Abstract
Aim:
FMS-like receptor tyrosine kinase (FLT3) is expressed in some normal hematopoietic cell types and plays an important role in the pathogenesis of acute myeloid leukemia (AML). In this study, we examined the effects of triazoloacridinone C-1305, an antitumor compound, on AML cells with different FLT3 status in vitro.
Methods:
A panel of human leukemic cell lines with different FLT3 status was used, including FLT3 internal tandem duplication mutations (FLT3-ITD, MV-4-11), wild-type FLT3 (RS-4-11) and null-FLT3 (U937) cells. Cell proliferation was estimated using MTT assays, and apoptosis was studied with flow cytometry and fluorescence microscopy. FLT3 kinase activity (phosphorylation of FLT3 at Tyr591) was determined with ELISA and Western blotting. FLT3 downstream signaling proteins involving AKT, MAPK and STAT5 were examined by Western blotting. RNA silencing was used to decrease the endogenous FLT3.
Results:
The mutant FLT3-ITD cells were more sensitive to C-1305 than the wild-type FLT3 and null-FLT3 cells (the IC50 values measured at 24 h were 1.2±0.17, 2.0±09, 7.6±1.6 μmol/L, respectively). C-1305 (1–10 μmol/L) dose-dependently inhibited the kinase activity of FLT3, which was more pronounced in the mutant FLT3-ITD cells than in the wild-type FLT3 cells. Furthermore, C-1305 dose-dependently decreased the phosphorylation of STAT5 and MAPK and the inhibitory phosphorylation of Bad, and induced time- and dose-dependent apoptosis in the 3 cell lines with the null-FLT3 cells being the least susceptible to C-1305-induced apoptosis. Knockdown of FLT3 with siRNA significantly decreased C-1305-induced cytotoxicity in the mutant FLT3-ITD cells.
Conclusion:
C-1305 induces apoptosis in FLT3-ITD-expressing human leukemia cells in vitro, suggesting that mutated FLT3 kinase can be a new target for C-1305, and C-1305 may be a drug candidate for the therapeutic intervention in FLT3-associated AML.
Keywords: acute myeloid leukemia, triazoloacridinone, C-1305, FLT3 kinase, FLT3-ITD, AKT, STAT5, MAPK, Bad, apoptosis
Introduction
FMS-like receptor tyrosine kinase 3 (FLT3), a member of the class III tyrosine kinase family, is expressed in some normal hematopoietic cell types1,2 and plays an important role in the pathogenesis of acute myeloid leukemia (AML)3. FLT3 mutations are among the most common abnormalities in acute myeloid leukemia, affecting more than one third of all AML patients4. Two major classes of mutation are associated with AML, internal tandem duplications (ITD) in the juxtamembrane domain of FLT3 and point mutations within the activation loop of the FLT3 tyrosine kinase domain (TKD)5,6. Internal tandem duplications lead to the constitutive, ligand-independent activation of FLT3 kinase7, which correlates with a poor prognosis8,9. Therefore, FLT3 kinase is one of the most attractive targets for therapeutic intervention in AML. Accordingly, various FLT3 inhibitors have been tested in clinical trials, both as monotherapy and in combination with chemotherapy10, but the responses are incomplete and are often limited by acquired resistance during treatment10,11. For this reason, the identification of novel FLT3 inhibitors with higher potency is required.
5-Dimethylaminopropyloamino-8-hydroxytriazoloacridinone (C-1305) (Figure 1A) is the most potent triazoloacridinone derivative synthesized in the Department of Pharmaceutical Technology and Biochemistry at the Gdańsk University of Technology12. This drug exhibited significant cytotoxic activity in vitro towards a panel of human cell lines in the NCI screening system (Bethesda, MD, USA) and displayed high antitumor activity against several experimental tumors in mice13. During biotransformation, C-1305 is not a substrate of cytochrome P450 but is metabolized by the flavin monooxygenases FMO1 and FMO314 and by UDP-glucuronyltransferases15. Selective cellular expression of UGT1A10 increases the cytotoxic response of C-130516, and this compound up-regulates selected cytochrome P450 isoenzymes in HepG2 cells17. Studies of the molecular mechanism of action revealed that C-1305 is a topoisomerase II inhibitor and binds covalently to DNA in tumor cells18. Interestingly, in contrast to other topoisomerase II poisons, C-1305 has a strong anti-proliferative effect against cells lacking functional poly(ADP-ribose)polymerase1 (PARP-1), which is involved in DNA repair19. On the cellular level, C-1305 induces irreversible arrest in the G2/M phase of the cell cycle followed by apoptosis in human leukemia cells20. C-1305 is the close structural analogue of the anticancer compound imidazoacridinone C-131121, which reached phase II clinical trials22. Among its many unique features (for review see23), C-1311 was found to be a selective inhibitor of FLT3 kinase in a cell-free kinase assay24.
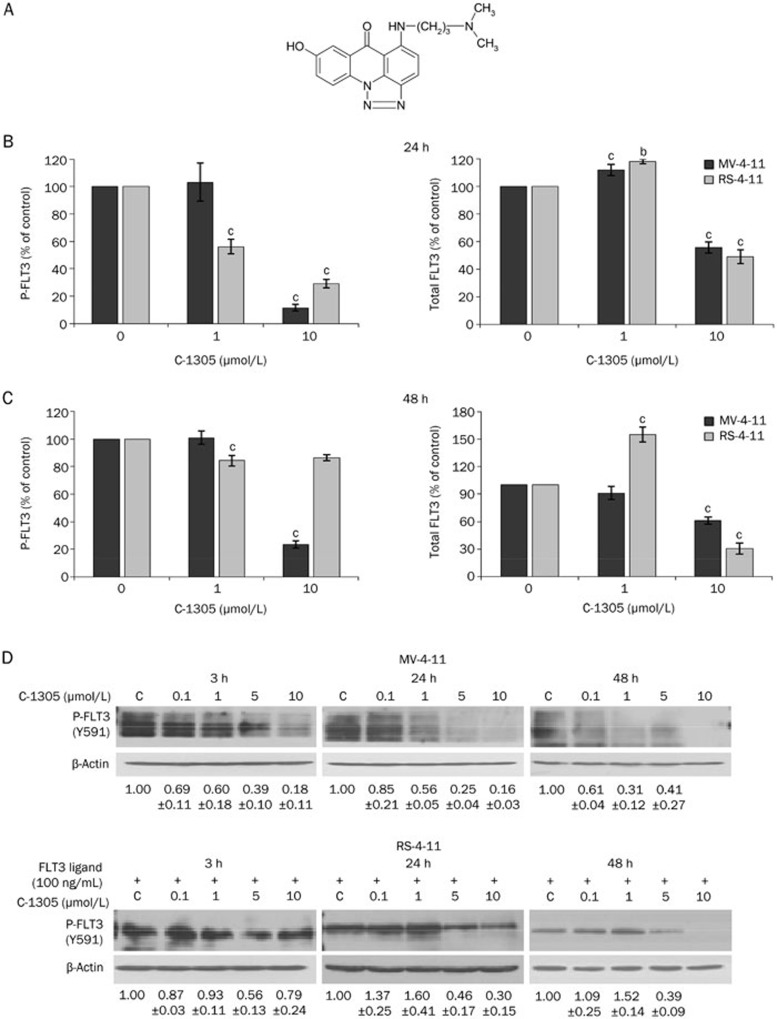
Figure 1.
C-1305 inhibits the autophosphorylation of FLT3. (A) The chemical structure of C-1305. (B) The phospho-FLT3 (Tyr591) (left panel) and total FLT3 (right panel) levels following treatment with varying concentrations of C-1305 or vehicle for 24 h and (C) for 48 h. The changes in the phosphorylated (Tyr591) and total forms of FLT3 were determined using ELISA. The levels of phospho- and total FLT3 in untreated cells were set as 100% (control), and the extent of FLT3 inhibition was expressed as the percent change from this baseline. The results are shown as the mean±SD. n=3. bP<0.05 vs untreated cells, cP<0.01 vs untreated cells. Student's t-test. (D) Inhibition of the constitutive autophosphorylation of ITD-mutated FLT3 and the ligand-dependent phosphorylation of wild-type FLT3 by C-1305. MV-4-11 cells were incubated with varying concentrations of C-1305 for 3, 24, and 48 h. RS-4-11 cells, after incubation with C-1305, were subsequently treated with 100 ng/mL FLT3 ligand for 15 min. Cell extracts were analyzed by Western blotting with antibody against FLT3 phosphorylated at Tyr591. The relative level of phospho-FLT3-ITD was determined by densitometric analysis of protein band intensities normalized to β-actin and represented relative to the level in untreated cells (c). The results are shown as the mean±SD. n=3.
In this study, we examined the cellular effects of C-1305 on human acute myeloid leukemia cells with different FLT3 status: MV-4-11 (FLT3-ITD), RS-4-11 (wild-type FLT3), and U937 (null-FLT3). Specifically, we asked whether C-1305 inhibited the FLT3 kinase activity in AML cells and if it did, whether the inhibition of FLT3 kinase activity by C-1305 had an impact on downstream FLT3 signaling pathways and apoptosis in the cells tested.
Materials and methods
Chemicals and antibodies
C-1305 was synthesized as the dihydrochloride in our Department. The compound was more than 98% pure as determined by HPLC and NMR. A stock solution of C-1305 was prepared in 50% (v/v) ethanol, stored at -20 °C, and freshly dissolved in water before use. The 5,5′,6,6′-tetra-chloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide, JC-1, was purchased from Molecular Probes (Eugene, OR, USA). All other chemicals, unless otherwise stated, were obtained from Sigma-Aldrich (St Louis, MO, USA).
Cell culture and growth inhibition assays
The MV-4-11, RS-4-11, and U937 cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells (mycoplasma-free as determined by using the Universal Mycoplasma Detection Kit, ATCC-30-1012K) were cultured in RPMI 1640 medium (Sigma-Aldrich, St Louis, MO, USA) containing 10% fetal bovine serum and antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin). Cells were maintained at 37 °C in a 5% CO2 atmosphere. All of the experiments were performed with cells in the exponential phase of growth.
To estimate cell viability, MTT assays were performed. Briefly, cells were seeded into 6-well plates (5×105 cells/well for 24 h, 2.5×105 cells /well for 48 h, 1×105 cells/well for 72 h) and were treated with increasing concentrations of C-1305. After treatment, 0.5 mg/mL MTT was added into each well, and the plates were incubated for 4 h at 37 °C. Medium was then removed by centrifugation, and the precipitated formazan crystals were dissolved in DMSO. Absorbance at 540 nm was measured with a microplate reader (iMARKTM, BIO-RAD, Hercules, CA, USA). The cytotoxic effect of C-1305 treatment was expressed as the concentration of the drug required to inhibit cell growth by 50% compared to untreated control cells (IC50 value). The IC50 value was determined by plotting survival as a function of dose.
Phospho-FLT3 and total-FLT3 ELISA
The levels of the total and phosphorylated FLT3 (Tyr591) were determined using PathScan Total FLT3 and PathScan Phospho-FLT3 (Tyr591) Sandwich ELISA Kits (Cell Signaling Technology, Danvers, MA, USA), respectively. Briefly, MV-4-11 and RS-4-11 cells were seeded at 5×106 cells per 100 mm Petri dish and treated with C-1305 for 24 and 48 h. At the end of the drug treatment, cells were lysed with the Cell Lysis Buffer (Cell Signaling Technology, Danvers, MA, USA) supplemented with a protease inhibitor cocktail (Roche, Mannheim, Germany), and phosphatase inhibitors (50 mmol/L β-glycerolophosphate, 1 mmol/L PMSF, 1 mmol/L sodium orthovanadate). After 20 min incubation on ice, lysates were centrifuged at 14 000 rpm for 15 min at 4 °C. Supernatants, containing equal amounts of total protein, were then analyzed to measure both phosphorylated-FLT3 (Tyr 591) and total FLT3 levels.
Cell cycle analysis
Analysis of DNA content was performed as described previously20. Briefly, cells (1×106) were stained with propidium iodide (PI) and analyzed by FACScan (Becton Dickinson, San Jose, CA, USA).
Morphological examination
The nuclear morphology of cells was examined with a fluorescence microscope (Olympus BX60, Tokyo, Japan) after staining with 4′,6-diamidino-2-phenylindole (DAPI). Following treatment with C-1305, cells (approximately 2×105) were spun onto microscope slides, fixed in methanol:acetic acid (3:1) for 15 min and stained with 1 μg/mL DAPI for 5 min. Slides were examined with a 40× objective and photographed using an AxioCam digital camera (Zeiss, Jena, Germany).
Annexin V/PI flow cytometry analysis
The Annexin V-FITC binding assay was carried out using an Annexin-V-Flous Staining Kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. Briefly, following treatment with C-1305, cells (1.5×106) were washed twice with ice-cold PBS, pelleted and resuspended in Annexin V-FITC binding buffer. FITC-conjugated Annexin V and PI were added at the manufacturer's recommended concentrations. After staining, cells were analyzed by flow cytometry within 1 h.
Measurement of mitochondrial membrane potential (ΔΨm)
Changes in mitochondrial membrane potential (ΔΨm) were analyzed by flow cytometry using JC-1 dye as described earlier25. Briefly, after C-1305 treatment, cells (1×106) were stained with JC-1 (10 μg/mL) for 15 min at room temperature in the dark. The loss of ΔΨm was monitored by flow cytometry based on the decrease in JC-1 red fluorescence accompanied by an increase in green fluorescence.
Caspase-3 activity
Caspase-3 activation was determined using the Active Caspase-3 Apoptosis Kit (BD Pharmingen, San Diego, CA, USA) according to the manufacturer's instruction. Briefly, cells (1×106) stained with FITC-conjugated anti-active caspase-3 antibody were analyzed by flow cytometry.
Western blot analysis
The analyses of protein expression and phosphorylation were conducted using standard protocols for whole-cell extract preparation as described previously26. Approximately 5×106 MV-4-11 and 1×107 RS-4-11 cells were exposed to varying concentrations of C-1305 for the indicated times. In the case of the RS-4-11 line, cells at the end of drug treatment were washed twice with serum-free medium and then incubated with 100 ng/mL of the FLT3 ligand (Cell Signaling Technology, Danvers, MA, USA) for 15 min at 37 °C. Cells were washed twice with ice-cold PBS and lysed with radioimmunoprecipitation assay buffer (50 mmol/L Tris-HCl (pH 7.4), 5 mmol/L EDTA, 1% Nonidet P-40, 150 mmol/L NaCl, 0.1% SDS, protease inhibitor cocktail (Roche Diagnostics), 0.5% sodium deoxycholate, 50 mmol/L NaF, 50 mmol/L β-glycerolophosphate, 1 mmol/L PMSF, 1 mmol/L sodium orthovanadate) for 20 min on ice with brief vortexing every 5 min. The lysates were then centrifuged at 14 000 rpm for 15 min at 4 °C. The same amount of protein (∼30 μg) was analyzed by SDS-polyacrylamide gel electrophoresis and then transferred onto a nitrocellulose membrane. The membrane was probed with the corresponding primary anti-phospho-FLT3 (Tyr591), anti-AKT, anti-phospho-AKT (Ser473), anti-p44/42 MAPK (Erk1/2), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), anti-Stat5, anti-phospho-Stat5 (Tyr694), anti-Bad, anti-phospho-Bad (Ser136), anti-PARP (Cell Signaling Technology), or anti-β-actin (Sigma-Aldrich, St Louis, MO, USA). The membrane was then incubated with a horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology) and visualized with the enhanced chemiluminescence kit, SuperSignal West PICO Chemiluminescent Substrate (Fisher Scientific, Pittsburgh, PA, USA). Densitometric analysis of protein bands was performed using ImageJ software (NIH, Bethesda, MD, USA).
Depletion of FLT3-ITD by RNA silencing
Transfection of cells with FLT3 small interfering RNA (siRNA) or scrambled RNAi oligonucleotide (Ambion Life Technologies, Grand Island, CA, USA) was performed using the DMIR-C reagent suitable for transfection of suspension cells (Invitrogen, Carlsbad, CA, USA). Briefly, cells were washed with serum-free RPMI medium, and then 2.5×106 cells were treated with a mixture of DMIR-C and siRNA (75 pmol). After 5 h, RPMI medium containing 15% FBS was added, and the cells were left to recover overnight. The next day, the cells were washed once with serum-free RPMI medium, suspended in fresh complete growth medium and exposed to C-1305 for 24 h. Cell viability was measured using an MTT assay as described above. The efficiency of siRNA-mediated depletion of FLT3 kinase was determined by Western blot analysis.
Statistical analysis
All of the data are reported as the mean±SD. Pairs of values were compared with Student's unpaired t-test, and the difference was considered significant if P<0.05. Analysis of variance (one-way ANOVA) was carried out using GraphPad Software (San Diego, CA, USA).
Results
C-1305 inhibits the phosphorylation of the internal tandem duplication mutant (MV-4-11) and the wild-type (RS-4-11) FLT3
To determine the ability of C-1305 to inhibit FLT3 in the cellular environment, we measured the inhibition of FLT3 autophosphorylation in the human leukemia cell line MV-4-11, which harbors a homozygous FLT3-ITD mutation, and in RS-4-11 cells, which express wild-type FLT3. Preliminary ELISA experiments (Figure 1B) showed that autophosphorylation of FLT3 was inhibited in MV-4-11 and RS-4-11 cells by C-1305 treatment for 24 h in a dose-dependent manner. Interestingly, at the lower drug concentration (1 μmol/L), this inhibitory effect was visible only in RS-4-11 cells. Simultaneously, at this concentration, there was no change in the total expression level of FLT3 protein in either cell line. At the higher dose, 10 μmol/L, FLT3 inhibition was more evident in the FLT3-ITD mutant cells than in the RS-4-11 cells. The expression level of the FLT3 protein was also decreased in both cell lines at the 10 μmol/L concentration. After a longer incubation time (48 h), C-1305 inhibited FLT3 autophosphorylation only in the mutant MV-4-11 cells, and it had a slight effect on the expression of total FLT3 in these cells (Figure 1C). In the wild-type RS-4-11 cells, the total expression level of FLT3 protein decreased significantly, but we did not observe the inhibition of FLT3 autophosphorylation (Figure 1C).
The observation that the phosphorylation of the wild-type FLT3 receptor, after the initial decrease following 24 h treatment, returned to the baseline level prompted us to assess whether such changes resulted from simple technical issues regarding the analysis of FLT3 phosphorylation or the treatment conditions. In RS-4-11 cells, phosphorylation of the wild-type FLT3 receptor takes place following the binding of the exogenous FLT3 ligand (FL)27. Thus, to achieve experimental conditions better reflecting the physiological situation, we treated RS-4-11 cells with C-1305, and the FL (100 ng/mL) was added for 15 min at 37 °C to prepare the cell lysates for Western blot analysis. Given that the MV-4-11 cells, due to the ITD mutation in the FLT3 receptor, have constitutively active, autophosphorylated FLT3 in the absence of exogenous ligand stimulation28, those cells were not exposed to FL. As shown in Figure 1D, C-1305 significantly blocked ligand-independent phosphorylation of FLT3-ITD kinase in a time- and dose-dependent manner. C-1305 inhibited the ITD-mutant protein phosphorylation even after a short 3-h exposure of MV-4-11 cells to the drug. This effect was intensified after prolonged drug treatment; there was a 50% inhibition of FLT3-ITD phosphorylation at approximately 1 μmol/L of C-1305 after a 24-h exposure. C-1305 also diminished FL-dependent phosphorylation of wild-type FLT3 kinase in RS-4-11 cells in a time- and dose-dependent manner (Figure 1D); however, higher concentrations were required for blocking the phosphorylation of wild-type FLT3 than for FLT3-ITD. Even after prolonged drug exposure (48 h), 50% inhibition of wild-type FLT3 phosphorylation was obtained between 1 and 5 μmol/L of C-1305.
C-1305 inhibits the proliferation of AML cells
To determine whether the inhibition of FLT3 contributes to cell growth inhibition, we tested the activity of C-1305 against MV-4-11 and RS-4-11 cells using MTT assays. As a cellular selectivity control, we measured the effect of C-1305 on the proliferation of U937 cells, which lack FLT3 expression. We showed that MV-4-11 cells (FLT3-ITD) were the most sensitive to C-1305 treatment among the AML cell lines tested, and this effect was visible after 24 h of drug exposure (Table 1). The RS-4-11 cells (wild-type FLT3) were less sensitive to C-1305, and the U937 cells (null-FLT3) showed the lowest sensitivity to the drug. However, following a longer incubation time (72 h), both the RS-4-11 and the U937 cell lines exhibited similar cytotoxic activity (IC50=0.3 μmol/L and 0.35 μmol/L, respectively), but they were still ∼4.5-fold less sensitive to C-1305 than the FLT3-ITD (MV-4-11) cells (IC50=0.07 μmol/L). These results suggest that blocking the mutated FLT3-ITD kinase may significantly contribute to overall C-1305 cytotoxicity.
Table 1. Cytotoxic activity of C-1305 against AML cells at different incubation time.
| Cell line | IC50 (μmol/L) |
||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| MV-4-11 | 1.2±0.17c | 0.19±0.03b,e | 0.07±0.011c,e |
| RS-4-11 | 2.0±0.9c | 0.38±0.06b | 0.30±0.074c |
| U937 | 7.6±1.6 | 1.90±0.29 | 0.35±0.11 |
Each value represented the mean±SD of three independent experiments.
bP<0.05,
cP<0.01 vs U937 cells.
eP<0.05 vs RS-4-11 cells. Student's t-test.
C-1305 inhibits the FLT3-dependent phosphorylation of AKT, MAPK, and STAT5
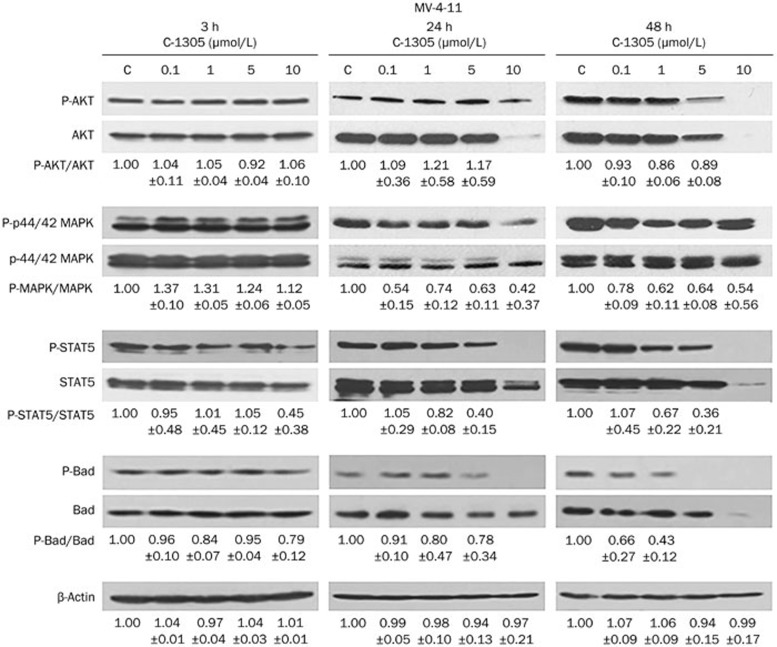
To characterize the effects of C-1305 on FLT3 inhibition, we investigated the modulation of AKT, MAPK, and STAT5, which are downstream targets of FLT3 and are key proteins in cell growth and proliferation29,30,31. In addition, we examined whether C-1305 affects Bad, a pro-apoptotic protein, which, apart from being a substrate for AKT and MAPK phosphorylation32, is also one of the principal molecules of the FLT3/ITD-mediated anti-apoptotic cell survival pathway in AML33. MV-4-11, RS-4-11, and U937 cells were treated with increasing concentrations of C-1305 for 3, 24, and 48 h, and Western blot analysis was used to detect phosphorylated and total levels of the AKT, MAPK, STAT5 and Bad proteins. To determinate whether the changes in protein phosphorylation occurred as a result of the disruption of cellular signaling or decreased protein expression or induced cell death, the ratio of phospho- to total level of the tested proteins was determined and normalized to that of untreated cells. As shown in Figure 2, short-term incubation (3 h) of MV-4-11 (FLT3-ITD) cells with C-1305 had no effect on the phosphorylation and the total expression of AKT and MAPK. In contrast, a profound reduction in the phosphorylation of STAT5 accompanied by a moderate decrease in the level of phosphorylated Bad was observed at a high concentration (10 μmol/L) of the drug. Total STAT5 and Bad protein expression was unaffected by the treatment. Prolonged incubation with C-1305 for 24 and 48 h resulted in a marked, dose-dependent decrease in the phosphorylation of AKT. However, an almost complete reduction of total AKT protein content was observed at a higher drug concentration (10 μmol/L) after 24 h exposure, suggesting that the inhibition of AKT phosphorylation resulted from a direct effect of C-1305 on the AKT level rather than from an interference with FLT3 signaling. In contrast, C-1305 decreased the phosphorylation of MAPK in a dose- and time-dependent manner but had no such effect on the overall expression of the MAPK protein in contrast to the effect on AKT. The decrease of STAT5 phosphorylation detected after 3 h of C-1305 incubation was more pronounced following 24 and 48 h of drug exposure, and the level of total STAT5 decreased only at 10 μmol/L (Figure 2). Nevertheless, a progressive decrease of the phospho-STAT5/STAT5 ratio following C-1305 treatment compared with untreated cells suggests that C-1305 initially inhibits STAT5 signaling by affecting its phosphorylation and then by down-regulating its total level. Similarly, a dose- and time-dependent inhibition of phosphorylation of Bad was observed. After 24 h exposure to C-1305, phosphorylation of Bad significantly decreased, with complete inhibition observed at a concentration of 10 μmol/L, while total Bad protein expression was largely unaffected even at a high dose. A decrease in total Bad protein was noticeable only after prolonged (48 h) exposure to 10 μmol/L of C-1305.
Figure 2.
C-1305 blocks the activation of signal transduction pathways downstream of constitutively active FLT3-ITD in MV-4-11 cells. Cells were cultured in the presence of varying concentrations of C-1305 or vehicle (c) for 3, 24, and 48 h. Cell extracts were analyzed by Western blots using antibodies against anti-AKT, anti-phospho-AKT (Ser473), anti-p44/42 MAPK (Erk1/2), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), anti-Stat5, anti-phospho-Stat5 (Tyr694), anti-Bad, and anti-phospho-Bad (Ser136). The relative levels of phospho-proteins were determined by densitometric analysis of protein band intensities normalized to the total level of the protein and represented relative to the level in untreated cells (c). Equal protein loading was demonstrated by blotting for β-actin. The results are shown as the mean±SD. n=3. Representative blots from at least three experiments are shown.
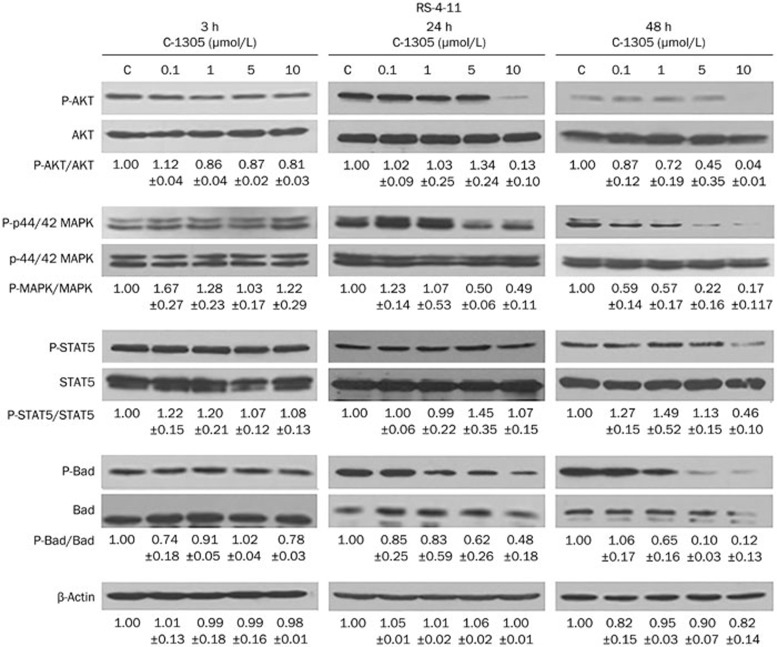
Consistent with the less potent inhibition of wild-type FLT3 kinase observed after 3-h exposure, the inhibitory effects of C-1305 on FLT3 downstream targets were attenuated in FLT3 ligand-stimulated RS-4-11 cells compared with MV-4-11 cells carrying constitutively active FLT3-ITD (Figure 3). C-1305, following 3-h administration did not significantly affect MAPK and STAT5 phosphorylation, although a slight inhibition of AKT and Bad phosphorylation at higher doses were observed. Total protein expression was unaffected by the treatment. The levels of phospho-AKT and phospho-MAPK progressively decreased following 24 and 48 h of C-1305 treatment, whereas the total level of these proteins did not significantly change. Consistent with the inhibition of AKT and MAPK phosphorylation, a time- and dose-dependent reduction of phospho-Bad was detected, although at a higher drug concentration. The total level of Bad clearly dropped following 48 h of incubation (Figure 3). Interestingly, in RS-4-11 cells, the levels of phospho- and total STAT5 remained unaffected even after extended treatment with C-1305 for 24 and 48 h. A significant decrease in STAT5 phosphorylation was noted only after 48 h exposure to 10 μmol/L C-1305.
Figure 3.
C-1305 blocks the activation of signal transduction pathways downstream of wild-type FLT3 in RS-4-11 cells. Cells were cultured in the presence of varying concentrations of C-1305 or vehicle (c) for 3, 24, and 48 h and were subsequently treated with 100 ng/mL FLT3 ligand (FL) for 15 min. Cell extracts were analyzed by Western blots using antibodies against anti-AKT, anti-phospho-AKT (Ser473), anti-p44/42 MAPK (Erk1/2), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), anti-Stat5, anti-phospho-Stat5 (Tyr694), anti-Bad, and anti-phospho-Bad (Ser136). The relative levels of the phospho-proteins were determined by densitometric analysis of protein band intensities normalized to the total level of the protein and represented relative to the level in untreated cells (c). Equal protein loading was demonstrated by blotting for β-actin. The results are shown as the mean±SD. n=3. Representative blots from at least three experiments are shown.
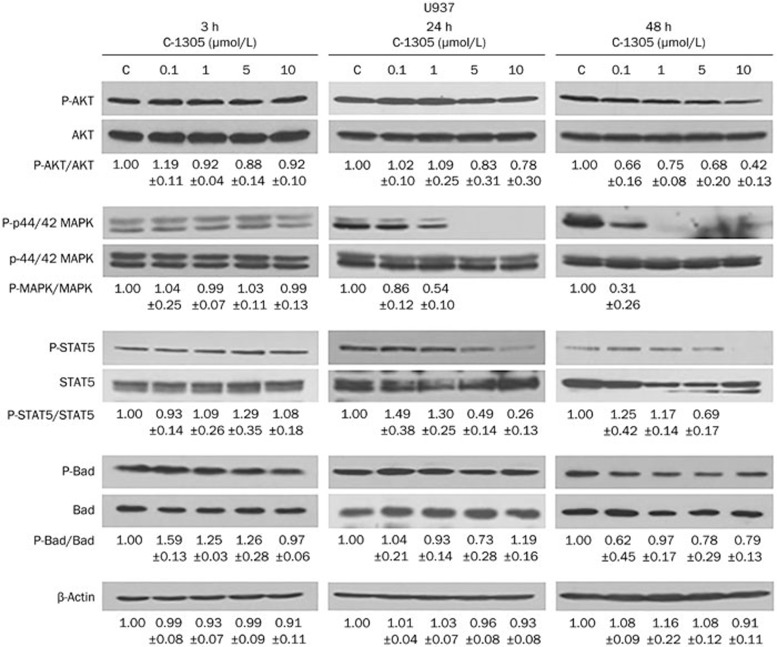
In U937 cells lacking FLT3 kinase, a short-term incubation (3 h) with increasing concentrations of C-1305 did not affect the phosphorylation and total expression of AKT, MAPK, STAT5, and Bad. Moreover, the levels of phospho-and total AKT and phospho- and total Bad remained unchanged even during longer incubation times (24 and 48 h) with C-1305. In turn, a reduction of STAT5 phosphorylation was detected following C-1305 treatment, but only at higher doses (5 and 10 μmol/L), whereas the total level of this protein remained unchanged. Interestingly, a marked, time- and dose-dependent decrease of the level of phospho-MAPK was detected, but the total MAPK level did not change.
C-1305 induces changes in the cell cycle progression of AML cells
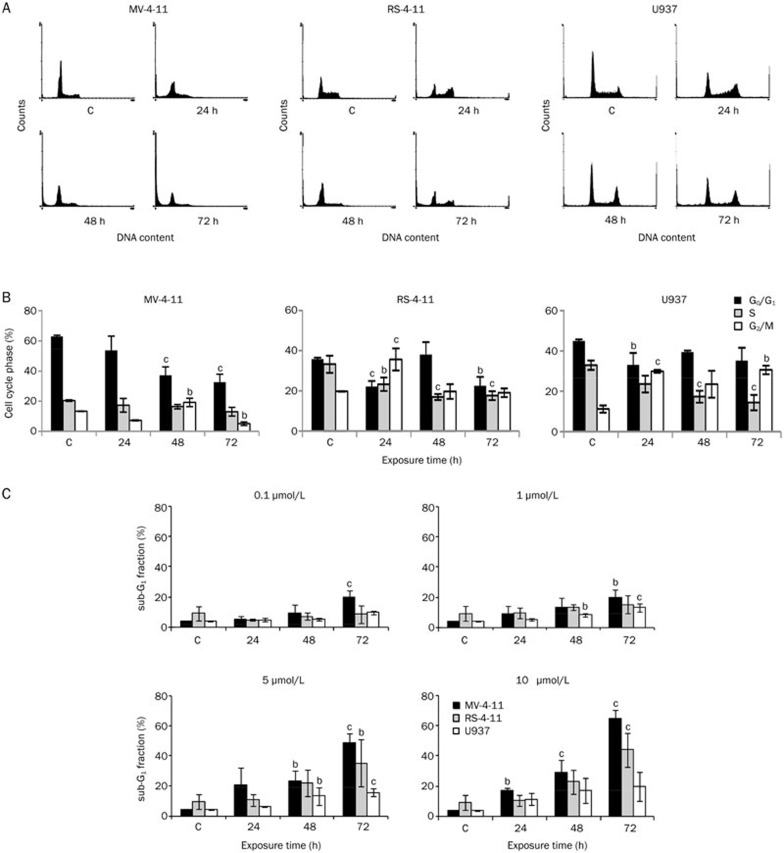
The progressive disappearance of Bad phosphorylation following C-1305 exposure indicated the induction of the apoptotic program in treated AML cells. Thus, we asked whether the inhibition by C-1305 of FLT3 kinase activity and its downstream targets (AKT, MAPK, and STAT5) had an impact on cell cycle progression and apoptosis of AML cells. As shown in Figure 5A and 5B, after C-1305 treatment at 5 μmol/L, the population of MV-4-11 cells (FLT3-ITD) in G1 phase began to decrease from ∼63% under control conditions (untreated cells) to ∼32% after 72 h. The G2/M phase population was reduced after 24 h of treatment (from ∼13% to ∼7%); however, after 48 h this population increased again (to 19%) and decreased again after the next 24 h compared to the control (to 5%). The S phase population at 5 μmol/L remained relatively unchanged, and the sub-G1 population progressively increased and reached ∼48% and ∼65% at 5 μmol/L and 10 μmol/L, respectively, after 72 h exposure (Figure 5C). Compared to FLT3-ITD cells, RS-4-11 cells (wild-type FLT3) underwent a transient G2/M arrest following 24 h of 5 μmol/L C-1305 exposure (36% compared to 19% in untreated, control cells, Figure 5A and 5B). However, after the next 24 h, some of the cells were able to enter and complete mitosis, despite the presence of the drug, and this led to an increase in the G1 phase cells (38%) after 48 h. Starting from 24 h of incubation with C-1305, a progressive accumulation of cells in the sub-G1 compartment (35% after 72 h, Figure 5C) was observed. In the U937 cells (null-FLT3) C-1305 treatment at 5 μmol/L resulted in a lower level of sub-G1 population (15% after 72 h) than was observed in the MV-4-11 and RS-4-11 cells (Figure 5C). Compared with the control, a time-dependent reduction of cells in the G1 and S phases with a concomitant increase in the G2/M phase population was observed following C-1305 exposure (from above 11% to ∼30% after 72 h) (Figure 5A and 5B).
Figure 5.
The influence of C-1305 on the cell cycle progression of AML cells. MV-4-11, RS-4-11, and U937 cells were treated with increasing concentrations of C-1305 for 24, 48, and 72 h or with the vehicle (c) and analyzed by flow cytometry. (A) Representative histograms from the treatment of cells with 5 μmol/L of C-1305 for the time indicated. Histograms are representative of three independent experiments. (B) The percentage of cells in the G0/G1, S, and G2/M phases following C-1305 treatment at 5 μmol/L for the time indicated. Values are the mean±SD. n=3. bP<0.05 vs untreated cells (c), cP<0.01 vs untreated cells (c), Student's t-test. (C) Cell populations in the sub-G1 fraction indicative of apoptosis. Values are the mean±SD. n=3. bP<0.05 vs untreated cells (c), cP<0.01 vs untreated cells (c), Student's t-test.
In summary, these data show a time- and dose-dependent increase in the sub-G1 population (<2n DNA content) following C-1305 treatment, which is indicative of apoptosis. Moreover, C-1305 induced apoptosis earlier and to a greater extent in the MV-4-11 (FLT3-ITD) cells than in the U937 cells (null-FLT3) and the RS-4-11 cells (wild-type FLT3).
C-1305 induces apoptosis in AML cells
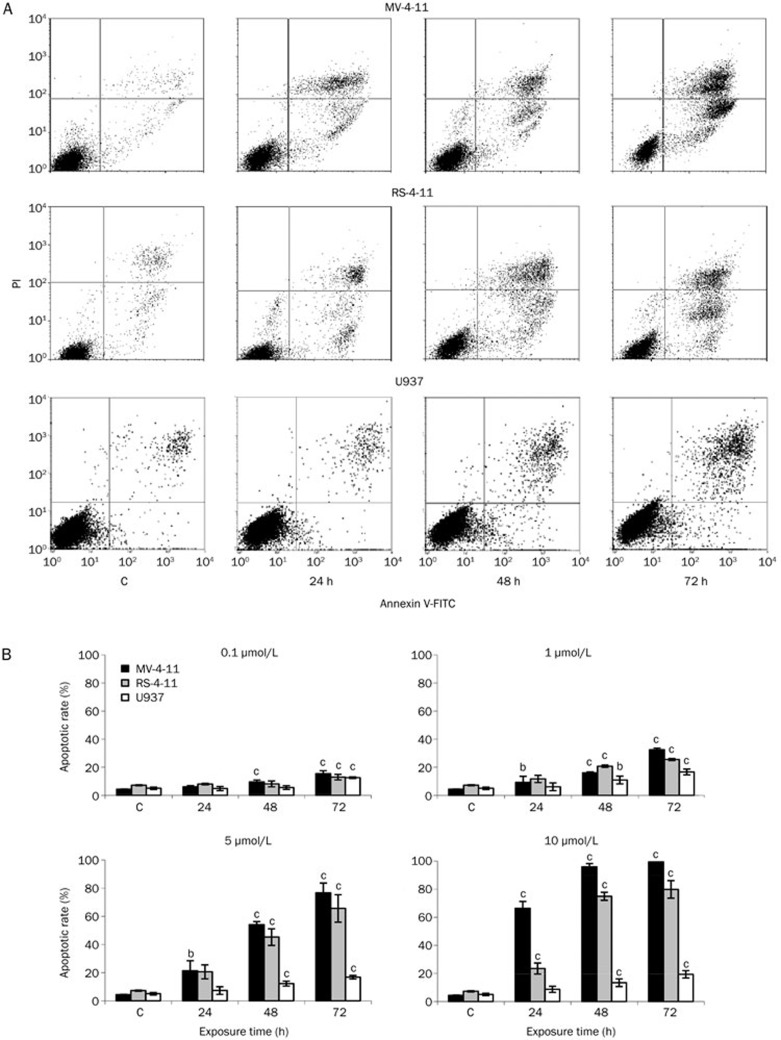
To confirm that apoptotic cell death was induced by C-1305 and to determine whether the cytotoxic effects of C-1305 were due to the induction of apoptosis in the AML cell lines, we conducted Annexin V binding assays using flow cytometry. We found a concentration- and a time-dependent induction of apoptosis in the cells after treatment with C-1305 (Figure 6A and 6B). The greatest increase in early (Annexin V positive, PI negative) and late (Annexin V positive, PI positive) apoptotic cells occurred in the MV-4-11 cells (FLT3-ITD) in 5 and 10 μmol/L C-1305 following 72-h exposure and amounted to 76.7% and 99.4%, respectively. The increase in early and late apoptotic cells was also observed in RS-4-11 cells (wild-type FLT3), and the total proportion of dead cells reached ∼80% following 72 h incubation with 10 μmol/L C-1305. The U937 cells (null-FLT3) underwent less apoptosis than the MV-4-11 and RS-4-11 cells. The fractions of early and late apoptotic cells progressively increased with the duration of C-1305 exposure but not with increasing drug concentrations, and after 72 h these populations together reached ∼19% (Figure 6B). These results indicate that the sensitivity to C-1305 of the MV-4-11 cells correlated with their increased susceptibility to apoptosis. The RS-4-11 and U937 cells were less sensitive to C-1305, underwent apoptosis more slowly and, particularly in the case of U937, to a more limited extent.
Figure 6.
Induction of apoptosis by C-1305 in AML cells. (A) Representative bivariate histograms of the Annexin V/PI staining of the cells cultured in the presence of 5 μmol/L C-1305 or vehicle (c) for the time indicated. The bottom left quadrant represents live unaffected cells (AV–/PI–), the bottom right quadrant represents early apoptotic cells (AV+/PI–), the top right quadrant represents late apoptotic and secondary necrotic cells (AV+/PI+) and the top left quadrant represents primary necrotic cells (AV–/PI+). (B) The percentage of early and late apoptotic cells among cells treated with increasing C-1305 concentrations for 24, 48, and 72 h based on flow cytometry analysis. Values are the mean±SD, n=3. bP<0.05 vs untreated cells (c), cP<0.01 vs untreated cells (c), Student's t-test.
Treatment with C-1305 results in morphological changes of AML cells
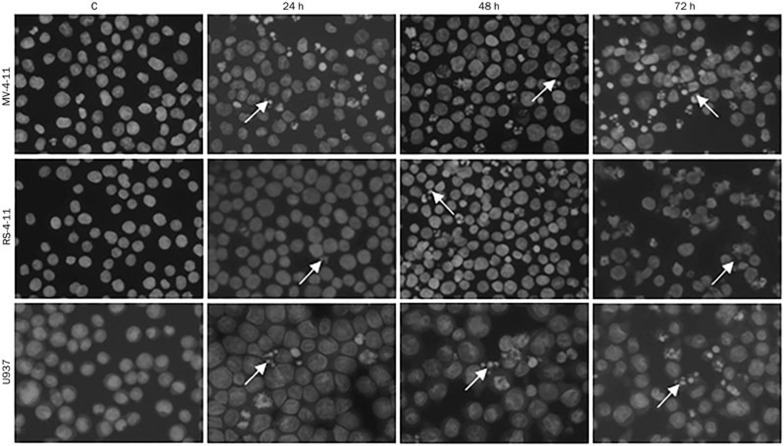
We examined the changes in morphology of the AML cells treated with C-1305 by staining with DAPI. A time-dependent appearance of typical morphologic features of apoptosis (cell shrinkage, condensation and fragmentation of nuclear chromatin) was observed (Figure 7). In the MV-4-11 cells, a few apoptotic nuclei appeared after 24-h treatment with 5 μmol/L C-1305, and after 72 h of incubation, half of the population exhibited apoptotic features. Among the RS-4-11 cells, the population of apoptotic cells was slightly lower than among the MV-4-11 cells, but it also increased with prolonged incubation time. In the U937 cells, under the same experimental conditions, we observed only a small population of apoptotic cells, consistent with a lower level of apoptosis.
Figure 7.
C-1305 induces changes in AML cell morphology. Representative pictures of cells stained with DAPI, exposed to 5 μmol/L C-1305 for the time indicated and examined with a fluorescence microscope (magnification ×400). Cells with condensed, intensely stained fragmented chromatin are typical of apoptosis (arrows).
Apoptosis induced by C-1305 is mitochondrial- and caspase-dependent
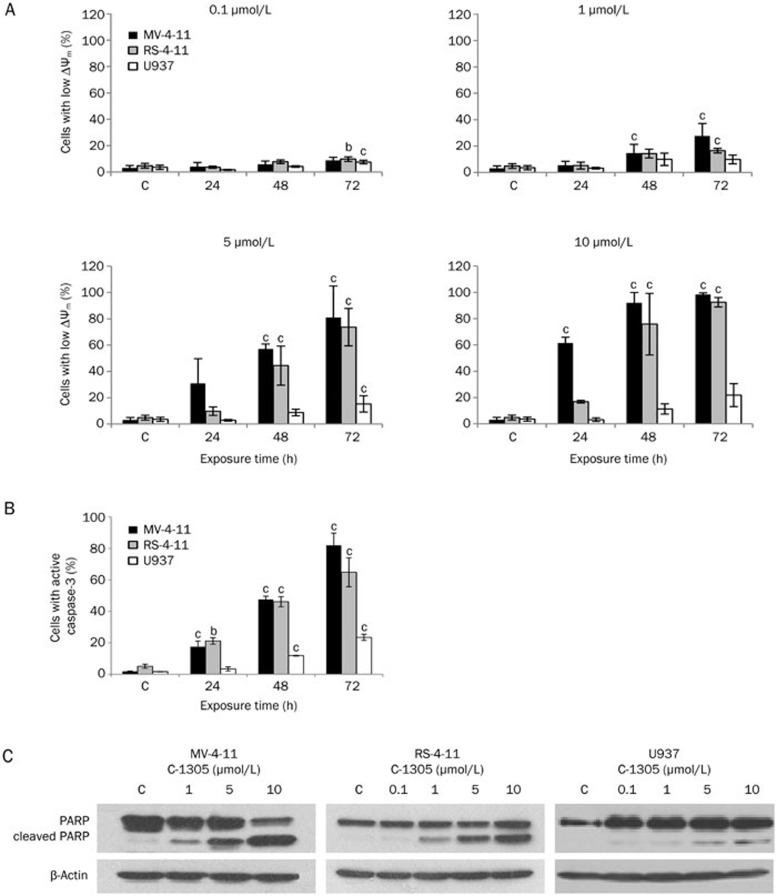
A drop in the mitochondrial membrane potential ΔΨm has been shown to participate in the induction of apoptosis34. To evaluate the role of mitochondria in C-1305-induced apoptosis, we investigated the ability of C-1305 to induce alterations in ΔΨm by measuring fluorescence of the cationic lipophilic dye, JC-1. Compared to the control (untreated) cells, a dose- and time-dependent decrease in ΔΨm was detected in the treated AML cell lines (Figure 8A). After 72 h of treatment with 5 μmol/L C-1305, the percentage of cells with low ΔΨm reached 81% in the FLT3-ITD cells (MV-4-11), 74% in wild-type FLT3 cells (RS-4-11) and only 15% in null-FLT3 cells (U937), which was consistent with low levels of apoptosis in the latter cell line. The permeabilization of the mitochondrial outer membrane allows the release of cytochrome c, which induces the activation of caspases35. Accordingly, treatment with 5 μmol/L C-1305 resulted in a time-dependent elevation of activated caspase-3 in MV-4-11 and RS-4-11 cells. After 72 h of drug exposure, caspase-3 activity was detected in 82% of FLT3-ITD cells (MV-4-11) and in 65% of cells with wild-type FLT3 (RS-4-11). In the U937 cells (null-FLT3), the population of cells with active caspsase-3 reached the level of 23% (Figure 8B). Consistent with the activation of caspase-3, a dose-dependent PARP cleavage (formation of the 89 kDa PARP fragment) was detected in MV-4-11 and RS-4-11 cells treated with increased C-1305 concentrations. In contrast, in U937 (null-FLT3) cells, PARP-cleavage was detected only at high doses of C-1305 and was less pronounced than in the two cell lines expressing FLT3 (Figure 8C).
Figure 8.
The involvement of mitochondria in C-1305-induced apoptosis. (A) The fraction of cells with low ΔΨm among AML cells exposed to increasing C-1305 concentrations based on flow cytometry analysis. (B) Quantitation of the percentage of caspase-3 positive cells following exposure to 5 μmol/L C-1305 for 24, 48 and 72 h based on flow cytometry analysis. Values are the mean±SD, n=2. bP<0.05 vs untreated cells (c), cP<0.01 vs untreated cells (c), Student's t-test. (C) PARP cleavage following C-1305 exposure. MV-4-11, RS-4-11, and U937 cells were treated with C-1305 for 48 h at the concentrations indicated. Whole-cell lysates were analyzed by Western blots for total and cleaved PARP. β-Actin was used as a loading control. Experiments were repeated at least three times.
FLT3-ITD knock-down decreases the sensitivity of AML cells to C-1305
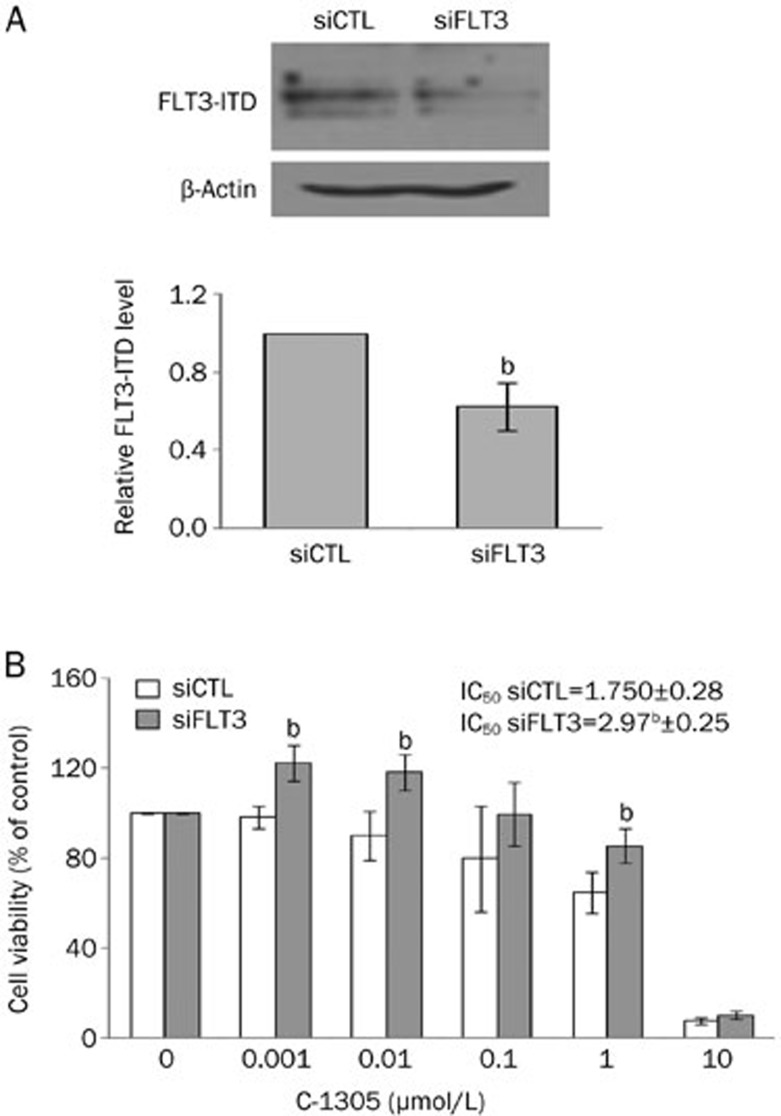
Data obtained thus far indicated that MV-4-11 cells (FLT3-ITD) were the most sensitive to C-1305 treatment among the AML cell lines tested, suggesting that blocking mutated FLT3-ITD kinase might contribute to overall C-1305 cytotoxicity. To determine whether FLT3-ITD kinase plays an important role in the response of AML cells to C-1305 exposure, we lowered the endogenous levels of FLT3-ITD in MV-4-11 cells using an siRNA technique. Western blot analysis confirmed an efficient siRNA-mediated downregulation of FLT3-ITD expression (Figure 9A). Although our transfection procedure produced only approximately a 40% reduction of FLT3-ITD, such a decrease was sufficient to demonstrate that C-1305 treatment, together with FLT3-ITD depletion, resulted in an increased viability of MV-4-11 cells. Cells with reduced FLT3-ITD expression were less sensitive to C-1305 than control cells transfected with nonspecific siRNA (Figure 9B). The IC50 value of C-1305 in MV-4-11 cells transfected with siRNA against FLT3 mRNA was approximately 3 μmol/L, whereas in control siRNA treated cells, the IC50 of C-1305 was 1.75 μmol/L. The observation that the siRNA-mediated knock-down of FLT3-ITD partially rescued MV-4-11 cells from C-1305-induced cytotoxicity supports the idea that the inhibition of FLT3-ITD kinase is important for the increased activity of C-1305 against leukemia cells carrying FLT3-ITD mutations.
Figure 9.
siRNA-mediated depletion of FLT3-ITD kinase increases the viability of AML cells treated with C-1305. (A) MV-4-11 cells were transfected with FLT3 siRNA or with nonspecific siRNA as a control (siCTL) for 5 h and were left to recover overnight. The expression of FLT3-ITD was analyzed by Western blots. β-Actin served as a loading control. The relative level of FLT3-ITD protein in FLT3 knock-down cells was determined by densitometric analysis of protein band intensities, normalized to β-actin and represented relative to the FLT3-ITD level in cells transfected with nonspecific siRNA (siCTL). The results are the mean±SD. n=3. bP<0.05 vs siCTL, Student's t-test. (B) MV-4-11 cells following transfection with FLT3 siRNA or nonspecific siRNA (siCTL) were exposed to increasing concentrations of C-1305 for 24 h. Cell viability was measured using MTT assays. The bar graph shows the percentage of viable cells relative to untreated controls. The results are the mean±SD. n=3. bP<0.05 siFLT3 vs siCTL, Student's t-test.
Discussion
It is estimated that one third of patients with acute myeloid leukemia have constitutively active mutant FLT3 receptor kinase (predominantly an ITD mutation), which is associated with a poor prognosis for AML treatment36. Therefore, receptor tyrosine kinase has become an attractive therapeutic target37. Several small molecule FLT3 tyrosine kinase inhibitors, including sunitinib, lestaurtinib, sorafenib, quizartinib, tandutinib, KW-2449, midostaurin and crenolanib are in development for the treatment of FLT3-mutated AML38. Although these inhibitors appear to have appreciable activity as single agents, and patients with FLT3-ITD mutations can respond if adequate drug levels are achieved, a large number of cancers are still resistant to the administration of single FLT3 inhibitors39. Therefore, to overcome the problem of this type of resistance, it is necessary to have a broad panel of FLT3 inhibitors that may be used in therapy interchangeably or in combination with standard chemotherapy. This in turn entails the need to search for new compounds that inhibit the activity of FLT3 tyrosine kinase40.
In this study, we show for the first time that a promising topoisomerase II inhibitor, triazoloacridinone C-1305, in addition to its DNA-damaging properties, also targets FLT3 kinase, which reveals a novel mechanism that may be relevant for C-1305 anticancer activity. A detailed analysis of the antiproliferative effect of C-1305 against AML cell lines with different FLT3 statuses indicated a more potent inhibition of cells with constitutively active FLT3-ITD kinase than of cells with the wild-type FLT3. Importantly, a comparison of the IC50 values of C-1305 in the cell lines tested showed that the increased sensitivity of FLT3-ITD mutants was sustained for an extended period of drug exposure (24–72 h), indicating the importance of FLT3-ITD inhibition for overall C-1305 cytotoxicity.
We also studied the molecular mechanisms underlying the enhanced susceptibility of FLT3-ITD mutants to C-1305. We found that C-1305 substantially inhibited FLT3-ITD tyrosine kinase activity by decreasing its phosphorylation at the crucial Tyr591 in a time- and dose-dependent manner. The inhibitory effect on FLT3-ITD phosphorylation was observed by 3 h and was potentiated by prolonged drug treatment. After a 24-h exposure, 50% inhibition of FLT3-ITD phosphorylation was achieved at approximately 1 μmol/L of C-1305. The ITD mutation in the FLT3 receptor results in constitutively activated FLT3, which manifests in its autophosphorylation in the absence of exogenous ligand stimulation28. In contrast, phosphorylation of the wild-type FLT3 receptor takes place following binding of the exogenous FLT3 ligand27. We show that, in addition to significant blocking of ligand-independent phosphorylation of the constitutively active FLT3-ITD, C-1305 also decreased ligand-dependent phosphorylation of the wild-type FLT3 receptor; however, higher concentrations (between 1 and 5 μmol/L) were required for modulation of wild-type FLT3 than for FLT3-ITD. Independently, C-1305 at a higher concentration (10 μmol/L) reduced the total FLT3 protein level to a similar level in wild-type and FLT3-ITD expressing cells. Importantly, however, substantial (50%) inhibition of FLT3 phosphorylation, regardless of the kinase mutation status, occurred at a C-1305 dose range lower than that affecting the total level of FLT3. This in turn suggests that inhibition of both ITD- and wild-type FLT3 phosphorylation may result from the direct interference of C-1305 with the FLT3 receptor.
FLT3-ITD mutations result in the loss of the autoinhibitory function, with subsequent constitutive activation of FLT3 kinase and its downstream proliferative signaling pathways, including the PI3K/AKT, Ras/MAPK, and STAT529,30,31. Therefore, we next examined the effect of C-1305 on the FLT3-mediated signal transduction pathway. We found that the inhibition of FLT3-ITD was accompanied by a quick and robust abrogation of STAT5 phosphorylation accompanied by a less pronounced inhibition of MAPK phosphorylation. These results are consistent with the notion that signaling through constitutively activated FLT3 is mediated, at least partially, by the STAT5 and MAPK pathways41. Importantly, a decrease in STAT5 and MAPK phosphorylation occurred at a C-1305 concentration range similar to that required to inhibit FLT3-ITD activity. Although C-1305 exposure also led to a substantial decrease in the phosphorylation of AKT, this effect resulted from a reduction in the total level of AKT protein. Because there is no evidence that FLT3 kinase controls the total amount of AKT42, most likely C-1305 blocks the AKT pathway independently from FLT3-ITD signaling.
Consistent with the less potent inhibition of wild-type FLT3 kinase, the inhibitory effect of C-1305 on FLT3 downstream targets was weaker than in the FLT3-ITD mutant. C-1305 primarily affected the wild-type FLT3/MAPK signaling axis and to a less extent the AKT pathway. In contrast to FLT3-ITD mutants, signal transduction involving STAT5 was unaffected for an extended time of treatment. These results are in agreement with observations that in AMLs the downstream signals of wild-type FLT3 and FLT3-ITD are not identical; for example, wild-type FLT3 following ligand stimulation transduces mainly MAPK but not STAT541. Unlike in cells expressing FLT3, C-1305 exposure did not evoke immediate changes in cellular signaling in cells lacking FLT3 kinase, consistent with the decreased sensitivity of those cells to C-1305. Drug treatment mainly affected FLT3-independent MAPK activation and to a less extent STAT5 signaling, whereas the AKT pathway remained constantly active despite prolonged drug exposure. Such evident differences in signal transduction clearly underlie the different sensitivities of FLT3-ITD, wild-type FLT3 and null-FLT3 leukemia cells to C-1305.
At the cellular level, C-1305-induced inhibition of pro-survival pathways led to a progressive decrease of FLT3-ITD mutants in the G1 phase of the cell cycle followed by massive apoptosis. The loss of FLT3-ITD activity resulting in STAT5 and MAPK inactivation correlated with a decrease in the inhibitory phosphorylation of the pro-apoptotic Bad protein and increases in caspase-3 activation, PARP cleavage, loss of mitochondrial membrane potential, and phosphatidylserine externalization. Moreover, the observation that an siRNA-mediated decrease in FLT3 expression partially rescued FLT3-ITD mutants from C-1305-induced cytotoxicity supports the idea that inhibition of FLT3-ITD is important for the increased activity of C-1305 against leukemia cells carrying FLT3-ITD mutations. Wild-type FLT3 mutants treated with C-1305 were temporarily arrested in the G2/M compartment, and their apoptotic response to C-1305, consistent with a less profound inhibition of wild-type FLT3-mediated MAPK and AKT signaling, was less intense than that of the FLT3-ITD mutant. Together, these results indicate that the effect of C-1305 on FLT3, regardless of its mutation status, causes the induction of apoptotic cell death. In contrast, C-1305 treatment of cells without FLT3 kinase led to an increase in cells in the G2/M phase, and despite the evident inhibition of the MAPK pathway, these cells were significantly less prone to undergo apoptosis in response to C-1305. This implies that the direct inhibition of the FLT3-independent MAPK signaling pathway is insufficient to induce apoptosis by C-1305. These observations are consistent with results of studies showing that a potent inhibitor of FLT3, sorafenib, triggered the death of null-FLT3 U937 cells independent of the inactivation of the MAPK cascade43.
Our results indicate that triazoloacridinone C-1305 inhibits ITD- and wild-type FLT3 kinase in leukemia cells when applied at micromolar concentrations, whereas several FLT3 inhibitors, including Lestaurtinib, Sunitinib, Sorafenib, and AC220 block FLT3 activity in vitro at concentrations between 1–50 nmol/L44. This comparison indicates that C-1305 is a less potent inhibitor of FLT3 than the clinically tested compounds. However, we emphasize that C-1305 is primarily a topoisomerase II inhibitor, and its major mechanism of action affects the structure and function of DNA19. The ability of C-1305 to inhibit FLT3 kinase is important, but it is not the only mode of action of this compound. Nevertheless, in this study, we have uncovered the multi-target potential of C-1305; affecting both topoisomerase II and FLT3 may be relevant for the overall activity of C-1305 as an anticancer compound.
In conclusion, our findings indicate that the FLT3 kinase seems to be a potential new target for triazoloacridinone C-1305. C-1305 was capable of inhibiting autophosphorylation of both ITD and wild-type FLT3, although blocking the FLT3-ITD activity was more persistent and translated into more apoptosis of cells carrying the FLT3-ITD mutation following long-term drug exposure. Significantly weaker apoptosis was observed in cells lacking FLT3 expression. Regardless of the FLT3 mutation status, the range of C-1305 doses required to inactivate FLT3, similar to that affecting the signaling pathways and triggering apoptosis, supports the notion that inhibition of FLT3 may account for the cytotoxic activity of C-1305 against leukemia cells. Further studies are needed to evaluate this drug as a promising antitumor agent for AML therapy.
Author contribution
Ewa AUGUSTIN designed and supervised the research and wrote the manuscript. Anna SKWARSKA performed the research, analyzed the data and revised the manuscript. Anna WERYSZKO, Iwona PELIKANT, and Ewa SANKOWSKA performed the research. Barbara BOROWA-MAZGAJ performed statistical analysis of the results and designed the figures.
Figure 4.
C-1305 blocks the activation of signal transduction pathways in U937 cells (null-FLT3). Cells were cultured in the presence of varying concentrations of C-1305 or vehicle (c) for 3, 24, and 48 h. Cell extracts were analyzed by Western blots with antibodies against anti-AKT, anti-phospho-AKT (Ser473), anti-p44/42 MAPK (Erk1/2), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), anti-Stat5, anti-phospho-Stat5 (Tyr694), anti-Bad, anti-phospho-Bad (Ser136). The relative levels of the phospho-proteins were determined by densitometric analysis of the protein band intensities normalized to the total level of the protein and represented relative to the levels in untreated cells (c). Equal protein loading was demonstrated by blotting for β-actin. The results are the mean±SD. n=3. Representative blots from at least three experiments are shown.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education (Poland) (Grant No N N401 073536). The authors thank Prof Jerzy from our Department for giving us an opportunity to perform studies using the triazoloacridinone C-1305. We also thank Dr Joanna POLEWSKA, for help in flow cytometry analysis.
References
- Stirewald D, Radich J. The role of FLT3 in haematopoietic malignancies. Nat Cancer Rev. 2003;3:650–65. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- McKenzie SB. Advances in understanding the biology and genetics of acute myeloid leukemia. Clin Lab Sci. 2005;18:28–37. [PubMed] [Google Scholar]
- Testa U, Pelosi E. The impact of FLT3 mutations on the development of acute myeloid leukemias. Leuk Res Treatment. 2013. 2013. p. 275760. [DOI] [PMC free article] [PubMed]
- Ortlepp C, Steudel Ch, Heiderich C, Koch S, Jacobi A, Ryser M, et al. Autotaxin is expressed in FLT3-ITD positive acute myeloid leukemia and hematopoietic stem cells and promotes cell migration and proliferation. Exp Hematol. 2013;41:444–61. doi: 10.1016/j.exphem.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashina K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–8. [PubMed] [Google Scholar]
- Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D85 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–9. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H, et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12:1333–7. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- Kiyoi H, Yanada M, Ozekia K. Clinical significance of FLT3 in leukemia. Int J Hematol. 2005;82:85–92. doi: 10.1532/IJH97.05066. [DOI] [PubMed] [Google Scholar]
- Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Reinhardt D, Kaspers GJL, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–61. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after these years. Blood. 2010;116:5089–102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
- Smith CC, Wang Q, Chin CS, Salermo S, Damon LE, Levis MJ, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myleoid leukaemia. Nature. 2012;485:260–3. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chołody WM, Martelli S, Konopa J. 8-Substituted 5-[(aminoalkyl)amino]-6H-v-triazolo[4,5,1-de]acridin-6-ones as potential antineoplastic agents. Synthesis and biological activity. J Med Chem. 1990;33:2852–6. doi: 10.1021/jm00172a028. [DOI] [PubMed] [Google Scholar]
- Kuśnierczyk H, Chołody WM, Paradziej-Łukowicz J, Radzikowski C, Konopa J. Experimental antitumor activity and toxicity of the selected triazolo- and imidazoacridinones. Arch Immunol Ther Exp. 1994;42:415–23. [PubMed] [Google Scholar]
- Fedejko-Kap B, Niemira M, Radominska-Pandya A, Mazerska Z. Flavin monooxygenases, FMO1 and FMO3, not cytochrome P450 isoenzymes, contribute to metabolism of anti-tumor triazoloacridinone, C-1305, in liver mocrosomes and HepG2 cells. Xenobiotica. 2011;41:1044–55. doi: 10.3109/00498254.2011.604743. [DOI] [PubMed] [Google Scholar]
- Fedejko-Kap B, Bratton SM, Finel M, Radominska-Pandya A, Mazerska Z. Role of human UDP-glucuronosyltransferases in the biotransformation of the triazoloacridinone and imidazoacridinone antitumor agents C-1305 and C-1311: highly selective substrates for UGT1A10. Drug Metabol Dispos. 2012;40:1736–43. doi: 10.1124/dmd.112.045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawłowska M, Chu R, Fedejko-Kap B, Augustin E, Mazerska Z, Radominska-Pandya A, et al. Metabolic transformation of antitumor acridinone C-1305 but not C-1311 via selective cellular expression of UGT1A10 increases cytotoxic response: implications for clinical use. Drug Metabol Dispos. 2013;41:414–21. doi: 10.1124/dmd.112.047811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemira M, Dastych J, Mazerska Z. Pregnane X receptor dependent up-regulation of CYP2C9 and CYP3A4 in tumor cells by antitumor acridine agents, C-1748 and C-1305, selectively diminished under hypoxia. Biochem Pharmacol. 2013;86:231–41. doi: 10.1016/j.bcp.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Koba M, Konopa J. Interactions of antitumor triazoloacridinones with DNA. Acta Biochim Pol. 2007;54:297–306. [PubMed] [Google Scholar]
- Węsierska-Gądek J, Schloffer D, Gueorguieva M, Uhl M, Składanowski A. Increased susceptibility of poly(ADP-ribose) polymerase-1 knockout cells to antitumor triazoloacridinone C-1305 is associated with permanent G2 cell cycle arrest. Cancer Res. 2004;64:4487–97. doi: 10.1158/0008-5472.CAN-03-3410. [DOI] [PubMed] [Google Scholar]
- Augustin E, Moś-Rompa A, Skwarska A, Witkowski JM, Konopa J. Induction of G2/M phase arrest and apoptosis of human leukemia cells by potent triazoloacridinone C-1305. Biochem Pharmacol. 2006;72:1668–79. doi: 10.1016/j.bcp.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Chołody WM, Martelli S, Paradziej-Łukowicz J, Konopa J. 5-[(aminoalkyl)amino]imidazo[4,5,1-de]acridin-6-ones as a novel class of antineoplastic agents. Synthesis and biological activity. J Med Chem. 1990b;33:49–52. doi: 10.1021/jm00163a009. [DOI] [PubMed] [Google Scholar]
- Capizzi RL, Roman LA, Tjulandin S, Smirnova I, Manikhas A, Paterson JS. Phase II trial of C-1311, a novel inhibitor of topoisomerase II in advanced breast cancer. J Clin Oncol. 2008;26:1055. [Google Scholar]
- Mazerska Z, Augustin E, Składanowski A, Bibby MC, Double JA, Konopa J. C-1311. Drugs Fut. 1998;23:702–6. [Google Scholar]
- Chau M, Otake Y, Christensen JL, Fernandes DJ, Ajami AM. The imidazoacridine, C-1311 (Symadex TM): the first of potent new class of FLT3 inhibitors. AACR Meeting Abstracts. 2006;B:35. [Google Scholar]
- Augustin E, Moś-Rompa A, Nowak-Ziatyk D, Konopa J. Antitumor 1-nitroacridine derivative C-1748, induces apoptosis, necrosis or senescence in human colon carcinoma HCT8 and HT29 cells. Biochem Pharmacol. 2010;79:1231–41. doi: 10.1016/j.bcp.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Skwarska A, Augustin E, Konopa J. Sequential induction of mitotic catastrophe followed by apoptosis in human leukemia MOLT4 cells by imidazoacridinone C-1311. Apoptosis. 2007;12:2245–57. doi: 10.1007/s10495-007-0144-y. [DOI] [PubMed] [Google Scholar]
- Yee KWH, O'Farrell AM, Smolich BD, Cherrington JM, McMahon G, Wait CL, et al. SU5416 and SU5614 inhibit kinase activity of wild-type and mutant FLT3 receptor tyrosine kinase. Blood. 2002;100:2941–9. doi: 10.1182/blood-2002-02-0531. [DOI] [PubMed] [Google Scholar]
- Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–91. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- Brandts ChH, Sargin B, Rode M, Biermann C, Lindtner B, Schwäble J, et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necesssary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65:9643–50. doi: 10.1158/0008-5472.CAN-05-0422. [DOI] [PubMed] [Google Scholar]
- Takahashi S. Downstream molecular pathways of FLT3 in the pathogenesis of acute myeloid leukemia: biology and therapeutic implications. J Hematol Oncol. 2011;4:1–13. doi: 10.1186/1756-8722-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Brandts Ch, Schwable J, Tickenbrock L, Sargin B, Ueker A, et al. Activation mechanisms of STAT5 by oncogenic Flt3-ITD. Blood. 2007;110:370–4. doi: 10.1182/blood-2006-05-024018. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KT, Levis M, Small D. Constitutively activated FLT3 phosphorylates BAD partially through Pim-1. Brit J Haematol. 2006;134:500–9. doi: 10.1111/j.1365-2141.2006.06225.x. [DOI] [PubMed] [Google Scholar]
- Ly JD, Grubb DR, Lawen A. The mitochondria membrane potential (ΔΨm) in apoptosis; un uptade. Apoptosis. 2003;8:115–28. doi: 10.1023/a:1022945107762. [DOI] [PubMed] [Google Scholar]
- Ricci JE, Gottlieb RA, Green DR. Caspase-mediated loss of mitochondria function and generation of reactive oxygen species during apoptosis. J Cell Biol. 2003;160:65–75. doi: 10.1083/jcb.200208089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottaridis PD, Gale RE, Linch DC. FLT3 mutations and leukemia. Br J Hematol. 2003;122:523–38. doi: 10.1046/j.1365-2141.2003.04500.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Ito F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol Pharm Bull. 2011;34:1774–80. doi: 10.1248/bpb.34.1774. [DOI] [PubMed] [Google Scholar]
- Grunwald MR, Levis MJ. FLT3 inhibitors for acute myeloid leukemia: a review of their efficacy and mechanisms of resistance. Int J Hematol. 2013;97:683–94. doi: 10.1007/s12185-013-1334-8. [DOI] [PubMed] [Google Scholar]
- Wiernik PH. FLT3 inhibitors for the treatment of acute myeloid leukemia. Clin Adv Hematol Oncol. 2010;8:429–44. [PubMed] [Google Scholar]
- Gunawardane RN, Nepomuceno RR, Rooks AM, Hunt JP, Ricono JM, Belli B, et al. Transient exposure to quizartinib mediates sustained inhibition of FLT3 signaling while specifically inducing apoptosis in FLT3-activated leukemia cells. Mol Cancer Ther. 2013;12:438–47. doi: 10.1158/1535-7163.MCT-12-0305. [DOI] [PubMed] [Google Scholar]
- Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19:624–31. doi: 10.1038/sj.onc.1203354. [DOI] [PubMed] [Google Scholar]
- Yao Q, Nishiuchi R, Li Q, Kumar AR, Hudson WA, Kersey JH. FLT3 expressing leukemias are selectively sensitive to inhibitors of the molecular chaperone heat shock protein 90 through destabilization of signal transduction-associated kinases. Clin Cancer Res. 2003;9:4483–93. [PubMed] [Google Scholar]
- Rahmani M, Davis EM, Crabtree TR, Habibi JR, Nguyen TK, Dent P, et al. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Mol Cell Biol. 2007;15:5499–513. doi: 10.1128/MCB.01080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi AT, Chabner BA. FLT3 inhibition as therapy in acute myeloid leukemia: a record of trials and tribulations. Oncologist. 2011;16:1162–74. doi: 10.1634/theoncologist.2011-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]