Abstract
A patient presenting with acute carpal tunnel syndrome and swelling and pain in the wrist and thumb is presented. An open carpal tunnel release and tenosynovectomy were performed with biopsy specimen revealing infection with Histoplasma capsulatum. The case is discussed in context of the prior scant literature of tenosynovitis of the wrist and hand caused by histoplasmosis.
Introduction
Histoplasma capsulatum is a dimorphic fungus found in cat and bat feces endemic to the Midwestern and Southeastern USA, including the Mississippi and Ohio River valleys, and regions of Central and South America. Most infections are subclinical and self-limited; disseminated infection is rare and when present is typically seen in immunocompromised patients where it can be fatal [5, 9, 13, 20, 25, 26]. The most common route of infection is pulmonary via inhalation of aerosolized spores, with lung involvement present in approximately 90 % of cases. Uncommonly other extrapulmonary sites of focal infection have been reported, including musculoskeletal manifestations of oligo- or monoarticular joint [5, 7, 9, 13, 20, 23, 27, 30, 31], bone [1, 10–12, 15, 22, 24], and rarely tenosynovial involvement [3, 4, 6, 8, 14, 16–19, 21, 25, 26]. We report a case of histoplasmosis of the wrist and thumb flexor tendons presenting as an acute carpal tunnel syndrome (CTS) and tenosynovitis. The patient was successfully treated with surgical debridement and long-term antifungal therapy. Few cases have been previously reported presenting as CTS and tenosynovitis, and this is the first case we are aware of in the literature involving the flexor tenosynovium extending into the level of the thumb.
Case Report
A 48-year-old right-hand dominant female legal transcriptionist presented to our institution in May 2012 with 5 months of progressive numbness in the right thumb, index, and middle finger with pain and swelling in the volar wrist, hand, and thumb. The patient had a history of Sjogren’s syndrome with interstitial lung disease treated with Cell Cept (Mycophenolate Mofetil, 1 g twice daily for 2 years). Early in the course of this presentation, the patient’s rheumatologist prescribed a course of oral prednisone (5 mg daily) with minimal relief. After several weeks of worsening symptoms, the patient was treated for a presumed diagnosis of CTS with night-time splinting, and she had a carpal tunnel corticosteroid injection 3 months later which provided 1 month of relief, but her numbness, swelling, and pain returned.
The patient described constant numbness and paresthesias as well as hand weakness and pain. Her numbness increased at night. A prior workup included an electromyogram which was interpreted as evidence of a mild median neuropathy at the wrist. An upper extremity ultrasound was also performed to rule out a deep venous thrombosis given her degree of swelling as well as standard radiographs of the hand and thumb (Figs. 1a–b and 2a–b), all of which were unremarkable.
Fig. 1.
a and b. PA and lateral radiographs of the hand and wrist
Fig. 2.
a and b. PA and lateral radiographs of the thumb
Physical examination revealed significant swelling of the thumb and volar distal forearm extending to the palm. The patient had a positive carpal tunnel compression test. Strength testing revealed weakness with grip strength measured with a Jamar dynamometer of only 4 kg on the affected side compared to 20 kg on the normal side and opposition pinch measured with a pinch dynamometer of 2.3 kg on the affected side compared to 4.6 kg on the normal side. Range of motion of the wrist was reduced with wrist flexion and extension of 65 and 65°, respectively, on the affected side compared to 75 and 70° on the unaffected side.
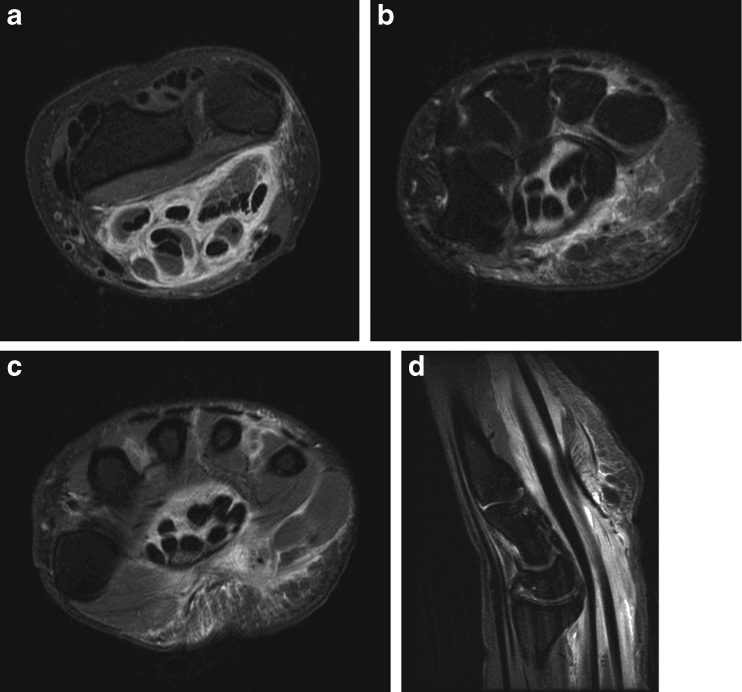
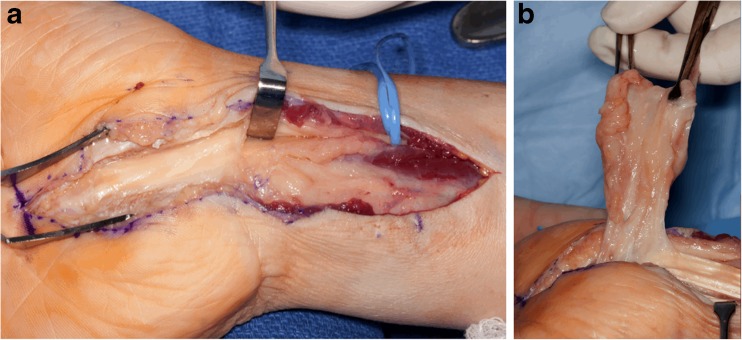
The patient was further worked up with a magnetic resonance imaging (MRI) scan of the wrist (Fig. 3a–d). The study revealed marked thickening and increased T2 signal surrounding the flexor tendons proximal to and within the carpal tunnel extending into the palm and thumb. There was also moderate thickening of the median nerve within the carpal tunnel. Inflammatory markers were obtained, including an erythrocyte sedimentation rate (ESR) of 6 mm/1 h (normal = 0–29), a C-reactive protein (CRP) of <3.0 mg/g (normal = <8.0 mg/g), and a leukocyte count of 7.2 × 109/L (normal = 2.5–10.5 × 109/L). In light of the presentation and findings, the patient was scheduled for an open carpal tunnel release with a tenosynovectomy. At the time of surgery, an extended carpal tunnel release was performed revealing remarkable thickening of the flexor tenosynovium, including the flexor digitorum profundus, flexor digitorum superficialis, and flexor pollicis longus tendons (FPL), with apparent compression of the median nerve by the surrounding tenosynovium (Fig. 4a–b). The affected flexor tenosynovium was comprised of abundant dense proliferative soft tissue resembling rheumatoid pannus. A separate Brunner’s approach to the thumb identified the FPL with an identical appearing abundant tenosynovial thickening to the level of the interphalangeal joint. Multiple cultures and pathology specimen were obtained, after which a dose of intravenous (IV) cefazolin was administered.
Fig. 3.
a–d. Axial T2 images of the right wrist at the level of the distal forearm (a) carpal tunnel (b) and palm (c) as well as sagittal images of the right wrist and hand (d) revealing marked flexor tendon thickening and edema
Fig. 4.
a and b. Intraoperative photographs of the right wrist and hand revealing abundant tenosynovitis at the level of the distal forearm, carpal tunnel, and palm
The patient reported some symptomatic relief on the first postoperative day. The pathology specimen revealed non-necrotizing granulomatous inflammation in both the wrist flexor tenosynovium as well as the FPL tenosynovium at the level of the thumb (Fig. 5). Initial cultures were negative, but 2 weeks later fungal cultures from multiple surgical specimens were positive for H. capsulatum. Once cultures became positive, the patient was readmitted for IV antifungal therapy and started on IV AmBisome (liposomal amphotericin B) at a dose of 186 mg IV daily. A peripherally inserted central catheter was placed for long-term IV antifungal therapy, but an overlapping course of oral itraconazole was also initiated to establish high serum levels of antifungals with the plan to transition solely to oral antifungal therapy. While in the hospital a serum histoplasma antibody screen was obtained and returned positive confirming the diagnosis. A chest radiograph did not reveal any evidence of pulmonary involvement.
Fig. 5.
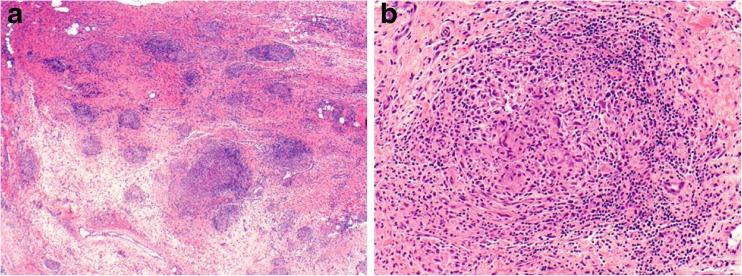
Biopsy specimen of the right wrist. Low-power view reveals a vaguely nodular process in a background of synovial and fibrovascular tissue (a). At high magnification, the nodules are comprised of clusters of multinucleated giant cells surrounded by a rim of lymphocytes, plasma cells, and histiocytes (b). These morphologic features are consistent with non-necrotizing granulomatous inflammation. Stains for microorganisms (Grocott’s methenamine silver, acid fast bacilli) were negative (not shown), and no foreign body material was appreciated under polarized light. Magnification ×40 (a), ×200 (b). Courtesy of Dr. Anja C. Roden, Mayo Clinic Rochester, MN
At 1 month postoperatively, the patient’s pain, swelling, and stiffness continued to improve, and her numbness was nearly completely resolved. At 6 months postoperatively, the patient was asymptomatic with a well-healed surgical wound and no swelling (Fig. 6). At 1 year postoperatively, the patient remained asymptomatic with no signs of recurrence. She rated her pain as a 0 out of 10. Physical examination revealed a grip strength of 24 kg bilaterally and an opposition pinch of 3.2 kg on the right compared to 4.1 kg on the unaffected left side. Range of motion of the wrist revealed wrist flexion and extension of 65 and 60°, respectively, on the affected side compared to 60 and 75° on the unaffected side. There were no sensory deficits and no provocative signs of median nerve irritation, and two-point discrimination testing was normal. Her course of oral itraconazole was completed at 1 year.
Fig. 6.
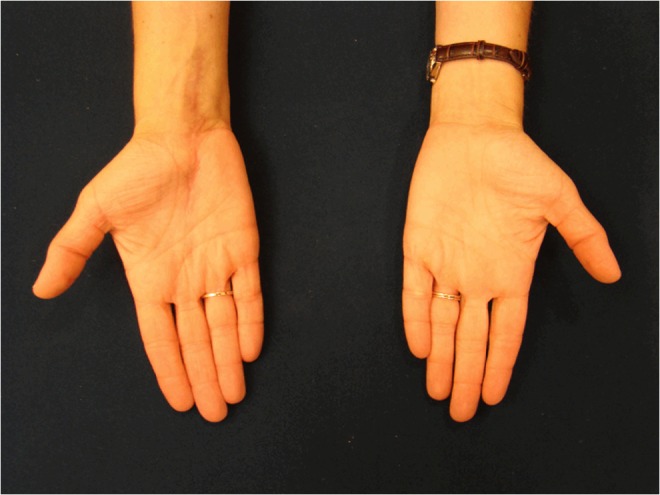
Clinical photograph of the appearance of the affected right hand at 6 months postoperatively compared to the unaffected left hand
Discussion
Disseminated histoplasmosis is rare, and musculoskeletal involvement is even more uncommon although it has been reported. Musculoskeletal manifestations of primary acute histoplasmosis have been previously described during a notable epidemic in Indianapolis in 1978 to include arthritis or arthralgias of the knees, ankles, wrists, or small joints of the hand in 6 % of reported cases [20]. Most cases were mild and resolved either without treatment or with nonsteroidal anti-inflammatory agents. No cases of tenosynovitis were reported during this outbreak. Another 15-year review of 111 patients with systemic histoplasmosis revealed no cases with joint, bone, or tenosynovial involvement [2]. Other authors have described single case reports of bone [1, 10–12, 15, 22, 24] or joint [5, 7, 9, 13, 20, 23, 27, 30, 31] involvement throughout the extremities, including unusual cases of joint [20, 25] or bone [12, 15] involvement of the fingers or wrist, but all of these cases occurred in the absence of tenosynovitis.
A literature review of cases of histoplasmosis presenting as tenosynovitis was performed using Pubmed (January 1946 to August 2013) revealing 11 isolated prior cases reported in the literature starting in 1963 [3, 4, 6, 8, 14, 16–19, 21, 25, 26]. Of these 11 cases, only 3 had evidence of disseminated disease [18, 19, 25]. In the other 8 cases, focal flexor or extensor tenosynovitis at the wrist or fingers, occasionally with adjacent carpal joint or carpal bone involvement, was the only site of involvement. While prior cases of primary extrapulmonary infection have been reported, they are rare and difficult to prove and have been attributed to histoplasmosis definitely in only a few cases of accidental inoculation of fungus in laboratory workers or health care providers [28, 29]. In the current case, we postulated that the patient may have had exposure through skin inoculation in the affected thumb or hand given the lack of pulmonary involvement, although this is impossible to prove.
Sites of involvement reported in the literature most commonly included the flexor tenosynovium at the level of the wrist in 8 cases [3, 6, 14, 16, 17, 19, 25, 26], digital flexor tenosynovitis in 2 cases [4, 18], and the extensor tenosynovium at the level of the wrist in two cases [3, 21]. One case initially involved only the flexor tenosynovium at the level of the carpal tunnel but 1 year later recurred involving the second through fourth extensor tendon compartments [3]. As far as we are aware, the current case is the only report of tenosynovitis caused by histoplasmosis extending into and including the FPL tenosynovium all the way into the thumb, although one prior case reported involvement of the FPL at the level of the carpal tunnel upon a second recurrence [17].
Fungal and mycobacterial infections are rare causes of median nerve compression produced by a mass effect within the carpal tunnel. Of the 11 previously reported cases of tenosynovitis caused by histoplasmosis, 8 of these caused an acute CTS [3, 6, 14, 16, 17, 19, 25, 26]. In most of these cases prior to the identification of a diagnosis of histoplasmosis, an initial corticosteroid injection produced transient relief but symptoms recurred as in our patient [4, 6, 17, 19, 26]. All 8 of these cases with carpal tunnel symptoms were treated with a carpal tunnel release, and median nerve symptoms resolved promptly in all instances, as in the current case.
H. capsulatum has been described as an opportunistic infection more commonly affecting immunocompromised hosts [5, 9, 13, 20, 25, 26]. Of the reported literature on tenosynovitis caused by histoplasmosis, only 4 of 11 cases had known immunocompromised states. Randall and colleagues in 1982 reported on a case of a 43-year-old female with breast cancer on fluorouracil, cyclophosphamide, and methotrexate with an acute CTS and flexor tenosynovitis of the wrist caused by H. capsulatum [19]. Pfaller et al. in 1985 reported on a 42-year-old female with systemic lupus erythematosus on oral prednisone and azathioprine who developed a flexor tenosynovitis of the middle finger and later disseminated disease attributed to histoplasmosis [18]. Schasfoort et al. in 1999 reported on a case of a 71-year-old male on chronic prednisone therapy for the treatment of emphysema who developed extensor tenosynovitis of the right hand, including the extensor digitorum communis, extensor carpi radialis, and brevis and extensor pollicis longus compartments [21]. Last, Smith and Shatford in 2005 reported on a 70-year-old male with insulin-dependent diabetes who developed flexor tenosynovitis of the wrist with a CTS caused by histoplasmosis [25]. In the current case, the patient did have an immunocompromised status having Sjogren’s syndrome with interstitial lung disease treated with Cell Cept.
Combined surgical tenosynovectomy and antifungal therapy appear to yield the best chance of clinical resolution in the treatment of histoplasmosis. Oral antifungal agents such as itraconazole and fluconazole have been used with success, with current recommendations for 200 mg of itraconazole once or twice daily for 6–18 months in non-immunocompromised hosts with disseminated disease with mild to moderate symptoms [32, 33]. Reviewing the previous literature revealed that of the 11 cases of Histoplasma-induced tenosynovitis, there were 3 recurrences—two of which had surgical debridements alone without any antifungal therapy initially [17, 21] and the third being treated only with oral ketoconazole for 4 months [3]. Interestingly, one case of isolated index finger flexor tenosynovitis resolved with oral itraconazole alone for 12 months without surgical debridement [4], perhaps suggesting that timely antifungal therapy is even more crucial than surgical debridement. In contrast to non-immunocompromised hosts with mild to moderate disease that respond to courses of PO antifungal therapy, for resistant cases or in immunocompromised hosts, treatment with IV amphotericin B is recommended followed by extended or even lifelong oral therapy [32, 33]. In the current case of a mildly immunocompromised host, a short-term course of liposomal form of IV amphotericin B was utilized in concert with a 1-year course of oral itraconazole after surgical debridement with complete resolution of symptoms apparent during a period of close surveillance, suggesting that lifelong oral therapy may not always be necessary in this setting.
In summary, the current case illustrates that the hand surgeon should consider atypical causes of CTS and tenosynovitis when the presentation is not classic, and histoplasmosis should be included in the differential in endemic areas particularly in immunocompromised hosts. Specifically, when there is significant swelling along the tendon sheaths and/or rapid development of symptoms, particularly pain, one should have a heightened suspicion of atypical causes of CTS and tenosynovitis. In such cases, the treating surgeon should consider imaging with an MRI and measurement of inflammatory markers including ESR, CRP, and a leukocyte count, ensure collection of both intraoperative cultures and pathology specimen when operative treatment is undertaken, and be prepared for an extended carpal tunnel exposure to provide adequate visualization of the soft tissues and to allow for a thorough debridement. The current case adds to the literature supporting that a strategy of aggressive surgical debridement followed by long-term oral or IV antifungal agents yields a high likelihood of complete resolution in the unusual case of tenosynovitis caused by H. capsulatum.
Acknowledgments
Author acknowledgement: M.A.V., A.C.R., and M.R.
Conflict of Interest
Mark A. Vitale declares that he has no conflict of interest.
Anja C. Roden declares that she has no conflict of interest.
Marco Rizzo declares that he has no conflict of interest.
Statement of Human and Animal Rights
Please note that this is a case report and the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Statement of Informed Consent
The authors have omitted any identifying information in this case report to the reported patient.
References
- 1.Allen JH., Jr Bone involvement with disseminated histoplasmosis. Am J Roentgenol. 1959;82:250–3. [PubMed] [Google Scholar]
- 2.Assi MA, Sandid MS, Baddour LM, Roberts GD, Walker RC. Systemic histoplasmosis: a 15-year retrospective institutional review of 111 patients. Medicine. 2007;86:162–9. doi: 10.1097/md.0b013e3180679130. [DOI] [PubMed] [Google Scholar]
- 3.Care SB, Lacey SH. Recurrent histoplasmosis of the wrist: a case report. J Hand Surg. 1998;23A:112–4. doi: 10.1016/S0363-5023(98)80025-7. [DOI] [PubMed] [Google Scholar]
- 4.Cucurull E, Sarwar H, Williams CD, IV, Espinosa LR. Localized tenosynovitis caused by Histoplasma capsulatum: case report and review of the literature. Arthritis Rheum. 2005;53:129–32. doi: 10.1002/art.20918. [DOI] [PubMed] [Google Scholar]
- 5.Darouiche RO, Cadle RM, Zenon GJ, Weinert MF, Hamill RJ, Lidsky MD. Articular histoplasmosis. J Rheumatol. 1992;19:1991–3. [PubMed] [Google Scholar]
- 6.Eglseder WA. Carpal tunnel syndrome associated with histoplasmosis: a case report and literature review. Mil Med. 1992;157:557–9. [PubMed] [Google Scholar]
- 7.Fowler VG, Nacinovich FM, Alspaugh JA, Corey GR. Prosthetic joint infection due to Histoplasma capsulatum: case report and review. Clin Infect Dis. 1998;26:1017. doi: 10.1086/517643. [DOI] [PubMed] [Google Scholar]
- 8.Houtman PM, Marck KW, Hol C. Histoplasmosis of the wrist: a case report. Rheumatol. 1999;38:906–7. doi: 10.1093/rheumatology/38.9.906. [DOI] [PubMed] [Google Scholar]
- 9.Jones PG, Rolston K, Hopfer RL. Septic arthritis due to Histoplasma capsulatum in a leukaemic patient. Ann Rheum Dis. 1985;44:128–9. doi: 10.1136/ard.44.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Key JA, Large AM. Histoplasmosis of the knee. J Bone Joint Surg. 1942;24:281–90. [Google Scholar]
- 11.Klingberg WG. Generalized histoplasmosis in infants and children, review of ten cases, one with apparent recovery. J Pediatr. 1950;36:728–41. doi: 10.1016/S0022-3476(50)80227-5. [DOI] [PubMed] [Google Scholar]
- 12.Lunn HF. A case of histoplasmosis of bone in East Africa. J Trop Med Hyg. 1960;63:175–80. [PubMed] [Google Scholar]
- 13.Makol A, Wieland CN, Ytterberg SR. Articular involvement in disseminated histoplasmosis in a kidney transplant patient taking azathioprine. J Rheumatol. 2011;38:2692–3. doi: 10.3899/jrheum.110776. [DOI] [PubMed] [Google Scholar]
- 14.Mascola JR, Rickman LS. Infectious causes of carpal tunnel syndrome: case report and review. Rev Infect Dis. 1991;13:911–7. doi: 10.1093/clinids/13.5.911. [DOI] [PubMed] [Google Scholar]
- 15.McCabe MP, Heck RK. Histoplasma osteomyelitis simulating giant-cell tumor of the distal part of the radius. J Bone Joint Surg. 2010;92A:708–14. doi: 10.2106/JBJS.H.01507. [DOI] [PubMed] [Google Scholar]
- 16.Omer GE, Lockwood RS, Travis LO. Histoplasmosis involving the carpal joint. J Bone Joint Surg. 1963;45A:1699–703. [PubMed] [Google Scholar]
- 17.Perlman R, Juberlirer RA, Schwartz J. Histoplasmosis of the common palmar tendon sheath. J Bone Joint Surg. 1971;54A:676. [PubMed] [Google Scholar]
- 18.Pfaller MA, Kyriakos M, Week PM, Kobayashi GS. Disseminated histoplasmosis presenting as an acute tenosynovitis. Siagn Microbiol Infect Dis. 1985;3:251–5. doi: 10.1016/0732-8893(85)90037-9. [DOI] [PubMed] [Google Scholar]
- 19.Randall G, Smith PW, Korbitz B, Owen DR. Carpal tunnel syndrome caused by Mycobacterium fortuitum and Histoplasma capsulatum: report of two cases. J Neurosurg. 1982;56:299–301. doi: 10.3171/jns.1982.56.2.0299. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal J, Brandt KD, Wheat LJ, Slama TG. Rheumatologic manifestations of histoplasmosis in the recent Indianapolis endemic. Arthritis Rheum. 1983;26:1065–70. doi: 10.1002/art.1780260902. [DOI] [PubMed] [Google Scholar]
- 21.Schasfoort RA, Marck KW, Houtman PM. Histoplasmosis of the wrist. J Hand Surg. 1999;24B:625–7. doi: 10.1054/jhsb.1999.0273. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz E. Regional roentgen manifestations of histoplasmosis. Am J Roentgenol. 1962;87:865–74. [PubMed] [Google Scholar]
- 23.Sen D, Birns J, Rahman A. Articular presentation of disseminated histoplasmosis. Clin Rheumatol. 2007;26:L823–4. doi: 10.1007/s10067-006-0331-1. [DOI] [PubMed] [Google Scholar]
- 24.Silverman FN, Schwarz J, Lahey ME, Carson RP. Histoplasmosis. Am J Med. 1955;19:410–59. doi: 10.1016/0002-9343(55)90129-7. [DOI] [PubMed] [Google Scholar]
- 25.Smith GD, Shatford RAD. Histoplasmosis infection presenting as an isolated subcutaneous periarticular upper limb swelling in the immunocompromised patient. J Hand Surg. 2005;30B:229–232. [DOI] [PubMed]
- 26.Strayer DS, Gutwein MB, Herbold D, Bresalier R. Histoplasmosis presenting as the carpal tunnel syndrome. Am J Surg. 1981;141:286–8. doi: 10.1016/0002-9610(81)90177-X. [DOI] [PubMed] [Google Scholar]
- 27.Sutton DA, Paghye AA, Standard PG, Rinaldi MG. An aberrant variant of Histoplasma capsulatum var. capsulatum. J Clin Microbiol. 1997;35:734–5. doi: 10.1128/jcm.35.3.734-735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesh RB, Schneidau JD., Jr Primary cutaneous histoplasmosis. N Engl J Med. 1966;275:597–9. doi: 10.1056/NEJM196609152751107. [DOI] [PubMed] [Google Scholar]
- 29.Tosh FE, Balhuizen J, Yates JL, Brasher CA. Primary cutaneous histoplasmosis: report of a case. Arch Intern Med. 1964;114:118–9. doi: 10.1001/archinte.1964.03860070164022. [DOI] [PubMed] [Google Scholar]
- 30.Van der Schee AC, Dinkla BA, Festen JJ. Gonarthritis as only manifestation of chronic disseminated histoplasmosis. Clin Rheumatol. 1990;9:92–4. doi: 10.1007/BF02030251. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg JM, Ali R, Badve S, Pelker RR. Musculoskeletal histoplasmosis: case report and review of the literature. J Bone Joint Surg. 2001;81A:1718–22. [PubMed] [Google Scholar]
- 32.Wheat J. Histoplasmosis: experience during outbreaks in Indianapolis and review of the literature. Medicine. 1997;76:339–54. doi: 10.1097/00005792-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Yates JL, Langeluttig HV, Brasher CA. Medical management of histoplasmosis. In: Sweeny HC, editor. Histoplasmosis. Illinois: Charles C. Thomas; 1960. pp. 459–71. [Google Scholar]