Abstract
Introduction
The anti-anginal efficacy of ivabradine is well established. We describe a post hoc analysis in the ADDITIONS database to investigate effectiveness and tolerability of ivabradine in combination with beta-blocker in patients with angina who have had a percutaneous coronary intervention (PCI).
Methods
ADDITIONS was a non-interventional, multicenter prospective study including 2,330 patients with stable angina. In addition to beta-blocker, patients were treated with ivabradine in approved dosages for 4 months. We divided the population according to whether they had previously had a PCI or not, and explored the effect of ivabradine on heart rate, number of weekly angina attacks, frequency of nitrate consumption, as well as quality of life (QoL) and tolerability.
Results
Data were available for 2,319 patients, of whom 51.4% had previously had a PCI. There was no difference in the effect of ivabradine on mean heart rate between patients with a previous PCI [64.4 ± 7.6 beats per minute (bpm)] than those without (66.8 ± 8.5 bpm) at 4 months (both P < 0.0001). Similarly, the number of angina attacks decreased from 1.9 ± 2.4 to 0.5 ± 1.5 per week in patients with a previous PCI and 1.5 ± 2.0 to 0.3 ± 1.0 per week in patients without a previous PCI (both P < 0.0001). The frequency of nitrate consumption fell from 2.7 ± 3.7 to 1.0 ± 1.9 per week and 1.8 ± 2.8 to 0.6 ± 1.5 per week (both P < 0.0001) in patients with and without a previous PCI, respectively. There was no difference in the improvements in Canadian Cardiovascular Society class of angina, QoL, and physicians’ assessment of effectiveness and tolerability between patients with a previous PCI and those without.
Conclusion
Ivabradine is an effective and well-tolerated anti-anginal treatment in patients with stable angina after PCI. Ivabradine reduced the frequency of weekly angina attacks and nitrate consumption, led to an improvement in Canadian Cardiovascular Society class and a substantial improvement in the QoL of stable angina patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-015-0182-8) contains supplementary material, which is available to authorized users.
Keywords: Angina pectoris, Cardiology, Ivabradine, Percutaneous coronary intervention, Quality of life
Introduction
Coronary artery disease (CAD) leads to the death of more than seven million people each year worldwide [1]. Guidelines for the treatment of CAD strongly encourage the use of optimal medical therapy as a first step in its management [2, 3]. Percutaneous coronary intervention (PCI) is also frequently used in the management of CAD to relieve symptoms of angina. Registry data suggest that more than half of CAD patients have undergone a PCI, though this figure can vary depending on sex and geographical location [4, 5].
Angina pectoris is a common symptom of CAD and affects around 112 million people globally [6]. PCI has been shown to substantially reduce angina symptoms particularly in patients with severe angina [7–9]. However, a randomized clinical study, COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation; ClinicalTrials.gov #NCT00007657), showed that the benefits of PCI regarding quality of life (QoL) can be short term [10] and that angina symptoms can persist in many patients after PCI [11–13]. In a study including post-myocardial infarction (MI) patients, angina symptoms were reported to have occurred more frequently after 1 year in patients with a clinical history of PCI (25.2%) than in those without (15.4%) [13]. COURAGE also showed that complementing optimal medical therapy with PCI does not lessen the risk of death, MI, or other major cardiovascular events in comparison to medical therapy alone, and may have no long-term or additional cardiovascular benefits [14]. More recent meta-analyses of randomized trials comparing PCI and medical therapy in patients with CAD showed that PCI did not reduce the risk of mortality, non-fatal MI, or revascularization [15, 16]. Two-year results from the FAME II (ClinicalTrials.gov #NCT01132495) trial showed a significant reduction in urgent revascularizations after fractional flow reserve-guided PCI plus medical therapy compared with medical therapy alone, but also with no significant effect on mortality or MI [17]. These results may indicate a relevance of objective assessment of ischemia in patients with PCI to improve outcomes.
Optimal medical therapy is currently under-prescribed for symptomatic CAD patients and less than half benefit from such an approach prior to PCI [18]. One such medical therapy, ivabradine, lowers heart rate by reducing the activity of the cardiac pacemaker through inhibition of the I f current, which in turn decreases cardiac workload and myocardial oxygen consumption [19]. It is effective at reducing ischemia and symptoms of angina [20–23]. The ADDITIONS (PrActical Daily efficacy anD safety of Procoralan® In combinaTION with betablockerS; Controlled-Trials.com #ISRCTN53233058) trial [24] showed that treating patients with stable angina with a combination of ivabradine and beta-blocker reduces heart rate, angina attacks, and nitrate consumption and improves Canadian Cardiovascular Society (CCS) classification along with QoL. Due to the fact that angina symptoms can persist in many patients after PCI, leading to significant impairment in QoL [10–13], we aimed to evaluate ivabradine as an option for such patients with high resting heart rate. In this paper, we present a post hoc analysis of ADDITIONS that assesses the effect of ivabradine as part of a medical therapy when treating stable angina in patients with a clinical history of PCI at baseline.
Methods
Study Design
ADDITIONS was a 4-month, non-interventional, prospective, open-label multicenter trial. A more detailed description of the methods used in the ADDITIONS trial is given by Werdan et al [24]. A total of 2,330 patients with stable angina pectoris were included in 818 centers in Germany. Investigators, comprising physicians specialized in internal medicine, general practitioners, or cardiologists in private practice, filled out a standardized questionnaire at each visit during the trial.
Patients with symptomatic chronic stable angina pectoris under current beta-blocker therapy were eligible for inclusion into the study. To be suitable for treatment with ivabradine, patients also had to have one of the following: (1) angina pectoris that was insufficiently controlled with beta-blocker alone or (2) insufficient effectiveness of—or intolerance to—another anti-anginal medicine leading to a change in treatment. Only patients who had given their written consent and who met the inclusion and exclusion criteria were eligible for inclusion. Patients commenced ivabradine treatment at 5 mg, twice daily. At the investigator’s discretion, after 4 weeks, the dose could be increased to 7.5 mg, twice daily. For patients aged ≥75 years or with resting heart rate <50 beats per minute (bpm) at follow-up visits, investigators also had the option of prescribing a lower dose of 2.5 mg, twice daily. The physician was free to change the ivabradine dose during the trial if considered necessary.
This study was carried out in accordance with the Declaration of Helsinki and in agreement with the ethical guidelines of the European Independent Ethics Committee. Ethical approval was granted by the ethical commission of Martin-Luther-University Halle-Wittenberg. The ADDITIONS study is registered at www.controlled-trials.com (No. ISRCTN53233058).
Clinical Examinations
There were three visits (at baseline, and around 1 and 4 months after baseline) programmed during this trial. At baseline, parameters including general medical and cardiovascular history (previous MI or revascularization using PCI), cardiac risk factors, concomitant diseases, and medication were recorded. Heart rate, the incidence of angina attacks (within the previous week), and the frequency of use of short-acting nitrates (within the previous week) in patients were documented at baseline and after 1 and 4 months. Patient QoL was also recorded using an EQ-5D questionnaire (single scores summarized using the EQ-5D index score) at each visit [25]. Information relating to safety such as suspected adverse drug reactions, reasons for discontinuing ivabradine and changes in concomitant medication was recorded after 1 and 4 months. The effectiveness and tolerance of ivabradine was further assessed by the treating physician and categorized into “very good”, “good”, “moderate”, or “poor”. Due to the non-interventional design of the study, the participating physicians were free to initiate any intervention they considered appropriate for the individual patient.
Analysis
In this post hoc analysis of the ADDITIONS population, we divided the population according to whether they had previously had a PCI or not. In the two subgroups, we evaluated the effect of ivabradine on heart rate and assessed its impact upon the incidence of angina attacks, frequency of use of short-acting nitrates, angina classification using the CCS class, and QoL using the EQ-5D index and visual analog scale (VAS) score. The previous PCI subgroup population was also divided according to whether patients had a baseline heart rate of <70 bpm or ≥70 bpm. The impact of ivabradine on the incidence of angina attacks, frequency of use of short-acting nitrates rate and heart rate in these two subgroups was then evaluated, and the mean daily dose of ivabradine was reported.
Statistics
The analysis of the data is essentially descriptive and was performed using SAS® software (version 9.1; SAS Institute Inc., Cary, NC, USA). Absolute and relative changes were calculated for continuous variables such as heart rate, number of angina attacks, consumption of short-acting nitrates and EQ-5D score, and described using the arithmetic mean and standard deviation. Details are given in Werdan et al [24]. Absolute changes of values between baseline and follow-up visits were assessed using Wilcoxon’s signed-rank test and Chi-square test. Corresponding P values should be interpreted descriptively.
Results
Baseline
Of the 2,330 patients who participated in the ADDITIONS study, 11 were not included in this post hoc analysis as their PCI status had not been recorded. Among the 2,319 patients with a recorded PCI status (Table 1), 51.4% had previously had a PCI. Two-thirds (67%) of the patients with PCI were male vs. 49% in patients without PCI. The mean age was 65.8 ± 9.9 years and 66.0 ± 11.5 years in patients with and without a previous PCI, respectively. The majority of patients with previous PCI had only one previous PCI (56%). Patients with a previous PCI status were more likely to have a history of MI, hypertension, dyslipidemia, diabetes, and peripheral artery disease (PAD), but less likely to have chronic obstructive pulmonary disease (COPD) or asthma. The mean resting heart rate was slightly lower in patients with a previous PCI than without (83.1 ± 11.0 bpm vs. 87.0 ± 13.2 bpm, respectively). The number of angina attacks was slightly higher in patients with a PCI status than without (1.9 ± 2.4 vs.1.5 ± 2.0 per week, respectively). A similar trend was observed in the use of nitrates (2.7 ± 3.7 vs. 1.8 ± 2.8 per week, respectively). A lower proportion of patients in the previous PCI subgroup were classified as CCS class I or II (75%), in comparison with the no previous PCI subgroup (86%). At baseline, the EQ-5D scores were similar in patients with and without a previous PCI at 0.65 ± 0.28 and 0.68 ± 0.26, respectively (Table 1). The EQ-5D VAS scores were also comparable at 56.2 ± 18.1 and 58.7 ± 18.5 for previous PCI and no previous PCI, respectively.
Table 1.
Baseline characteristics of the stable CAD population with angina in ADDITIONS according to PCI status
| Characteristic | Patients with previous PCI (n = 1,193) | Patients with no previous PCI (n = 1,126) |
|---|---|---|
| Demographic characteristics | ||
| Age (years) | 65.8 ± 9.9 | 66.0 ± 11.5 |
| Men | 803 (67%) | 547 (49%) |
| Body mass index (kg/m2) | 28.1 ± 4.3 | 28.5 ± 4.9 |
| Medical history | ||
| Previous PTCA with stent (PCI) | ||
| One | 669 (56%) | – |
| Two or more | 311 (26%) | – |
| Unknown | 213 (18%) | – |
| Previous myocardial infarction | 681 (58%) | 157 (13%) |
| Hypertension | 1,072 (90%) | 988 (88%) |
| Dyslipidemia | 889 (75%) | 638 (57%) |
| Diabetes mellitus | 409 (34%) | 368 (33%) |
| Peripheral artery disease | 125 (10%) | 79 (7%) |
| COPD | 171 (14%) | 173 (15%) |
| Asthma | 37 (3%) | 76 (7%) |
| Cardiovascular medication | ||
| Beta-blockers | 1,193 (100%) | 1,126 (100%) |
| ACE inhibitors | 698 (59%) | 500 (44%) |
| AT1 antagonists | 341 (29%) | 319 (28%) |
| Calcium antagonists | 244 (20%) | 185 (16%) |
| Long-acting nitrates/molsidomin | 374 (31%) | 204 (18%) |
| Ranolazine | 12 (1%) | 4 (<1%) |
| Diuretics | 477 (40%) | 398 (35%) |
| Aspirin | 1,059 (89%) | 804 (71%) |
| Clopidogrel | 336 (28%) | 84 (7%) |
| Statins | 1,001 (84%) | 689 (61%) |
| Clinical findings | ||
| Heart rate (bpm) | 83.1 ± 11.0 | 87.0 ± 13.2 |
| Weekly number of angina attacks | 1.9 ± 2.4 | 1.5 ± 2.0 |
| Weekly use of nitrates | 2.7 ± 3.7 | 1.8 ± 2.8 |
| Systolic blood pressure (mm Hg) | 135.1 ± 15.1 | 140.0 ± 16.1 |
| Diastolic blood pressure (mm Hg) | 81.5 ± 9.3 | 83.5 ± 9.5 |
| Ejection fraction (%) | 54.9 ± 12.9 | 57.2 ± 12.9 |
| Canadian Cardiovascular Society class | ||
| Class I | 214 (20%) | 391 (40%) |
| Class II | 609 (55%) | 452 (46%) |
| Class III | 264 (24%) | 139 (14%) |
| Class IV | 12 (1%) | 4 (<1%) |
| Quality of life | ||
| Visual analog scale | 56.2 ± 18.1 | 58.7 ± 18.5 |
| Quality of life index | 0.65 ± 0.28 | 0.68 ± 0.26 |
Values are presented as mean ± standard deviation or numbers and percentages (%)
Bpm beats per minute, CAD coronary artery disease, COPD chronic obstructive pulmonary disease, PCI percutaneous coronary intervention, PTCA percutaneous transluminal coronary angioplasty
Beta-Blocker Dosage
Every patient in the study was prescribed optimal dosages of beta-blocker at the discretion of the investigator. Metoprolol and bisoprolol were prescribed for the majority of patients, at similar rates in the two subgroups (previous PCI, 45% and 36%, respectively; no previous PCI, 41% and 39%, respectively) and at similar doses (previous PCI, 110.6 ± 50.6 mg and 7.2 ± 3.4 mg, respectively; no previous PCI, 101.5 ± 48.9 mg and 6.8 ± 3.5 mg, respectively). Other beta-blockers, which were prescribed for <13% of patients in each subgroup, in decreasing order of frequency were, nebivolol, carvedilol, and atenolol. The recommended maximum beta-blocker doses were defined for metoprolol at 190 mg/day, bisoprolol and nebivolol each at 10 mg/day, and atenolol and carvedilol each at 100 mg/day. A slightly higher proportion of patients in the previous PCI subgroup (81%) were taking ≥50% to <100% or ≥100% of the recommended maximum doses in comparison with the no previous PCI group (75%).
Effect of Ivabradine
At baseline, patients with and without a previous PCI were on comparable doses of ivabradine (mean daily dose, 9.6 ± 1.3 mg/day and 9.5 ± 1.6 mg/day, respectively). An initial rapid decrease in heart rate was similarly observed in both subgroups over the first month (Fig. 1). The heart rate of patients with a previous PCI decreased from 83.1 ± 11.0 bpm to 69.4 ± 8.8 bpm, while the heart rate in patients without a previous PCI dropped from 87.0 ± 13.2 bpm to 72.5 ± 10.6 bpm. The decrease in heart rate was maintained in the 2 subgroups over the study, and the heart rates for the previous PCI and no previous PCI subgroups at 4 months were 64.4 ± 7.6 bpm and 66.8 ± 8.5 bpm, respectively (P < 0.0001 for all changes in both subgroups). The mean daily dose of ivabradine increased in both subgroups over the 4-month study period to 12.6 ± 2.9 mg/day and 12.1 ± 3.0 mg/day for patients with and without a PCI, respectively.
Fig. 1.
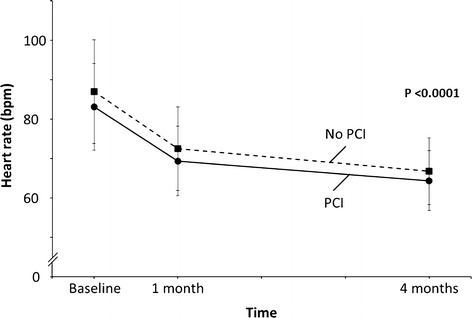
Reduction in resting heart rate with initiation of ivabradine in patients with stable angina on top of optimal medical therapy including beta-blockers who underwent previous percutaneous coronary intervention (PCI) and those who did not (No PCI). P value describes change between baseline and month 1 and baseline and month 4 for both subgroups. bpm beats per minute
The number of angina attacks experienced by patients with a history of PCI was slightly higher at baseline at 1.9 ± 2.4 per week vs. 1.5 ± 2.0 per week in patients without. However, a substantial and similar decrease in the number of weekly angina attacks was observed in both subgroups after 1 month dropping 2.7-fold to 0.7 ± 1.4 and threefold to 0.5 ± 1.1 for the previous PCI and no previous PCI subgroups, respectively (Fig. 2). By 4 months, the number of angina attacks had further dropped to comparable levels in patients with a PCI (0.3 ± 1.0 attacks per week) and patients without (0.2 ± 0.7 attacks per week; P < 0.0001 for all changes in both subgroups). The drop in the number of angina attacks was reflected by a similar decrease in the use of short-acting nitrates. At baseline, patients with a previous PCI used nitrate more often (2.7 ± 3.7 times per week) than patients without (1.8 ± 2.8 times per week). After 1 month, nitrate usage dropped to 1.0 ± 1.9 and 0.6 ± 1.5 times per week in patients with and without a PCI, respectively (Fig. 2). The decrease in nitrate usage continued at a comparable rate in both subgroups, so that by 4 months patients with and without a PCI were using nitrates at similar frequencies, 0.5 ± 1.5 and 0.3 ± 1.0 times per week (P < 0.0001 for all changes in both subgroups).
Fig. 2.
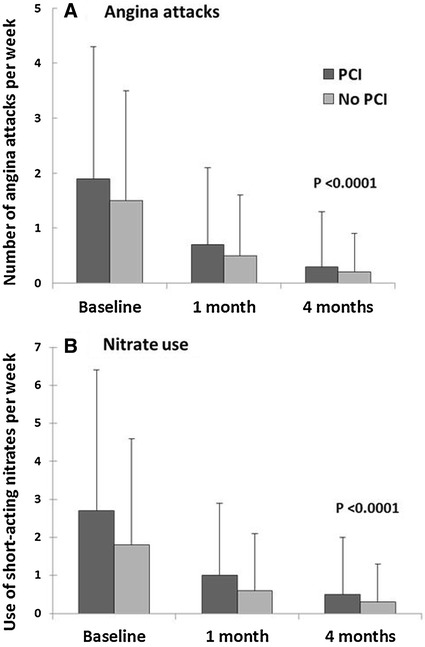
Number of angina attacks (a) and use of short-acting nitrates (b) per week in post-PCI and no PCI stable angina patients. P value describes change between baseline and month 1 and baseline and month 4 for both subgroups. PCI percutaneous coronary intervention
The severity of angina was also assessed in both groups using the CCS scale. At baseline, twice the number of patients without a previous PCI (39.7%) had CCS class I angina than patients with a previous PCI (19.5%; Fig. 3). Most patients were in CCS class II angina (55.4% with a PCI and 45.8% without). After 4 months of treatment, there was a substantial shift of patients to CCS class I in both subgroups from 19.5% to 65.2% in patients with a PCI and 39.7% to 71.1% in patients without. Notably, the combined proportion of patients in the previous PCI subgroup who moved into CCS class III and IV dropped by 21.2% after 4 months in comparison with an 11.1% drop in the no previous PCI subgroup (P < 0.0001 for changes in each CCS Class).
Fig. 3.
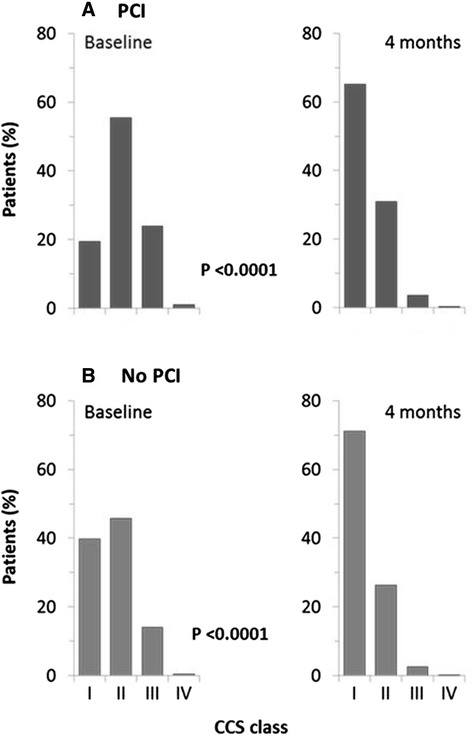
Change in severity of angina from baseline to 4 months, according to CCS class, in post-PCI and no PCI stable angina patients. P value describes the change between baseline and month 1 (data not shown) and baseline and month 4 for each CCS class. CCS Canadian cardiovascular society, PCI percutaneous coronary intervention
After 4 months, the EQ-5D score indicated that patient QoL had improved in both subgroups increasing from 0.65 ± 0.28 to 0.83 ± 0.20 in patients with a previous PCI and 0.68 ± 0.26 to 0.82 ± 0.20 for patients without (P < 0.0001 for all changes in both subgroups; Fig. 4). QoL improved slightly more in the previous PCI subgroup (27.8%) than in the no previous PCI group (21.9%). The final VAS scores indicated an improvement in QoL in all patients with slightly better improvement in scores in patients with a previous PCI (28.7%) vs. those without (24.5%; 56.2 ± 18.1 to 72.3 ± 15.2 vs. 58.7 ± 18.5 to 73.1 ± 15.6, respectively).
Fig. 4.
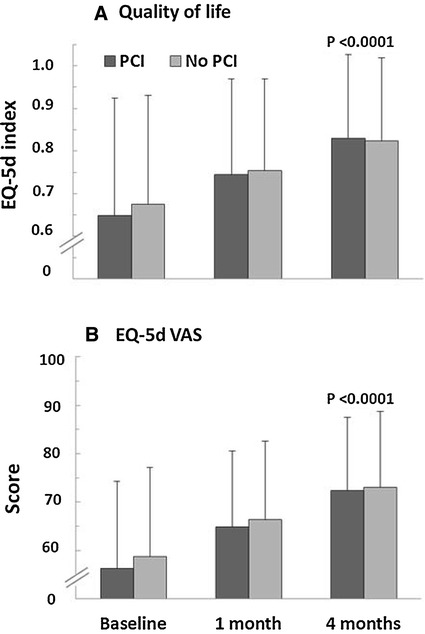
Change in quality of life index (EQ-5d) (a) and EQ-5d visual analog scale score (b) in post-PCI and no PCI stable angina patients. P value describes change between baseline and month 1 and baseline and month 4 for both subgroups. PCI percutaneous coronary intervention, VAS Visual analog scale
The efficacy of ivabradine in patients with a baseline heart rate of <70 bpm vs. ≥70 bpm, in terms of the absolute and relative reduction in the frequency of angina attacks, nitrate consumption, and heart rate, was also evaluated. In the previous PCI subgroup, patients with a baseline heart rate ≥70 bpm had similar absolute decrease in angina attacks at month 1 (−1.3 ± 1.7 vs. −1.1 ± 2.4 per week) and month 4 (−1.6 ± 1.9 vs. −1.7 ± 2.9 per week) than those with a baseline heart rate <70 bpm (Table 2). However, there was a more pronounced absolute reduction in the use of nitrates per week at month 4 for patients with a baseline heart rate of ≥70 bpm than those with a baseline heart rate of <70 bpm (−2.3 ± 3.1 vs. −1.7 ± 5.0). There was a large difference in the absolute change in heart rate for patients with a baseline heart rate of ≥70 bpm vs. <70 bpm at month 1 (−14.6 ± 8.7 vs. −2.9 ± 7.3 bpm) and at month 4 (−19.9 ± 9.8 vs. −5.0 ± 6.9 bpm). Patients with a baseline heart rate of ≥70 bpm were on a higher daily dose of ivabradine (12.7 ± 2.8 mg) during the study than patients with a baseline heart rate of <70 bpm (10.9 ± 2.8 mg). There was a greater relative reduction in the number of angina attacks per week in patients with a baseline heart ≥70 bpm in comparison with patients who had a baseline heart rate of <70 bpm at month 1 (70.7% vs. 49.2%) and at month 4 (87.5% vs. 67.0%; Fig. 5). A larger relative reduction in weekly nitrate consumption was also observed for patients with a baseline heart rate ≥70 bpm vs. <70 bpm at month 1 (70.0% vs. 57.3%) and 4 (86.9% vs. 60.7%). Patients with a baseline heart rate ≥70 bpm also had a considerably greater relative reduction in heart rate than patients with a baseline heart rate of <70 bpm at month 1 (16.8% vs.4.1%) and month 4 (22.9% vs. 7.3%).
Table 2.
Absolute reduction in angina attacks, nitrate use, and heart rate and mean daily ivabradine dose in the previous PCI subgroup according to patient baseline heart rate
| Absolute reduction | Baseline heart rate | |
|---|---|---|
| <70 bpm (n = 94) | ≥70 bpm (n = 1,095) | |
| Angina attacks (per week) | ||
| Month 1 |
−1.1 ± 2.4 n = 85 |
−1.3 ± 1.7 n = 1,006 |
| Month 4 |
−1.7 ± 2.9 n = 85 |
−1.6 ± 1.9 n = 970 |
| Nitrate use (per week) | ||
| Month 1 |
−1.6 ± 3.8 n = 83 |
−1.8 ± 2.6 n = 987 |
| Month 4 |
−1.7 ± 5.0 n = 82 |
−2.3 ± 3.1 n = 956 |
| Heart rate | ||
| Month 1 |
−2.9 ± 7.3 n = 93 |
−14.6 ± 8.7 n = 1,087 |
| Month 4 |
−5.0 ± 6.9 n = 91 |
−19.9 ± 9.8 n = 1,073 |
| Daily ivabradine dose (mg) | ||
| Month 1 |
10.7 ± 2.8 n = 91 |
12.7 ± 2.8 n = 1,073 |
| Month 4 |
10.9 ± 2.8 n = 88 |
12.7 ± 2.8 n = 1,057 |
Values are presented as mean ± standard deviation
bpm beat per minute, PCI percutaneous coronary intervention
Fig. 5.
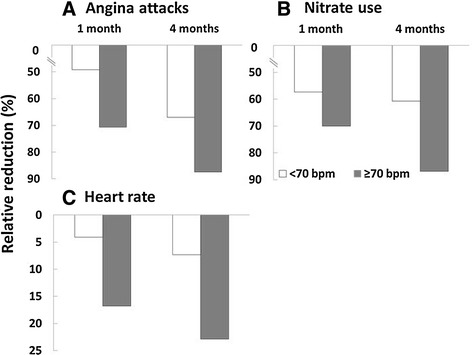
Relative reduction in the number of angina attacks per week (a) and use of short-acting nitrates (b) per week and heart rate (c) in patients with stable angina in the post-PCI group according to baseline heart rate. PCI percutaneous coronary intervention
Investigators Assessment
The effectiveness and tolerability of ivabradine were assessed as either “very good” or “good” by treating physicians for over 96% of all patients. No differences in either effectiveness or tolerability were observed by the physicians between the two subgroups (Table 3). For the majority of patients with and without a previous PCI (97.8% and 96.6%, respectively), treating physicians categorized ivabradine effectiveness as either “very good” or “good”. Similarly, physicians classified ivabradine tolerability as either “very good” or “good” for over 99% of patients in each of the two subgroups.
Table 3.
Physician assessment of the effectiveness and tolerability of ivabradine after 4 months in the ADDITIONS population, according to PCI status
| Ivabradine | Patients with previous PCI (n = 1,193) | Patients with no previous PCI (n = 1,126) |
|---|---|---|
| Effectiveness | 1,170 (98%) | 1,109 (98%) |
| Very good | 732 (63%) | 662 (60%) |
| Good | 412 (35%) | 409 (37%) |
| Moderate | 25 (2%) | 36 (3%) |
| Bad | 1 (< 1%) | 2 (< 1%) |
| Tolerability | 1,136 (95%) | 1,065 (95%) |
| Very good | 805 (71%) | 768 (72%) |
| Good | 328 (29%) | 295 (28%) |
| Moderate | 2 (< 1%) | 2 (< 1%) |
| Bad | 1 (< 1%) | 0 (0%) |
Values are patient numbers and percentages (%)
PCI percutaneous coronary intervention
Safety
Fourteen patients (0.6%; see Table 4 for subgroup data) experienced an adverse drug reaction during the 4-month study period in the total cohort, the most common of which were the presence of phosphenes in the eye (0.13%) and dizziness (0.13%). Other reported adverse drug reactions were palpitations (0.09%), headaches (0.04%), and bradycardia (0.04%). There were no reported cases of death or non-fatal MI. No serious adverse drug reactions were reported during the study.
Table 4.
The most frequently reported adverse drug reactions, according to PCI status, classified using MedDRA (Medical Dictionary for Regulatory Activities)
| Adverse drug reaction | Patients with previous PCI (n = 1,193) | Patients with no previous PCI (n = 1,126) |
|---|---|---|
| All | 10 (0.8%) | 4 (0.4%) |
| Phosphenes (visual symptoms) | 3 (0.3%) | 0 (0.0%) |
| Dizziness | 0 (0.0%) | 3 (0.3%) |
| Palpitations | 1 (0.1%) | 1 (0.1%) |
| Bradycardia | 1 (0.1%) | 0 (0.0%) |
Values are patient numbers and percentages (%)
PCI percutaneous coronary intervention
Discussion
This analysis has shown that treating stable angina pectoris patients with a combination of ivabradine and beta-blockers lowered heart rate and reduced the frequency of angina symptoms to the extent that CCS classification and QoL improved independently of PCI status (P < 0.0001 for all changes). Ivabradine was well tolerated by patients in both subgroups. These results are consistent with the findings of a pooled analysis of randomized trials assessing the effect of ivabradine in various subpopulations of angina pectoris patients including those with a previous PCI [26].
PCI alleviates symptoms of angina by a local approach, as it directly removes defined, hemodynamically compromising lesions [27]. It has an important role to play in the treatment of lifestyle-limiting angina [28]. However, as our results indicate, angina symptoms can recur in a large proportion (51%) of post-PCI patients [24]. In this study, 26% of the previous PCI group had received 2 or more stents and despite this they still suffered symptoms of angina. Recurrent symptoms were also reported in a study in a Swedish population where more than half of the women and a third of the men experienced angina symptoms 4 years after undergoing a PCI [12]. This was also confirmed by findings from the COURAGE trial, where 41% of patients had angina 3 years after PCI treatment [10].
As CAD is a diffuse disease process, frequently involving the entire coronary vasculature, it is more likely to benefit from systemic treatments such as medical therapy [29]. This, combined with the fact that PCI does not lessen the risk of death or other cardiovascular outcomes in comparison with medical therapy alone [14], suggests that optimal medical therapy for the treatment of stable angina should be pursued from the outset [14, 18, 30] and is a course of action for symptom control currently recommended by the European and American guidelines for the treatment of CAD [2, 3].
Heart rate is now believed to have a fundamental role in ischemia and angina. An elevated heart rate increases myocardial oxygen demand and reduces diastolic time, these effects can limit myocardial perfusion leading to ischemia and, in turn, triggering angina [20, 31]. In patients with stable CAD, an elevated mean heart rate often precedes myocardial ischemia and is associated with a higher risk of cardiovascular events [32–34]. Therefore, medical therapies that effectively reduce heart rate merit serious consideration, but are currently under-prescribed in the treatment of CAD and stable angina [35].
Ivabradine reduces heart rate by specifically inhibiting the I f current, but does not affect blood pressure or other cardiac parameters. In reducing heart rate, ivabradine leads to a decrease in myocardial oxygen consumption and an increase in myocardial perfusion, thereby improving cardiac efficiency [36]. Furthermore, ivabradine maintains the coronary vasodilation that occurs during exercise [37]. The results of a placebo-controlled randomized study showed that ivabradine improves coronary collateral function in patients with stable CAD, which was accompanied by diminished ECG signs of ischemia [38]. In contrast, beta-blockers—owing to their negative effect on myocardial contractility—prolong systole, reducing their beneficial effect on diastolic time. Beta-blockers may also affect vasomotion in the coronary circulation by unmasking alpha-adrenergic vasoconstriction resulting in constriction of large and small coronary arteries during exercise [39]. Ivabradine has been shown to be an effective anti-anginal agent either as a stand-alone or as a combination therapy including beta-blockers [21, 40]. Despite the fact that several guidelines recommend a target heart rate below 60 bpm in patients with angina [2, 3], the CLARIFY (Controlled-Trials.com #ISRCTN43070564) registry revealed that only 22% of angina patients on beta-blockers achieved an optimal heart rate. Thus, many patients with still elevated heart rate could benefit from the addition of ivabradine to their treatment [41].
In the ADDITIONS study, the addition of ivabradine led to a further substantial reduction in resting heart rate without excessive bradycardia. The effect was significant within the first 4 weeks of treatment despite prior and ongoing beta-blocker therapy. The effect of ivabradine was more pronounced in patients who had a baseline heart rate of ≥70 bpm. The relative reduction in the number of angina attacks and short-acting nitrate consumption was substantially larger in patients with a baseline heart rate of ≥70 bpm than <70 bpm at 1 month and 4 months. These data are in line with the pharmacological use-dependent properties of ivabradine, and therefore patients with a higher heart rate will benefit most from therapy with ivabradine [42]. This is also in line with new recommendations from the European Medicines Agency (EMA) to initiate ivabradine therapy in patients with angina with a heart rate of ≥70 bpm.
QoL has been shown to be worse in patients with stable angina than in those without [43]. In this post hoc analysis, treatment with ivabradine was associated with improved QoL in patients with stable angina. The CADENCE (Coronary Artery Disease in General Practice; ANZCTR.org.au #ACTRN12608000347369) study reported that 29% of stable angina patients experienced weekly angina (≥1 episode per week) and had a poorer QoL than patients who experienced angina less than once a week [44]. These results correlate with our findings where the mean frequency of angina attacks dropped from >1 to <1 attack per week and patient QoL improved congruently. QoL is an important factor to be kept in mind when assessing the effectiveness of CAD treatment. The Society of Cardiovascular Angiography recommends that QoL be considered as an outcome in clinical trials and physicians care [45]. While PCI improves patient QoL in the short term, it is often proportional to the severity of angina symptoms and there is less evidence supporting the benefits of PCI in the presence of severe comorbidities [45]. The results of this post hoc analysis of ADDITIONS have demonstrated that combining ivabradine with beta-blocker in daily clinical practice reduces symptoms, improves CCS classification and also the QoL in patients with stable angina pectoris after PCI.
The recent data from the SIGNIFY (Study assessInG the morbidity–mortality beNefits of the If inhibitor ivabradine in patients with coronarY artery disease, Controlled-Trials.com #ISRCTN61576291) trial, conducted in patients with CAD on optimal medical treatment without clinical heart failure, did not demonstrate an effect of ivabradine on the incidence of the primary combined endpoint of cardiovascular death and non-fatal MI [46]. In a pre-specified subgroup of symptomatic angina patients, there was a small, but statistically significant, increase in the incidence of the primary endpoint with no significant effects for the individual components of the primary endpoint. The incidence of bradycardia was the highest ever reported in any ivabradine trial (ivabradine, 18.0% vs. placebo, 2.3%; P < 0.001). However, in the SIGNIFY trial a therapeutic regimen that is not approved for clinical use was applied, which included a higher initiation and uptitration dose (7.5 and 10 mg bid, respectively). The EMA conducted a review of ivabradine and made recommendations aimed at reducing the risk when using ivabradine in patients with angina. These recommendations included the importance of respecting the recommended dosage regimen—the starting dose should not exceed 5 mg, twice daily, and the maximum dose should not exceed 7.5 mg, twice daily. Furthermore, ivabradine should only be prescribed for patients with a resting heart rate of ≥70 bpm, with a contraindication for the concomitant use of ivabradine with diltiazem or verapamil [47].
Regarding the safety profile of ivabradine in our study, only mild adverse drug reactions were reported in 0.6% of all patients. There were no reported cases of death, non-fatal MI or serious adverse drug reactions during the study.
A limitation of the study was its short duration (4 months) and that it was open label. There was also no placebo group to support the observed effect of treatment. Adherence to the treatment was not formally evaluated, though the significant reduction in heart rate observed throughout the study indicates good adherence. The frequency of weekly angina attacks and nitrate consumption was measured for only 1 week prior to the study visits. This was due, in part, to the non-interventional design of the study but also to avoid poor or inaccurate symptom recall by patients, which could occur over a longer period of time. Nevertheless, reporting/recall bias to some extent cannot be ruled out, but this may in part also be compensated using different measures of effectiveness (number of attacks, nitrate use, QoL). The missing placebo group, presence of possible reporting bias, and also relatively high resting heart rate at baseline in both patient groups (ivabradine effects are more pronounced with higher heart rate) can lead to an overestimation of the treatment benefit. On the other hand, ivabradine is often used in these high heart rates in practice, thus reflecting current clinical use of the compound. Only half of the patients were uptitrated to the recommended maximal dose (7.5 mg twice daily). Although it is not possible to say to which extent the symptomatic benefit may have increased in this study population by intensified uptitration, it has been demonstrated that heart rate lowering and anti-anginal effects of ivabradine are clearly dose dependent up to 7.5 mg twice daily [20]. Another limitation of the study regarding specifically the sub-analysis of patients with PCI according to baseline heart rate (<70 bpm or ≥70 bpm) is the small number of patients with baseline heart rate <70 bpm (n = 94). But it should be noted that ivabradine is not to be used in angina patients with baseline heart rate below 70 bpm according to recent EMA recommendations and European Union indication. Focus of this analysis is therefore clearly the patient cohort with heart rate ≥70 bpm. The main strength of this study was that it was undertaken in a large population of stable angina patients, who were already receiving optimized medical therapy, in over 800 centers in Germany. Moreover, the non-interventional study design supported an evaluation of treatment effects under conditions of routine clinical practice, taking into account patient subgroups that are usually excluded from clinical trials, such as elderly patients or patients with multiple comorbidities.
Conclusions
The addition of ivabradine to medical therapy, including beta-blockers, for the treatment of stable angina was effective and well tolerated, independent of PCI status. Many patients with a previous PCI have recurring symptoms of angina. Ivabradine should be considered as an important therapeutical option in the treatment of symptomatic stable angina as it reduced the frequency of weekly angina attacks and nitrate usage, led to an improvement in CCS class and a substantial improvement in the QoL of patients with stable angina.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The study was supported by Servier Deutschland GmbH, Munich, Germany. Sponsorship and article processing charges for this study were funded by Servier Deutschland. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. The authors would like to thank all investigators for their contributions to the study. Subsets of these data were presented during poster sessions at the Autumn Congress of the German Society of Cardiology in 2013.
Conflict of interest
K. Werdan, has been engaged in RCTs with ivabradine fully or partly supported by Servier (BEAUTIFUL, SHIFT, SIGNIFY, MODIFY and others), has received honoraria for lectures from Servier, is a member of the German Procoralan advisory board of Servier, and receives research grants for experimental and clinical ivabradine research from Servier. H. Ebelt has been engaged in RCTs with ivabradine fully or partly supported by Servier (BEAUTIFUL, SHIFT, SIGNIFY, MODIFY and others). S. Nuding has been engaged in RCTs with ivabradine fully or partly supported by Servier (BEAUTIFUL, SHIFT, SIGNIFY, MODIFY and others). Dr Stöckl is an employee of Servier (Medical Affairs). U. Müller-Werdan and F. Höpfner report no conflict of interest.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
On behalf of the ADDITIONS Study Investigators.
Trial registration: Controlled-Trials.com #ISRCTN53233058.
References
- 1.World Health Organization. Types of cardiovascular disease. 2014. Available from: http://www.who.int/cardiovascular_diseases/en/cvd_atlas_01_types.pdf?ua=1. Accessed July 31, 2014.
- 2.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 3.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126(25):e354–e471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 4.Steg PG, Greenlaw N, Tardif JC, et al. Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective CLARIFY registry. Eur Heart J. 2012;33(22):2831–2840. doi: 10.1093/eurheartj/ehs289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrari R, Ford I, Greenlaw N, et al. Geographical variations in the prevalence and management of cardiovascular risk factors in outpatients with CAD: data from the contemporary CLARIFY registry. Eur J Prev Cardiol. 2014. Epub ahead of print. [DOI] [PubMed]
- 6.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coronary angioplasty versus medical therapy for angina: the second Randomised Intervention Treatment of Angina (RITA-2) trial. RITA-2 trial participants. Lancet. 1997;350(9076):461–8. [PubMed]
- 8.Henderson RA, Pocock SJ, Clayton TC, et al. Seven-year outcome in the RITA-2 trial: coronary angioplasty versus medical therapy. J Am Coll Cardiol. 2003;42(7):1161–1170. doi: 10.1016/S0735-1097(03)00951-3. [DOI] [PubMed] [Google Scholar]
- 9.Parisi AF, Folland ED, Hartigan P. A comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease. Veterans Affairs ACME Investigators. N Engl J Med. 1992;326(1):10–16. doi: 10.1056/NEJM199201023260102. [DOI] [PubMed] [Google Scholar]
- 10.Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359(7):677–687. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 11.Holubkov R, Laskey WK, Haviland A, et al. Angina 1 year after percutaneous coronary intervention: a report from the NHLBI Dynamic Registry. Am Heart J. 2002;144(5):826–833. doi: 10.1067/mhj.2002.125505. [DOI] [PubMed] [Google Scholar]
- 12.Brorsson B, Bernstein SJ, Brook RH, Werko L. Quality of life of patients with chronic stable angina before and four years after coronary revascularisation compared with a normal population. Heart. 2002;87(2):140–145. doi: 10.1136/heart.87.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maddox TM, Reid KJ, Spertus JA, et al. Angina at 1 year after myocardial infarction: prevalence and associated findings. Arch Intern Med. 2008;168(12):1310–1316. doi: 10.1001/archinte.168.12.1310. [DOI] [PubMed] [Google Scholar]
- 14.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 15.Pursnani S, Korley F, Gopaul R, et al. Percutaneous coronary intervention versus optimal medical therapy in stable coronary artery disease: a systematic review and meta-analysis of randomized clinical trials. Circ Cardiovasc Interv. 2012;5(4):476–490. doi: 10.1161/CIRCINTERVENTIONS.112.970954. [DOI] [PubMed] [Google Scholar]
- 16.Stergiopoulos K, Brown DL. Initial coronary stent implantation with medical therapy vs medical therapy alone for stable coronary artery disease: meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(4):312–319. doi: 10.1001/archinternmed.2011.1484. [DOI] [PubMed] [Google Scholar]
- 17.De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208–1217. doi: 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]
- 18.Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention. JAMA. 2011;305(18):1882–1889. doi: 10.1001/jama.2011.601. [DOI] [PubMed] [Google Scholar]
- 19.Summary of product characteristics. Procoralan. European Medicines Agency. 2005. Available from: http://www.ema.europa.eu. Accessed 15 April 2014.
- 20.Borer JS, Fox K, Jaillon P, Lerebours G. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107(6):817–823. doi: 10.1161/01.CIR.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- 21.Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26(23):2529–2536. doi: 10.1093/eurheartj/ehi586. [DOI] [PubMed] [Google Scholar]
- 22.Ruzyllo W, Tendera M, Ford I, Fox KM. Antianginal efficacy and safety of ivabradine compared with amlodipine in patients with stable effort angina pectoris: a 3-month randomised, double-blind, multicentre, noninferiority trial. Drugs. 2007;67(3):393–405. doi: 10.2165/00003495-200767030-00005. [DOI] [PubMed] [Google Scholar]
- 23.Koester R, Kaehler J, Ebelt H, Soeffker G, Werdan K, Meinertz T. Ivabradine in combination with beta-blocker therapy for the treatment of stable angina pectoris in every day clinical practice. Clin Res Cardiol. 2010;99(10):665–672. doi: 10.1007/s00392-010-0172-4. [DOI] [PubMed] [Google Scholar]
- 24.Werdan K, Ebelt H, Nuding S, Hopfner F, Hack G, Muller-Werdan U. Ivabradine in combination with beta-blocker improves symptoms and quality of life in patients with stable angina pectoris: results from the ADDITIONS study. Clin Res Cardiol. 2012;101(5):365–373. doi: 10.1007/s00392-011-0402-4. [DOI] [PubMed] [Google Scholar]
- 25.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Tendera M, Borer J, Tardif J. Efficacy of I(f) inhibition with ivabradine in different subpopulations with stable angina pectoris. Cardiology. 2009;114(2):116–125. doi: 10.1159/000219938. [DOI] [PubMed] [Google Scholar]
- 27.Holmes DR, Jr, Gersh BJ, Whitlow P, King SB, III, Dove JT. Percutaneous coronary intervention for chronic stable angina: a reassessment. JACC Cardiovasc Interv. 2008;1(1):34–43. doi: 10.1016/j.jcin.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Abrams J. Clinical practice. Chronic stable angina. N Engl J Med. 2005;352(24):2524–2533. doi: 10.1056/NEJMcp042317. [DOI] [PubMed] [Google Scholar]
- 29.Trikalinos TA, Sheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM. Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis. Lancet. 2009;373(9667):911–918. doi: 10.1016/S0140-6736(09)60319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang JC. Underestimating medical therapy for coronary disease… again. N Engl J Med. 2011;364(17):1671–1673. doi: 10.1056/NEJMe1103414. [DOI] [PubMed] [Google Scholar]
- 31.Fox KM, Ferrari R. Heart rate: a forgotten link in coronary artery disease? Nat Rev Cardiol. 2011;8(7):369–379. doi: 10.1038/nrcardio.2011.58. [DOI] [PubMed] [Google Scholar]
- 32.Deedwania PC, Carbajal EV. Role of myocardial oxygen demand in the pathogenesis of silent ischemia during daily life. Am J Cardiol. 1992;70(16):19F–24F. doi: 10.1016/0002-9149(92)90185-2. [DOI] [PubMed] [Google Scholar]
- 33.Hinderliter A, Miller P, Bragdon E, Ballenger M, Sheps D. Myocardial ischemia during daily activities: the importance of increased myocardial oxygen demand. J Am Coll Cardiol. 1991;18(2):405–412. doi: 10.1016/0735-1097(91)90593-X. [DOI] [PubMed] [Google Scholar]
- 34.McLenachan JM, Weidinger FF, Barry J, et al. Relations between heart rate, ischemia, and drug therapy during daily life in patients with coronary artery disease. Circulation. 1991;83(4):1263–1270. doi: 10.1161/01.CIR.83.4.1263. [DOI] [PubMed] [Google Scholar]
- 35.Daly CA, Clemens F, Sendon JL, et al. Inadequate control of heart rate in patients with stable angina: results from the European heart survey. Postgrad Med J. 2010;86(1014):212–217. doi: 10.1136/pgmj.2009.084384. [DOI] [PubMed] [Google Scholar]
- 36.Colin P, Ghaleh B, Monnet X, et al. Contributions of heart rate and contractility to myocardial oxygen balance during exercise. Am J Physiol Heart Circ Physiol. 2003;284(2):H676–H682. doi: 10.1152/ajpheart.00564.2002. [DOI] [PubMed] [Google Scholar]
- 37.Simon L, Ghaleh B, Puybasset L, Giudicelli JF, Berdeaux A. Coronary and hemodynamic effects of S 16257, a new bradycardic agent, in resting and exercising conscious dogs. J Pharmacol Exp Ther. 1995;275(2):659–666. [PubMed] [Google Scholar]
- 38.Gloekler S, Traupe T, Stoller M, et al. The effect of heart rate reduction by ivabradine on collateral function in patients with chronic stable coronary artery disease. Heart. 2014;100(2):160–166. doi: 10.1136/heartjnl-2013-304880. [DOI] [PubMed] [Google Scholar]
- 39.Heusch G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol. 2008;153(8):1589–1601. doi: 10.1038/sj.bjp.0707673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tardif JC, Ponikowski P, Kahan T. Efficacy of the I(f) current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: a 4 month, randomized, placebo-controlled trial. Eur Heart J. 2009;30(5):540–548. doi: 10.1093/eurheartj/ehn571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steg PG, Ferrari R, Ford I, et al. Heart rate and use of beta-blockers in stable outpatients with coronary artery disease. PLoS One. 2012;7(5):e36284. doi: 10.1371/journal.pone.0036284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borer JS, Heuzey JY. Characterization of the heart rate-lowering action of ivabradine, a selective I(f) current inhibitor. Am J Ther. 2008;15(5):461–473. doi: 10.1097/MJT.0b013e3181758855. [DOI] [PubMed] [Google Scholar]
- 43.Gardner AW, Montgomery PS, Ritti-Dias RM, Thadani U. Exercise performance, physical activity, and health-related quality of life in participants with stable angina. Angiology. 2011;62(6):461–466. doi: 10.1177/0003319711399897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beltrame JF, Weekes AJ, Morgan C, Tavella R, Spertus JA. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: the Coronary Artery Disease in General Practice (CADENCE) Study. Arch Intern Med. 2009;169(16):1491–1499. doi: 10.1001/archinternmed.2009.295. [DOI] [PubMed] [Google Scholar]
- 45.Blankenship JC, Marshall JJ, Pinto DS, et al. Effect of percutaneous coronary intervention on quality of life: a consensus statement from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;81(2):243–259. doi: 10.1002/ccd.24376. [DOI] [PubMed] [Google Scholar]
- 46.Fox K, Ford I, Steg PG, Tardif JC, Tendera M, Ferrari R. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371(12):1091–1099. doi: 10.1056/NEJMoa1406430. [DOI] [PubMed] [Google Scholar]
- 47.European Medicines Agency recommends measures to reduced the risk of heart problems with Corlentor/Procoralan (ivabradine. EMA: London, UK. 2014. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2014/11/news_detail_002207.jsp&mid=WC0b01ac058004d5c1. Accessed December 5, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


