Abstract
Background
The prevalence of urolithiasis in Germany is 4.7%; its incidence has trebled in the last three decades. The risk of recurrence is 50–80%, depending on the type of stone, unless secondary prevention is instituted. Risk-adapted secondary prevention lowers this risk to 10–15%.
Method
This review is based on publications retrieved by a selective search in PubMed uisng the key words “urolithiasis,” “urinary stones,” “epidemiology,” “lithogenesis,” “biominerals,” “risk factors,” and “diagnosis, therapy, metaphylaxis.“ These publications were evaluated with the aid of the urolithiasis guideline of the European Association of Urology.
Results
Acute renal colic can usually be diagnosed without sophisticated equipment. Stones can be dealt with by a variety of techniques depending on their size and location, including extracorporeal shock-wave lithotripsy, ureterorenoscopy, percutaneous nephrolitholapaxy, and open surgery. Most ureteric stones of diameter up to 5 mm pass spontaneously. 75% of patients have no complications. The basic evaluation needed for secondary prevention can be carried out by any physician on an ambulatory basis. In the 25% of patients who have complications, a more extensive interdisciplinary evaluation of metabolic parameters should be performed in a clinical center for urinary stones.
Conclusion
Urolithiasis has many causes and can be treated in many different ways. An extensive metabolic work-up is often necessary for secondary prevention. The various treatment options must be considered for their suitability in each individual patient. Robust data are now available on surgical and interventional methods, but there are as yet no high-quality trials of secondary prevention. Further research should concentrate on the etiology and pathogenesis of urolithiasis.
In the year 2006 urolithiasis was second only to diseases of the prostate as the most frequent diagnosis at urological centers in Germany (1).
Urinary stones are polycrystalline concretions occurring in the urinary tract of humans and animals. Like bones and teeth, they are biominerals. While the non-pathological products of biomineralization, formed in genetically determined processes, display a high degree of biological organization, uroliths are a special case. Their formation is governed by pathoanatomical and physicochemical factors (2).
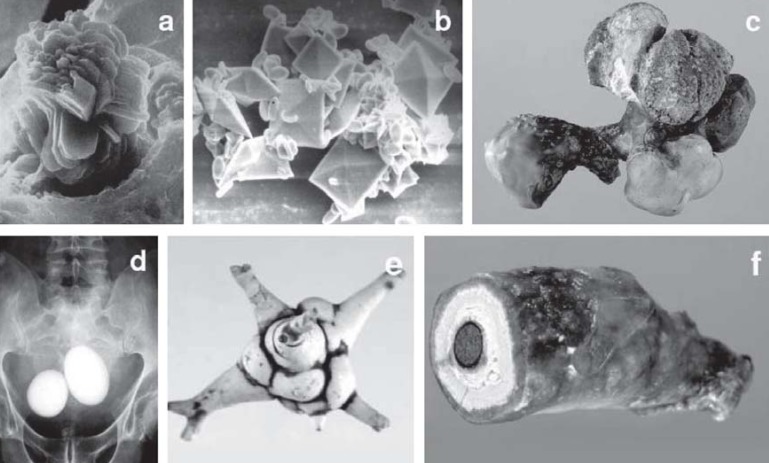
Around 97% of urinary stones are found in the kidneys and ureters (kidney stones), the remaining 3% in the urinary bladder and urethra (3). Urinary stones can range in size from micrometers to several centimeters in diameter. They frequently remain unnoticed for long periods before manifesting themselves—often very painfully—or being discovered incidentally on radiography or ultrasound (Figure 1).
Figure 1.
Starting with pathological processes in the formation of urine, mineralization can occur as high as the renal tubule (a). Crystals just a few micrometers in diameter form further downstream in the urinary tract, preferentially attaching themselves to the urothelium in sheltered niches, where—sometimes with the participation of organic cementing substances—they grow into larger aggregates (b). Under unfavorable conditions, e.g., severe metabolic disease, acute urinary tract infection, or drug-induced alteration of urinary composition, urinary stones several centimeters in diameter can form in a matter of weeks in the kidney (c), the bladder (d), or even the urethra (f). In most cases, however, the concrements (e) are flushed from the kidney into the ureter when they are just a few millimeters in diameter. They cause excruciating colicky pain and if they are too large to pass into the bladder spontaneously they have to be surgically removed.
Urinary stones are a symptom of exo- and endogenous factors and are usually of multifactorial origin. Natural passing or surgical removal of the stone does not eliminate the cause, and many patients suffer recurrences (4).
Owing to the etiological diversity of urinary stones, many different compositions are found. Calcium oxalate (whewellite, weddellite; prevalence >80%), calcium phosphate (carbonate apatite, 5%), magnesium ammonium phosphate (“infectious stones”; struvite, 5%), and uric acid (13%) are the most commonly occurring biominerals, while cystine, ammonium urate, and brushite are rare (all ≤1%) (5, 6).
Changes in lifestyle and improvements in diagnosis have led to growing prevalence and incidence of urinary stones. In Germany, a nationwide survey showed an increase in both incidence (from 0.54% to 1.47%) and prevalence (from 4.0% to 4.7%) between 1979 and 2001 (4). Urolithiasis is thus a widespread disease. Fifty percent of patients suffer at least one recurrence, and 10 to 20% experience three or more further episodes of urolithiasis (4, 6). Prevalence of 12% has been reported in the USA (6). Affluence-related urolithiasis is also on the increase in the emerging economies, due among other reasons to highly calorific diets combined with low levels of exercise. As early as the 12thcentury, Hildegard of Bingen (1098–1179) recognized the connection between rich meals, wine, and urinary stones and urged her contemporaries to modify their diets accordingly (7, 8). In the year 2000, 9.7% of German men but only 5.9% of German women in the age group 50 to 64 years had experienced an episode of urolithiasis. In the past two decades the incidence has risen predominantly between the ages of 40 and 49 years (4, 6, 9). There are differences in sex distribution and among different regions of Germany: uric acid stones are more common in the eastern and southern states, infectious stones in the east of the country (9). Calcium phosphate stones are demonstrated more often in younger patients, while uric acid stones and stones with atypical compositions are more frequent in older individuals (10). Occupation can be a risk factor: among other professions, the risk is elevated in physicians—particularly surgeons (11). Poor fluid balance is one of the factors responsible.
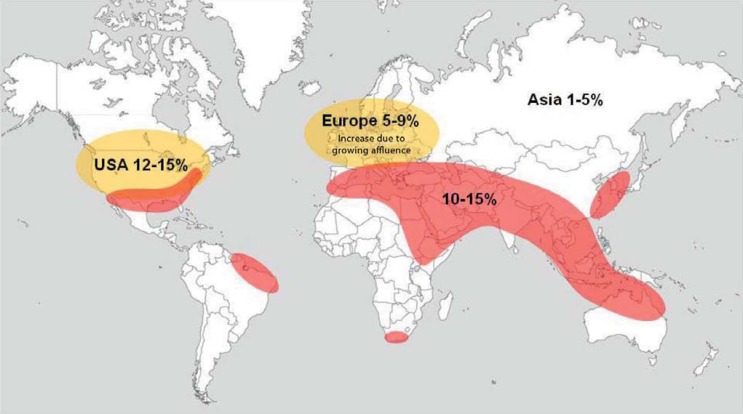
The essential factors accounting for variations in the prevalence of urolithiasis include dietary habits, climate, environment, ethnicity, and heredity (Figure 2).
Figure 2.
The so-called stone belt (red) extends all the way around the world and is characterized by urinary stone prevalence of 10 to 15%.
In this zone the climatic and social conditions are conducive to stone formation. Some stones are associated with poverty, others with affluence. In Europe and the USA, there has been a sharp, almost exclusively affluence-related rise in the occurrence of calcium oxalate and uric acid stones. Climate simulations for the USA indicate that the stone belt will move northwards in the coming two decades (12).
Exogenic risk factors such as nutritional patterns and lifestyles characterized by low levels of physical activity combined with high energy intake from food rich in fats, proteins, carbohydrates, and purines (13, 14) are growing in importance, as are smoking, alcohol abuse, and chronic stress (15). Increasing incidence and prevalence of urolithiasis is therefore expected, especially in Europe and the USA (7).
Acute renal colic
The most commonly occurring leading symptom is radiating colicky pain in the hypochondrium. The pain varies depending on the position of the stone in the ureter and may attain excruciating intensity (16). The worst pain is caused by high-lying concretions located in the costovertebral angle. The pain from lower-lying stones is perceived in the hypogastric region, possibly radiating as far as the genitals (16). The patients are restless and cannot find any position that relieves their pain. Accompanying vegetative reactions such as nausea and vomiting may occur. Depending on the presentation, the differential diagnoses include those of acute abdomen, i.e., pyelonephritis, diverticulitis, appendicitis, cholecystitis, and pancreatitis; however, extrauterine pregnancy and ovarian cyst with torsion, vertebrogenic symptoms, pneumonia, abdominal aortic aneurysm, and myocardial infarction also have to be taken into consideration owing to their potential consequences.
Before any diagnostic investigations are instituted, the patient suffering from colic should be given appropriate pain-relieving medication. The options are non-steroidal antirheumatics, e.g., diclofenac and metamizole (level of evidence 1b, recommendation grade A), and opioids, e.g., tramadol (evidence level 4, recommendation grade C). These are given in combination (19– 21).
Analgesia should be followed by a symptom- or differential diagnosis-oriented physical examination, which must include palpation of the renal bed and the abdomen. This should be followed by investigation of spontaneously excreted urine with a urine test strip (dipstick). Microhematuria is a strong sign of renal colic. Sonography is a valuable non-invasive diagnostic test, with sensitivity of 61 to 93% and specificity of 84 to 100%, and should follow next (6, 22). In most cases of stones in the ureter, the only sonographic finding is accumulation of urine. The stone is often not visualized directly owing to overlying intestinal gas. The triad of colicky flank pain, sonographically diagnosed ectasia of the renal calyces, and microhematuria is practically pathognomonic for ureterolithiasis. The sensitivity of microhematuria in the context of this triad is 0.95 in the acute phase (23).
If available, low-dose plain abdominal CT is the diagnostic imaging method of choice (evidence level 1a, recommendation grade A), with specificity and sensitivity of almost 99%. Stones not detected on radiography are visualized, and the density of the stone in Hounsfield units gives a first indication of its composition and helps with differential diagnosis (6, 24– 26). Alternative imaging procedures are plain radiography and excretion urography. However, in acute colic the latter involves the risk of rupture of the renal calyces owing to contrast medium-induced diuresis (6).
Clinical chemistry should include electrolytes, uric acid, creatinine, C-reactive protein (CRP), complete blood count without differential, and the global parameters of coagulation (recommendation grade A) (6).
If a urinary concretion is confirmed as cause of the patient’s symptoms, the treatment options depend on the location and size of the stone (6). The following are available:
Conservative treatment
Extracorporeal shock-wave lithotripsy (ESWL)
Ureterorenoscopy (URS)
Percutaneous nephrolithotomy (PCNL)
Laparoscopy
Open surgery
In extreme cases nephrectomy may be necessary. Laparoscopic or open surgery is usually performed in combination with the treatment of comorbidities, e.g., renal pelvic stenosis.
Oral medicinal chemolitholysis, in which the concretion is dissolved in situ, is applicable only to uric acid stones (recommendation grade A) (6).
The German and European urolithiasis guidelines recommend the following treatment options:
Conservative stone treatment
The most frequent strategy for treatment of acute renal colic is conservative management with the aim of achieving spontaneous passage of the urinary stone (medical expulsive therapy) (evidence level 1a, recommendation grade A). Caution should be applied in the case of elevated parameters of retention or parameters of infection. Moreover, conservative treatment is not appropriate if despite adequate analgesia the patient continues to suffer pain or vegetative symptoms such as nausea and vomiting persist. Alpha-blockers promote spontaneous passage and reduce episodes of colic (evidence level 1a, recommendation grade A) (6, 20, 27– 29). Rates of spontaneous passage of 71 to 98% for distal stones ≤5 mm and 25 to 79% for stones between 6 and 10 mm are reported in the literature. Stones in the proximal ureter ≤5 mm are passed spontaneously in 29 to 98%, stones ≤10 mm in 10 to 53% of cases (30).
Other forms of conservative treatment are chemolitholysis of uric acid stones and “watchful waiting” in the case of asymptomatic kidney stones (30).
Interventional stone treatment
Renal pelvis and upper/intermediate calyces
Stones in the renal pelvis and upper/intermediate calyces can be treated by ESWL, PCNL, and flexible URS. In patients with stones ≤20 mm ESWL is the preferred method, dealing successfully with 56 to 94% of stones in the upper/intermediate calyces and 79 to 85% in the renal pelvis (recommendation grade B). For uroliths >20 mm ESWL entails the risk of leaving a trail of fragments (“steinstrasse”) in the ureter and achieves lower rates of complete freedom from stones, so PCNL should be preferred (recommendation grade B) (6).
Lower calyces
For reasons of anatomy, ESWL yields lower rates of complete freedom from stones in the lower calyces. Depending on previous treatment, risk of recurrence, comorbidities, and anatomical circumstances, among other factors, mini-PCNL with a diameter of 11 to 21 Charrière is an increasingly common option for stones as small as 10 mm (recommendation grade B) (6). Flexible URS competes with ESWL for the treatment of stones up to 10 mm (6). The April 2014 revision of the EAU guidelines accords greater importance to endoscopy than in 2013. Endoscopic interventions, i.e., URS and PCNL, now seem to be just as valid as ESWL for the treatment of stones of any size in this location. Since, however, no relevant randomized studies exist, the recommendation for this upgrade of endoscopic techniques is strength B, resting on expert consensus.
Staghorn stones
Kidney stones that occupy large portions of the renal pelvis or fill at least one calyx are termed staghorn stones. The treatment options are PCNL, possibly in combination with ESWL and flexible URS, or in occasional cases nephrolithotomy. If the kidney is no longer functional, nephrectomy can be considered (6).
Proximal ureter
The preferred treatment method for stones ≤10 m in the proximal ureter is ESWL, attaining complete freedom from stones in 70 to 90% of cases (recommendation grade A) (6). If primary in-situ ESWL is not possible or is contraindicated by laboratory findings, e.g., renal failure or urinary tract infection, ureteral splinting followed by ESWL is an option. It should be noted that approximately 20% of patients have to take time off work because of the ureteral splinting alone (31).
URS is the method of choice for ureteral stones >10 mm. Recent advances in semirigid and flexible URS, including smaller instrument diameters and higher angles of flexion, have transformed the treatment of stones in the proximal ureter. Complete freedom from stones can now be achieved in up to 82% of cases with low complication rates (6).
Distal ureter
ESWL and endoscopy are both valid treatment options for stones ≤10 mm in the distal ureter, with complete freedom from stones achieved in 86% and 97% of cases respectively. Endoscopy is preferable for concretions >10 mm, with complete elimination of stones in 93% of patients, versus 74% for ESWL (recommendation grade A) (6).
Metaphylaxis
If successful primary treatment is to be followed by effective prevention of recurrence, the stone material must be subjected to Fourier transform infrared spectroscopy (FTIR) or X-ray diffraction (XRD) as described in the guidelines. Without analysis of the stone, no specific prophylaxis can be carried out (evidence level 2, recommendation grade A) (6, 32).
This analysis should be performed after every stone event, because the composition of consecutive stones in the same patient can change to a clinically relevant extent (evidence level 2, recommendation grade B) (6). In practice this is frequently forgotten, however, so that a long-term patient may end up receiving treatment that is no longer appropriate.
In how much detail should a stone event be investigated? Critical voices may question the point of complex postinterventional diagnostics if treatment then consists solely of the advice to increase fluid intake. However, a renewed episode of urolithiasis may require surgical intervention, and potential complications such as acute renal failure and urosepsis must be considered, along with sometimes serious comorbidities such as chronic and terminal renal insufficiency (33). Numerous other comorbidities have been described. Rule and colleagues showed that urinary stone formation is associated with an elevated risk of myocardial infarction (34). Comparing 4564 patients with 10 860 controls, the risk was 38% higher for the former after 9 years’ observation. Following adjustment for risk factors, e.g., renal insufficiency, the increase in risk was 31%. Moreover, Sun et al. described an elevated risk of urothelial cancer in urinary stone formers (35).
Postinterventional diagnosis and metaphylaxis—ideally instituted when the patient is free of stone—must be individualized and adapted to the risk constellation. The spectrum ranges from watchful waiting to interdisciplinary metabolic testing. Around 25% of stone patients belong to the high-risk group (36, 37). In an estimated 75% of cases, further stone episodes can be effectively prevented by basic metabolic diagnosis followed by general urinary stone metaphylaxis (38).
Knowledge of the patient’s history and dietetic–medicinal treatment specific to stone type are, along with biochemically oriented investigation and detection of possible anatomical causes, essential for postinterventional metaphylaxis. This is true both for patients with a first stone event and for those with recurrent urolithiasis. The recurrence rate is significantly reduced by targeted treatment (10–15% vs. 50–80%) (39, 40).
The main associated factors are enzyme defects, hormonal disorders, malabsorption in the gastrointestinal tract, renal insufficiency, disorders of urodynamics, recurring urease-positive urinary tract infections, and unfavorable urinary pH. In particular, the consequences of the modern western lifestyle are risk factors for metabolic syndrome (e1– e3) and are increasingly responsible for formation of urinary stones. Overweight (body mass index [BMI] ≥ 25 kg/m2) and obesity (BMI ≥ 30 kg/m2) increase the risk of urinary stone formation significantly (e4, e5).
An exhaustive discussion of the pathomechanisms of biomineralization and their investigation would considerably exceed the scope of this article. Therefore the authors will confine ourselves to general orientation (36, 37).
Uncomplicated urolithiasis
Around 75% of patients with urinary stones can be categorized as uncomplicated. The basis for classifying a case of urolithiasis as complicated or uncomplicated is the patient’s medical history (Table 1). In simple terms, every patient who does not fulfill at least one of the criteria listed in Table 2 is at low risk of recurrence.
Table 1. Classification of urinary stone patients as uncomplicated on the basis of their medical history (6, 37).
| Findings | Action |
|---|---|
| First episode | Cave: History of "frequent kidney pain" in childhood, but unclear origin |
| Age: adult | |
| No anatomic abnormalities | Exclusion of, for example, horseshoe kidney and outlet stenosis |
| Probable correlation with lifestyle | For instance, stone formation at or soon after a time of unusual stress and specific compensation reactions |
| Negative family history of urolithiasis | Cave: Hints of possibly undiscovered stones in family members through statements such as "There was something, but I can’t quite remember…" |
| Single stone | Assessment with suitable imaging procedures |
Table 2. Classification of urinary stone patients as high risk (6, 37).
| Finding | Action |
|---|---|
| Age; child or adolescent | Consider assessing siblings for risk of lithogenesis |
| Brushite, uric acid/urate, infectious stones | Bear other accompanying minerals in mind in diagnosis and treatment |
| Chronic psychovegetative stress | Establish severity, perhaps with aid of validated stress-assessment systems |
| Single kidney | |
| Malformation of the urinary tract | |
| Disorders of gastrointestinal function | E.g., Crohn disease, ulcerative colitis, sprue, chronic pancreatitis, liver cirrhosis, small bowel resection |
| High recurrence rate | More than three stones in 3 years. Changes in stone type (principal and subsidiary mineral phase) or composition may indicate alterations in metabolic conditions |
| Hyperparathyroidism (HPT) | Five forms of HPT, primary to quinary |
| Nephrocalcinosis | Numerous causes, e.g., following renal tubular acidosis, primary hyperoxaluria, sarcoidosis, HPT, chronic glomerulitis |
| Positive family history | Consider assessing patient’s children for risk of lithogenesis |
| Primary hyperoxaluria | Two types, autosomal-recessive hereditary disease |
| Renal tubular acidosis | Test by means of urinary pH curve, blood gas analysis, and ammonium chloride load test |
| Residual stone fragments | Possibly consider endoscopic means of stone removal, particularly when the concrement is of a type that resists disintegration by ESWL, e.g., brushite, cystine, whewellite |
| Cystine, 2,8-dihydroxyadenine, xanthine stones | Stone formation genetically determined; lifelong metaphylaxis is mandatory |
ESWL, extracorporeal shockwave lithotripsy
Extensive metabolic diagnostics are unnecessary in uncomplicated urinary stone patients; depending on the type of stone, however, some general measures should be carried out. This includes a comprehensive anamnesis to record potential risk factors as early as possible:
Familial disposition
Characteristics of metabolic syndrome, e.g., obesity, hypertension, dyslipoproteinemia, hyperglycemia.
Mental and physical condition, e.g., mental problems such as restlessness, desensitization, disinterest and loss of motivation, and physical problems such as restricted mobility.
Recurrent urinary tract infection
Social and occupational factors such as partnership, unemployment, shift work, rest periods, meal breaks, frequent business trips and travel.
Metabolic disorders, e.g. renal reabsorption and transport disorders: renal leak (calcium, phosphate); aciduria (urinary pH permanently <6.0; associated for instance with metabolic syndrome and favored by excessive consumption of animal protein); cystinuria; enteral hyperabsorption of lithogenic substances (e.g., calcium, oxalate); hormonal disorders (e.g., of parathormone and cortisol levels); elevated vitamin D3 level; enzyme deficiencies.
Urodynamic anomalies: The history may include flank pain after increased fluid intake, a feeling of incomplete emptying of the bladder, flank pain on micturition, recurring urinary tract infections, or incidental sonographic detection of urinary transport disorders, possibly even in the uterus during routine antenatal examination. If any of these are found, further investigation for urodynamic anomalies is indicated.
Causative comorbidities, for example Crohn disease, short bowel syndrome, cystic fibrosis, osteoporosis, or catabolic metabolism, e.g., owing to tumor, pancreatic, or hepatic disease.
Screening for severe metabolic disorders is mandatory in high-risk patients, and because there is no clear dividing line between low and high risk in clinical practice, we also recommend it for low-risk patients:
For at least 3 days the patient should keep a nutritional diary, recording all food and drink consumed with details of amount and time. This readily reveals any special features of the diet, e.g., whether the person is omnivorous, carnivorous, vegetarian, or vegan.
A sample of urine should be taken from every micturition for a week under normal conditions and tested for pH. The resulting urinary pH profile can exclude, for example, renal tubular acidosis (RTA). Patients with RTA have a tubular disorder of proton excretion or bicarbonate resorption, leading to metabolic acidosis. The urinary pH is typically permanently <5.8. Furthermore, weekday-related or lifestyle-associated fluctuations in pH (e.g., workdays versus weekend) can be detected.
Blood testing (standard blood count plus calcium, creatinine, and uric acid) (recommendation grade A); careful determination of parathormone in all patients barring completely uncomplicated cases (e6).
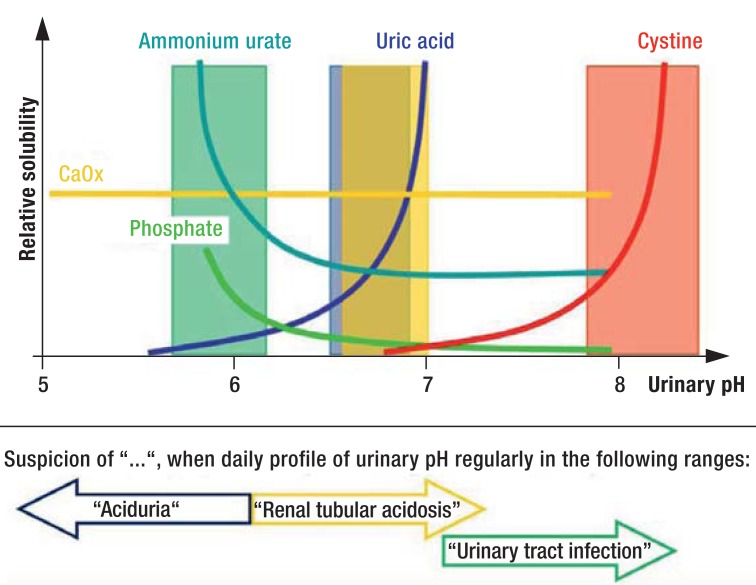
Investigation of at least one 24-h urine sample (e7): volume, pH (Figure 3), sodium, potassium, calcium, magnesium, ammonia, chloride, oxalate, citrate, phosphate, uric acid, and creatinine (recommendation grade A). If any signs of infection are noted, a sample of urine must be prepared for bacterial culture (14).
Calculation of empirical risk indices for urinary stone formation from the above-mentioned urinary parameters (e8, e9) and/or additional determination of the crystallization risk using the BONN Risk Index (BRI) (e10– e12). This enables more detailed evaluation of both the risk profile and the disease course.
Figure 3.
Dependence of lithogenesis on urinary pH (modified from Laube and Berg (e13).
If the basic diagnostic measures confirm a stone patient’s classification as uncomplicated, this metabolic screening need not be carried out. General urinary stone metaphylaxis with regular follow-ups is sufficient (6, 36, 37, 39).
Complicated urolithiasis
Around 25% of urinary stone patients are categorized as complicated. These patients, classified by the guidelines as high risk, show at least one of the characteristics listed in Table 2.
The basic investigation as carried out in uncomplicated patients is followed by the extended metabolic screening described above. This involves complex interdisciplinary diagnostics that should be performed at a specialized center. When an individual patient’s urinary stone formation has been fully characterized and medicinal treatment is required, various agents are available. The most important of them are listed in Table 3.
Table 3. Principal substances used in medicinal prophylaxis of urinary stones (e13).
| Substance | Goal | Dosage | Remarks | Stone types amenable to treatment |
|---|---|---|---|---|
| Alkaline citrates |
|
5–12 g/day (14–36 mmol/day), children: 0.1–0.15 g/kg BW/day
|
Dose size and frequency depend on urinary pH or need to compensate acidosis. Cave: Phosphate precipitation possible in cystine metaphylaxis (→ high rinary pH) |
|
| Allopurinol | Lowering of
|
100–300 mg/day, children: 1–3 mg/kg BW/day |
Cave: high-dose allopurinol treatment can lead to xanthinuria |
|
| Calcium (Ca) | Lowering of enteral hyperoxaluria | 160 mg corresponding to 100 mg Mg) with each meal, maximum 500 mg/day | Intake 30 min before each main meal Cave: hypercalciuria (→ testig) |
Calcium oxalate |
| L-Methionine | Urinary acidification | 600–1500 mg/day to urinary pH 5.8–6.2 | Cave: contraindicated in RTA
|
|
| Magnesium (Mg) |
|
200–400 mg/day, children: 6 mg/kg BW/day | Dose reduction in renal insufficiency, intake with main meals |
|
| Sodium carbonate |
|
4.5 g/day, target urinary pH: see alkaline citrates | Dose depends on urinary pH or need to compensate acidosis |
|
| Pyridoxine (vitamin B6) | Lowering of endogenous hyperoxaluria | Initially 5 mg/kg BW/day, maximum 20 mg/kg BW/day | If no effect, discontinue after 1 year at latest Cave: polyneuropathy |
|
| Thiazide (hydrochlorothiazide) | Increase in tubular Ca reabsorption in hypercalciuria (>8 mmol/day) so that renal Ca excretion goes down | 12.5–50 mg/day (gradually increase dosage), children: 0.5–1 mg/kg BW/day) |
|
|
| Tiopronin | Intermediate conversion of poorly soluble cystine to cysteine + cysteine–drug complex (readily soluble) | Initially 250 mg/day, maximum 2000 mg/day | Cave:
|
|
RTA, renal tubular acidosis
Conclusion
Urolithiasis is already widespread and is growing in prevalence. A wide range of options are available for surgical treatment. The EAU guidelines provide information on surgery and metaphylaxis. A useful German-language overview of the differential diagnoses is provided by a practical chart, oriented on the urinary stone guidelines, in which details of pathogenesis, metabolic diagnosis, and metaphylaxis are summarized (e13).
Key Messages.
Urolithiasis is a symptom of many renal, endocrine, intestinal, and occasionally even malignant diseases. Stone formation is often triggered and influenced by external risk factors.
The choice and success of methods to remove urinary stones depend on the site, size, and composition of the concretion and on any comorbidities.
There are various types of urinary stone, some of which form under mutually exclusive physicochemical conditions. Analysis of uroliths is therefore mandatory.
Targeted anamnesis permits every patient to be categorized as high or low risk. The subsequent diagnostic and therapeutic measures depend on this classification.
Individualized, risk-adapted metaphylaxis reduces the risk of recurrence from ca. 50% to around 15%.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Koch H, Brenner G, Kerek-Bodden H. Die 50 häufigsten Diagnosestellungen (ICD-10-Schlüsselnummern) des Gesamtjahres 2006 für 12 ausgewählte Fachgebiete. Zentralinstitut für die kassenärztliche Versorgung in der Bundesrepublik Deutschland. 2007 [Google Scholar]
- 2.Hesse A, Bach D. Stuttgart: Thieme Verlag; 1982. Harnsteine. [Google Scholar]
- 3.Bichler K, Strohmaier WL, Eipper E, Lahme S. Bichler K, editor. Epidemiologie: Das Harnsteinleiden. GEK-Edition. Lehmanns Media - LOB.de. 2007;52:31–44. [Google Scholar]
- 4.Hesse A, Brandle E, Wilbert D, Kohrmann KU, Alken P. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs 2000. Eur Urol 2003. 44:709–713. doi: 10.1016/s0302-2838(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 5.Schubert G. Stone analysis. Urol Res. 2006;34:146–150. doi: 10.1007/s00240-005-0028-y. [DOI] [PubMed] [Google Scholar]
- 6.Türk C, Knoll T, Petrik A. EAU guidelines on urolithiasis. www.uroweb.org. 2014 (last accessed on 16 October 2014) [Google Scholar]
- 7.Trinchieri A. Epidemiology of urolithiasis: an update. Clin Cases Miner Bone Metab. 2008;5:101–106. [PMC free article] [PubMed] [Google Scholar]
- 8.Miller K. Jocham D, Miller K, editors. Interventionelle Steintherapie. Praxis der Urologie. Thieme-Verlag. 2003:25–45. [Google Scholar]
- 9.Knoll T, Schubert AB, Fahlenkamp D, Leusmann DB, Wendt-Nordahl G, Schubert G. Urolithiasis through the ages: data on more than 200,000 urinary stone analyses. J Urol. 2011;185:1304–1311. doi: 10.1016/j.juro.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 10.Krambeck AE, Lieske JC, Li X, Bergstralh EJ, Melton LJ III, Rule AD. Effect of age on the clinical presentation of incident symptomatic urolithiasis in the general population. J Urol. 2013;189:158–164. doi: 10.1016/j.juro.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linder BJ, Rangel LJ, Krambeck AE. The effect of work location on urolithiasis in health care professionals. Urolithiasis. 2013;41:327–331. doi: 10.1007/s00240-013-0579-2. [DOI] [PubMed] [Google Scholar]
- 12.Brikowski TH, Lotan Y, Pearle MS. Climate-related increase in the prevalence of urolithiasis in the United States. Proc Natl Acad Sci U S A. 2008;105:9841–9846. doi: 10.1073/pnas.0709652105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorensen MD, Chi T, Shara NM, Wang H, Hsi RS, Orchard T, et al. Activity, energy intake, obesity, and the risk of incident kidney stones in postmenopausal women: a report from the Women’s Health Initiative. J Am Soc Nephrol. 2014;25:362–369. doi: 10.1681/ASN.2013050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straub M, Hautmann RE. Developments in stone prevention. Curr Opin Urol. 2005;15:119–126. doi: 10.1097/01.mou.0000160627.36236.6b. [DOI] [PubMed] [Google Scholar]
- 15.Berg W, Uhlemann C, Meissner A, Laube N. [Stress-related alteration of urine compositions: idiopathic CaOx stone formers, patients with chronic inflammatory bowel disease (CIBD) and healthy controls] Urologe A. 2011;50:1606–1613. doi: 10.1007/s00120-011-2706-4. [DOI] [PubMed] [Google Scholar]
- 16.Hautmann R, Gschwend JE, editors. Urologie. 5th revised edition. Springer. 2014 [Google Scholar]
- 17.Phillips E, Kieley S, Johnson EB, Monga M. Emergency room management of ureteral calculi: current practices. J Endourol. 2009;23:1021–1024. doi: 10.1089/end.2008.0615. [DOI] [PubMed] [Google Scholar]
- 18.Micali S, Grande M, Sighinolfi MC, De Carne C, De Stefani S, Bianchi G. Medical therapy of urolithiasis. J Endourol. 2006;20:841–847. doi: 10.1089/end.2006.20.841. [DOI] [PubMed] [Google Scholar]
- 19.Ramos-Fernandez M, Serrano LA. Evaluation and management of renal colic in the emergency department. Bol Asoc Med P R. 2009;101:29–32. [PubMed] [Google Scholar]
- 20.Dellabella M, Milanese G, Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol. 2005;174:167–172. doi: 10.1097/01.ju.0000161600.54732.86. [DOI] [PubMed] [Google Scholar]
- 21.Cohen E, Hafner R, Rotenberg Z, Fadilla M, Garty M. Comparison of ketorolac and diclofenac in the treatment of renal colic. Eur J Clin Pharmacol. 1998;54:455–458. doi: 10.1007/s002280050492. [DOI] [PubMed] [Google Scholar]
- 22.Ray AA, Ghiculete D, Pace KT, Honey RJ. Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology. 2010;76:295–300. doi: 10.1016/j.urology.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Nishizawa K, Mitsumori K, Ogura K. Impact of date of onset on the absence of hematuria in patients with acute renal colic. J Urol. 2003;170:1093–1096. doi: 10.1097/01.ju.0000080709.11253.08. [DOI] [PubMed] [Google Scholar]
- 24.Kennish SJ, Bhatnagar P, Wah TM, Bush S, Irving HC. Is the KUB radiograph redundant for investigating acute ureteric colic in the non-contrast enhanced computed tomography era? Clin Radiol. 2008;63:1131–1135. doi: 10.1016/j.crad.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Worster A, Preyra I, Weaver B, Haines T. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Ann Emerg Med. 2002;40:280–286. doi: 10.1067/mem.2002.126170. [DOI] [PubMed] [Google Scholar]
- 26.Jellison FC, Smith JC, Heldt JP, Spengler NM, Nicolay LI, Ruckle HC, et al. Effect of low dose radiation computerized tomography protocols on distal ureteral calculus detection. J Urol. 2009;182:2762–2767. doi: 10.1016/j.juro.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 27.Resim S, Ekerbicer H, Ciftci A. Effect of tamsulosin on the number and intensity of ureteral colic in patients with lower ureteral calculus. Int J Urol. 2005;12:615–620. doi: 10.1111/j.1442-2042.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- 28.Seitz C, Liatsikos E, Porpiglia F, Tiselius HG, Zwergel U. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455–471. doi: 10.1016/j.eururo.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Liatsikos EN, Katsakiori PF, Assimakopoulos K, Voudoukis T, Kallidonis P, Constantinides C, et al. Doxazosin for the management of distal-ureteral stones. J Endourol. 2007;21:538–541. doi: 10.1089/end.2006.0107. [DOI] [PubMed] [Google Scholar]
- 30.Knoll T. [S2 guidelines on diagnostic, therapy and metaphylaxis of urolithiasis : Part 1: Diagnostic and therapy] Urologe A. 2009;48:917–924. doi: 10.1007/s00120-009-2047-8. [DOI] [PubMed] [Google Scholar]
- 31.Davenport K, Kumar V, Collins J, Melotti R, Timoney AG, Keeley FX., Jr New ureteral stent design does not improve patient quality of life: a randomized, controlled trial. J Urol. 2011;185:175–178. doi: 10.1016/j.juro.2010.08.089. [DOI] [PubMed] [Google Scholar]
- 32.Joshi VS, Vasant SR, Bhatt JG, Joshi MJ. Some critical aspects of FT-IR, TGA, powder XRD, EDAX and SEM studies of calcium oxalate urinary calculi. Indian J Biochem Biophys. 2014;51:237–243. [PubMed] [Google Scholar]
- 33.Keddis MT, Rule AD. Nephrolithiasis and loss of kidney function. Curr Opin Nephrol Hypertens. 2013;22:390–396. doi: 10.1097/MNH.0b013e32836214b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rule AD, Roger VL, Melton LJ III, Bergstralh EJ, Li X, Peyser PA, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. 2010;21:1641–1644. doi: 10.1681/ASN.2010030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun LM, Lin CL, Chang YJ, Liang JA, Liu SH, Sung FC, et al. Urinary tract stone raises subsequent risk for urinary tract cancer: a population-based cohort study. BJU Int. 2013;112:1150–1155. doi: 10.1111/bju.12402. [DOI] [PubMed] [Google Scholar]
- 36.Straub M. [Recurrence prevention of kidney stone disease] Urologe A. 2011;50:1323–1332. doi: 10.1007/s00120-011-2640-5. [DOI] [PubMed] [Google Scholar]
- 37.Straub M. [What kind of stone prevention for whom? Risk-adjusted metaphylaxis following urinary stone disease] Urologe A. 2006;45:1387–1381. doi: 10.1007/s00120-006-1224-2. [DOI] [PubMed] [Google Scholar]
- 38.Straub M, Strohmaier WL, Berg W, Beck B, Hoppe B, Laube N, et al. Diagnosis and metaphylaxis of stone disease. Consensus concept of the National Working Committee on Stone Disease for the upcoming German Urolithiasis Guideline. World J Urol. 2005;23:309–323. doi: 10.1007/s00345-005-0029-z. [DOI] [PubMed] [Google Scholar]
- 39.Siener R, Hesse A. [Modern general metaphylaxis of stone disease. New risks, new evidence, new recommendations] Urologe A. 2006;45 doi: 10.1007/s00120-006-1219-z. 1392, 1394-2. [DOI] [PubMed] [Google Scholar]
- 40.Siener R. Impact of dietary habits on stone incidence. Urol Res. 2006;34:131–133. doi: 10.1007/s00240-005-0025-1. [DOI] [PubMed] [Google Scholar]
- e1.Cho ST, Jung SI, Myung SC, Kim TH. Correlation of metabolic syndrome with urinary stone composition. Int J Urol. 2013;20:208–213. doi: 10.1111/j.1442-2042.2012.03131.x. [DOI] [PubMed] [Google Scholar]
- e2.Kohjimoto Y, Sasaki Y, Iguchi M, Matsumura N, Inagaki T, Hara I. Association of metabolic syndrome traits and severity of kidney stones: results from a nationwide survey on urolithiasis in Japan. Am J Kidney Dis. 2013;61:923–929. doi: 10.1053/j.ajkd.2012.12.028. [DOI] [PubMed] [Google Scholar]
- e3.Kohjimoto Y, Iba A, Sasaki Y, Hara I. [Metabolic syndrome and nephrolithiasis] Hinyokika Kiyo. 2011;57:43–47. [PubMed] [Google Scholar]
- e4.Daudon M, Lacour B, Jungers P. Influence of body size on urinary stone composition in men and women. Urol Res. 2006;34:193–199. doi: 10.1007/s00240-006-0042-8. [DOI] [PubMed] [Google Scholar]
- e5.Eisner BH, Eisenberg ML, Stoller ML. Relationship between body mass index and quantitative 24-hour urine chemistries in patients with nephrolithiasis. Urology. 2010;75:1289–1293. doi: 10.1016/j.urology.2009.09.024. [DOI] [PubMed] [Google Scholar]
- e6.Julka S, Gupta SK, Srivastava A. Protocol-based metabolic evaluation in high-risk patients with renal stones in North India. Indian J Endocrinol Metab. 2012;16:283–287. doi: 10.4103/2230-8210.93754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Laube N, Berg W. [Carefully conducted preanalytic and postanalytic procedures for urine samples. Often neglected in urolithiasis treatment] Urologe A. 2014;53:48–54. doi: 10.1007/s00120-013-3265-7. [DOI] [PubMed] [Google Scholar]
- e8.Tiselius HG. Risk formulas in calcium oxalate urolithiasis. World J Urol. 1997;15:176–185. doi: 10.1007/BF02201855. [DOI] [PubMed] [Google Scholar]
- e9.Yang B, Dissayabutra T, Ungjaroenwathana W, et al. Calcium oxalate crystallization index (COCI): an alternative method for distinguishing nephrolithiasis patients from healthy individuals. Ann Clin Lab Sci. 2014;44:262–271. [PubMed] [Google Scholar]
- e10.Kavanagh JP, Laube N. Why does the Bonn Risk Index discriminate between calcium oxalate stone formers and healthy controls? J Urol. 2006;175:766–770. doi: 10.1016/S0022-5347(05)00145-X. [DOI] [PubMed] [Google Scholar]
- e11.Laube N, Berg W, Bernsmann F, Gravius S, Klein F, Latz S, et al. Induced urinary crystal formation as an analytical strategy for the prediction and monitoring of urolithiasis and other metabolism-related disorders. EPMA J. 2014;5 doi: 10.1186/1878-5085-5-13. doi: 10.1186/1878-5085-5-13. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Porowski T, Zoch-Zwierz W, Konstantynowicz J, Taranta-Janusz K. A new approach to the diagnosis of children’s urolithiasis based on the Bonn Risk Index. Pediatr Nephrol. 2008;23:1123–1128. doi: 10.1007/s00467-008-0786-1. [DOI] [PubMed] [Google Scholar]
- e13.Laube N, Berg W. Praxisorientiertes Kompendium. Zweitauflage der Harnsteinlehrtafel. Uro-News. 2013;11:47–49. [Google Scholar]