus générale, nous préconisions que l’identification de cibles moléculaires, cellulaires, et/ou phénotypiques communes au rat et à l’homme précéde le choix d’un paramètre critique utilisant le rat comme modèle en toxicologie réglementaire.
Mots-clés: Perturbateurs endocriniens, Phtalates, Cellules de Leydig, Masculinisation, Santé environnementale, Évaluation du risque, Test de toxicité, Développement, Fœtus, Testicule, Testostérone
Introduction
The risk of chemicals for human health is routinely assessed by epidemiological and experimental studies. The latters used a traditional risk assessment paradigm based on the integration of both exposure assessment and hazard characterization. Hazard characterization is based on in vivo exposure of animals, particularly rats and establishment of a dose response curve allowing the identification of the lowest Non Observed Adverse Effect Level (NOAEL). Then values obtained by this methodology are extrapolated to human to define the Tolerable Daily Intake (TDI). Regulatory Agencies are aware of the difficulties due to the uncertainties linked to extrapolation of experimental data from rodent studies to human health assessment. Therefore, the NOAEL measured in rodent models is divided by a safety factor of 100 to define the regulatory acceptable dose for human health. This safety factor is based on a factor of 10 to account for the differences between rodents and humans multiplied by an additional uncertainty factor of 10 to account for inter-individual human differences in susceptibility.
However, new data show that this rule may be completely inappropriate for Endocrine Disruptor (ED) risk assessment. By comparing the effects of various compounds in rat, mouse and human foetal testes, we have recently demonstrated that the extrapolation to human species of data obtained in rodents is « illogical » in one third of the analyses, whatever the security factor used, because the effect observed in animals does not exist in humans or vice versa [1]. Indeed, whereas fundamental processes in biology, such as apoptosis, cell cycle or cell differentiation, and their pathological disturbances (cancer) are sustained by common basic mechanisms in various species, hormonal regulations are often species-specific in relation with evolution and the large variations in life style. Differences are observed in many hormonal regulatory processes: biochemical nature of the hormone, production level, control of the secretion, metabolism, hormone receptors, signalling molecules… These differences are even more important in reproduction, the most variable function from one species to another. As an example linked with this paper, foetal Leydig cells are stimulated by Chorionic Hormone (CG) as soon as they differentiated in human species whereas there is no CG physiologically active in the rat [2, 3].
Many studies indicate that the incidence of male reproductive function abnormalities in humans is increasing over the years [4–7]. Although differences are observed according to the country and even within regions of the same country, human sperm count has been markedly decreasing over the past four decades. A recent work showed that sperm concentration in France has been declining by 1.9% per year from 1996 to 2005 [8]. Moreover, all studies show that the rate of testicular cancer has been clearly increasing over the last decades. The prevalence rates of cryptorchidism and hypospadias are also probably increasing. According to the most common hypothesis, named the «Testis Dysgenesis Syndrome», these abnormalities result from defaults in the development of the testis during foetal life [9, 10]. Furthermore, many epidemiological, clinical and experimental data suggest that these male reproductive disorders are, at least in part, due to the effects of EDs, which are progressively becoming more concentrated and widespread in our environment [5, 6, 9, 11–20].
ED effects are often more severe when exposure occurs during foetal life than in adulthood [21–26]. Foetal testis thus appears to be a crucial ED target. Accordingly, the tolerable dose intake of a given ED is often derived from the measurement of its effects on the male reproductive functions following foetal exposure. This is the case for phthalates.
It is well established that some phthalates decrease testosterone production by foetal testes in the rat and this anti-androgenic effect is routinely used for human health risk assessment. The aim of this review is to question the validity of this practice because recent data indicate that phthalates do not have detectable anti-androgenic effect in human foetal testes. After a rapid overview of the effects of phthalates on foetal Leydig cell functions and development in the rat, this review will detail data obtained in human foetal testes and finally will extend the analysis to other species.
Review
Human exposure to phthalates
Phthalates are phthalic acid esters the use of which has been steadily increasing since 1930. Their worldwide production grew from 1.8 to 4.3 million tons between 1970 and 2006. Phthalates are plasticisers that are added to polymers and principally to PVC to make them softer and more flexible. They are widely used in many soft PVC products, including building and construction materials, such as cabling, flooring, wall covering, profiles and roofs. They are components of medical equipment, hoses, shower curtains, films and plastic gloves, household furnishings, toys, car interiors, clothing, food and beverage packaging, pharmaceutical products, etc. Phthalates are also used as solvents for oil-soluble dyes, insecticides, peroxides and other organic products. They are added to paints and lacquers, adhesives and sealants, cosmetics, lubricants, putty, perfumes, deodorants, sprays. Di(2-ethylhexyl) phthalate (DEHP) was the most widely used phthalate until recently. Now, it is progressively replaced by di-iso-nonyl phtalate (DiNP) which is less biologically active [21].
Phthalates are not covalently bound to the product matrix and can leak out over time. For instance, they are detected in domestic dust [27]. Dermal exposure may also occur via clothes, cosmetics and medical equipment, such as catheters and plastic pockets, for instance. Thus, humans are constantly exposed to phthalates through the oral, dermal and inhalation routes [28, 29]. In the body, phthalates are rapidly hydrolysed by esterases in the gut and other tissues into monoesters that are the active molecules [30, 31]. For example, DEHP is metabolized to its monoester metabolite mono-(2-ethylhexyl) phthalate (MEHP), and di-butyl phthalate (DBP) is converted into mono-butyl phthalate (MBP).
The half-life of phthalates does not exceed 36 h in the body. In humans, 75% of ingested DEHP is metabolized and excreted in the urine within two days [32]. However, phthalates are so widespread in the environment that humans are constantly and largely exposed. According to a study published in 2003, 12% of the German population has a daily DEHP intake that exceeds the European recommendations of 37 micrograms / kg body weight / day [33]. Moreover, all biomonitoring studies highlight the continuous co-exposure to different phthalates. For instance, a North American study estimated that the 95th percentiles of daily exposure to DEHP, DBP, DEP and butylbenzyl phthalate (BBzP) are 9.32, 2.68, 112.3 and 2.47 μg/kg/day, respectively [34]. The concentration of phthalates in human biological fluids shows large individual variations [35–42]. In pregnant women, the mean values in urinary and amniotic fluid samples are 4.10-7 M for MBP and 8.10-8 M for MEHP. The maximum MEHP concentrations in urinary and amniotic fluid samples are 5.10-6 M and 8.10-6 M, respectively.
Effect of phthalates in the rat
A very large body of works demonstrated that some phthalates, those with a C3 to C7 lateral chain such as DEHP, DBP, DiNP, BBzP, di-propyl phthalate (DprP), dipentyl phthalate (DPeP), dihexyl phthalate (DHP), dicyclohexyl phthalate (DCHP), butylbenzyl phthalate (BBzP), and diisoheptyl phthalate (DIHepP), can reduce the functions and development of foetal Leydig cells and consequently impair masculinization in the rat.
Disturbances in foetal Leydig cell development and function
In all mammalian species, the first foetal Leydig cells differentiate shortly after the differentiation of seminiferous cords [3, 43–45]. In human testes, the functional differentiation of Leydig cells begins at 6–7 weeks of gestation (GW) and testosterone secretion is highest at around 14 GW and then decreases [46, 47]. In the rat, the first functional Leydig cells differentiate at 15 day post-conception (dpc) and foetal testis steroidogenic activity is highest at around 18.5 dpc (i.e., 3 days before birth) [48]. Then, foetal Leydig cells progressively regress, at least functionally, during the first two weeks of extrauterine life [49–51]. It is important to note that the morphological, functional and molecular characteristics of foetal Leydig cells are different from those of adult Leydig cells, which will differentiate at puberty. For instance, differently from adult cells, foetal Leydig cells are not desensitized by high concentrations of luteinizing hormone (LH)/human chorionic gonadotropin (hCG) [52, 53], their initial differentiation does not depend on LH/hCG [54, 55] and their in vivo response to LH/hCG is weak [56]. Furthermore, intratesticular regulations of Leydig cell functions differ between foetus and adult [3]. Moreover, aromatase, a key enzyme in steroidogenesis, is strongly expressed by Sertoli cells in adult testes, while it is essentially expressed by Leydig cells in the foetus [57]. Similarly, FSH, which regulates Leydig cells activity via paracrine factors produced by Sertoli cells, inhibits testosterone production in the foetus and stimulates its production in adult testes [58]. In the rat, exposure to phthalates during the last week of pregnancy induces morphological, molecular and functional alterations in foetal Leydig cells [16, 43, 59, 60]. In response to phthalates, foetal Leydig cells form clusters, develop inside the seminiferous cords and change in size (but not in number) [23, 61–67]. Testis production of testosterone is reduced in a dose-dependent manner due to decreased expression of genes involved in cholesterol metabolism and transport (Star, HMG-CoA synthase and Srb1) and in testosterone biosynthesis (Cyp11a, 3beta-Hsd and Cyp17a1) [68–70]. Recently, Sharpe et al. hypothesized that these changes are the consequence of the prevention of Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) down-regulation, a competitive inhibitor of steroidogenic factor-1 (SF-1), which is required for the development and maintenance of Leydig cell differentiated function [71].
Foetal Leydig cells also secrete insulin like-3 (INSL-3). This hormone induces the transabdominal descent of foetal testes from their initial mesonephrotic position to the entrance of the inguinal duct, whereas the inguino-scrotal descent is androgen-dependent [72]. Exposure to some phthalates also reduces Insl-3 expression in the rat foetal testis [73, 74].
A comparison of the effect of various phthalates on testosterone production by rat foetal testes showed that DBP, DEHP, BzBP and diisobutylphtalate (DiBP) are equipotent, dipentyl phthalate (DPP) is about three-fold more potent and diethylphthalate (DEP) has no effect [75].
Masculinization defects in the rat
A reduction in foetal Leydig cell function variably impairs masculinization in the rat and these effects are used as endpoints for phthalate risk assessment.
In the male foetus, under the influence of androgens, the Wolffian ducts differentiate into epididimydes, vas deferens and seminal vesicles; the urogenital sinus gives rise to prostate and bulbourethral glands, and scrotum and penis are formed from the urogenital tubercle. In the female foetus, in the absence of androgens, the basic programme of differentiation is maintained: Wolffian ducts regress, while urogenital sinus and tubercle form the lower part of vagina, labia majora and labia minora. Furthermore, the fallopian tubes, uterus and upper part of the vagina spontaneously differentiate from the Müllerian ducts. Conversely in the male, Sertoli cells produce Anti-Müllerian Hormone (AMH) that induces Müllerian duct regression [76].
In the rat, the masculinization/defeminisation of internal and external genitalia begins at 18.5 dpc [77] and therefore many studies on the anti-androgenic effects of phthalates focused on the interval between 18.5 and 21.5 dpc. However, an important period for androgen-dependent masculinization precedes this phenotypic differentiation [78, 79]. Indeed, a reduction of testosterone synthesis or action between 15.5 and 18.5 dpc causes masculinization defects, such as hypospadias, cryptorchidism, incomplete development or agenesis of prostate and seminal vesicles, reduction of the ano-genital distance (AGD) and penis length. It was thus concluded that the development of genitalia is programmed between 15.5 and 18.5 dpc, a period that was named the « masculinization programming window ». A decrease in androgen production after this period does not affect the ontogenesis of male reproductive organs, but reduces their size and the AGD. By analogy with the rat, if a « masculinization programming window » exists in humans, it should be between 7 and 14 GW.
Exposure to phthalates during foetal life causes many masculinization defects in the rat [16, 20, 43, 59, 80–88]. Specifically, phthalates induce hypospadias, cryptorchidism, gubernaculum alterations, defects in differentiation or growth of epididymis, seminal vesicles, deferent ducts, prostate, levator ani and bulbocavernosus muscles, Cowper’s glands, and reduced AGD. Finally, whereas androgens induce nipple regression at 12 days post-partum (dpp) in the rat, phthalates induce nipples retention [89, 90].
All these defects can be easily quantified and are the usual endpoints to establish the NOAEL/lowest observed adverse effect level (LOAEL) of phthalates. For instance, the LOAEL for DEHP as been established to be 11 mg/kg/d based on the reproductive organ malformations in adults after exposure from 8.5 dpc to 17 dpp [91] and 10 mg/kg/d based on AGD measurement at birth and on nipple retention at 12 dpp after exposure from 7.5 to 16.5 dpc [92].
Comparisons of the effect of various phthalates on masculinization showed that DEHP = BBP > DINP>> > DEP, DOTP, DMP [21] and DBP, BBP, DPeP, and DEHP >> > DMP, DEP, and DOTP [93].
Effect of phthalates in humans
In contrast with the multitude of studies on phthalate anti-androgenic effects in the rat, few data are available on humans [13, 16, 43, 94–96].
Epidemiological studies
In 2005, Swann et al. found a positive correlation between AGD (measured from the centre of the anus to the anterior base of the penis) and penis volume, testis descent and scrotum volume in 2 to 36 month/old boys in the USA [37]. Therefore, they proposed to use AGD as an easily accessible endpoint to evaluate the androgenic activity of foetal and neonatal testes in humans, similarly to what is done in the rat. They then investigated whether the AGD or the ano-scrotal distance (ASD; measured between the centre of the anus and the posterior basis of the scrotum) in 85 young boys aged from 2 to 36 months was inversely correlated with the concentration of various phthalate metabolites in maternal urine samples collected during the last trimester of gestation. Three years later they strengthened their results by increasing to 106 the number of mother-son pairs [97]. The main result of these two works is the existence of an inverse correlation between AGD and the concentration of MBP, monoisobutyl phthalate (MiBP) and several DEHP metabolites in maternal urine samples. Conversely, AGD was not associated with dibenzyl phtalate (DBzP) or di-n-octyl phtalate (DNOP) metabolites. ASD was not associated with any of these phthalate metabolites. Surprisingly both AGD and ASD were associated with monoethyl phtalate (MEP) concentration, a metabolite of DEP, which does not have any anti-androgenic effect in the rat [21, 75]. Finally, the authors observed an inverse correlation between the penis diameter (but not the length) and metabolites of DEHP, but not of other phthalates.
Bustamante-Montes et al. [42] confirmed these results [42]. They observed an inverse correlation between MEHP concentration in maternal urinary samples during the last trimester of pregnancy and AGD but not ASD, measured 24-48 h after birth, in 73 mother-son pairs in Mexico State. Moreover, they found a significant inverse correlation between the cumulative MEHP, MBzP, MEP and MBP urinary levels and AGD as well as penis width and stretched length. However, MEHP was undetectable in the urine samples of 24 mothers, while mBzP, MEP and MBP urinary levels were above detection level only in 8–10 mothers.
Choi et al. [98] searched for an increase of the concentrations of DEHP, DBP, MEHP, MBP and phthalic acid in the plasma or the urine of 40 children with hypospadias or in the urine of their mother as compared with a control group in Korea [98]. Only DEHP in the urine and phthalic acid in the plasma were increased in the patient boys. Interestingly, bisphenol A plasma concentration was sharply increased (×7) in the plasma of the patient group.
Similarly, Suzuki et al. [41] did not find any correlation between AGD at birth and the concentrations of DBP, DMP, DEP, DBzP and DEHP (MEHP and MEOHP) metabolites in maternal urine samples collected between the 9th and the 40th GW in 111 Japanese mother-son pairs [41]. Only AGD, but not ASD, correlation with MEHP concentrations was almost significant.
Lastly, Huang et al. [40] did not detect any association between ASD at birth and MEHP or MBP concentration in maternal urine samples collected at 16–20 GW in 33 mother-son pairs in Taiwan [40].
The difficulty to establish an association between exposure to phthalates and androgenic deficiency could be explained by the fact that maternal urine samples are most often collected during the third trimester of pregnancy (i.e., after the masculinization programming window, between 7 and 14 GW). Significant higher risk of hypospadias was found in boys whose mothers were professionally exposed to phthalates (and thus particularly during the first trimester of pregnancy), including hairdressers, beauty therapists, research chemists, line operators, pharmaceutical operators, electrical assemblers, factory assistants, compared to boys whose mothers were not exposed to phthalates at work [99]. However, people who are professionally exposed to phthalates are also exposed to many other confounding anti-androgenic factors.
Another difficulty is linked to the fact that maternal urine levels of a compound or its metabolites may not be indicative of a direct effect of these compounds on the testis in the fetal compartment. This is particularly the case for phtalathes since phthalates are rapidly hydrolyzed by esterases in the gut and other tissues to monoesters, which are the active molecules [30].
The strength of these works could be also limited by the fact that phthalate exposure was always evaluated in a single sample. Nevertheless, according to Teitelbaum et al. [100], a single urine sample is often representative of exposure over a 6-month period to warrant its use as an exposure estimate in epidemiological studies [100].
Moreover, phthalates may exert phenotypical anti-androgenic effects by acting on targets different from the fetal testis as suggested by some epidemiological studies that reported an effect also in the female foetuses. Huang et al. [40] observed an inverse correlation between the distance from the anus to the posterior convergence of the fourchette in female new-borns and the concentration of MBP in amniotic fluid samples [40]. Furthermore, Sathyanarayana et al. [101] reported the association between lower circulating testosterone levels and increased DEHP and DBP exposure in women carrying female foetuses during the second and the third trimester of pregnancy [101]. This association was found also in women carrying male foetuses, but only for DEHP and not DBP.
The main limit of these epidemiological studies is the presence of confounding factors. Specifically, exposure to phthalates is often associated with exposure also to bisphenol A (BPA), a powerful inhibitor of human foetal Leydig cell activity [102]. High levels of plasma BPA have been shown to be strongly associated with hypospadias in Korean boys [98]. The bias resulting from confounding factors could explain the inverse association between AGD and exposure to MEP, a compound without anti-androgenic effects, reported by Swan’s group.
In conclusion, these epidemiological studies do not allow confirming or infirming the existence of an association between phthalate exposure and reduced androgenic activity of foetal testes in humans.
Experimental studies
Three independent research groups (headed by K. Boekelheide, R. Habert and R. Sharpe) failed to find any anti-androgenic effect of phthalates in foetal human testes using experimental in vitro and in vivo models.
Studies using in vitro models Historically, the first report on the absence of detection of phthalate anti-androgenic effects in human testes was by the group directed by Richard Sharpe using an organotypic culture model. However, they did not conclude that humans are not responsive to phthalate anti-androgenic effects and attributed this observation to the inability of their in vitro system to detect such an effect [63]. Human foetal testes at 15–19 GW were cut in small pieces and the same number of pieces was deposited on filters immersed in culture medium with or without 10-3 M MBP for 24 hours. MBP did not change basal, hCG-stimulated and 22R-hydroxycholestorol-stimulated testosterone secretion. As a positive control the authors used rat testes from 19.5 dpc foetuses (a developmental stage comparable with 15–19 GW human foetuses because testis testosterone production is highest at 18.5 dpc in the rat and at 14 GW in humans) [3]. They confirmed that oral gavage in pregnant rats with 500 mg/kg/day DBP at 19.5 dpc reduced testosterone production by foetal testes in vivo. Conversely, in their in vitro system, 10-3 M MBP did not reduce basal and 22R-hydroxycholesterol-stimulated testosterone secretion, but only decreased hCG-stimulated secretion in cultured rat testes. The authors concluded that their in vitro approach was not suitable for toxicological purposes and that their observation that phthalates did not alter testosterone production in humans was explained by the limitations of the used in vitro method.
Using an organotypic culture system that we developed and named Foetal Testis Assay (FeTA) [16, 47, 103–108] (Figure 1), we affirmed for the first time in January 2009 that phthalates do not reduce testosterone production (and INSL3 expression) in human foetal testes [109] (Figure 2). We validated this culture system as a powerful in vitro model to test the effects of EDs [1, 103, 110].
Figure 1.
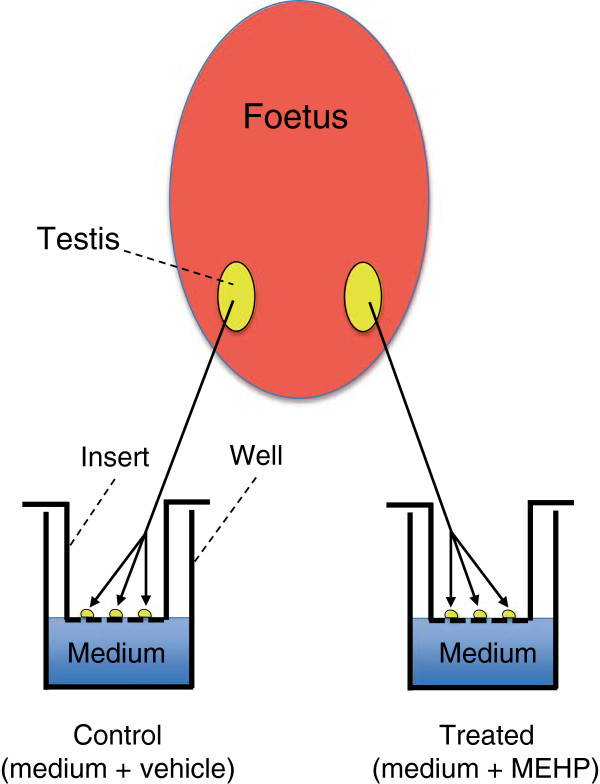
The Foetal Testis Assay (FeTA) as a usefull tool to measure the effects of Endocrine Disruptors. Foetal Testis Assay (FeTA) was developed by our laboratory for rat, mouse and human species [47, 103–108, 126]. Human, rat or mouse foetal testes are cut in small pieces of a volume of about 0.2 mm3/each piece, at all developmental stages and in all studied species. One to four pieces are randomly placed on Millicell-CM Biopore membranes (pore size 0.4 μm, Millipore, Billerica, MA) floating on 320 μL culture medium in tissue culture dishes. One to eight wells per testis are thus prepared, depending on the species and the age at explantation. The culture medium is phenol red-free Dulbecco modified Eagle medium/Ham F12 (1:1) without addition of any biological factor or hormone. Testes are cultured at 37°C in a humidified atmosphere containing 95% air/5% CO2 for three or four days. Medium is completely changed every 24 h. The androgenic and masculinizing activities of cultured testes are evaluated based on the amount of testosterone that is secreted daily by foetal Leydig cells of each testis, on the total number of foetal Leydig cells per testis, or the expression of Leydig cell markers (steroidogenic enzymes or actors, INSL3, LH Receptors).
Figure 2.
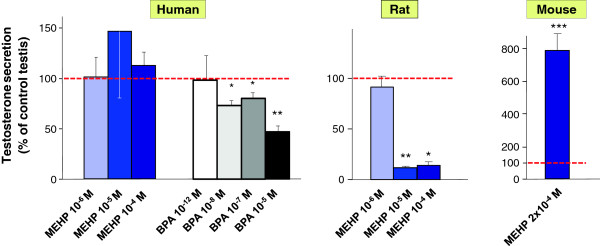
Comparison of the effects of one phthalate as a function of the species. Testes at similar stage of development i.e. from 7–11 GW human foetuses, 14.5 day-old rat fetuses, or 13.5 day-old mouse foetuses were cultured using the FeTA system described in Figure 1. For each foetus, one testis was cultured in the absence (control) and the other one in the presence of MEHP at concentrations ranging from 10-6 to 2×10-4 M for 3 days. The daily testosterone secretion was measured by radioimmunoassay. Only values on the third day are presented here but results are similar for the other two days of treatment. Values (means ± SEM) are expressed as the percentage of the secretion of the treated testis compared with that of the control testis. MEHP had negative, positive or no effect with rat, mouse or human fetal testes respectively. Bisphenol A (BPA) at various concentrations was used as positive control for human fetal testes *p < 0.05, **p < 0.01, ***p < 0.001 compared with control testis using the Wilcoxon’s non-parametric paired test. From [1, 102, 109, 121].
Testis explants were deposited on an insert at the air-medium interface to allow the survival and development of all testis cell types. Human foetal testes were issued from legal abortions performed between 7–12 GW, a period comparable to 15.5-18 dpc in the rat and corresponding to the « Masculinization Programming Window ». All pieces from one testis were cultured with MEHP concentrations ranging from 10-6 M to 10-4 M for 3 days. All pieces from the other testis were cultured without MEHP and served as control. Using this system, we showed that, at all concentrations tested, MEHP does not change the daily basal and LH-stimulated testosterone secretion or the expression of genes involved in testosterone biosynthesis (StAR, CYP11A and CYP17A1). Similarly, also INSL3 expression was unaffected [103].
Many positive and negative controls validated these conclusions:
In these testis explants, 10-4 M MEHP reduced by 40% the number of gonocytes and by 50% AMH mRNA levels. Apoptosis of human gonocytes was significantly increased by exposure to MEHP doses as low as 10-5 M [110]. Furthermore, MEHP increased the size of lipid droplets and the expression of LXR alpha, of downstream genes, such as SREBP1, SREBP2, and of genes involved in cholesterol and lipid biosynthesis [111].
Using the FeTA system we showed that human foetal Leydig cells can respond to other EDs as indicated by the finding that exposure to 10-8 M BPA reduces testosterone production and INSL3 expression [102].
In the FeTA system, exposure to 10-4 and 10-5 M MEHP reduced the basal testosterone production of rat testes explanted at 14.5 dpc and cultured for three days [1]. Importantly, 14.5 to 17.5 dpc is a developmental period comparable with 7–12 GW in humans. The finding by Hallmark et al. [63] that MBP did not have a negative effect on basal testosterone production in foetal rat testes in vitro can be explained by the protocol used by these authors who deposited pieces of testes from different animals in the same well [63], whereas in the FeTA system we compare all pieces of one testis to all pieces of the other testis from the same foetus. Moreover, rat foetal testes are less susceptible to phthalates at 19.5 dpc (stage used by Hallmark et al.) than at 14.5 dpc. Indeed, addition of 10-3 M MBP reduced basal secretion of testosterone by 41% in 19.5 dpc rat testes and by 60% in 14.5 dpc testes cultured in the FeTA system for two days (personal data).
Other EDs such as diethylstylbestrol reduced testosterone production by the fetal testis in rodents but not in human [11, 102, 112].
In conclusion, although our in vitro approach displays intrinsic limits (absence of vascularization, cell survival limited to a few days) it allows the sensitive, direct, rapid, not labour-intensive and relatively cheap evaluation of the toxic effects of a given compound on foetal testis development and functions [1]. Using this method, we never detected any anti-androgenic effect of phthalates in human foetal testes, whereas we always did in rat foetal testes.
Studies using xenograft models Three recent studies from the Sharpe’s and Boekelheide’s laboratories described in vivo models in which human testes were xenografted in rodents.
Richard Sharpe’s group was the first to develop this approach [113]. Human foetal testes at 14–20 GW were cut in small pieces that were grafted under the skin of castrated CD1 nude mice. In this model, the different testis cell types can survive and develop for six weeks. Specifically, foetal Leydig cell activity (based on the host testosterone concentration or seminal vesicle size) was maintained when the host mice were treated with hCG to mimic normal pregnancy. To evaluate the anti-androgenic effect of phthalates, human foetal testes were xenografted in castrated male nude mice that were then treated with hCG and vehicle, or 500 mg/kg/day of DBP or MBP for 4–21 days [114]. Serum testosterone and seminal vesicle weight did not differ in vehicle-, MBP- and DBP-treated host mice. Conversely, in mice xenografted with 17.5 dpc rat foetal testes (positive control), DBP treatment significantly reduced seminal vesicle weight in the host as well as Cyp11a1 and StAR expression in the graft.
Kim Boekelheide’s group used another approach [115]. Human foetal testes at 10–24 GW were cut in small pieces and grafted in the renal capsule of adult nude male rats that were then force-fed with 0, 100, 250 or 500 mg DBP/kg/day for two or three days. DBP treatment did not change the expression of INSL3 and key genes in cholesterol metabolism and steroidogenesis (SCARB1, STAR, CYP11A1 and CYP17A1) in the grafts. As internal positive control, the authors reported that the number of multinucleated gonocytes in the grafts was increased in response to all DBP doses used. However, they did not observe a reduction in gonocyte density, whereas phthalates decreased gonocyte number by increasing their apoptosis in the FeTA system [109, 110]. As another positive control, the authors grafted 16.5 dpc foetal rat testes in nude rats. Gavage of the host with 500 mg DBP/kg/day for two days reduced by half the ability of the graft to produce testosterone and to express Cyp17a1, Scarb1 and Insl3.
A recent paper from this group confirmed these data [116]. Testis pieces from human foetuses at 10–24 GW were grafted in the renal subcapsular space of castrated adult nude male mice. Animals were then treated with hCG and gavaged with vehicle or 500 mg DBP/kg/day or 75 mg/kg/day of abiraterone acetate (a CYP17A1, inhibitor). The plasma testosterone concentration of the mice was unaffected by DBP, whereas it was dramatically reduced by abiraterone acetate. Furthermore, DBP treatment did not affect the host accessory sex organ weight, whereas the weight of seminal vesicles and levator ani and bulbocavernosus muscle complex was reduced in hosts treated with abiraterone acetate.
In conclusion, although the xenograft model displays intrinsic limits (variability in the survival of grafted pieces, host compensatory reactions, differences between the metabolism in the host and in human…), this approach allows the long-term evaluation of the toxic effects of one chemical compound on foetal testis development and functions. The three mentioned papers used different methods and endpoints, but they all confirmed and extended the results obtained with the FeTA model that phthalates are not anti-androgenic in human foetal testes in experimental settings.
Effect of phthalates in other species
In stark contrast with the many works on phthalate effects in the rat, very few studies focused on other mammals.
Effect of phthalates in the rabbit
In the only available study [22], 400 mg/kg/day of DBP was administered orally to pregnant rabbits from 15 dpc (i.e., just after testis differentiation) to 29 dpc (i.e., three days before birth). DBP treatment decreased serum testosterone levels in male offspring (n = 17) at 6 weeks post-partum, but not thereafter; the weight of testes and accessory sex glands was reduced at 12 weeks post-partum. Furthermore, one rabbit (1/17) had hypospadias, hypoplastic prostate and cryptorchid testes with carcinoma in situ-like cells. After reaching adulthood, these rabbits showed a qualitative and quantitative decrease in sperm.
This study suggests that phthalates have anti-androgenic effects in male rabbit foetuses. However, the incidence of reproductive tract malformations following exposure to DBP is lower in rabbits than in rats [43].
Effect of phthalates in the mouse
The first study was performed by Boekelheide’s group [117]. Gavage of pregnant mice with DBP (1500 mg/kg/day), MBP (1000 mg/kg/day) or MEHP (1000 mg/kg/day) from 14.5 to 16.5 dpc or from 15.5 to 17.5 dpc did not change foetal testis testosterone concentration and Scarb1 and Cyp11a1 mRNA expression at 17.5 dpc. Importantly, like in the rat, these treatments caused an increase in multinucleate gonocytes. Gavage with an unique dose of DBP (500 mg/kg) at 18.5 dpc did not reduce the expression of key genes involved in cholesterol transport and metabolism (Scarb1, Dhcr7, Star) and in testosterone biosynthesis (Cyp11a1, Cyp17a1) measured 4–8 h after gavage. These genes were all down-regulated in rat foetuses treated using a similar protocol [68]. The authors concluded that, differently from the rat, phthalates do not have an anti-androgenic effect in mouse foetal testes.
On the other hand, Liu et al. [118] observed a dose-dependent increase in hypospadias in new-born mice from pregnant animals that received 100, 200 or 500 mg/kg/day of DEHP from 12.5 dpc (i.e., when the first foetal Leydig cells differentiate) to 17.5 dpc (i.e., two days before birth) [118]. AGD was also decreased in animals exposed to the highest dose.
Do et al. [119] gave 0, 0.0005, 0.001, 0.005, 0.5, 50 or 500 mg/kg/day of DEHP to pregnant mice from 9 to 18.5 dpc [119]. With the exception of the 500 mg/kg/day dose that did not have any effect, plasma and testis testosterone levels and AGD measured 2–4 hours after the last gavage were increased in male foetuses according to an inversed U-shaped dose–response curve in agreement with the results by Gaido et al. [117].
In another study, 100, 200 or 500 mg/kg/day DEHP given to mice from 12.5 dpc to 3 dpp decreased testis Insl3 expression measured at 5 dpp in a dose-dependent manner [120].
Heger et al. [115] analyzed mouse testes from 15.5 dpc mouse implanted into adult male immunodeficient host rats, which were then given for 2 days to 0, 250 or 500 mg/kg/day DBP by oral gavage [115]. Quantitative RT-PCR analysis did not reveal any change of the expression of the Leydig cell–specific genes Ex vivo testicular incubation showed slight DBP-induced increase in testosterone production. On the opposite, all these parameters were decreased by DBP in 16.5 dpc rat grafted fetal testes.
Finally, an in vitro study highlighted the complexity of the response to phthalates in the mouse and tried to reconcile the contradictory data described above [121]. Mouse foetal testes were cultured using the FeTA system in the presence or not of 2.10-4 M MEHP for three days. MEHP increased basal testosterone production by testes explanted at 13.5 or 18.5 dpc, but decreased LH-stimulated testosterone secretion by testes explanted at 18.5 dpc. As LH secretion physiologically appears in late foetal life, these in vitro data explain why phthalates have antiandrogenic effects in the mouse only during late foetal or neonatal life, while they do not have any effect or show positive effects before late foetal life in the in vivo experiments described above.
Effect of phthalates in the marmoset
The group headed by Richard Sharpe tested the effects of phthalates also in marmosets. Indeed, this non-human primate (Callithrix jacchus) could be a better model for assessing the effects on humans than rodents [122]. Pregnant female marmosets received 500 mg/kg/day of MBP from the 7th to 15th week of gestation and male offspring were studied at birth. This period overlaps with the human «masculinization programming window» because the formation of the gonad occurs during week 6 and the duration of pregnancy is of 21 weeks in this species [123]. MBP did not affect gross testicular morphology, reproductive tract development or testosterone levels at birth. Conversely, a similar treatment in rats causes 17% hypospadias and 70% cryptorchidism [64]. Differently from what observed in human and rat testes, neither the number nor the differentiation of Sertoli cells and gonocytes was affected by MBP. Multinucleated gonocytes were not observed in both control and MBP-treated new-born marmosets, but abnormally aggregated gonocytes were detected in 33% of treated new-borns, as previously reported in the rat [124].
Although this study did not include positive controls and did not demonstrate that with this protocol DBP/MBP could reach the foetal testes, it suggests that Leydig cells are not affected by phthalates during foetal life in marmosets.
Table 1 schematically summarizes the differences observed in the various studied mammals.
Table 1.
Effect of DEHP/MEHP and DBP/MBP on fetal Leydig cell functions in human and various species
| Species | Stage of development of the fetal testis at exposure | Observation | References | |
|---|---|---|---|---|
| Epidemiological studies | Human | Evidence for an association between a reduction of masculinization and phthalates exposure | [37], [42, 97, 99] | |
| Weak evidence for an association between a reduction of masculinization and phthalates exposure | [41], [98, 101] | |||
| Not any association between masculinization and phhtalates exposure | [40] | |||
| Experimental studies | Human | Early stages | No effect of phthalates | [109], [110] |
| Late stage | No effect of phthalates | [63], [114–116] | ||
| Rat | Early stages | Strong negative effect of phthalates | [1], [23, 25, 61, 63–70, 75, 80–88, 90–93] | |
| Late stages | Negative effect of phthalates | [63], [89] | ||
| Mouse | Early stages | Positive effect of phthalates | [115], [118, 119, 121] | |
| Late stages | Negative or no effect of phthalates | [117], [121] | ||
| Rabbit | Early + late stages | Negative effect of phthalates | [22] | |
| Marmoset | Early + late stages | No effect of phthalates | [122] |
Conclusion
The toxicological reference values of various phthalates are mostly based on their anti-androgenic effects in the rat foetus; however, more and more studies show suggest that these effects do not exist in humans. Similarly, phthalates do not reduce foetal Leydig cell activity in the mouse during the beginning of testis development and in foetal marmoset, whereas they might have a weak anti-androgenic effect in rabbits. This suggests that phthalates suppress foetal Leydig cell steroidogenesis specifically in the rat.
The explanation for this species difference is not yet established. However, Veeramachaneni and Klinefelter recently observed that, DEHP exposure induced an increase in circulating oestradiol in the rat and they supposed that aromatase is one of the primary targets of phthalates [96]. Since oestradiol reduces testosterone production by the rat fetal testis [112] but not by the human fetal testis during the first or the second week of pregnancy [102, 125] this could explain the species difference concerning phthalate anti-androgenic effects. The species difference in the oestrogen anti-androgenic effect has been explained as follow : estradiol reduced fetal testicular testosterone production via ESR1 [11, 102, 126] and ESR1 expression (mRNA and protein) is not detected in human fetal testes [127].
This review focused only on phthalate exposure during foetal life, because this period is particularly sensitive to endocrine disruption and, thus, intrauterine exposure to phthalates is normally used to evaluate phthalate hazards. The critical susceptibility to EDs extends to neonatal life, but no experimental data on the adverse effects of phthalates in humans during this developmental period are presently available. An anti-androgenic effect of phthalates during this period cannot be excluded because foetal and neonatal Leydig cells are different in human [3] and exposure to DBP reduces plasma testosterone levels in marmoset neonates, but not in foetuses [63].
While phthalate effects on Leydig cell development and function are highly variable from one species to another, their adverse effect on the seminiferous compartment has been reported in foetuses of all studied species. However, it is difficult to perform a precise inter-species comparison in this case because the number of studies is very limited. Furthermore the analysed endpoints differ from one study to another. It is clearly established that phthalates increase the development of multinucleated gonocytes in the rat during late foetal life [23, 64, 124, 128, 129] and in the mouse [117, 130]. However, this was not observed in marmosets [122], and in humans phthalate-induced multinucleated gonocytes were not detected during the first trimester of gestation [109] but only later, starting from the second trimester [115, 116]. Gonocyte apoptosis may be another phthalate-responsive endpoint common to different species because it was observed in vitro [131] and in vivo [129] in the rat, and in vitro in mouse [121] and human foetal testes [103, 110]. Unfortunately, this endpoint has not been studied in xenografted human foetal testes. It is not clear whether phthalate-induced disturbances in gonocyte development result from a direct effect on this cell type or whether they are mediated by Sertoli cells. Indeed, Sertoli cell proliferation and/or gene expression are altered by phthalates both in rat and human foetuses [103, 132, 133]. In conclusion, as gonocytes and Sertoli cells are common targets of phthalates in different species, including humans, efforts should be made to define a set of genes the expression of which is changed by phthalates both in rat and human testes in order to establish new endpoints for human risk assessment.
More generally, the identification of common molecular, cellular or/and phenotypic targets in both rat and human models should precede the choice of a toxicological endpoint in the rat to accurately assess the safety threshold of any ED in humans.
Acknowledgements
We are grateful to René Frydman and Alexandra Benachi for their efficient collaboration throughout the last ten years, Claire Beausoleil (ANSES, France), Christophe Rousselle (ANSES, France) and Paul Fowler (University of Aberdeen, Scotland) for helpful discussions, Aurélie Gouret for skillfull secretarial assistance and E. Andermarcher for editing the English manuscript. Our work on phthalates was supported by Université Paris Diderot-Paris 7, the Atomic Energy Comittee (CEA), the National Institute for Health and Medical Research (INSERM), the Agence Nationale de la Recherche (ANR, contrat Phtalatestis) and by the Ministère de l’Ecologie, du Développement Durable, des Transports et du Logement (Programme ANTIOPES – Storm). All the authors never received any personal or professional advantages, remunerations, financial supports or fundings from the Chemical Industry. This review is dedicated to Jose M. Saez who was René Habert’s mentor.
Footnotes
Competing interests
All the authors declare that they have no competing interests in relation to this manuscript. All the authors never received any personal financial remuneration or funding for research studies from any chemical industrial company.
Authors’ contributions
R.H. wrote the manuscript that was critically reviewed by GL and VRF. All authors read and approved the final manuscript.
Contributor Information
René Habert, Email: rene.habert@cea.fr.
Gabriel Livera, Email: gabriel.livera@cea.fr.
Virginie Rouiller-Fabre, Email: virginie.rouiller-fabre@cea.fr.
References
- 1.Habert R, Muczynski V, Grisin T, Moison D, Messiaen S, Frydman R, Benachi A, Delbes G, Lambrot R, Lehraiki A, N’Tumba-Byn T, Guerquin MJ, Levacher C, Rouiller-Fabre V, Livera G. Concerns about the widespread use of rodent models for human risk assessments of endocrine disruptors. Reproduction. 2014;147:R119–129. doi: 10.1530/REP-13-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habert R, Picon R. Attempts for identification of a chorionic gonadotrophin-like bioactivity in the rat placenta which stimulates the testosterone secretion of the fetal testis in vitro. Biol Neonate. 1990;58:24–31. doi: 10.1159/000243227. [DOI] [PubMed] [Google Scholar]
- 3.Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol. 2001;179:47–74. doi: 10.1016/S0303-7207(01)00461-0. [DOI] [PubMed] [Google Scholar]
- 4.Leridon H, Slama R. The impact of a decline in fecundity and of pregnancy postponement on final number of children and demand for assisted reproduction technology. Hum Reprod. 2008;23:1312–1319. doi: 10.1093/humrep/den106. [DOI] [PubMed] [Google Scholar]
- 5.Main KM, Skakkebaek NE, Virtanen HE, Toppari J. Genital anomalies in boys and the environment. Best Pract Res Clin Endocrinol Metab. 2010;24:279–289. doi: 10.1016/j.beem.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe RM, Irvine DS. How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? BMJ. 2004;328:447–451. doi: 10.1136/bmj.328.7437.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jr, Jegou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Muller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek NE. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104(Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Moal J, Rolland M, Goria S, Wagner V, De Crouy-Chanel P, Rigou A, De Mouzon J, Royere D. Semen quality trends in French regions are consistent with a global change in environmental exposure. Reproduction. 2014;147:567–574. doi: 10.1530/REP-13-0499. [DOI] [PubMed] [Google Scholar]
- 9.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 10.Olesen IA, Sonne SB, Hoei-Hansen CE, Rajpert-De Meyts E, Skakkebaek NE. Environment, testicular dysgenesis and carcinoma in situ testis. Best Pract Res Clin Endocrinol Metab. 2007;21:462–478. doi: 10.1016/j.beem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Delbes G, Levacher C, Habert R. Estrogen effects on fetal and neonatal testicular development. Reproduction. 2006;132:527–538. doi: 10.1530/rep.1.01231. [DOI] [PubMed] [Google Scholar]
- 12.Toppari J, Virtanen HE, Main KM, Skakkebaek NE. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res Part A Clin Mol Teratol. 2010;88:910–919. doi: 10.1002/bdra.20707. [DOI] [PubMed] [Google Scholar]
- 13.Adamo C, Antignac J, Auger J, Balaguer P, Bourc’his D, Bujan L, Chevrier C, Cotinot C, Cravedi J, Laudet V, Livera G, Slama R. Reproduction et Environnement. Paris: INSERM edn; 2011. [Google Scholar]
- 14.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Myers JP, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT. Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod Toxicol. 2013;38:1–15. doi: 10.1016/j.reprotox.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giwercman A, Giwercman YL. Environmental factors and testicular function. Best Pract Res Clin Endocrinol Metab. 2011;25:391–402. doi: 10.1016/j.beem.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Habert R, Muczynski V, Lehraiki A, Lambrot R, Lecureuil C, Levacher C, Coffigny H, Pairault C, Moison D, Frydman R, Rouiller-Fabre V. Adverse effects of endocrine disruptors on the foetal testis development: focus on the phthalates. Folia Histochem Cyto. 2009;47:S67–74. doi: 10.2478/v10042-009-0056-5. [DOI] [PubMed] [Google Scholar]
- 17.Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–1395. doi: 10.1016/0140-6736(93)90953-E. [DOI] [PubMed] [Google Scholar]
- 18.Juul A, Almstrup K, Andersson AM, Jensen TK, Jorgensen N, Main KM, Meyts ER, Toppari J, Skakkebaek NE. Nat Rev Endocrinol. 2014. Possible fetal determinants of male infertility. [DOI] [PubMed] [Google Scholar]
- 19.Svechnikov K, Stukenborg JB, Savchuck I, Soder O. Similar causes of various reproductive disorders in early life. Asian J Androl. 2014;16:50–59. doi: 10.4103/1008-682X.122199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008;89:e33–38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi TT, Palmer JS, Gray LE, Jr, Veeramachaneni DN. Effects of dibutyl phthalate in male rabbits following in utero, adolescent, or postpubertal exposure. Toxicol Sci. 2003;72:301–313. doi: 10.1093/toxsci/kfg036. [DOI] [PubMed] [Google Scholar]
- 23.Mahood IK, Scott HM, Brown R, Hallmark N, Walker M, Sharpe RM. In utero exposure to di(n-butyl) phthalate and testicular dysgenesis: comparison of fetal and adult end points and their dose sensitivity. Environ Health Perspect. 2007;115(Suppl 1):55–61. doi: 10.1289/ehp.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mylchreest E, Foster PM. DBP exerts its antiandrogenic activity by indirectly interfering with androgen signaling pathways. Toxicol Appl Pharmacol. 2000;168:174–175. doi: 10.1006/taap.2000.9032. [DOI] [PubMed] [Google Scholar]
- 25.Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE., Jr The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 26.Wine RN, Li LH, Barnes LH, Gulati DK, Chapin RE. Reproductive toxicity of di-n-butylphthalate in a continuous breeding protocol in Sprague–Dawley rats. Environ Health Perspect. 1997;105:102–107. doi: 10.1289/ehp.97105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, Rosskamp E, Schluter C, Seifert B, Ullrich D. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207:409–417. doi: 10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- 28.Koch HM, Becker K, Wittassek M, Seiwert M, Angerer J, Kolossa-Gehring M. Di-n-butylphthalate and butylbenzylphthalate - urinary metabolite levels and estimated daily intakes: pilot study for the German Environmental Survey on children. J Expo Sci Environ Epidemiol. 2007;17:378–387. doi: 10.1038/sj.jes.7500526. [DOI] [PubMed] [Google Scholar]
- 29.Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26:803–824. doi: 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 30.Latini G. Monitoring phthalate exposure in humans. Clin Chim Acta. 2005;361:20–29. doi: 10.1016/j.cccn.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Saravanabhavan G, Murray J. Human biological monitoring of diisononyl phthalate and diisodecyl phthalate: a review. J Environ Public Health. 2012;2012:810501. doi: 10.1155/2012/810501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79:367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- 33.Koch HM, Drexler H, Angerer J. An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int J Hyg Environ Health. 2003;206:77–83. doi: 10.1078/1438-4639-00205. [DOI] [PubMed] [Google Scholar]
- 34.Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect. 2006;114:805–809. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111:1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL, Study for Future Families Research T Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, Schmidt IM, Suomi AM, Virtanen HE, Petersen DV, Andersson AM, Toppari J, Skakkebaek NE. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Hakansson H. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect. 2008;116:334–339. doi: 10.1289/ehp.116-a334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang PC, Kuo PL, Chou YY, Lin SJ, Lee CC. Association between prenatal exposure to phthalates and the health of newborns. Environ Int. 2009;35:14–20. doi: 10.1016/j.envint.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int J Androl. 2012;35:236–244. doi: 10.1111/j.1365-2605.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 42.Bustamante-Montes LP, Hernandez-Valero MA, Flores-Pimentel D, Garcia-Fabila M, Amaya-Chavez A, Barr DB, Borja-Aburto VH. Prenatal exposure to phthalates is associated with decreased anogenital distance and penile size in male newborns. J Dev Orig Health Dis. 2013;4:300–306. doi: 10.1017/S2040174413000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- 44.O’Shaughnessy PJ, Fowler PA. Endocrinology of the mammalian fetal testis. Reproduction. 2011;141:37–46. doi: 10.1530/REP-10-0365. [DOI] [PubMed] [Google Scholar]
- 45.Svechnikov K, Landreh L, Weisser J, Izzo G, Colon E, Svechnikova I, Soder O. Origin, development and regulation of human Leydig cells. Horm Res Paediatr. 2010;73:93–101. doi: 10.1159/000277141. [DOI] [PubMed] [Google Scholar]
- 46.Tapanainen J, Kellokumpu-Lehtinen P, Pelliniemi L, Huhtaniemi I. Age-related changes in endogenous steroids of human fetal testis during early and midpregnancy. J Clin Endocrinol Metab. 1981;52:98–102. doi: 10.1210/jcem-52-1-98. [DOI] [PubMed] [Google Scholar]
- 47.Lambrot R, Livera G, Coffigny H, Pairault C, Frydman R, Habert R, Rouiller-Fabre V. A new method for toxicity assays on human and mouse fetal testis. Biochimie. 2006;88:1831–1835. doi: 10.1016/j.biochi.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 48.Habert R, Picon R. Testosterone, dihydrotestosterone and estradiol-17 beta levels in maternal and fetal plasma and in fetal testes in the rat. J Steroid Biochem. 1984;21:193–198. doi: 10.1016/0022-4731(84)90383-2. [DOI] [PubMed] [Google Scholar]
- 49.Tapanainen J, Kuopio T, Pelliniemi LJ, Huhtaniemi I. Rat testicular endogenous steroids and number of Leydig cells between the fetal period and sexual maturity. Biol Reprod. 1984;31:1027–1035. doi: 10.1095/biolreprod31.5.1027. [DOI] [PubMed] [Google Scholar]
- 50.Kerr JB, Knell CM. The fate of fetal Leydig cells during the development of the fetal and postnatal rat testis. Development. 1988;103:535–544. doi: 10.1242/dev.103.3.535. [DOI] [PubMed] [Google Scholar]
- 51.Habert R, Brignaschi P. Developmental changes in in vitro testosterone production by dispersed Leydig cells during fetal life in rats. Arch Androl. 1991;27:65–71. doi: 10.3109/01485019108987654. [DOI] [PubMed] [Google Scholar]
- 52.Huhtaniemi I. Endocrine function and regulation of the fetal and neonatal testis. Int J Dev Biol. 1989;33:117–123. [PubMed] [Google Scholar]
- 53.Habert R, Veniard B, Brignaschi P, Gangnerau MN, Picon R. Absence of development of late steroidogenic lesions in rat testis during the end of fetal life. Arch Androl. 1989;22:41–48. doi: 10.3109/01485018908986749. [DOI] [PubMed] [Google Scholar]
- 54.Habert R, Picon R. Control of testicular steroidogenesis in foetal rat: effect of decapitation on testosterone and plasma luteinizing hormone-like activity. Acta Endocrinol (Copenh) 1982;99:466–473. doi: 10.1530/acta.0.0990466. [DOI] [PubMed] [Google Scholar]
- 55.Migrenne S, Pairault C, Racine C, Livera G, Geloso A, Habert R. Luteinizing hormone-dependent activity and luteinizing hormone-independent differentiation of rat fetal Leydig cells. Mol Cell Endocrinol. 2001;172:193–202. doi: 10.1016/S0303-7207(00)00339-7. [DOI] [PubMed] [Google Scholar]
- 56.Habert R. In vivo acute testicular testosterone response to injection of luteinizing hormone in the rat fetus. Acta Endocrinol (Copenh) 1993;128:268–273. doi: 10.1530/acta.0.1280268. [DOI] [PubMed] [Google Scholar]
- 57.Borday C, Merlet J, Racine C, Habert R. Expression and localization of aromatase during fetal mouse testis development. Basic Clin Androl. 2013;23:13. doi: 10.1186/2051-4190-23-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Migrenne S, Moreau E, Pakarinen P, Dierich A, Merlet J, Habert R, Racine C. Mouse testis development and function are differently regulated by follicle-stimulating hormone receptors signaling during fetal and prepubertal life. PLoS ONE. 2012;7:e53257. doi: 10.1371/journal.pone.0053257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svechnikov K, Izzo G, Landreh L, Weisser J, Soder O. Endocrine disruptors and Leydig cell function. J Biomed Biotechnol. 2010;2010:Article ID684504. doi: 10.1155/2010/684504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez-Arguelles DB, Campioli E, Culty M, Zirkin BR, Papadopoulos V. Fetal origin of endocrine dysfunction in the adult: the phthalate model. J Steroid Biochem Mol Biol. 2013;137:5–17. doi: 10.1016/j.jsbmb.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Barlow NJ, Foster PM. Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to Di(n-butyl) phthalate. Toxicol Pathol. 2003;31:397–410. doi: 10.1080/01926230390202335. [DOI] [PubMed] [Google Scholar]
- 62.Mahood IK, Hallmark N, McKinnell C, Walker M, Fisher JS, Sharpe RM. Abnormal Leydig Cell aggregation in the fetal testis of rats exposed to di (n-butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology. 2005;146:613–623. doi: 10.1210/en.2004-0671. [DOI] [PubMed] [Google Scholar]
- 63.Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, Coutts S, Anderson RA, Greig I, Morris K, Sharpe RM. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ Health Perspect. 2007;115:390–396. doi: 10.1289/ehp.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human 'testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18:1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- 65.Lehmann KP, Phillips S, Sar M, Foster PM, Gaido KW. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol Sci. 2004;81:60–68. doi: 10.1093/toxsci/kfh169. [DOI] [PubMed] [Google Scholar]
- 66.Howdeshell KL, Rider CV, Wilson VS, Gray LE., Jr Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res. 2008;108:168–176. doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Saillenfait AM, Sabate JP, Robert A, Rouiller-Fabre V, Roudot AC, Moison D, Denis F. Dose-dependent alterations in gene expression and testosterone production in fetal rat testis after exposure to di-n-hexyl phthalate. J Appl Toxicol. 2013;33:1027–1035. doi: 10.1002/jat.2896. [DOI] [PubMed] [Google Scholar]
- 68.Thompson CJ, Ross SM, Gaido KW. Di(n-butyl) phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism. Endocrinology. 2004;145:1227–1237. doi: 10.1210/en.2003-1475. [DOI] [PubMed] [Google Scholar]
- 69.Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology. 2006;223:144–155. doi: 10.1016/j.tox.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 70.Culty M, Thuillier R, Li W, Wang Y, Martinez-Arguelles DB, Benjamin CG, Triantafilou KM, Zirkin BR, Papadopoulos V. In utero exposure to di-(2-ethylhexyl) phthalate exerts both short-term and long-lasting suppressive effects on testosterone production in the rat. Biol Reprod. 2008;78:1018–1028. doi: 10.1095/biolreprod.107.065649. [DOI] [PubMed] [Google Scholar]
- 71.van den Driesche S, Walker M, McKinnell C, Scott HM, Eddie SL, Mitchell RT, Seckl JR, Drake AJ, Smith LB, Anderson RA, Sharpe RM. Proposed role for COUP-TFII in regulating fetal Leydig cell steroidogenesis, perturbation of which leads to masculinization disorders in rodents. PLoS ONE. 2012;7:e37064. doi: 10.1371/journal.pone.0037064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anand-Ivell R, Ivell R. Insulin-like factor 3 as a monitor of endocrine disruption. Reproduction. 2014;147:R87–95. doi: 10.1530/REP-13-0486. [DOI] [PubMed] [Google Scholar]
- 73.Wilson VS, Lambright C, Furr J, Ostby J, Wood C, Held G, Gray LE., Jr Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicol Lett. 2004;146:207–215. doi: 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 74.McKinnell C, Sharpe RM, Mahood K, Hallmark N, Scott H, Ivell R, Staub C, Jegou B, Haag F, Koch-Nolte F, Hartung S. Expression of insulin-like factor 3 protein in the rat testis during fetal and postnatal development and in relation to cryptorchidism induced by in utero exposure to di (n-Butyl) phthalate. Endocrinology. 2005;146:4536–4544. doi: 10.1210/en.2005-0676. [DOI] [PubMed] [Google Scholar]
- 75.Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, Gray LE., Jr A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague–Dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105:153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- 76.Jost A, Vigier B, Prepin J, Perchellet JP. Studies on sex differentiation in mammals. Recent Prog Horm Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- 77.Jost A. Hormonal factors in the sex differentiation of the mammalian foetus. Philos T Roy Soc B. 1970;259:119–130. doi: 10.1098/rstb.1970.0052. [DOI] [PubMed] [Google Scholar]
- 78.Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118:1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macleod DJ, Sharpe RM, Welsh M, Fisken M, Scott HM, Hutchison GR, Drake AJ, van den Driesche S. Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl. 2010;33:279–287. doi: 10.1111/j.1365-2605.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 80.Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 81.Foster PM, Mylchreest E, Gaido KW, Sar M. Effects of phthalate esters on the developing reproductive tract of male rats. Hum Reprod Update. 2001;7:231–235. doi: 10.1093/humupd/7.3.231. [DOI] [PubMed] [Google Scholar]
- 82.Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 83.Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- 84.Gray LE, Jr, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, Howdeshell K, Ankley GT, Guillette L. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int J Androl. 2006;29:96–104. doi: 10.1111/j.1365-2605.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- 85.Toppari J, Virtanen H, Skakkebaek NE, Main KM. Environmental effects on hormonal regulation of testicular descent. J Steroid Biochem Mol Biol. 2006;102:184–186. doi: 10.1016/j.jsbmb.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 86.Ge RS, Chen GR, Tanrikut C, Hardy MP. Phthalate ester toxicity in Leydig cells: developmental timing and dosage considerations. Reprod Toxicol. 2007;23:366–373. doi: 10.1016/j.reprotox.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Hu GX, Lian QQ, Ge RS, Hardy DO, Li XK. Phthalate-induced testicular dysgenesis syndrome: Leydig cell influence. Trends Endocrinol Metab. 2009;20:139–145. doi: 10.1016/j.tem.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Struve MF, Gaido KW, Hensley JB, Lehmann KP, Ross SM, Sochaski MA, Willson GA, Dorman DC. Reproductive toxicity and pharmacokinetics of di-n-butyl phthalate (DBP) following dietary exposure of pregnant rats. Birth Defects Res B Dev Reprod Toxicol. 2009;86:345–354. doi: 10.1002/bdrb.20199. [DOI] [PubMed] [Google Scholar]
- 89.Lee KY, Shibutani M, Takagi H, Kato N, Takigami S, Uneyama C, Hirose M. Diverse developmental toxicity of di-n-butyl phthalate in both sexes of rat offspring after maternal exposure during the period from late gestation through lactation. Toxicology. 2004;203:221–238. doi: 10.1016/j.tox.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 90.Clewell RA, Thomas A, Willson G, Creasy DM, Andersen ME. A dose response study to assess effects after dietary administration of diisononyl phthalate (DINP) in gestation and lactation on male rat sexual development. Reprod Toxicol. 2013;35:70–80. doi: 10.1016/j.reprotox.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Gray LE, Jr, Barlow NJ, Howdeshell KL, Ostby JS, Furr JR, Gray CL. Transgenerational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicol Sci. 2009;110:411–425. doi: 10.1093/toxsci/kfp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christiansen S, Boberg J, Axelstad M, Dalgaard M, Vinggaard AM, Metzdorff SB, Hass U. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod Toxicol. 2010;30:313–321. doi: 10.1016/j.reprotox.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 93.Liu K, Lehmann KP, Sar M, Young SS, Gaido KW. Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol Reprod. 2005;73:180–192. doi: 10.1095/biolreprod.104.039404. [DOI] [PubMed] [Google Scholar]
- 94.Witorsch RJ, Thomas JA. Personal care products and endocrine disruption: a critical review of the literature. Crit Rev Toxicol. 2010;40(Suppl 3):1–30. doi: 10.3109/10408444.2010.515563. [DOI] [PubMed] [Google Scholar]
- 95.Johnson KJ, Heger NE, Boekelheide K. Of mice and men (and rats): phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicol Sci. 2012;129:235–248. doi: 10.1093/toxsci/kfs206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Veeramachaneni DN, Klinefelter GR. Phthalate-induced pathology in the foetal testis involves more than decreased testosterone production. Reproduction. 2014;147:435–442. doi: 10.1530/REP-13-0441. [DOI] [PubMed] [Google Scholar]
- 97.Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi H, Kim J, Im Y, Lee S, Kim Y. The association between some endocrine disruptors and hypospadias in biological samples. J Environ Sci Health A Tox. 2012;47:2173–2179. doi: 10.1080/10934529.2012.680387. [DOI] [PubMed] [Google Scholar]
- 99.Ormond G, Nieuwenhuijsen MJ, Nelson P, Toledano MB, Iszatt N, Geneletti S, Elliott P. Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: case–control study. Environ Health Perspect. 2009;117:303–307. doi: 10.1289/ehp.11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 101.Sathyanarayana S, Barrett E, Butts S, Wang C, Swan SH. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction. 2014;147:401–409. doi: 10.1530/REP-13-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.N’Tumba-Byn T, Moison D, Lacroix M, Lecureuil C, Lesage L, Prud’homme SM, Pozzi-Gaudin S, Frydman R, Benachi A, Livera G, Rouiller-Fabre V, Habert R. Differential effects of bisphenol A and diethylstilbestrol on human, rat and mouse fetal leydig cell function. PLoS ONE. 2012;7:e51579. doi: 10.1371/journal.pone.0051579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Habert R, Devif I, Gangnerau MN, Lecerf L. Ontogenesis of the in vitro response of rat testis to gonadotropin-releasing hormone. Mol Cell Endocrinol. 1991;82:199–206. doi: 10.1016/0303-7207(91)90032-N. [DOI] [PubMed] [Google Scholar]
- 104.Lambrot R, Coffigny H, Pairault C, Donnadieu AC, Frydman R, Habert R, Rouiller-Fabre V. Use of organ culture to study the human fetal testis development: effect of retinoic acid. J Clin Endocrinol Metab. 2006;91:2696–2703. doi: 10.1210/jc.2005-2113. [DOI] [PubMed] [Google Scholar]
- 105.Lecerf L, Rouiller-Fabre V, Levacher C, Gautier C, Saez JM, Habert R. Stimulatory effect of follicle-stimulating hormone on basal and luteinizing hormone-stimulated testosterone secretions by the fetal rat testis in vitro. Endocrinology. 1993;133:2313–2318. doi: 10.1210/endo.133.5.8404683. [DOI] [PubMed] [Google Scholar]
- 106.Olaso R, Pairault C, Boulogne B, Durand P, Habert R. Transforming growth factor beta1 and beta2 reduce the number of gonocytes by increasing apoptosis. Endocrinology. 1998;139:733–740. doi: 10.1210/endo.139.2.5765. [DOI] [PubMed] [Google Scholar]
- 107.Livera G, Rouiller-Fabre V, Durand P, Habert R. Multiple effects of retinoids on the development of Sertoli, germ, and Leydig cells of fetal and neonatal rat testis in culture. Biol Reprod. 2000;62:1303–1314. doi: 10.1095/biolreprod62.5.1303. [DOI] [PubMed] [Google Scholar]
- 108.Livera G, Delbes G, Pairault C, Rouiller-Fabre V, Habert R. Organotypic culture, a powerful model for studying rat and mouse fetal testis development. Cell Tissue Res. 2006;324:507–521. doi: 10.1007/s00441-006-0167-7. [DOI] [PubMed] [Google Scholar]
- 109.Lambrot R, Muczynski V, Lecureuil C, Angenard G, Coffigny H, Pairault C, Moison D, Frydman R, Habert R, Rouiller-Fabre V. Phthalates impair germ cell development in the human fetal testis in vitro without change in testosterone production. Environ Health Perspect. 2009;117:32–37. doi: 10.1289/ehp.11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Muczynski V, Cravedi JP, Lehraiki A, Levacher C, Moison D, Lecureuil C, Messiaen S, Perdu E, Frydman R, Habert R, Rouiller-Fabre V. Effect of mono-(2-ethylhexyl) phthalate on human and mouse fetal testis: In vitro and in vivo approaches. Toxicol Appl Pharmacol. 2012;261:97–104. doi: 10.1016/j.taap.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 111.Muczynski V, Lecureuil C, Messiaen S, Guerquin MJ, N’Tumba-Byn T, Moison D, Hodroj W, Benjelloun H, Baijer J, Livera G, Frydman R, Benachi A, Habert R, Rouiller-Fabre V. Cellular and molecular effect of MEHP Involving LXRalpha in human fetal testis and ovary. PLoS ONE. 2012;7:e48266. doi: 10.1371/journal.pone.0048266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Delbes G, Duquenne C, Szenker J, Taccoen J, Habert R, Levacher C. Developmental changes in testicular sensitivity to estrogens throughout fetal and neonatal life. Toxicol Sci. 2007;99:234–243. doi: 10.1093/toxsci/kfm160. [DOI] [PubMed] [Google Scholar]
- 113.Mitchell RT, Saunders PT, Childs AJ, Cassidy-Kojima C, Anderson RA, Wallace WH, Kelnar CJ, Sharpe RM. Xenografting of human fetal testis tissue: a new approach to study fetal testis development and germ cell differentiation. Hum Reprod. 2010;25:2405–2414. doi: 10.1093/humrep/deq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mitchell RT, Childs AJ, Anderson RA, van den Driesche S, Saunders PT, McKinnell C, Wallace WH, Kelnar CJ, Sharpe RM. Do phthalates affect steroidogenesis by the human fetal testis? Exposure of human fetal testis xenografts to di-n-butyl phthalate. J Clin Endocrinol Metab. 2012;97:E341–348. doi: 10.1210/jc.2011-2411. [DOI] [PubMed] [Google Scholar]
- 115.Heger NE, Hall SJ, Sandrof MA, McDonnell EV, Hensley JB, McDowell EN, Martin KA, Gaido KW, Johnson KJ, Boekelheide K. Human fetal testis xenografts are resistant to phthalate-induced endocrine disruption. Environ Health Perspect. 2012;120:1137–1143. doi: 10.1289/ehp.1104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spade DJ, Hall SJ, Saffarini CM, Huse SM, McDonnell EV, Boekelheide K. Differential response to abiraterone acetate and di-n-butyl phthalate in an androgen-sensitive human fetal testis xenograft bioassay. Toxicol Sci. 2014;138:148–160. doi: 10.1093/toxsci/kft266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gaido KW, Hensley JB, Liu D, Wallace DG, Borghoff S, Johnson KJ, Hall SJ, Boekelheide K. Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol Sci. 2007;97:491–503. doi: 10.1093/toxsci/kfm049. [DOI] [PubMed] [Google Scholar]
- 118.Liu X, He DW, Zhang DY, Lin T, Wei GH. Di(2-ethylhexyl) phthalate (DEHP) increases transforming growth factor-beta1 expression in fetal mouse genital tubercles. J Toxic Environ Health A. 2008;71:1289–1294. doi: 10.1080/15287390802114915. [DOI] [PubMed] [Google Scholar]
- 119.Do RP, Stahlhut RW, Ponzi D, Vom Saal FS, Taylor JA. Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod Toxicol. 2012;34:614–621. doi: 10.1016/j.reprotox.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song XF, Wei GH, Liu X, Zhang DY, Chen X, Deng YJ. Effects of diethylhexyl phthalate (DEHP) on INSL3 mRNA expression by Leydig cells derived from mouse embryos and in newborn mice. J INT MED RES. 2008;36:512–521. doi: 10.1177/147323000803600316. [DOI] [PubMed] [Google Scholar]
- 121.Lehraiki A, Racine C, Krust A, Habert R, Levacher C. Phthalates impair germ cell number in the mouse fetal testis by an androgen- and estrogen-independent mechanism. Toxicol Sci. 2009;111:372–382. doi: 10.1093/toxsci/kfp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McKinnell C, Mitchell RT, Walker M, Morris K, Kelnar CJ, Wallace WH, Sharpe RM. Effect of fetal or neonatal exposure to monobutyl phthalate (MBP) on testicular development and function in the marmoset. Hum Reprod. 2009;24:2244–2254. doi: 10.1093/humrep/dep200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li LH, Donald JM, Golub MS. Review on testicular development, structure, function, and regulation in common marmoset. Birth Defects Res B Dev Reprod Toxicol. 2005;74:450–469. doi: 10.1002/bdrb.20057. [DOI] [PubMed] [Google Scholar]
- 124.Kleymenova E, Swanson C, Boekelheide K, Gaido KW. Exposure in utero to di(n-butyl) phthalate alters the vimentin cytoskeleton of fetal rat Sertoli cells and disrupts Sertoli cell-gonocyte contact. Biol Reprod. 2005;73:482–490. doi: 10.1095/biolreprod.104.037184. [DOI] [PubMed] [Google Scholar]
- 125.Mitchell RT, Sharpe RM, Anderson RA, McKinnell C, Macpherson S, Smith LB, Wallace WH, Kelnar CJ, van den Driesche S. Diethylstilboestrol exposure does not reduce testosterone production in human fetal testis xenografts. PLoS ONE. 2013;8:e61726. doi: 10.1371/journal.pone.0061726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Delbes G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R. Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor alpha. Endocrinology. 2005;146:2454–2461. doi: 10.1210/en.2004-1540. [DOI] [PubMed] [Google Scholar]
- 127.Gaskell TL, Robinson LL, Groome NP, Anderson RA, Saunders PT. Differential expression of two estrogen receptor beta isoforms in the human fetal testis during the second trimester of pregnancy. J Clin Endocrinol Metab. 2003;88:424–432. doi: 10.1210/jc.2002-020811. [DOI] [PubMed] [Google Scholar]
- 128.Mylchreest E, Sar M, Wallace DG, Foster PM. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod Toxicol. 2002;16:19–28. doi: 10.1016/S0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 129.Ferrara D, Hallmark N, Scott H, Brown R, McKinnell C, Mahood IK, Sharpe RM. Acute and long-term effects of in utero exposure of rats to di(n-butyl) phthalate on testicular germ cell development and proliferation. Endocrinology. 2006;147:5352–5362. doi: 10.1210/en.2006-0527. [DOI] [PubMed] [Google Scholar]
- 130.Saffarini CM, Heger NE, Yamasaki H, Liu T, Hall SJ, Boekelheide K. Induction and persistence of abnormal testicular germ cells following gestational exposure to di-(n-butyl) phthalate in p53-null mice. J Androl. 2012;33:505–513. doi: 10.2164/jandrol.111.013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li H, Kim KH. Effects of mono-(2-ethylhexyl) phthalate on fetal and neonatal rat testis organ cultures. Biol Reprod. 2003;69:1964–1972. doi: 10.1095/biolreprod.103.018895. [DOI] [PubMed] [Google Scholar]
- 132.Hutchison GR, Scott HM, Walker M, McKinnell C, Ferrara D, Mahood IK, Sharpe RM. Sertoli cell development and function in an animal model of testicular dysgenesis syndrome. Biol Reprod. 2008;78:352–360. doi: 10.1095/biolreprod.107.064006. [DOI] [PubMed] [Google Scholar]
- 133.Mazaud-Guittot S. Dissecting the phthalate-induced Sertoli cell injury: the fragile balance of proteases and their inhibitors. Biol Reprod. 2011;85:1091–1093. doi: 10.1095/biolreprod.111.095976. [DOI] [PubMed] [Google Scholar]


