Abstract
Objective
Factors governing events between exposure of male genital mucosa surfaces and the establishment of infection are poorly understood. Furthermore, little is known about the safety and efficacy of microbicides on male genital mucosa.
Design
Here we present a novel penile tissue explant model to characterise the mechanisms of HIV-1 infection of male genital tissue and evaluate candidate microbicides.
Methods
Mucosal explant culture conditions were determined for glans, urethra and foreskin obtained from gender reassignment and circumcision. Density and distribution of CD4+ and CD1a+ cells were visualized by microscopy. In vitro HIV-1 infection was determined by measuring p24 release, while microbicide biocompatibility and efficacy were assessed by measurement of tissue viability, cytokine expression and p24 production.
Results
Cultured glans and foreskin showed comparable epithelial thickness but some differences in CD4+ and CD1a+ cell density. All tissue sites examined (foreskin, glans, meatus, urethra) were equally susceptible to R5 HIV-1 infection, which was productively disseminated by migratory cells emigrating from tissue. In contrast, X4 HIV-1 failed to infect mucosal tissue and dissemination by migratory cells was less efficient. The three candidate microbicides PMPA, PRO 2000 and Cyanovirin-N, showed good tissue compatibility and efficient prevention of HIV-1 infection, causing only minor changes in tissue cytokine profile.
Conclusion
The described model provides a useful model to study the determinants of HIV-1 infection of male genital tissue and is likely to be an important tool for the future development of microbicide candidates and concepts.
Keywords: HIV-1, glans, foreskin, tissue explant, microbicide
Introduction
Thirty-three million people are living with HIV, half of them adult men [1]. Differences in the efficiency of male-to-female HIV transmission versus female-to-male are controversial [2–8], and may depend on several factors thought to influence the risk of female-to-male transmission [6, 7, 9], including circumcision [7, 10–12]. Recent prophylactic trials demonstrate that circumcision could provide > 50% protection against HIV infection [13–15], but provides no protection to female partners of HIV+ men [16, 17]. Explaining the association between circumcision and reduced HIV acquisition may provide important insight into the mechanism of transmission and development of intervention strategies. Previous reports suggest that a higher density and a more superficial presence of Langerhans cells together with reduced keratinisation of the inner layer of foreskin, might increase HIV acquisition in uncircumcised men [18–21]. However, determinants of HIV infection in men are not fully understood, and their characterization may aid microbicide development. Current microbibicides have been designed to prevent female acquisition [22, 23], assuming bi-directional protection [24]; however, no studies have tested the efficacy of microbicides against penile infection and their potential role in protecting men from insertive vaginal or rectal intercourse.
Using male genital tissue as an ex vivo model of HIV-1 transmission, we evaluate the frequency of HIV target cells in foreskin, glans and urethra and their differential susceptibility to infection. Furthermore, we evaluate candidate microbicides for safety and efficacy against HIV transmission.
Materials and methods
Patients and tissue
Penile tissue was obtained following gender reassignment at Charing Cross Hospital, London, UK. All subjects had ceased hormonal therapy a minimum of six weeks prior to surgery. Foreskin tissue was obtained following elective circumcision at St. George’s University of London, UK. All tissue was collected with written consent according to LRC guidelines. Penis and foreskin were cut into 2–3 mm3 explants comprising both epithelium and stroma. Tissue explants were cultured in RPMI 1640 medium supplemented with glutamax, 10% FCS, penicillin and streptomycin.
Measurement of tissue viability
Tissue explants were cultured for 10 days, with half medium replacement every 2–3 days. Tissue viability was measured by MTT dye reduction as previously described [25].
Immunohistochemistry
Tissue blocks were embedded in OCT, sectioned (12µm) and stained. LCs were identified by CD1a (OKT 6 hybridoma (ATCC)), CD4+ cells by anti-CD4 (clone Q4120, Sigma). Donkey anti-mouse secondary antibodies were conjugated to Cy3, Cy5, Rhodamine Red-X conjugated (Jackson Immunolabs), and Oregon Green (Molecular Probes). Zenon Alexa Fluor 647 (Molecular Probes) was utilized for antibody illumination. Epithelium was stained with WGA conjugated with Alexa fluor 594 (Molecular Probes).
Imaging of tissue slices
Images were collected on a DeltaVision RT system using a 40× oil objective, an Olympus IX71 microscope, and analysed using deconvolution microscopy software. Thirty z-sections, 0.5µm apart, were collected per image field. All measurements were obtained through the “Measure Distances” tool, using a standard two point method.
Determination of microbicides cytotoxicity
Compounds used in this study were Cyanovirin-N (Biosyn Inc.; Huntingdon Valley, USA), PRO 2000 (Indevus Pharmaceutical, USA) and PMPA (Gilead Sciences, USA). Cyanovirin-N was provided unformulated, while PRO 2000 and PMPA were gel formulated compounds. Placebo formulations containing the same excipients as the active product were also provided. All compounds were diluted into culture media. Potential toxicity of microbicides was measured as previously described [25].
Cells and viral cultures
PM1 cells (AIDS reagent project, NIBSC, Potters Bar, UK) were cultured in RPMI 1640 medium supplemented with glutamax, 10% FCS, penicillin and streptomycin. HIV-1BaL and HIV-1LaV were grown on PBMCs as previously described [26] and the TCID50 determined.
HIV infection of human male genital tract tissue explants, and dissemination by migratory cells
Tissue explants were exposed to HIVBaL or HIVLaV (104 TCID50/explant) for 2 hours. Aldithriol-2 (10 mM) treated virus and medium only were used as negative controls. After washing with PBS, explants were resuspended in medium ± PHA (10µg/ml) overnight. The day after, tissue explants were removed and cultured ± PHA for 3 days, replaced by IL-2 (100 IU/ml) for the following 8 days. Migratory cells present in overnight culture plates were washed with PBS and co-cultured with PM1 (40.000 cells/well) for 7 days. Culture supernatants were harvested every 2–3 days and assessed for p24 antigen level (Beckman Coulter, UK). Statistical analysis was performed using two tail T-test.
Screening of candidate microbicides
Tissue explants were treated with medium or compound just prior to exposure to HIV-1BaL (104 TCID50) for 2 h in presence of the compound. The samples were then washed with PBS in absence of compound. The day after migratory cells were separated from tissue as described above. Cultures were assessed for viral replication by p24 ELISA as described.
Cytokine quantification
Tissue samples were cultured with or without compound for 2 hours, washed with PBS and cultured in medium overnight. Supernatant was then collected and 23 cytokines quantified by in house multiplex bead immunoassay as described [27].
Results
Establishment of optimal culture conditions
Human male genital explant cultures were established by adapting previous methods developed for culture of cervicovaginal tissue [26]. Viability of explants in culture was assessed (data not shown). Glans, meatus and urethra maintained high viability for up to 7 days (average viability: 76.5% – 97.8%), with a slight decline by day 10 (62.7% – 76.7%). Inner and outer foreskin demonstrated shorter viability (75% at day 3 but 38% by day 7).
HIV target cell distribution
Genitourinary tissue was sectioned and stained with fluorescent antibodies to identify Langerhans cells (LCs) and CD4+ cells following 0, 3 or 7 days in culture (Figure 1a, b). Fluorescent WGA was used to reveal tissue structure and integrity.
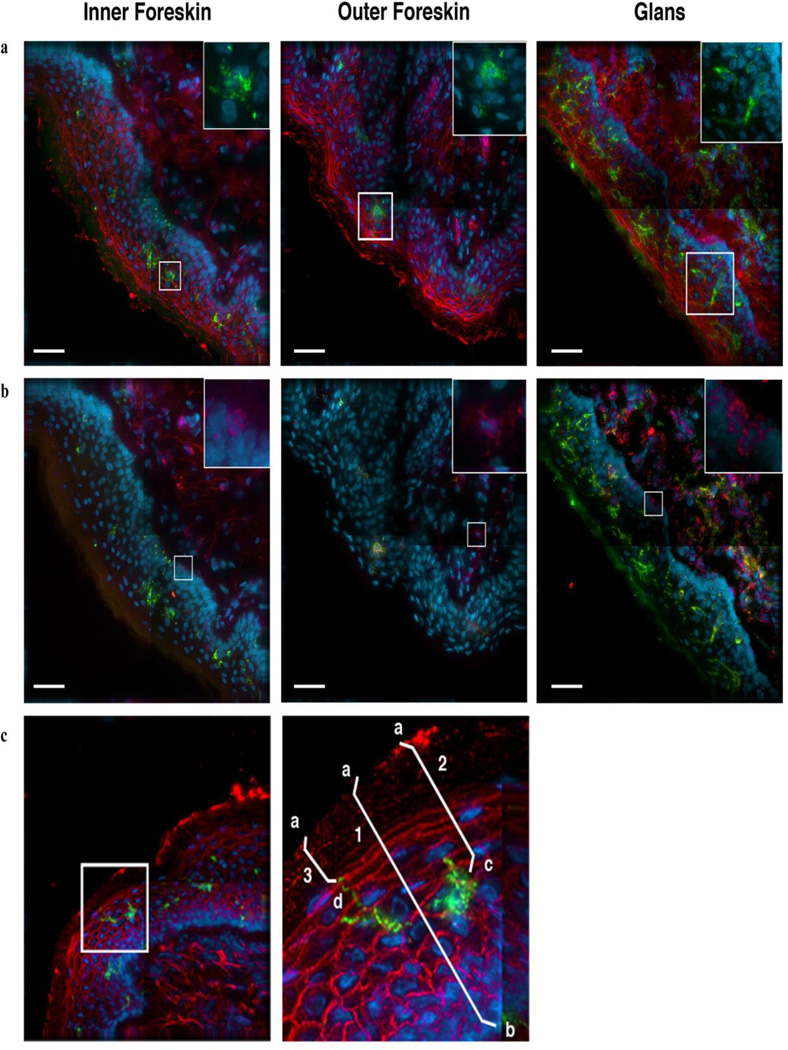
Figure 1. Identification of LCs and CD4+ cells in epithelial tissue and illustration of measurements taken to characterize cell localization.
Tissue specimens were collected from healthy donors and cultured for up to 1 week. These samples were frozen in OCT, then sectioned and stained with fluorescent antibodies to identify LCs and CD4+ cells. Images are representative of inner foreskin, outer foreskin and glans tissue specimens that were kept for up to 3 days in culture. The same image is shown in both (a) and (b), with different staining patterns. (a) LCs are identified through staining for CD1a (green) and nuclei (blue). Tissue integrity is displayed through staining with fluorescent wheat germ agglutinin (WGA, red). The boxed area, enlarged in the upper right corner, shows LCs (green) and nuclei (blue). (b) CD4+ cells are identified through positive staining for CD4 (red). LCs (green) and nuclei (blue) can also be seen, as before. The boxed area is enlarged in the upper right corner and shows characteristic CD4+ cells (red) and nuclei (blue). Scale bars, 40µm.
(c). LCs (green) can be observed in this inner foreskin specimen, which has also been stained with WGA (red) and for nuclei (blue). The boxed area is enlarged at right to show the LC in greater detail. For each cell studied, we measured (1) epithelial thickness, (2) the distance of the cell body to the surface, and, in the case of LCs, (3) the proximity of the closest LC projection to the surface. (1) Epithelial thickness was calculated from the tissue surface (a) to below the epithelial basement membrane (b). (2, 3) The proximity of cell bodies and LC projections to surface were determined by measuring the distance between the tissue surface (a) and either the cell body (c) or LC projection (d).
For each target cell identified, we measured epithelial thickness, distance of the cell body to tissue surface and distance of the closest LC projection to tissue surface (Figure 1c). Epithelial thickness was determined by measuring the distance between the basement membrane and tissue surface, crossing through each cell body. Following 7 days in culture, specimens of inner foreskin were severely fragmented and lacked semblance of integrity. We therefore focused on differences in tissue from the initiation to 3 days in culture.
Overall, average epithelial thickness for each tissue type remained similar throughout the 3-day culture period (Figure 2a), although inner foreskin decreased (100.4 – 92.4µm) and glans epithelium increased in thickness (95.8µm – 106.9µm) after 3 days.
Figure 2. Density and distribution of LCs and CD4+ cells in genital epithelium.
Tissue samples at the initiation of culture (2h, black bars) and 3 days in culture (grey bars) were stained to identify LCs and CD4+ cells and compared. (a) Average thickness of epithelium. (b) Average distance of LC cell bodies to tissue surface. (c) Average distance of LC projections to tissue surface. (d) Average number of LCs found in epithelium per 100µm2 imaged. (e) Average number of CD4+ T cells detected in each tissue type per 100µm2 imaged (stroma + epithelium). (f) Average number of CD4+ T cells found within the surface epithelium per 100µm2 imaged. (g) Average distance of CD4+ cells, found within surface epithelium, to tissue surface. Error bars denote standard error of the mean. P values on top of grey bars indicate significance between 2 hours and 3 days in culture. P values spanning black bars indicate significance between different tissue sites after 2 hours in culture. P values < 0.05 marked with *, <0.01 marked with **, <0.001 marked with ***.
Similarly, the average distance of LC bodies to tissue surface decreased in both inner (73.4µm – 66.2µm) and outer foreskin (71.6µm – 61.3µm), and increased in glans (57.3µm – 66.7µm) epithelium after 3 days in culture (Figure 2b). Upon examination, we found no change in LCs projection distance to tissue surface over time for inner foreskin (Figure 2c). However, LCs projections appeared to move closer to the tissue surface in outer foreskin (59.5µm – 47.7µm) and farther in glans (41.4µm – 54.8µm). These changes most likely reflect increase in epithelial thickness observed with time.
Next we enumerated the number of LCs observed in each image. While no difference in LCs number was observed in inner or outer foreskin samples after 3 days (Figure 2d), for glans tissue we observed that the value decreased from 0.033 LCs/100µm2 to 0.017 LCs/100µm2 after 3 days. In addition, a significantly higher number of LCs were observed in glans compared to inner and outer foreskin, and in inner compared to outer foreskin, with an average of 0.023 and 0.017 LCs/100µm2 in inner and outer foreskin, respectively. These values decreased to 0.02 and 0.016 LCs/100µm2 by day 3.
The majority of CD4+ cells observed in the tissue specimens typically reside below the basement membrane in mucosal tissue, but may infiltrate surface epithelium under inflammatory conditions [28], while CD4+ LCs exclusively reside in the epithelium. No change was observed for CD4+ cell number for inner foreskin over 3 days in culture, but we detected a slight increase in outer foreskin, from 0.01 to 0.016 cells/100µm2 (Figure 2e). In contrast, significantly fewer CD4+ cells were found in glans following 3 days in culture. Here we observed 0.037 CD4+ cells/100µm2 imaged in glans tissue at the initiation of culture, decreasing to 0.013 cells/100µm2 by day 3.
When the density of CD4+ cells was compared between tissue sites, glans tissue was found to contain the most CD4+ cells/100µm2 at the initiation of the culture (Figure 2e), while inner foreskin had 0.026 cells/100µm2 or 2.6 times more than outer foreskin. By 3 days in culture, the only difference still detected was between inner foreskin and glans, with inner foreskin containing 1.6 times more CD4+ cells/100µm2.
This trend continued for CD4+ cells observed within surface epithelium (Figure 2f). While no significant differences were observed in the number of CD4+ cells/100µm2 of surface epithelium for either inner or outer foreskin, glans tissue exhibited a 2.7 fold decrease, from 0.0097 to 0.0037 after 3 days. In addition, glans showed significantly greater numbers of CD4+ cells in the epithelium compared to both inner (0.0019 CD4+ cells/100µm2) and outer foreskin (0.0009 CD4+ cells/100µm2) at the initiation of culture.
Despite these differences in cell number, the average distance of epithelial CD4+ cells to tissue surface remained unchanged in all tissue types throughout the culture period (Figure 2g).
HIV infection of male genital tissue
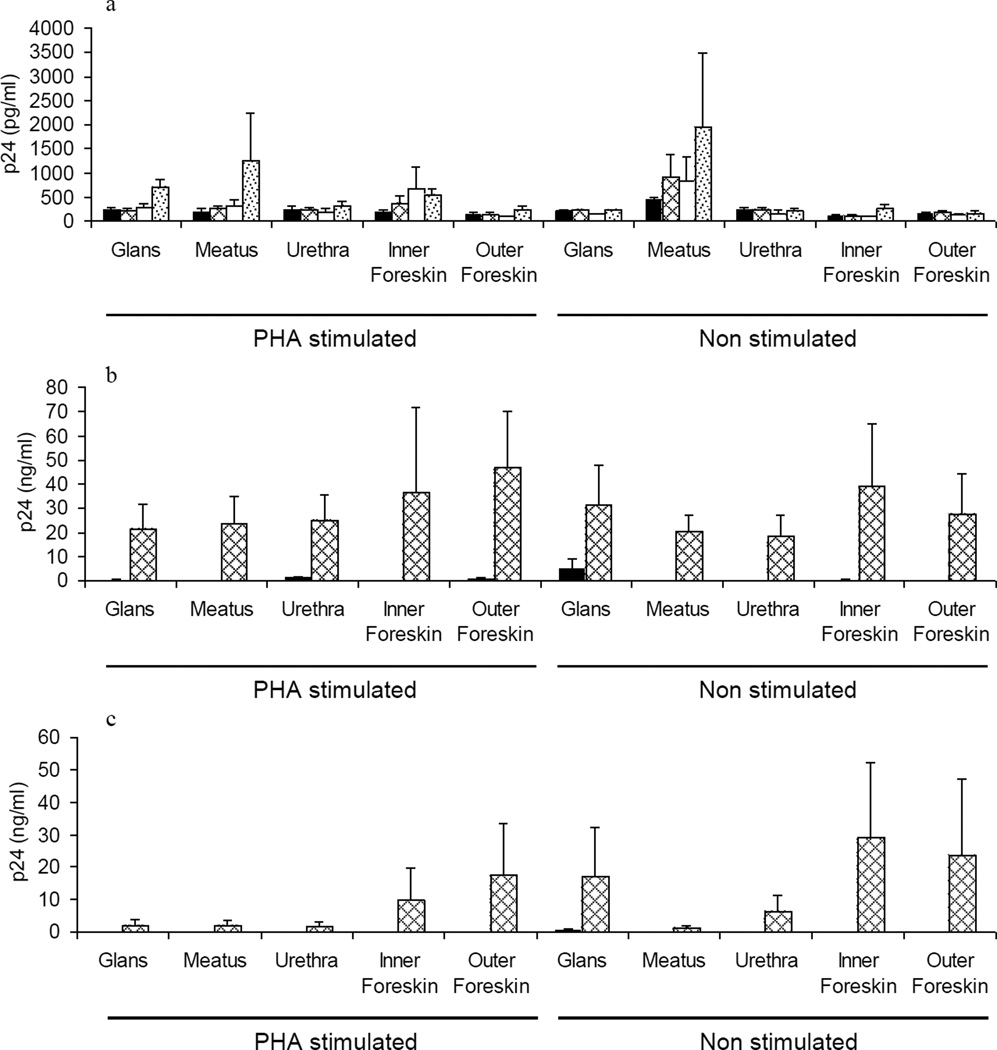
Ex vivo susceptibility to HIV-1 infection and relative efficiency of HIV dissemination by migratory cells was determined by exposing glans, urethra and foreskin to either HIV-1BaL (R5 isolate) or HIV-1LAV (X4 isolate). HIV-1BaL productively infected all tissue sites investigated (Figure 3a), but no infection was detected with HIV-1LAV. Cellular emigrants from all tissue sites (± PHA) were able to disseminate HIV (BaL and LAV), but the level of HIV-1LAV p24 in culture supernatant was generally lower compared to HIV-1BaL (Figure 3b,c). No significant differences in the level of HIV-1 infection either between different tissue sites or between activated versus non-activated tissue and migratory cells were detected.
Figure 3. Replication of HIVBaL and HIVLAV in penile tissue explants and dissemination of the infection by migratory cells.
Figure 3a shows the time course of HIVBaL replication in tissue explants at days 4 (black bars), 7 (grey net bars), 9 (white bars) and 11 (grey dotted bars) days post infection. Figure 3b and 3c show dissemination of HIVBaL (Figure 3b) and HIVLAV (Figure 3c) infection by migratory cells isolated from different tissue explants, folowing 4 (black bars) and 7 (grey net bars) day co-culture with indicator T cells. Data represent the mean p24 ± standard error of the mean of 3 separate donors; each condition was tested in triplicate. P24 levels for uninfected controls and in the samples exposed to AT-2 treated virus were undetectable (data not shown).
Cytokine release profile of male genital tissue explants
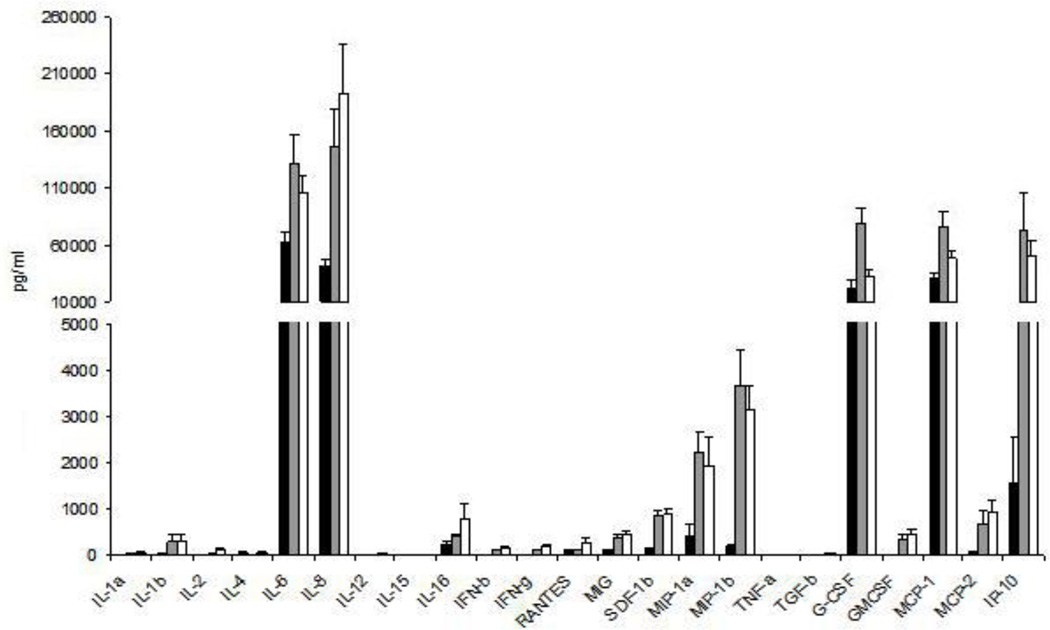
As cytokines can significantly modulate tissue susceptibility to HIV infection, the pattern of cytokine release from glans and foreskin after 24 hours in culture was analysed (Figure 4). IL-1α, IL-4, IL-12, IL-15, TNF-α and TGF-β were all below or at the limit of detection for all 3 tissue sites. Among the cytokines produced at moderate levels, IFN-β, IFN-γ, MIG, SDF-1β, GM-CSF and MCP-2 levels were significantly higher for both inner and outer foreskin compared to glans (p<0.05), while IL-2 levels were only higher for outer foreskin compared to glans (p=0.035). The same was observed for IL-6, IL-8, MIP-1β, MCP-1, and IP-10 (p<0.05) released at higher concentrations, while MIP-1α and G-CSF were only released at significantly higher levels by inner foreskin (p<0.01) when compared to glans. Inner and outer foreskin had a comparable pattern of cytokine release, with the exception of G-CSF, where secretion by inner foreskin was significantly higher than outer foreskin (p=0.006). Overall, both inner and outer foreskin demonstrated a significantly higher level of production of most secreted cytokines when compared to glans.
Figure 4. Cytokine production by male genital tissue.
Cytokine expression was quantified in glans (black bars), inner foreskin (grey bars) and outer foreskin (white bars) after culturing tissue explants for 24 hours in medium alone. Data represent the mean ± standard error of 9 separate donors, where each donor was tested in triplicate.
Evaluation of microbicide safety and efficacy
PRO 2000, PMPA and Cyanovirin-N were tested for their ability to inhibit HIV-1BaL infection and dissemination (Figure 5). PMPA and PRO 2000 gel placebo demonstrated no activity against HIV infection (data not shown). No toxicity was detected at the highest concentrations tested for any of the compounds (data not shown).
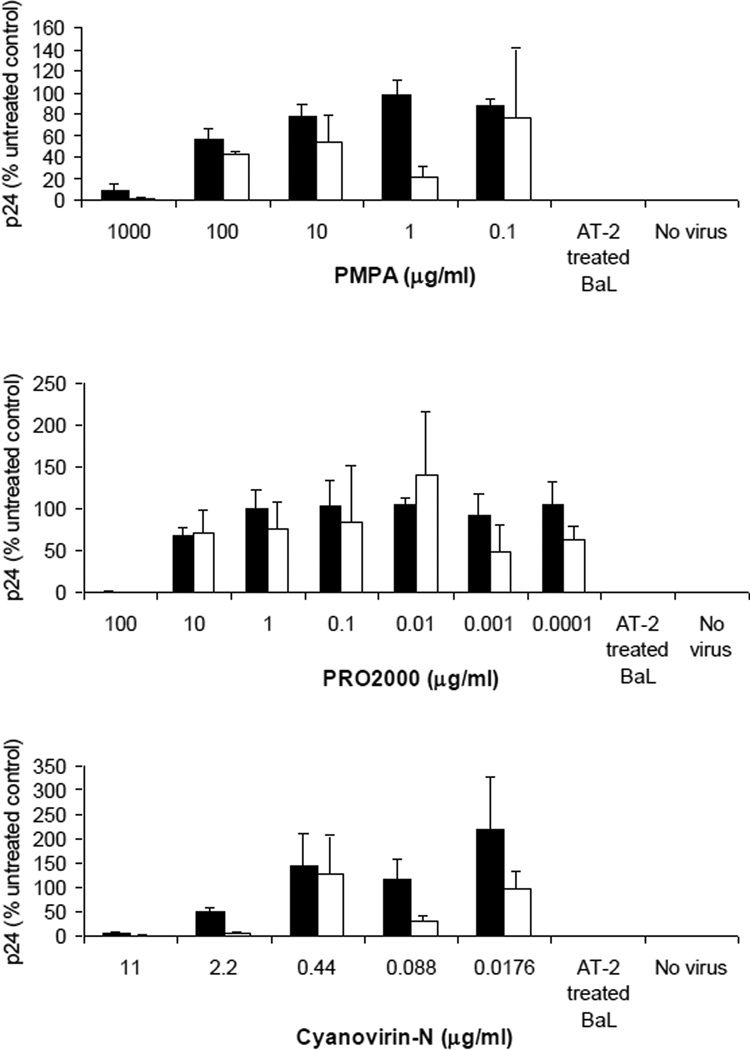
Figure 5. Inhibition of HIV infection and viral dissemination by candidate microbicides.
Penile tissue explants were exposed to compound (PMPA (Figure 5a), PRO 2000 (Figure 5b) and Cyanovirin-N (Figure 5c)) just prior to exposure to HIV-1BaL in the presence of compound for 2 hours. Virus and compound were removed and explants cultured overnight. Explants were then separated from any cells that had migrated from the tissue and cultured separately for 10 days (black bars). Viral replication was determined by measurement of p24 release into culture supernatant and is expressed as % of the untreated control. Viral dissemination by migratory cells (white bars) was determined by p24 release following 4 day co-culture with indicator T cells. Data represent the mean ± standard error of 3 separate donors, where each donor was tested in triplicate.
PMPA at 1 mg/ml (10 fold dilution of the original 1% stock gel) inhibited HIV infection of glans by 90%, and completely inhibited dissemination by migratory cells (Figure 5a). PRO 2000 at 100 µg/ml (400 fold dilution of the original 4% stock gel) inhibited infection by 99.4%, and dissemination of infection was totally suppressed (Figure 5b). Cyanovirin-N at 11 µg/ml prevented HIV replication in tissue (95%) and propagation of the virus by migratory cells (99%) (Figure 5c). Similar results were obtained when tested on foreskin (data not shown).
PMPA, PRO 2000 and CV-N had little effect on the 23 cytokines tested and none affected cytokine release by outer foreskin. However, Cyanovirin-N at 11 µg/ml increased production of SDF-1β (2.8 fold) and MIP-1β (2.9 fold) in glans, and IL-2 (2 fold), IFN-β (2.4 fold), IFN-γ (1.6 fold), MIG (21.2 fold), GM CSF (1.4 fold), IP-10 (2.7 fold) and MCP-2 (1.8 fold) in inner foreskin (p<0.05). PRO 2000 at 100 µg/ml did not induce any modification in cytokine release by both inner and outer foreskin, but reduced the production of SDF-1β 2.3 fold (p<0.01), MIP-1β (6 fold), IP-10 (3.1 fold) and MCP-2 (2.7 fold) (p<0.05) in glans. PMPA 1 mg/ml did not exert any significant variation in cytokine release for all tissue sites tested, with the only exception of inner foreskin, where SDF-1β release increased 2 fold (p<0.05).
Discussion
All male genital tissue sites examined adapted well to explant culture, with the exception of foreskin, that lost tissue integrity after 3 days, most likely reflecting that foreskin epithelium is supported by a thinner layer of stroma compared to the other tissue sites. Interestingly, foreskin explants released higher levels of cytokines compared to glans, suggesting a higher level of immune activation. At the initiation of culture, the average number of LCs and CD4+ cells was greatest for glans > inner foreskin > outer foreskin, in agreement with a previous report [19] comparing inner and outer foreskin. However, another study reported a higher density of CD4+ cells and LCs in outer foreskin > inner foreskin > glans [18]. These discrepancies might reflect that in the latter study tissue was obtained post-mortem or that foreskin and glans tissue in this study were not taken from the same donors.
The distance of dendritic projections emanating from LCs bodies were closest to the epithelial surface for glan > inner = outer foreskin. These observations also differ to those of McCoombe et al., where LCs bodies and projections were particularly superficial in inner foreskin. In agreement with both previous studies [18, 19], CD4+ cells in all tissue sites were predominantly distributed within the stroma underlying the epithelium. Significant changes in cell number and dendritic projections were observed in culture. The most pronounced being the decrease in CD4+ and CD1a+ cells in glans by day 3, suggesting a considerable migration of both CD4+ cells and LCs, out of the tissue. In contrast, the number of LCs and CD4+ cells in the epithelium of both inner and outer foreskin were fairly stable.
All genital tissue sites examined were susceptible to R5 HIV-1BaL infection, independent of immune activation. In contrast, no infection was observed with X4 HIV-1LAV. These data confirm a previous study of human foreskin [19] and reflects the predominant CCR5 expression in foreskin and cervical tissue [19, 29, 30]. However, this is not the case for penile glans and urethral meatus, reported to equal levels of CCR5 and CXCR4 expression [18, 19]. Interestingly, migratory cells from all male tissue sites also preferentially transmitted HIV-1BaL over HIV-1LAV, in agreement with previous observations that in vitro derived LCs and DCs preferentially transmit R5 virus [31–38].
To investigate the potential role of male genital explants as a model for microbicide development, we selected three microbicide candidates: PRO 2000, which is in phase III efficacy trials [39–41]; PMPA gel (tenofovir), which is in phase II trials [42, 43]; Cyanovirin-N, which is in preclinical development [44–47]. PRO 2000 at 100 µg/ml, (1/50 of the vaginal gel) provided nearly complete suppression of viral infection and dissemination in penile tissue, with no toxicity and little modulation of cytokine expression. These data are in agreement with previous studies of cervical and rectal tissue [25, 26, 48] and with a clinical study demonstrating safety for 4% gel applied daily on the penis [49]. PMPA at 1 mg/ml (1/10 of the vaginal gel) demonstrated potent activity against R5 HIV-1BaL infection of glans tissue and dissemination of virus. However, it was less active then a previous study using colorectal explants [48]. PMPA demonstrated no toxicity for penile tissue and little modulation of cytokine expression, in agreement with human studies demonstrating good tolerability of PMPA gel 1% applied vaginally [50]. Cyanovirin-N conferred 95% protection against HIV-1BaL at 11 µg/ml similar to that seen in cervical explants [46] and at a dose 1/500 of that shown to be protective in macaques [46, 47]. The ability to block HIV-1 transfer by migratory cells was is in agreement with the ability of CV-N to impair DC-SIGN mediated HIV-1 transmission in vitro [51]. Although CV-N was not toxic for male genital tissue explants, we observed a degree of cytokine disregulation following 2 hours exposure to CV-N, in line with previous reports [52].
In summary, this study describes an ex vivo model to study the vulnerability of male genital mucosa to HIV infection. While we observed some differences in the frequency of target cells (CD4+ and LCs) between tissue sites, all sites examined were susceptible to HIV-1 (R5) infection. We found no evidence to support enhanced susceptibility of inner foreskin relative to glans and outer foreskin. Nevertheless, circumcision would remove 2/3 of the exposed surface area of the penis, reducing the chance of virus coming in contact with susceptible target cells. The susceptibility to infection and the observed inhibition by 3 different microbicides, 2 of which are already in clinical trials, offer new perspectives for the development of compounds able to protect male genital tissue from HIV transmission.
Acknowledgments
We are most grateful to Mr James Bellringer, Consultant Urologist and Gender Surgeon of Charing Cross Hospital, London, for provision of penile tissue samples and to St. George’s Hospital, London for providing the foreskin tissue, as well as the many tissue donors that have contributed to this work. We also would like to thank Ms Naomi Louise Armanasco for coordinating the tissue specimen collection.
Sponsorship: This work was funded by the National Institutes for Health Grant R21 HDO 48391-01. We also acknowledge the generous grant from the Fondation Dormeur that facilitated purchase of equipment used in this project.
Footnotes
Author contributions
RJS and LF designed the experiments; LF performed the cellular and explant experiments; SMB and TJH performed all the Immunohistochemistry and imaging analysis; RJS, LF and TJH wrote the manuscript.
References
- 1.WHO. AIDS epidemic update. 2007. 2007 [Google Scholar]
- 2.European Study Group on Heterosexual Transmission of HIV. Comparison of female to male and male to female transmission of HIV in 563 stable couples. BMJ. 1992;304:809–813. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hugonnet S, Mosha F, Todd J, Mugeye K, Klokke A, Ndeki L, et al. Incidence of HIV infection in stable sexual partnerships: a retrospective cohort study of 1802 couples in Mwanza Region, Tanzania. J Acquir Immune Defic Syndr. 2002;30:73–80. doi: 10.1097/00042560-200205010-00010. [DOI] [PubMed] [Google Scholar]
- 4.Nicolosi A, Correa Leite ML, Musicco M, Arici C, Gavazzeni G, Lazzarin A. The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus: a study of 730 stable couples. Italian Study Group on HIV Heterosexual Transmission. Epidemiology. 1994;5:570–575. doi: 10.1097/00001648-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Padian NS, Shiboski SC, Jewell NP. Female-to-male transmission of human immunodeficiency virus. JAMA. 1991;266:1664–1667. [PubMed] [Google Scholar]
- 6.Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 7.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 8.Serwadda D, Gray RH, Wawer MJ, Stallings RY, Sewankambo NK, Konde-Lule JK, et al. The social dynamics of HIV transmission as reflected through discordant couples in rural Uganda. AIDS. 1995;9:745–750. doi: 10.1097/00002030-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 10.Gray RH, Kiwanuka N, Quinn TC, Sewankambo NK, Serwadda D, Mangen FW, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. Rakai Project Team. AIDS. 2000;14:2371–2381. doi: 10.1097/00002030-200010200-00019. [DOI] [PubMed] [Google Scholar]
- 11.Siegfried N, Muller M, Deeks J, Volmink J, Egger M, Low N, et al. HIV and male circumcision--a systematic review with assessment of the quality of studies. Lancet Infect Dis. 2005;5:165–173. doi: 10.1016/S1473-3099(05)01309-5. [DOI] [PubMed] [Google Scholar]
- 12.Weiss HA, Quigley MA, Hayes RJ. Male circumcision and risk of HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. AIDS. 2000;14:2361–2370. doi: 10.1097/00002030-200010200-00018. [DOI] [PubMed] [Google Scholar]
- 13.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 15.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 16.Wawer MJ, Kigozi G, Serwadda D, Makumbi F, Nalugoda F, Watya S, et al. Trial of male circumcision in HIV+ men, Rakai, Uganda: effects in HIV+ men and in women partners; 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, US. 2008. [Google Scholar]
- 17.Turner AN, Morrison CS, Padian NS, Kaufman JS, Salata RA, Chipato T, et al. Men's circumcision status and women's risk of HIV acquisition in Zimbabwe and Uganda. AIDS. 2007;21:1779–1789. doi: 10.1097/QAD.0b013e32827b144c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–1495. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 19.Patterson BK, Landay A, Siegel JN, Flener Z, Pessis D, Chaviano A, Bailey RC. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donoval BA, Landay AL, Moses S, Agot K, Ndinya-Achola JO, Nyagaya EA, et al. HIV-1 target cells in foreskins of African men with varying histories of sexually transmitted infections. Am J Clin Pathol. 2006;125:386–391. [PubMed] [Google Scholar]
- 21.Hussain LA, Lehner T. Comparative investigation of Langerhans' cells and potential receptors for HIV in oral, genitourinary and rectal epithelia. Immunology. 1995;85:475–484. [PMC free article] [PubMed] [Google Scholar]
- 22.Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6:371–382. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- 23.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 24.Watts C, Kumaranayake L, Vickerman P, Terris-Prestholt F. Public Health Working Group. New York: Rockefeller Foundation; 2002. The public health benefits of microbicides in lower-income countries. [Google Scholar]
- 25.Fletcher PS, Wallace GS, Mesquita PM, Shattock RJ. Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants. Retrovirology. 2006;3:46. doi: 10.1186/1742-4690-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biancotto A, Grivel JC, Iglehart SJ, Vanpouille C, Lisco A, Sieg SF, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 29.Patterson BK, Landay A, Andersson J, Brown C, Behbahani H, Jiyamapa D, et al. Repertoire of chemokine receptor expression in the female genital tract: implications for human immunodeficiency virus transmission. Am J Pathol. 1998;153:481–490. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeaman GR, Asin S, Weldon S, Demian DJ, Collins JE, Gonzalez JL, et al. Chemokine receptor expression in the human ectocervix: implications for infection by the human immunodeficiency virus-type I. Immunology. 2004;113:524–533. doi: 10.1111/j.1365-2567.2004.01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaitseva M, Blauvelt A, Lee S, Lapham CK, Klaus-Kovtun V, Mostowski H, et al. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 32.Kawamura T, Cohen SS, Borris DL, Aquilino EA, Glushakova S, Margolis LB, et al. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J Exp Med. 2000;192:1491–1500. doi: 10.1084/jem.192.10.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David SA, Smith MS, Lopez GJ, Adany I, Mukherjee S, Buch S, et al. Selective transmission of R5-tropic HIV type 1 from dendritic cells to resting CD4+ T cells. AIDS Res Hum Retroviruses. 2001;17:59–68. doi: 10.1089/088922201750056799. [DOI] [PubMed] [Google Scholar]
- 34.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman RM. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J Virol. 1998;72:2733–2737. doi: 10.1128/jvi.72.4.2733-2737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamura T, Gulden FO, Sugaya M, McNamara DT, Borris DL, Lederman MM, et al. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc Natl Acad Sci U S A. 2003;100:8401–8406. doi: 10.1073/pnas.1432450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reece JC, Handley AJ, Anstee EJ, Morrison WA, Crowe SM, Cameron PU. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J Exp Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fahrbach KM, Barry SM, Ayehunie S, Lamore S, Klausner M, Hope TJ. Activated CD34-derived Langerhans cells mediate transinfection with human immunodeficiency virus. J Virol. 2007;81:6858–6868. doi: 10.1128/JVI.02472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turville SG, Santos JJ, Frank I, Cameron PU, Wilkinson J, Miranda-Saksena M, et al. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- 39.Moulard M, Lortat-Jacob H, Mondor I, Roca G, Wyatt R, Sodroski J, et al. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J Virol. 2000;74:1948–1960. doi: 10.1128/jvi.74.4.1948-1960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neurath AR, Strick N, Li YY. Anti-HIV-1 activity of anionic polymers: a comparative study of candidate microbicides. BMC Infect Dis. 2002;2:27. doi: 10.1186/1471-2334-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fletcher PS, Shattock RJ. PRO-2000, an antimicrobial gel for the potential prevention of HIV infection. Curr Opin Investig Drugs. 2008;9:189–200. [PubMed] [Google Scholar]
- 42.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 43. www.microbicides.org. [Google Scholar]
- 44.Botos I, O'Keefe BR, Shenoy SR, Cartner LK, Ratner DM, Seeberger PH, et al. Structures of the complexes of a potent anti-HIV protein cyanovirin-N and high mannose oligosaccharides. J Biol Chem. 2002;277:34336–34342. doi: 10.1074/jbc.M205909200. [DOI] [PubMed] [Google Scholar]
- 45.Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O'Keefe BR, Mori T, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A, et al. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20:11–18. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- 47.Tsai CC, Emau P, Jiang Y, Tian B, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2003;19:535–541. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- 48.Fletcher PS, Elliott J, Grivel JC, Margolis L, Anton P, McGowan I, Shattock RJ. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS. 2006;20:1237–1245. doi: 10.1097/01.aids.0000232230.96134.80. [DOI] [PubMed] [Google Scholar]
- 49.Tabet SR, Callahan MM, Mauck CK, Gai F, Coletti AS, Profy AT, et al. Safety and acceptability of penile application of 2 candidate topical microbicides: BufferGel and PRO 2000 Gel: 3 randomized trials in healthy low-risk men and HIV-positive men. J Acquir Immune Defic Syndr. 2003;33:476–483. doi: 10.1097/00126334-200308010-00008. [DOI] [PubMed] [Google Scholar]
- 50.Mayer KH, Maslankowski LA, Gai F, El-Sadr WM, Justman J, Kwiecien A, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20:543–551. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 51.Balzarini J, Van Herrewege Y, Vermeire K, Vanham G, Schols D. Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIV-1 transmission to T lymphocytes. Mol Pharmacol. 2007;71:3–11. doi: 10.1124/mol.106.030155. [DOI] [PubMed] [Google Scholar]
- 52.Balzarini J, Van Laethem K, Peumans WJ, Van Damme EJ, Bolmstedt A, Gago F, Schols D. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J Virol. 2006;80:8411–8421. doi: 10.1128/JVI.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]