Abstract
Background
Peripheral nerve transfers are being used to improve upper extremity function in cervical spinal cord injury (SCI) patients. The purpose of this study was to evaluate feasibility and perioperative complications following these procedures.
Methods
Eligible SCI patients with upper extremity dysfunction were assessed and followed for a minimum of 3 months after surgery. Data regarding demographics, medical history, physical examination, electrodiagnostic testing, intraoperative nerve stimulation, recipient nerve histomorphometry, surgical procedure, and complications were collected.
Results
Seven patients had surgery on eight limbs, mean age of 28 ± 9.9 years and mean time from SCI injury of 5.1 ± 5.2 years. All patients had volitional elbow flexion and no volitional hand function. The nerve to the brachialis muscle was used as the expendable donor, and the recipients included the anterior interosseous nerve (AIN) (for volitional prehension), nerve branches to the flexor carpi radialis, and flexor digitorum superficialis. Two patients underwent additional nerve transfers: (1) supinator to extensor carpi ulnaris or (2) deltoid to triceps. No patients had any loss of baseline upper extremity function, seven of eight AIN nerve specimens had preserved micro-architecture, and all intraoperative stimulation of recipient neuromuscular units was successful further supporting feasibility. Four patients had perioperative complications; all resolved or improved (paresthesias).
Conclusion
Nerve transfers can be used to reestablish volitional control of hand function in SCI. This surgery does not downgrade existing function, uses expendable donor nerve, and has no postoperative immobilization, which might make it a more viable option than traditional tendon transfer and other procedures.
Keywords: Spinal cord injury, Tetraplegia, Nerve transfer, Peripheral nerve, Surgery
Introduction
Nerve transfers have transformed the management of peripheral nerve injury (PNI) and have allowed remarkable restoration of motor function [9, 10, 15, 33]. This innovative surgery has been applied to patients with cervical spinal cord injury (SCI) [5–8, 16, 19, 23]. Nerve transfer in SCI comprises the following: An expendable donor nerve, over which the patient has volitional control (above the level of SCI), is coapted to an intact but nonfunctional recipient nerve below the level of SCI to restore volitional control to these muscles. Nerve transfers have several advantages over traditional tendon transfer surgery including no prolonged postoperative immobilization or weight-bearing limitations and less biomechanical limitations.
There are case reports of nerve transfers in SCI patients to restore elbow extension, wrist extension, and pinch [6–8, 16, 23]. While the preliminary functional results in these reports are encouraging, it is vital to establish the safety of nerve transfers in this new population. Patients with SCI present unique challenges to the upper extremity surgeon [2, 12, 22, 34]. Spasticity and joint contractures make examination difficult. Furthermore, it can be difficult to discern whether muscles are nonfunctional due to lack of cortical control (upper motor neuron injury) or due to denervation secondary to direct lower motor neuron or concomitant PNI [12, 34]. Many patients with SCI demonstrate asymmetric, incomplete patterns of injury, which can make it difficult to discern the zone of injury to the spinal cord [12]. Autonomic lability, chronic wounds, and recurrent infections also render these patients at higher risk for perioperative complications [2, 22].
In prior reports of treatment of lower brachial plexus injury, a commonly used donor for nerve transfer procedures is the nerve to brachialis because elbow flexion can be provided by the biceps muscle, with or without the contribution of brachioradialis [29, 33]. Similarly, both biceps and brachialis function are usually present in a C6-7 level SCI. Although there is evidence from the brachial plexus literature that biceps reinnervation alone can provide functional elbow flexion [11, 27, 32], the converse functional impact of losing brachialis function in the tetraplegia population following a nerve transfer procedure has not been carefully examined. With the unique challenges and limitations of the SCI population, it is imperative that we ascertain whether sacrificing brachialis results in any objective or subjective loss of function.
The morbidity associated with nerve transfer surgery requires careful evaluation before widely applying this surgical option to this unique and complex patient population. The primary purpose of this study was to prospectively review postoperative complications and donor morbidity in our initial case series of nerve transfers for SCI. A secondary objective was to further confirm the feasibility of performing nerve transfers even years post-SCI by histomorphometric analysis of recipient nerve specimens. We hypothesized that the novel of use of peripheral nerve transfers in this setting would have a low frequency of perioperative complications and that the recipient nerve architecture would be preserved and available for nerve transfer thus providing the opportunity to restore hand function even years after initial SCI.
Materials and Methods
A prospective cohort study was performed of all patients with cervical SCI presenting for consideration of surgery to restore upper extremity function between March 2012 and March 2013. Research ethics approval was obtained from the local institutional review committee, and written consent was obtained from all participants. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 as revised in 2008. Informed consent was obtained from all patients for being included in the study (of note, one patient was <18 years of age and therefore did not meet study inclusion criteria; that patient and parent provided media consent to allow for sharing of clinical information).
Baseline data were collected on all referred patients, including demographics, prior hand dominance, mechanism and level of SCI, comorbidities, prior surgery, functional evaluation (including manual muscle testing and range of motion measures), and electrodiagnostic testing. Appropriate patients were subsequently offered surgery to improve upper extremity function. Both traditional tendon transfers/tenodesis and nerve transfer procedures were discussed. In this cohort, no patients wanted to proceed with the tendon procedures either alone or in combination with a nerve transfer.
For those patients undergoing a nerve transfer procedure, the following variables were collected: operative procedure(s), operative time, length of hospitalization, postoperative complications, including the need for readmission and reoperation, and donor morbidity related to the nerve transfer. Postoperative evaluations were performed at a minimum of 2, 4, and 12 weeks postoperatively.
Nerve transfer procedures were selected based on available donor nerves and preoperative functional and electrodiagnostic assessment of the upper extremity. At a minimum, all patients required intact volitional elbow flexion (Medical Research Council (MRC) ≥4) so that the brachialis nerve could be utilized as a donor nerve. Prioritization of functional restoration of the hand and wrist was similar to that described for tendon transfer reconstruction in tetraplegia according to the International Classification for Surgery of the Hand in Tetraplegia (ICSHT) [24, 25] with some variation. When no wrist extension was present, the extensor carpi radialis or extensor carpi ulnaris nerves were targeted as recipients. When wrist extension was present, the anterior interosseous nerve (AIN) was utilized as a recipient nerve for restoration of pinch. The surgical technique for the brachialis-to-AIN transfer has been previously described [23].
Nerve tissue was collected intraoperatively for all patients who underwent nerve transfer. The recipient AIN was sectioned, prepared, and analyzed using previously described histomorphometric techniques [18].
Patients were observed overnight and then discharged home with instructions to avoid full weight bearing on the operative extremity until edema and pain had resolved (usually 2 weeks postoperatively). Feeding, grooming, pressure-relieving, and other light activities including elbow flexion were permitted immediately postoperatively. Patients were instructed to use their electric wheelchair and to use a slider board or other assistance for transfers for 2 to 4 weeks postsurgery. No splints, casts, or special dressings were required as all nerve transfer coaptations were performed without tension. A simple airstrip dressing (to avoid circumferential wraps and the risk of propagating episodes of autonomic dysreflexia) was removed on postoperative day 2, the incision was left open to air, and normal bathing was resumed at that time.
All data were inputted into a prospectively maintained SCI database. Descriptive statistics (means, standard deviations, frequencies) were performed for all variables. The main outcomes were the proportion of patients with a postoperative complication and the proportion of patients with functional loss related to the nerve transfer. The nature and severity of complications were analyzed descriptively. Secondary intraoperative nerve stimulation (presence or absence of distal muscle function) and histomorphometry data were also collected.
Results
Seven SCI patients underwent surgical treatment (six males, mean age of 28.2 ± 9.9 years and a mean time from SCI of 5.4 ± 5.4 years) (Table 1). All patients had documented cervical level SCI, and all patients had experienced comorbidities including urinary or pulmonary infections and pressure sores. Patients were noted to have varying degrees of spasticity that was being managed with medication, botulinum toxin type A injection, or baclofen bump. None had substantial contractures or previous surgery on the operative side.
Table 1.
Patient information
| Patient #/side | Sex | Age at surgery (years) | SCI levela | Time since SCI (years) | ICHST group | Nerve transfer(s) done | Complications | Donor morbidity |
|---|---|---|---|---|---|---|---|---|
| 1-Left | M | 22 | C6 ASIA-A |
0.9 | 4 | Brachialis to AIN Brachialis to FCR/FDS Supinator to PIN (2nd surgery) |
None | None |
| 1-Right | M | 22 | C6 ASIA-A |
0.9 | 4 | Brachialis to AIN Brachialis to FCR/FDS |
Minor—hypesthesia thumb | None |
| 2-Right | M | 31 | C4 ASIA-C |
10.4 | 3 | Brachialis to AIN Brachialis to FCR |
None | None |
| 3-Left | F | 15 | C4 ASIA-A |
3.5 | 0 | Exploration No transfer done |
Insufficient donors available | n/a |
| 4-Left | M | 47 | C6 ASIA-A |
0.6 | 3 | Brachialis to AIN/FCR Deltoid to triceps |
Major Systemic—urosepsis (1 week postoperatively) Minor—paresthesia thumb |
None |
| 5-Right | M | 22 | C5 ASIA-B |
1.7 | 1 | Brachialis to AIN Supinator to ECU |
Minor—seroma (drained in office) | None |
| 6-Right | M | 28 | C5 ASIA-A |
12.4 | 1 | Brachialis to AIN Brachialis to FCR |
Major Systemic—prolonged stay due to concern for urinary tract infection Minor—paresthesia thumb |
None |
| 7-Right | M | 34 | C6 ASIA-B |
12.6 | 4 | Brachialis to AIN/FDS | None | None |
Patient age at time of surgery, level of spinal cord injury (SCI), surgery performed, and complication information is reported.
ICSHT International Classification for Surgery of the Hand in Tetraplegia
aThis is the level given at the time of initial SCI
Thirteen nerve transfers were performed in these seven patients. All patients had biceps and brachialis function (MRC grade ≥4), and six of eight extremities had brachioradialis function. No patients demonstrated volitional independent hand function preoperatively; however, six of the eight extremities had some use of the hand via wrist extension and the tenodesis effect.
In one patient where a brachialis to extensor carpi radialis longus (ECRL) nerve transfer was planned, the procedure was abandoned at the time of surgery due to insufficient donor nerve. Both the biceps and brachialis nerves were stimulated intraoperatively, and the biceps muscle was found to be atrophied and did not provide antigravity elbow flexion, while the brachialis did. To avoid downgrading elbow flexion strength, no nerve transfer was completed.
The remaining patients all underwent a brachialis to AIN transfer for restoration of pinch; in two of these, some recipient fascicles to flexor carpi radialis (FCR) and flexor digitorum superficialis (FDS) were also incorporated. Four patients underwent a separate transfer from brachialis to FCR/FDS, one patient had supinator to extensor carpi ulnaris (ECU) transfer, and one patient had deltoid to triceps transfer. In some cases, incorporation of FCR nerve recipient fascicles was unavoidable from a technical standpoint. A representative schematic of the nerve transfers is shown in Fig. 1.
Fig. 1.
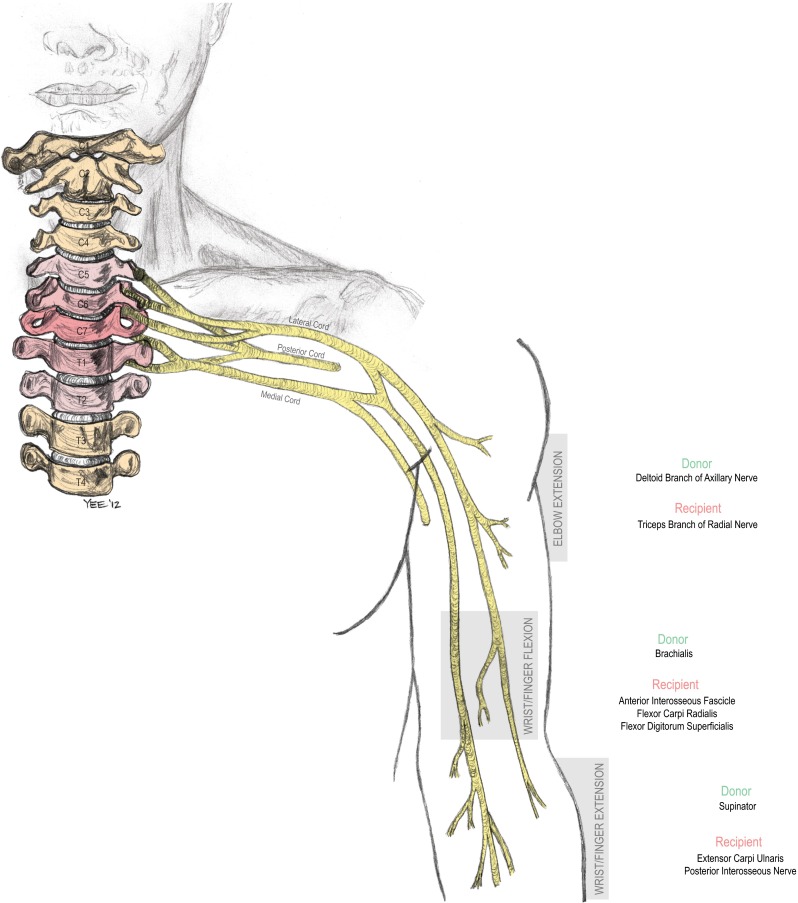
Illustrative diagram showing the use of the combinations of nerve transfers completed in these patients with cervical spinal cord injury and include brachialis to anterior interosseous nerve transfer to restore volitional control over prehension, nerve transfers to restore elbow extension (posterior head of deltoid to triceps), and improve wrist extension (supinator to extensor carpi ulnaris). Additional brachialis nerve fibers were also coapted to restore wrist flexion (to flexor carpi radialis) or finger flexion (to flexor digitorum superficialis) in some cases (©2012, nervesurgery.wustl.edu, Washington University School of Medicine)
Mean operative time was 231 ± 95 min, and length of hospital stay was 1.3 ± 0.5 days. Perioperative complications are listed in Table 1. The most common complication was thumb paresthesias; most resolved but one patient continues to have mild symptoms. The patient with the seroma required in-office percutaneous drainage. The patient readmitted for urosepsis was stabilized and treated and has returned to work and other normal activities.
No patients lost baseline function, with MRC grade elbow flexion strength at 2–4 weeks postoperative equivalent to preoperative. One patient reported subjective weakness on the operative versus nonoperative side with biceps curl weight lifting but had not tried this previously; it is unclear whether this asymmetry is postsurgical or preexisting. The patient notes no change in other activities of daily living due to this. Another patient who underwent a posterior deltoid-to-triceps transfer had postoperative weakness of the deltoid (MRC grade 4) that returned to baseline (MRC grade 5) at later follow-up.
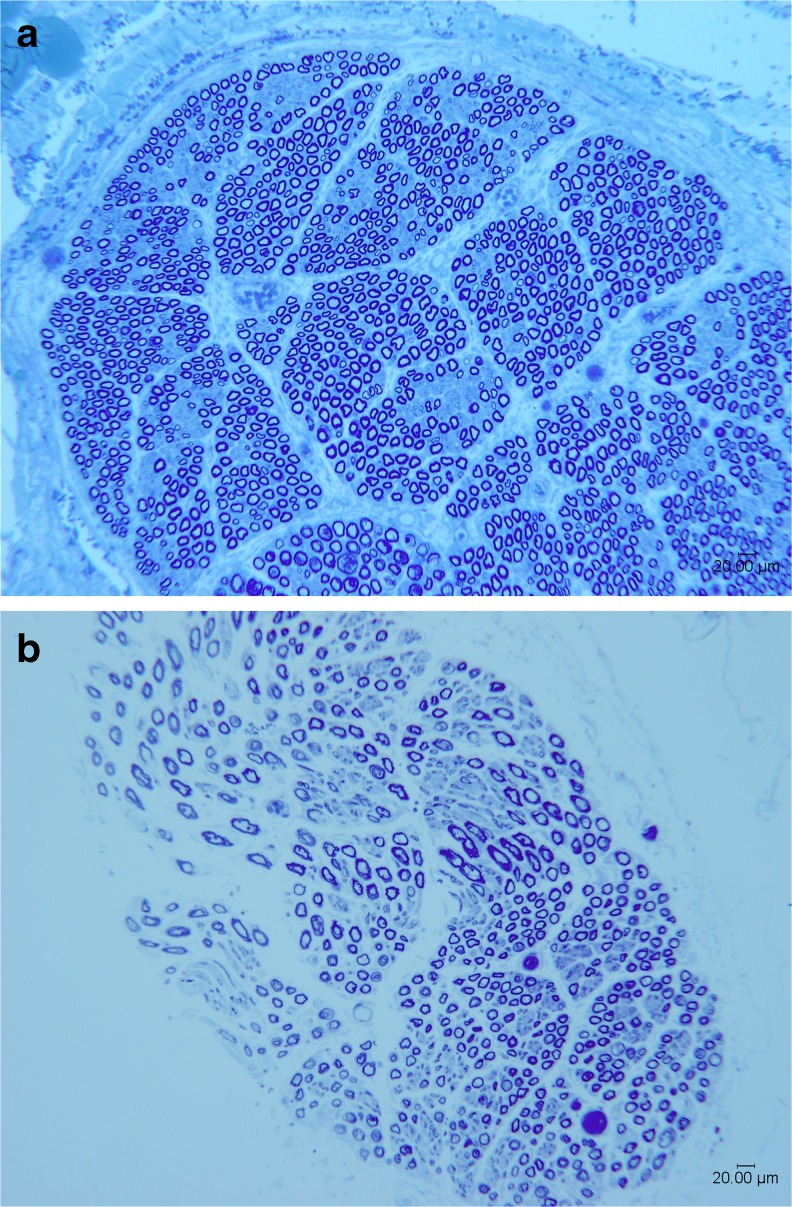
Histomorphometric analysis of the recipient AIN further supports feasibility of this transfer (Fig. 2 and Table 2). In addition, intraoperative nerve stimulation of all patients who were greater than 1 year postinjury demonstrated excellent function of the AIN innervated musculature. Specifically, intraoperative stimulation using a handheld disposable nerve stimulator (Vari-Stim, Medtronic, Jacksonville, FL) of the putative recipient nerve fascicle was performed prior to transection. As suggested by the perioperative electrodiagnostic testing, recipient neuromuscular units were intact such that stimulation at 2 mA provided antigravity flexor pollicis longus and flexor digitorum profundus muscle function.
Fig. 2.
Representative toluidine blue-stained histologic sections from the recipient anterior interosseus nerve section for patient 2 (a) and patient 4 (b), ×100, scale bar is 100 μm. a shows images from patient 2 who is 12 years post-SCI. Note the high number of mature fibers with intact myelin sheaths and preserved architecture indicating preserved lower motor neurons below the level of the SCI. b by comparison shows reduced fiber density, distorted architecture, and heterogeneity of fibers consistent with known partial lower motor neuron involvement in this patient (who underwent surgery to restore motor function within 8 months of the original SCI)
Table 2.
Histomorphometry
| Patient #/side | 1-Left | 1-Right | 2-Right | 4-Left | 5-Right | 6-Right | 7-Right |
|---|---|---|---|---|---|---|---|
| Fiber width (μm) | 5.63 | 5.45 | 5.69 | 7.32 | 7.95 | 6.09 | 5.85 |
| Fiber area (μm2) | 44.27 | 43.31 | 43.92 | 67.46 | 75.73 | 54.92 | 47.15 |
| Fiber density/mm2 | 9,943 | 8,878 | 10,072 | 4,810 | 5,206 | 7,099 | 9,606 |
| Total area of nerve (μm2) | 1,467,738 | 2,784,680 | 2,668,090 | 640,158 | 1,361,937 | 559,089 | 3,178,361 |
| Total fibers | 14,593 | 24,723 | 26,872 | 3,079 | 7,090 | 3,969 | 30,533 |
| Percent nerve (%) | 44.01 | 38.45 | 44.24 | 32.45 | 39.42 | 38.99 | 45.30 |
Histomorphometric analysis of recipient anterior interosseus nerve tissue. Note the difference in total fibers between patient 4 (with known lower motor neuron involvement at time of surgery, which was completed within 1 year of SCI) and the remaining patients. No data are reported for patient 3; this patient did not undergo a nerve transfer procedure and no nerve tissue was collected.
One patient (patient #4 in Table 1) was less than 1 year post-SCI and had absent intraoperative stimulation in keeping with known lower motor neuron injury and the plan to both restore volitional function and reinnervate. In this patient, more extensive dissection into the forearm to confirm the AIN recipient revealed nonfibrosed normal appearing musculature in the forearm.
Clinically, some patients have early functional gains as early as 6 months postsurgery. For the deltoid to triceps and supinator to ECU transfers, there was a flicker (MRC 1) of function at 6 months. For the brachialis to AIN transfer, there was some appreciable gain in function by approximately 8–12 months postsurgery, and this is primarily due to augmented tenodesis effect use of the hand. Most gains are expected after 12 months, and these patients are still early in their course (see Table 3).
Table 3.
Preliminary results
| Patient #/side | Time to last follow-up (months) | Nerve transfer(s) done | Medical Research Council (MRC) for muscle strength | Functional subjective results |
|---|---|---|---|---|
| 1-Left | 1 | Brachialis to AIN Brachialis to FCR | Not measured | ↑ grasp strength (via phone follow-up) |
| 1-Right | Brachialis to AIN Brachialis to FCR/FDS | |||
| 2-Right 1st surgery | 18 | Brachialis to AIN Brachialis to FCR |
FCR 2-, FPL 2, FDP IF/LF 2- | ↑ use of hand for feeding, holding TV remote, cell phone ; new ability to self-catheterize |
| 2nd surgery | 4 | Supinator to PIN | N/A | See above |
| 3-Left | 3 | No transfer done (exploration for brachialis to ECRL) | N/A | No change in baseline function |
| 4-Left | 11 | Brachialis to AIN/FCR Deltoid to Triceps | FPL 2-, FDP IF/LF 2-, triceps 2+ |
Improved pinch activities, ↑ arm use for reaching out and grabbing files |
| 5-Right | 10 | Brachialis to AIN Supinator to ECU |
ECU 2- (overall wrist extension including ECR function went from 2- to 3-), FPL, FDP IF 1+ | ↑ wrist stability when reaching hand into chip snack bag, better tenodesis grip |
| 6-Right | 9 | Brachialis to AIN Brachialis to FCR |
FDP IF 1 | No appreciable subjective changes reported |
| 7-Right | 11 | Brachialis to AIN/FDS | FPL 2-, FDP IF 2-, FDS 2- |
↑ grasp strength, able to self-catheterize without use of clip assist device |
FCR flexor carpi radialis, FDS flexor digitorum superficialis, AIN anterior interosseous nerve, N/A not available, ECRL extensor carpi radialis longus, ECU extensor carpi ulnaris, AIN anterior interosseous nerve
Discussion
Restoration of hand function in people with cervical SCI is critical to independence and improved health-related quality of life [1, 3, 4, 26, 31, 35]. Nerve transfer surgery improves function in patients with PNI [15]. Extrapolation of this well-established surgical technique for those with cervical SCI is a valid approach to augment upper extremity function [6–8, 16, 23, 28, 36]. These preliminary results suggest that sacrifice of the brachialis, which is a redundant elbow flexor, does not significantly downgrade function in this uniquely vulnerable patient population.
Nerve transfers provide a means to reestablish volitional control of hand function in people with cervical SCI when performed by skilled microsurgery/nerve surgeons trained in this transfer. Complete evaluation and treatment of patients also require significant hand surgery expertise to evaluate for joint stiffness and contractures, which would interfere with subsequent restored volitional function, and properly discuss other treatment options such as tendon transfers, tenodesis, and anti-claw procedures. This surgery does not downgrade existing function. The procedure uses expendable donor nerve and has minimal perioperative downtime for patients unlike traditional tendon transfer surgery. Nerve transfers may serve as a more accepted, feasible option compared to the traditional tendon transfer and tenodesis techniques whose use is limited, particularly in the USA [13, 14, 35, 37].
With this surgery, careful patient selection, evaluation, and management are imperative; SCI patients have limited treatment options, and any downgrade of function is unacceptable. Meticulous preoperative physical and electrodiagnostic examination as well as intraoperative confirmation of both donor and recipient nerve intact neuromuscular unit function are critical. Patients should be informed preoperatively that if intraoperative stimulation is unfavorable; the transfer will not be performed as this could unacceptably downgrade function.
In one illustrative case, the patient had MRC grade 4—elbow flexion and no wrist extension or hand function. In retrospect, the electrodiagnostic studies (while not quantitative) suggested significant lower motor neuron and/or concomitant peripheral nerve injury, and subsequent intraoperative stimulation corroborated that sacrifice of one of the two (biceps and brachialis) functioning elbow flexors would be unacceptable. The nerve transfer (to restore wrist extension by transfer of the brachialis to ECRL) was not performed. This case, done early in our experience, has informed subsequent patient evaluation such that only patients with MRC grade ≥4 elbow flexion and normal electromyography of the biceps and brachialis muscles (no fibrillations and normal motor unit potentials) are considered for this nerve transfer in our center.
One theoretical risk of sacrificing the brachialis is that the biceps is no longer available for use in a tendon transfer to restore triceps function in patients with absent elbow extension [20, 21, 30]. We have carefully considered this and have discussed this with each patient. In this cohort, no patient wanted to undergo tendon transfer (due to the required postoperative immobilization) to restore elbow extension; in addition, all patients did have the posterior deltoid muscle available for the alternative tendon transfer used to restore elbow extension. One patient in this series elected to proceed with a redundant deltoid fascicle to triceps nerve transfer at the time of the brachialis transfer surgery; perioperative deltoid weakness was noted but the deltoid function has since returned to normal baseline.
We have built on decades of nerve transfer surgery experience in peripheral nerve injury treatment at our institution to carefully assemble a multidisciplinary team to appropriately evaluate and treat SCI patients. We have successfully evaluated 14 patients and have performed 8 surgeries on 7 patients with no downgrade of baseline function. Through this experience, we have maximized appropriate patient selection and surgical technique. Early results in all patients who are more than 6 months postsurgery are promising. This includes early evidence of volitional control of wrist flexion at 3 months and some thumb and index (+/−long) finger flexion at 6 months postsurgery. However, meaningful antigravity function typically takes over 12 months to occur, and these patients are all relatively early in their course.
Histomorphometric analysis of the recipient AIN corroborates feasibility of these transfers even years after initial SCI and tracks with intraoperative nerve stimulation and early return of function results.
Future work to comprehensively evaluate functional outcomes, health-related quality of life, and patient perceptions is warranted to investigate the efficacy and utility of nerve transfer in the tetraplegia patient population. As our results and others suggest, additional options for using nerve transfers to restore wrist extension, elbow extension, and other functions are promising [6–8, 16, 23]. A deliberate and cautious approach is required in this unique patient population, in first building on the established principles of treatment of the tetraplegic upper extremity [17], and avoiding any downgrade of baseline function.
The precise role of nerve transfers to improve upper extremity function in SCI is in flux at this time. Significant work to formally define the appropriate patient selection criteria, assess outcomes after reinnervation occurs, and compare results with tendon transfer and tenodesis procedures is required. Nerve transfers may be combined with tendon transfers to augment options in surgery of the tetraplegic hand. They may also be used as an alternate for patients who lack the desire or ability to comply with the perioperative splinting and nonweight bearing required of tendon transfers and may open the door to better serving this deserving patient population.
Acknowledgments
Additional work was done by the following individuals, and without their input, this research would have been impossible. Mr. AY provided preoperative and intraoperative videography and photography and created the schematic figures. Mr. DH performed the nerve specimen histomorphometry. Dr. CZ performed and interpreted the electrodiagnostic testing and was instrumental in developing the protocol for the preoperative workup of these patients.
Conflict of interest
IF and LK have received travel/accommodation reimbursement from the Paralyzed Veterans of America organization for attendance and lecture at the 2013 Annual PVA Expo and Summit. SM has received NIH funding for unrelated research.
Funding
This study has not received grant funding.
Statement of informed consent
Additional informed consent was obtained from all patients for which identifying information is included in this article in the form of a formal and signed media consent.
Statement of human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. This article does not contain any studies with animal subjects.
References
- 1.Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21(10):1355–70. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- 2.Allieu Y. General indications for functional surgery of the hand in tetraplegic patients. Hand Clin. 2002;18(3):413–21. doi: 10.1016/S0749-0712(02)00021-5. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–83. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KD, Friden J, Lieber RL. Acceptable benefits and risks associated with surgically improving arm function in individuals living with cervical spinal cord injury. Spinal Cord. 2009;47(4):334–8. doi: 10.1038/sc.2008.148. [DOI] [PubMed] [Google Scholar]
- 5.Benassy J. Transposition of the musculo-cutaneous nerve upon the median nerve. Case report. Med Serv J Can. 1966;22(7):695–7. [PubMed] [Google Scholar]
- 6.Bertelli JA, Ghizoni MF, Tacca CP. Transfer of the teres minor motor branch for triceps reinnervation in tetraplegia. J Neurosurg. 2011;114(5):1457–60. doi: 10.3171/2010.12.JNS101519. [DOI] [PubMed] [Google Scholar]
- 7.Bertelli JA, Mendes Lehm VL, Tacca CP, Winkelmann Duarte EC, Ghizoni MF, Duarte H. Transfer of the distal terminal motor branch of the extensor carpi radialis brevis to the nerve of the flexor pollicis longus: an anatomic study and clinical application in a tetraplegic patient. Neurosurgery. 2012;70(4):1011–6. doi: 10.1227/NEU.0b013e3182367642. [DOI] [PubMed] [Google Scholar]
- 8.Bertelli JA, Tacca CP, Ghizoni MF, Kechele PR, Santos MA. Transfer of supinator motor branches to the posterior interosseous nerve to reconstruct thumb and finger extension in tetraplegia: case report. J Hand Surg [Am] 2010;35(10):1647–51. doi: 10.1016/j.jhsa.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Boyd KU, Nimigan AS, Mackinnon SE. Nerve reconstruction in the hand and upper extremity. Clin Plast Surg. 2011;38(4):643–60. doi: 10.1016/j.cps.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Brown JM, Shah MN, Mackinnon SE. Distal nerve transfers: a biology-based rationale. Neurosurg Focus. 2009;26(2):E12. doi: 10.3171/FOC.2009.26.2.E12. [DOI] [PubMed] [Google Scholar]
- 11.Carlsen BT, Kircher MF, Spinner RJ, Bishop AT, Shin AY. Comparison of single versus double nerve transfers for elbow flexion after brachial plexus injury. Plast Reconstr Surg. 2011;127(1):269–76. doi: 10.1097/PRS.0b013e3181f95be7. [DOI] [PubMed] [Google Scholar]
- 12.Coulet B, Allieu Y, Chammas M. Injured metamere and functional surgery of the tetraplegic upper limb. Hand Clin. 2002;18(3):399–412. doi: 10.1016/S0749-0712(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 13.Curtin CM, Gater DR, Chung KC. Upper extremity reconstruction in the tetraplegic population, a national epidemiologic study. J Hand Surg [Am] 2005;30(1):94–9. doi: 10.1016/j.jhsa.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Curtin CM, Hayward RA, Kim HM, Gater DR, Chung KC. Physician perceptions of upper extremity reconstruction for the person with tetraplegia. J Hand Surg [Am] 2005;30(1):87–93. doi: 10.1016/j.jhsa.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Fox IK, Mackinnon SE. Adult peripheral nerve disorders: nerve entrapment, repair, transfer, and brachial plexus disorders. Plast Reconstr Surg. 2011;127(5):105e–18. doi: 10.1097/PRS.0b013e31820cf556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friden J, Gohritz A. Brachialis-to-extensor carpi radialis longus selective nerve transfer to restore wrist extension in tetraplegia: case report. J Hand Surg [Am] 2012;37(8):1606–8. doi: 10.1016/j.jhsa.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Hentz VR, Ladd AL. In: Functional reconstruction of the upper extremity in tetraplegia. Piemer CA, editor. New York: McGraw Hill; 1996. [Google Scholar]
- 18.Hunter DA, Moradzadeh A, Whitlock EL, Brenner MJ, Myckatyn TM, Wei CH, et al. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166(1):116–24. doi: 10.1016/j.jneumeth.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiwerski J. Recovery of simple hand function in tetraplegia patients following transfer of the musculo-cutaneous nerve into the median nerve. Paraplegia. 1982;20(4):242–7. doi: 10.1038/sc.1982.44. [DOI] [PubMed] [Google Scholar]
- 20.Kozin SH. Biceps-to-triceps transfer for restoration of elbow extension in tetraplegia. Tech Hand Upper Extrem Surg. 2003;7(2):43–51. doi: 10.1097/00130911-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Kozin SH, D’Addesi L, Chafetz RS, Ashworth S, Mulcahey MJ. Biceps-to-triceps transfer for elbow extension in persons with tetraplegia. J Hand Surg [Am] 2010;35(6):968–75. doi: 10.1016/j.jhsa.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Landi A, Mulcahey MJ, Caserta G, Della Rosa N. Tetraplegia: update on assessment. Hand Clin. 2002;18(3):377–89. doi: 10.1016/S0749-0712(02)00024-0. [DOI] [PubMed] [Google Scholar]
- 23.Mackinnon SE, Yee A, Ray WZ. Nerve transfers for the restoration of hand function after spinal cord injury. J Neurosurg. 2012;117(1):176–85. doi: 10.3171/2012.3.JNS12328. [DOI] [PubMed] [Google Scholar]
- 24.McDowell CL, Moberg E, House JH. The second international conference on upper limb rehabilitation of the upper limb in tetraplegia. J Hand Surg [Am] 1986;11:604–8. doi: 10.1016/S0363-5023(86)80213-1. [DOI] [PubMed] [Google Scholar]
- 25.McDowell CL, Moberg EA, Smith AG. International conference on surgical rehabilitation of the upper limb in tetraplegia. J Hand Surg [Am] 1979;4(4):387–90. doi: 10.1016/S0363-5023(79)80083-0. [DOI] [PubMed] [Google Scholar]
- 26.Meiners T, Abel R, Lindel K, Mesecke U. Improvements in activities of daily living following functional hand surgery for treatment of lesions to the cervical spinal cord: self-assessment by patients. Spinal Cord. 2002;40(11):574–80. doi: 10.1038/sj.sc.3101384. [DOI] [PubMed] [Google Scholar]
- 27.Oberlin C, Ameur NE, Teboul F, Beaulieu JY, Vacher C. Restoration of elbow flexion in brachial plexus injury by transfer of ulnar nerve fascicles to the nerve to the biceps muscle. Tech Hand Upper Extrem Surg. 2002;6(2):86–90. doi: 10.1097/00130911-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Oppenheim JS, Spitzer DE, Winfree CJ. Spinal cord bypass surgery using peripheral nerve transfers: review of translational studies and a case report on its use following complete spinal cord injury in a human. Experimental article. Neurosurg Focus. 2009;26(2):E6. doi: 10.3171/FOC.2009.26.2.E6. [DOI] [PubMed] [Google Scholar]
- 29.Ray WZ, Yarbrough CK, Yee A, Mackinnon SE. Clinical outcomes following brachialis to anterior interosseous nerve transfers. J Neurosurg. 2012;117(3):604–9. doi: 10.3171/2012.6.JNS111332. [DOI] [PubMed] [Google Scholar]
- 30.Revol M, Briand E, Servant JM. Biceps-to-triceps transfer in tetraplegia. The medial route. J Hand Surg (Br) 1999;24(2):235–7. doi: 10.1054/jhsb.1998.0184. [DOI] [PubMed] [Google Scholar]
- 31.Snoek GJ, IJzerman MJ, Hermens HJ, Maxwell D, Biering-Sorensen F. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord. 2004;42(9):526–32. doi: 10.1038/sj.sc.3101638. [DOI] [PubMed] [Google Scholar]
- 32.Teboul F, Kakkar R, Ameur N, Beaulieu JY, Oberlin C. Transfer of fascicles from the ulnar nerve to the nerve to the biceps in the treatment of upper brachial plexus palsy. J Bone Joint Surg Am. 2004;86-A(7):1485–90. doi: 10.2106/00004623-200407000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Tung TH, Mackinnon SE. Nerve transfers: indications, techniques, and outcomes. J Hand Surg [Am] 2010;35(2):332–41. doi: 10.1016/j.jhsa.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Van Heest A. Tetraplegia. In: Wolfe SW, HOtchkiss RN, Pederson WC, Kozin SH, editors. Green’s operative hand surgery. 5. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 1271–95. [Google Scholar]
- 35.Wagner JP, Curtin CM, Gater DR, Chung KC. Perceptions of people with tetraplegia regarding surgery to improve upper-extremity function. J Hand Surg [Am] 2007;32(4):483–90. doi: 10.1016/j.jhsa.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Wang Y, Johnston L. Restoration of function in complete spinal cord injury using peripheral nerve rerouting: a summary of procedures. Surg Technol Int. 2008;17:287–91. [PubMed] [Google Scholar]
- 37.Zlotolow DA. The role of the upper extremity surgeon in the management of tetraplegia. J Hand Surg [Am] 2011;36(5):929–35. doi: 10.1016/j.jhsa.2011.03.001. [DOI] [PubMed] [Google Scholar]