Summary
Maximal exercise-associated oxidative capacity is strongly correlated with health and longevity in humans. Rats selectively bred for high running capacity (HCR) have improved metabolic health and are longer-lived than their low capacity counterparts (LCR). Using metabolomic and proteomic profiling, we show that HCR efficiently oxidize fatty acids (FA) and branched-chain amino acid (BCAA), sparing glycogen and reducing accumulation of short- and medium-chain acylcarnitines. HCR mitochondria have reduced acetylation of mitochondrial proteins within oxidative pathways at rest, and there is rapid protein deacetylation with exercise, which is greater in HCR than LCR. Fluxomic analysis of valine degradation with exercise demonstrates a functional role of differential protein acetylation in HCR and LCR. Our data suggest efficient FA and BCAA utilization contribute to high intrinsic exercise capacity and the health and longevity benefits associated with enhanced fitness.
Introduction
Exercise capacity and cardiovascular fitness are highly predictive of metabolic health, including lower fat mass, higher insulin sensitivity, lower blood pressure and, importantly, age adjusted mortality (Blair et al., 1996; Church et al., 2004; Dvorak et al., 2000; Kodama et al., 2009). The mechanisms underlying these associations are not fully understood. One important link between exercise capacity and overall metabolic health is the fuel selection for energy production. Higher exercise capacity is associated with increased fatty acid (FA) oxidation during exercise (Hall et al., 2010; Morris et al., 2013; Nordby et al., 2006; Venables et al., 2005), while poor metabolic health is associated with high basal use of carbohydrates and impaired fuel switching during the fast-fed transition (Kelley and Mandarino, 2000). The glucose-fatty acid cycle described by Randle et al. (1963) states that fat availability will drive fat oxidation and reciprocally lead to decreased glucose oxidation; however, this theory cannot explain instances when fat availability is high but carbohydrates are preferentially oxidized, as is the case during high-intensity exercise and insulin resistance (Kelley and Mandarino, 2000; Mittendorfer and Klein, 2001; Sidossis et al., 1997).
Recent advances in metabolomics and proteomics allow the quantification of tens to thousands of metabolites or peptides in a single biological sample. Integrating these techniques can provide insight into the changes in nutrient utilization under different physiological conditions. In these studies, we employed a combination of metabolomics and proteomics to investigate fuel selection in rats selectively bred for high and low intrinsic running capacity (HCR and LCR). The HCR-LCR rat model was derived from a heterogeneous founder population (N:NIH) with breeder selection based solely on intrinsic (untrained) treadmill running capacity (Koch and Britton, 2001). In this model, as in humans, exercise capacity is a heritable trait (Fagard et al., 1991; Ren et al., 2013), and like humans who differ in running capacity, HCR and LCR diverge in susceptibility to metabolic and related disease traits (Koch et al., 2011; Naples et al., 2010; Noland et al., 2007; Novak et al., 2010; Wisloff et al., 2005). Compared to LCR, HCR animals diverge more strongly in running capacity from the founder stocks and show a 2.4-fold increased running capacity over the highest capacity observed in inbred lines (Ren et al., 2013). HCR weigh significantly less than LCR throughout their lifespan, despite similar food consumption, and there is evidence of increased capacity of substrate oxidation (Rivas et al., 2011). A recent study (Gavini et al., 2014) showed that HCR and LCR have similar resting energy expenditure, but HCR have small elevations in exercise energy expenditure and greater exercise-induced heat production from their skeletal muscle. The phenotype of HCR is coincident with a host of health benefits (Wisloff et al., 2005), including a 28–40% increased lifespan (Koch et al., 2011).
In this study, we found that the respiratory quotient (RQ) is lower at rest in HCR compared to LCR, indicative of enhanced FA oxidation, and FA oxidation is even more markedly enhanced in HCR during exercise. Metabolomic and fluxomic profiling demonstrate that during exercise, HCR use FA and branched chain amino acids (BCAA) more efficiently than LCR. Assessment of the muscle mitochondrial proteome of HCR and LCR, as well as post-translational modifications (phosphorylation and acetylation), show specific differences between HCR and LCR within oxidative pathways of FA and BCAA metabolism and provide evidence that rapid changes in protein acetylation during exercise could play a role in augmenting the fuel selection differences. These differences in fuel selection and proteome modification mirror those found in caloric restriction (Hallows et al., 2011) and implicate fuel selection and mitochondrial oxidative efficiency as mechanisms linking enhanced exercise capacity with improved metabolic status and longevity.
Results
HCR efficiently use fat during exercise
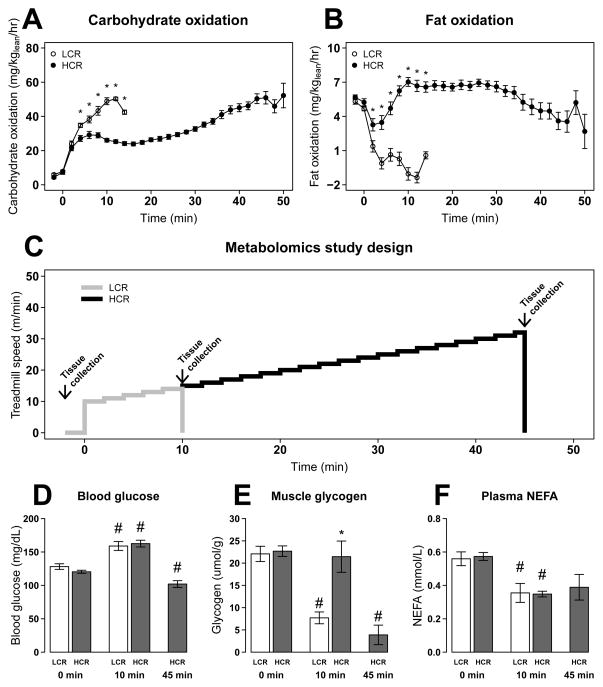
Using a protocol identical to that used for selecting breeders, HCR have 1.4-fold higher VO2max and run 4.3-fold longer distance than LCR (Table S1). To estimate fuel use, we determined VO2 and VCO2 (normalized to lean mass) at rest and during exercise in HCR and LCR. Carbohydrate utilization rose rapidly in both HCR and LCR with onset of exercise (Figure 1A). In LCR, carbohydrate utilization continued to increase until exhaustion, with a concomitant decrease in fat oxidation. In contrast, HCR maintained comparatively high levels of fat oxidation throughout exercise. As HCR approached exhaustion, carbohydrate oxidation increased and fat oxidation decreased (Figures 1A–B). Similarly, at maintained submaximal exercise (75% VO2max), the rate of fat oxidation was significantly higher in HCR than LCR (Figure S1), providing further evidence that HCR, compared to LCR, have enhanced capacity to oxidize FA.
Figure 1. HCR have lower glycogen utilization and higher fat oxidation during exercise.
Carbohydrate (A) and fat oxidation (B) were estimated from VO2 and VCO2 during an exhaustive exercise test for LCR (○) (n = 16) and HCR (●) (n = 23). Animals were separated into groups (n=4–6) and run for 0, 10, and 45 min (HCR only)(C). Blood glucose concentration (D), muscle glycogen (E), plasma non-esterified fatty acid (NEFA) (F) are presented as mean ± SEM for each group. * p<0.05 between HCR and LCR at a specific time point; # p<0.05 difference from rest (0 min).
We evaluated metabolites in plasma and gastrocnemius muscle from these same animals following a separate exercise bout (Table S2A). Subsets of rats (n=5–6) were sampled at rest (0 min), at 10 min (mean time to exhaustion for LCR), and at 45 min (mean time to exhaustion for HCR) (Figure 1C). At rest, LCR and HCR show no difference in levels of primary fuel sources blood glucose (Figure 1D), muscle glycogen (Figure 1E), or plasma non-esterified fatty acid (NEFA)(Figure 1F). Blood glucose increased in both LCR and HCR at 10 min of exercise but was reduced in HCR at 45 min (Figure 1D). Muscle glycogen decreased with exhaustion in LCR (10 min) and HCR (45 min), but glycogen levels were not significantly changed in HCR from 0 to 10 min of exercise (Figure 1E). These data suggest that muscle glycogen contributes significantly to the higher whole-body carbohydrate oxidation in LCR during the first 10 min of exercise (Figure 1A), while HCR have delayed mobilization of glycogen.
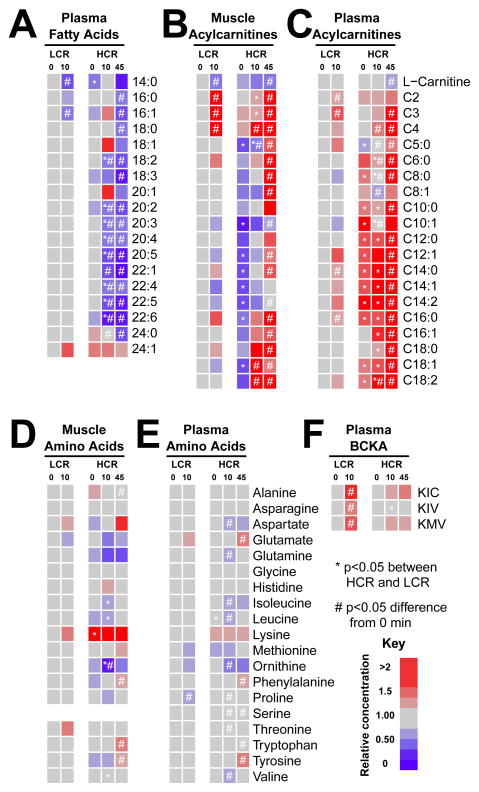
Although HCR have significantly greater fat oxidation throughout exercise, plasma NEFA declined similarly in HCR and LCR at 10 minutes of exercise (Figure 1F). However, plasma lipids (primarily triglycerides) declined more in HCR than LCR at 10 min of exercise (Figure 2A). Alterations in fat metabolism can also be observed through changes in acylcarnitines, which are derived from fatty acyl-chains exchanging CoA with L-carnitine in the mitochondria. At rest, muscle medium- and long-chain acylcarnitine species were lower in HCR than LCR (Figure 2B), while the same acylcarnitine species were higher in plasma of HCR than LCR (Figure 2C). With exercise, LCR muscle long-chain acylcarnitines changed minimally, and we observed only small rises in plasma long-chain acylcarnitines at 10 min, consistent with reduced FA use. In contrast, HCR muscle long-chain acylcarnitines increased at 10 min of exercise, indicative of increased FA import. In addition, minimal changes in muscle medium-chain acylcarnitines (C6–C12) were found, suggesting efficient oxidation of the FA. Only near exhaustion do HCR muscle and plasma medium- and long-chain acylcarnitines increase above resting levels (Figure 2B–C and S2). The increases in plasma acylcarnitines in HCR and LCR near exhaustion likely reflect the limit of mitochondrial FA oxidation, and the delayed rise in plasma acylcarnitines in HCR reflects their greater capacity to oxidize FA during exercise.
Figure 2. Muscle and plasma long-chain acyl-carnitines increase more with exercise in HCR.
Metabolite values at 0, 10 and 45 min of exercise (LCR and HCR) are expressed relative to LCR rest (0 min) for each group (n = 4–6). Plasma fatty acids (primarily triglycerides) (A) and muscle and plasma acylcarnitines (B and C) are listed by carbon chain-length. Muscle and plasma amino acids (D and E) are less dynamic with exercise, but branched-chain keto acids (F) α-ketoisocaproic acid (KIC), α-ketoisovaleric acid (KIV), and α-ketomethylvaleric acid (KMV) show similar trends as short-chain acylcarnitines. * p<0.05 between HCR and LCR at a specific time point; # p<0.05 difference from baseline.
Although FA and carbohydrates account for 85–95% of the fuel used during exercise, amino acids also contribute to overall energy production (Horton et al., 1998; Wagenmakers et al., 1991). At rest, HCR have higher muscle lysine and lower plasma leucine levels than LCR (Figures 2D–E). With exercise, there are greater changes in amino acids in HCR than LCR. Muscle BCAA (leucine, isoleucine, valine) and ornithine were lower in HCR than LCR at 10 min; these changes were paralleled by a fall in plasma BCAA and ornithine in HCR but not LCR (Figure 2D–E), suggesting increased utilization in HCR during exercise. Of all amino acids, BCAA contribute the most toward energy production, and BCAA metabolism increases with exercise (Wagenmakers et al., 1991). The first step in BCAA degradation is loss of nitrogen through transamination to yield branched-chain keto-acids (BCKA). We found that plasma BCKA, α-ketoisocaproic acid (KIC), α-ketomethlyvaleric acid (KMV), and α-ketoisovaleric acid (KIV), were significantly increased in LCR at 10 min but not in HCR (Figure 2F). Further metabolism of BCKA results in the formation of CoA derivatives that can be transferred to carnitine, primarily as C3-, C4- and C5-carnitine. These short-chain acylcarnitines were also elevated in LCR muscle and plasma near exhaustion (10 min) but only C4-carnitine increased in HCR at 10 min, likely reflecting increased n-butyryl-carnitine derived from fat oxidation. Muscle and plasma C3-, C4-, C5-carnitine similarly increased near exhaustion (45 min) in HCR (Figure 2B–C). As with FA oxidation, these results are consistent with greater utilization of BCAA in HCR during exercise.
Metabolite changes coincident with exhaustion
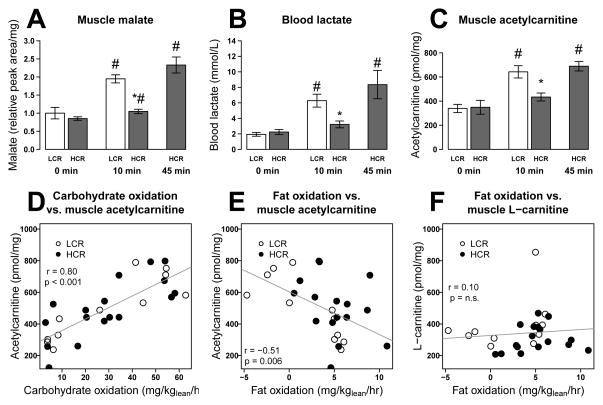
Carbohydrate use increases and FA use decreases with approach to exhaustion in both HCR and LCR. To identify metabolite changes that are coincident with fuel selection and exhaustion, we determined correlation coefficients (Pearson r) of metabolites with estimated FA use, carbohydrate use, and percent exhaustion (time at collection/previous time to exhaustion). We found 19 metabolites to be correlated with both fat and carbohydrate oxidation and coincident with exhaustion; most of these increased with longer exercise duration (Table S2B). Six of these metabolites were confirmed to change significantly with exhaustion in a second independent metabolomic data set (Table S2B) in which HCR and LCR were run to quarter, half, and full exhaustion (Table S2C). Muscle malate consistently increased with approach to exhaustion, as did blood lactate and acetylcarnitine (Figure 3A–C). In parallel, muscle glycogen levels declined with approach to exhaustion (Figure 1E). Muscle acetylcarnitine levels were positively correlated with carbohydrate oxidation and inversely correlated with fat oxidation (Figure 3D–E). A correlation between acetylcarnitine levels and fuel selection (RQ) has been reported previously (Kiens, 2006), but unlike previous reports, we found no association between depletion of muscle L-carnitine and the decline in fat oxidation (Figure 3F), suggesting that in this model, carnitine availability is not limiting fat oxidation. Rather, accumulation of acetylcarnitine indicates overproduction of acetyl units relative to downstream utilization through the citric acid cycle at exhaustion in both HCR and LCR. The similar pattern of change in metabolite levels relative to exhaustion in HCR and LCR suggests that the mechanism of exhaustion is similar in both strains, but is simply delayed in HCR.
Figure 3. Citric acid cycle intermediates, blood lactate and short-chain acylcarnitines increase with exhaustion.
Muscle malate (A), blood lactate (B) and muscle acetylcarnitine (C) values are shown at 0, 10 and 45 min of exercise as mean ± SEM for each group (n = 4–6). Muscle acetylcarnitine (C2) is also strongly correlated with previous estimates of carbohydrate oxidation (D) and fat oxidation (E) at 0, 10, and 45 min of exercise. Muscle L-carnitine (F) was not correlated with fat oxidation. * p<0.05 between HCR and LCR at a specific time point; # p<0.05 difference from baseline.
Specific upregulation and differential acetylation of mitochondrial proteins in HCR and LCR
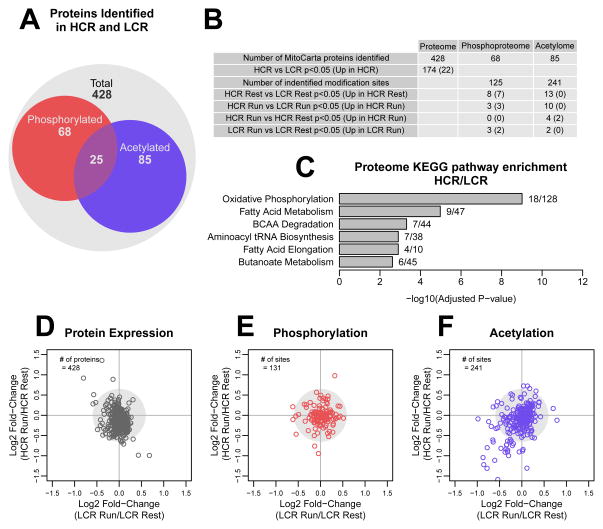
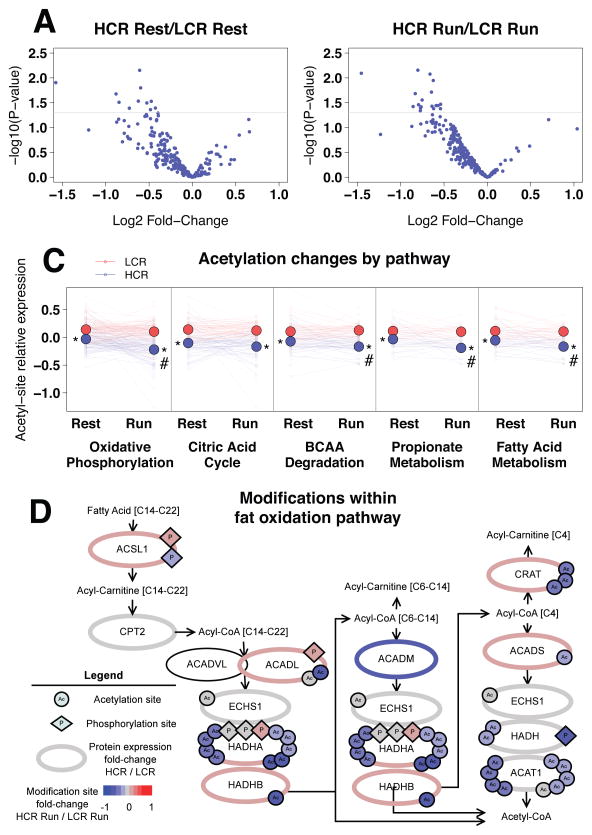
Changes in fuel use, though dependent on fuel availability, are largely controlled by enzyme availability and activity. Previous studies have demonstrated increased mRNA and protein expression in pathways related to oxidative metabolism in HCR vs. LCR (Burniston et al., 2011; Kivela et al., 2010). To investigate potential changes in the proteome and post-translation modifications in skeletal muscle mitochondria with exercise, we performed quantitative proteomic analysis. Mitochondria were isolated from HCR and LCR extensor digitorum longus muscle at rest and after 10 min of exercise, and isobaric tags were used for multiplexed quantification (Hebert et al., 2013). We identified 428 mitochondrial proteins (Table S3A), of which 73 were phosphorylated and 85 were acetylated (Figure 4A–B; Table S3A–C). In prior studies, HCR were reported to have no change (Naples et al., 2010) or a fiber type-specific upregulation of mitochondrial mass (Rivas et al., 2011) when compared to LCR rats; thus we normalized our proteomic data to mitochondrial protein. In the mitochondrial proteome, 174 proteins were significantly different (p<0.05, permutation t-test) between HCR and LCR. Pathway analysis is a common tool to understand global changes in phenotypes in cells and tissues. Due to our focus on metabolism, we chose to use KEGG Pathway Ontology for analysis, which has well annotated metabolic pathways. KEGG pathway analysis by Enrichr (Chen et al., 2013) showed that the differentially expressed proteins were enriched in oxidative phosphorylation, FA metabolism, BCAA degradation, and tRNA biosynthesis pathways (Figure 4C). Of the 174 proteins, only 22 were significantly higher in HCR and included multiple enzymes involved in FA oxidation (Figure 4C, Table S3A). When we examined changes with exercise, we found few alterations in the proteome or phosphoproteome in either strain (Figure 4D–E). However, we found evidence for decreased acetylation in both HCR and LCR mitochondria following exercise (Figure 4F).
Figure 4. Mitochondrial protein acetylation is dynamic with exercise.
We identified 428 mitochondrial proteins, of which 73 were phosphorylated and 85 were acetylated (A–B). For pathway enrichment, log2 fold-change values of differentially expressed proteins between HCR and LCR were input into Enrichr; the numbers of identified proteins out of total number of proteins in the ontology are listed to the right of the bars (C). Log2 fold-change (Run/Rest) values were used to plot changes with exercise in the proteome (D), phosphoproteome (E), and acetylome (F).
Overall, mitochondrial acetylation was lower in HCR at rest (Figure 5A), and the difference was amplified following exercise (Figure 5B). KEGG pathway analysis revealed that acetylation sites were enriched within the oxidative phosphorylation, citric acid cycle, BCAA degradation, propionate metabolism and FA metabolism pathways. By estimating the relative level of acetylation of all peptides within each of these pathways, we found that there was significantly lower mitochondrial acetylation in each pathway in HCR compared to LCR, both at rest and following exercise (Figure 5C). In addition, HCR had significant deacetylation in 4 of the 5 acetyl-rich pathways with 10 min of exercise, while LCR had no significant pathway changes in acetylation. These pathways are targets of the mitochondrial lysine deacetylase SIRT3 (Rardin et al., 2013). In particular, MDH2 K239 is a major Sirt3 target (Hebert et al., 2013) and is significantly differentially acetylated between HCR and LCR at 10 min of exercise (p=0.02) and becomes less acetylated in HCR with exercise (p=0.057; see Table S3C), suggesting a possible role of SIRT3 in mediating the differences in acetylation. However, we found no difference in the SIRT3 levels between HCR and LCR in mitochondrial extracts by proteomic analysis (Table S3A) or Western blotting (Figure S3). If SIRT3 is mediating these differences, then it is likely due to differential activation of SIRT3.
Figure 5. HCR have less mitochondrial protein acetylation than LCR.
Log2 fold-change (HCR/LCR) values for the 141 acetyl-lysine sites were plotted against p-values at rest (A) and at 10 min run (B, n=5). Acetylation sites were enriched within oxidative pathways; for each enriched pathway, HCR proteins are less acetylated than LCR proteins (C). Average protein acetylation (small circles) is dynamic with exercise, and average pathway acetylation (large circles) is lower in HCR than LCR at 10 min of exercise. The fat oxidation pathway (D) is less acetylated in HCR with exercise. Proteins (ellipses) are labeled by gene symbol with identified acetylation (circle) and phosphorylation (diamond) sites. The color scale designates log2 fold-change (HCR/LCR comparison for protein; or HCR Run/LCR Run comparison for acetylation and phosphorylation).
To assess the role of differential acetylation of MDH2, we measured MDH activity, previously shown to be modulated by acetylation state (Hebert et al., 2013; Zhao et al., 2010), in isolated mitochondria from HCR and LCR. While MDH2 protein level were similar in HCR and LCR (Table S3A), there was a significant inverse relationship between average acetylation state of MDH2 and MDH activity, but less significant inverse relationship between individual acetylation sites and MDH activity (Figure S4). These data suggest a complex interplay between sites, and potentially other post-translational modifications, in regulation of individual enzyme activity.
Deacetylation of proteins in the BCAA pathway is associated with increased BCAA catabolism during exercise
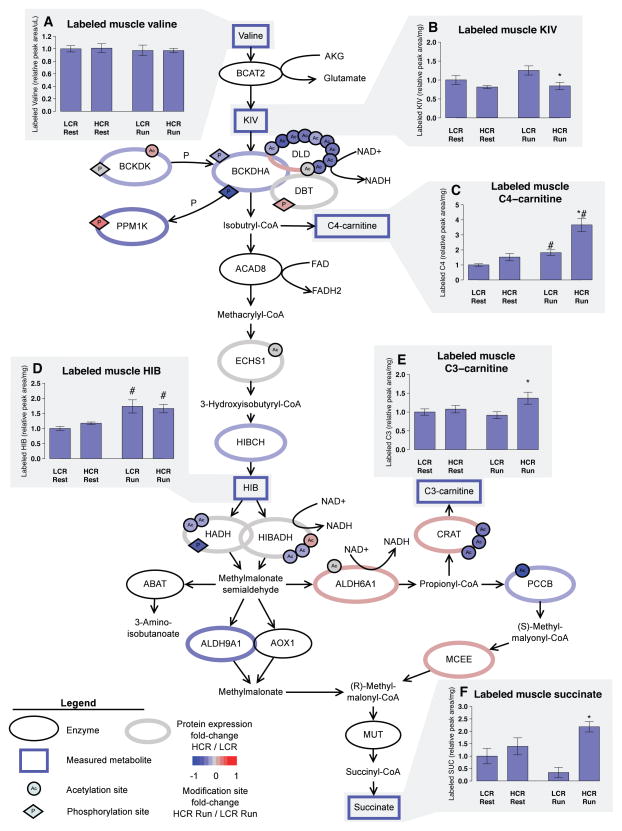
To further assess the functional consequence of differential acetylation in HCR and LCR, we estimated flux through the BCAA degradation pathway by intraperitoneal injection of U-13C15N valine. We determined the isotopic enrichment of downstream metabolites in serum and gastrocnemius muscle at rest and immediately after 10 min of exercise (Table S4A–B, Figure 6). We confirmed that valine injection does not significantly alter running capacity or estimated fuel preference (Figure S5) and that serum and muscle isotopic enrichment of valine in was not significantly different between HCR and LCR (Table S4A, Figure 6A). U-13C15N valine (mass shift of 6Da; M+6 isotope) is reversibly transaminated to M+5 KIV (Figure 6B) and can be reaminated to M+5 valine by the incorporation of 14N. HCR-Run had a greater M+5:M+6 ratio of valine in serum and muscle (Table S4A), indicating greater valine transamination-reamination in HCR. Muscle glutamate nitrogen enrichment (M+1) was also significantly greater in HCR-Run, supporting the conclusion that HCR have greater valine transamination in skeletal muscle (Table S4B).
Figure 6. HCR have greater valine metabolism with exercise than LCR.
Proteins involved in BCAA metabolism (ellipses) are labeled by gene symbol with identified acetylation (circle) and phosphorylation (diamond) sites. The color scale designates log2 fold-change (HCR/LCR comparison for protein; or HCR Run/LCR Run comparison for acetylation and phosphorylation). Animals were injected with U-13C15N valine and rested for 10 min before an additional 10 min rest or 10 min run. LCR and HCR have similar amounts of labeled valine in gastrocnemius (A), but show differences in amount of labeled metabolites downstream of valine: α-ketoisovaleric acid (KIV, B), isobutyryl-carnitine (C4, C), β-hydroxyisobutyrate (HIB, D), propionyl-carnitine (C3, E) and succinate (F). Values are mean ± SEM relative to LCR rest for each group (n = 4–6). * p<0.05 between HCR and LCR; # p<0.05 difference within strain between Rest and Run.
The irreversible branched-chain keto-acid dehydrogenase (BCKDH) complex is the rate-limiting step of BCAA degradation. With exercise, there is greater 13C-labeled C4-carnitine and β-hydroxyisobutryate (HIB) in muscle in both HCR and LCR (Figure 6C–D), indicating increased metabolism of valine. HCR showed greater flux through the BCKDH complex and downstream enzymes, as demonstrated by greater accumulation of 13C-labeled C4- and C3-carnitines and succinate in muscle of HCR-Run vs. LCR-Run (Figure 6C, E and F). However, accumulation of 13C-labeled succinate was quantitatively quite small (Table S4A), suggesting that valine contributes minimally to citric acid cycle anaplerosis.
The BCKDH complex can be activated by dephosphorylation, but the role of dephosphorylation in exercise-associated increase in BCAA utilization is unclear (Howarth et al., 2007). Our proteomics analysis showed a non-significant decrease in Ser293 phosphorylation of BCKDHA subunit with exercise in HCR (Figure 6, Table S3B). Notably, dihydrolipoamide dehydrogenase (DLD), a subunit of the BCKDH complex, contained 10 acetylation sites that were all less acetylated in HCR compared to LCR with exercise (Figure 6, Table S3C). As DLD is a subunit of the pyruvate dehydrogenase and α-ketoglutarate dehydrogenase complex, future studies are warranted to dissect the potential modulation of these complexes secondary to changes in DLD acetylation.
Discussion
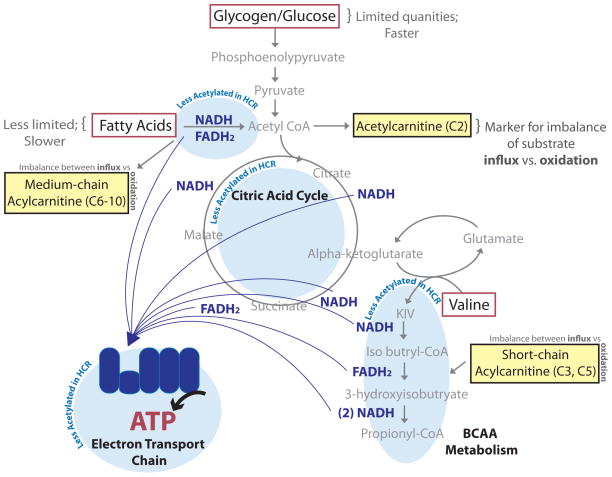
The selection for enhanced exercise capacity in HCR recapitulates many of the health benefits observed in humans (Blair et al., 1996; Church et al., 2004; Dvorak et al., 2000; Kodama et al., 2009) and provides a model to understand the underlying mechanisms linking oxidative capacity and metabolic health. Based on our results, we propose that animals with enhanced oxidative capacity have increased FA and BCAA utilization and ATP generation through upregulation and deacetylation of proteins within these oxidative pathways (Figure 7). In conditions of increasing energy demand such as exercise, HCR are able to continue production of ATP through oxidative phosphorylation, at a time when LCR have reached their maximal oxidative capacity. When the ability to oxidize substrate becomes limiting, glycogen is mobilized and lactate is generated through anaerobic glycolysis. The buildup of acetylcarnitine and other substrates in muscle at exhaustion in both HCR and LCR points to a profound imbalance between the influx of substrates and ability to oxidize these substrates.
Figure 7. Increased fuel efficiency supports enhanced oxidative capacity.
HCR have more efficient oxidation of fatty acid (FA) and branched-chain amino acid (BCAA); this is supported by lower protein acetylation within FA and BCAA metabolic pathways, as well as the citric acid cycle and the electron transport chain. Enhanced oxidative efficiency leads to slower accumulation of metabolic intermediates such as short- and medium- chain acylcarnitines, which indicate imbalances between substrate supply and downstream oxidation.
Like others (Hall et al., 2010; Morris et al., 2013; Nordby et al., 2006; Venables et al., 2005), we show that higher exercise capacity is associated with increased FA oxidation during exercise. Akin to adaptations observed with exercise training (Battaglia et al., 2012; Holloszy et al., 1998; Kiens et al., 1993), non-trained HCR have upregulation of enzymes within the FA oxidation pathway (Table S3A), increased FA oxidation, slower lactate production and delayed glycogen utilization (Figure 1). Accumulated evidence suggest that deacetylation of enzymes within FA and BCAA pathways increases their activity (Hallows et al., 2011; Hirschey et al., 2010; Rardin et al., 2013; Still et al., 2013). Our finding of mitochondrial protein deacetylation within specific catabolic pathways with exercise provides an additional potential mechanism by which muscle can increase substrate oxidation in response to increased energy demand. Similar to observations in mice (Hirschey et al., 2010), we find that reduced acetylation of enzymes in FA oxidation pathways (Figure 5D) is associated with increased whole-animal FA oxidation (Figure 1B) and increased skeletal muscle efficiency (delayed medium-chain acylcarnitine accumulation) in HCR vs. LCR. Differential acetylation of BCAA pathway enzymes also parallels differential valine flux (Figure 6). The finding that mitochondrial MDH activity was inversely correlated with average MDH2 acetylation (Figure S4), but not as well correlated with individual acetyl-sites, supports a complex rheostat-like role for acetylation (and likely other modifications) of lysine residues (Choudhary et al., 2014).
Change in lysine acetylation of mitochondrial proteins is a balance between NAD+-dependent SIRT3 deacetylase activity (Lombard et al., 2007) and non-enzymatic addition of acetyl groups to lysine (Wagner and Payne, 2013). In contrast to the lower acetylation observed with exercise training and calorie restriction (Palacios et al., 2009), the lower level of acetylation observed in HCR is not explained by SIRT3 content (Table 3A and Figure S3). Rather, the dynamic deacetylation could be due to SIRT3 activation by increases in mitochondrial NAD+ that occurs with exercise (White and Schenk, 2012). In addition, the differences in acetyl-unit availability may also contribute to the observed differences in acetylation between HCR and LCR. Acetylcarnitine accumulation with increasing exercise intensity is a consistent phenomenon (Constantin-Teodosiu et al., 1991; Gollnick et al., 1974; Hiatt et al., 1989; Sahlin, 1990; van Loon et al., 2001), and like others (Kiens, 2006), we show that acetylcarnitine levels correlate positively with carbohydrate oxidation and negatively with FA oxidation (Figure 3D–E). Thus, HCR mitochondria appear to be exposed to less acetyl groups at rest and during activity, contributing to the lower mitochondrial protein acetylation when compared to LCR.
Importantly, metabolic differences between HCR and LCR during exercise inform us about altered metabolic pathways that underlie risk for metabolic disease. Decreased fat oxidation during exercise (Hall et al., 2010) and impaired switching between glucose and FA oxidation are both linked to insulin resistance and diabetes (Kelley and Mandarino, 2000). Recent studies highlight a link between altered BCAA metabolism to the development of insulin resistance and diabetes, reviewed in (Lynch and Adams, 2014). Notably, resting plasma BCAA levels are also inversely correlated with exercise capacity (Morris et al., 2013), and disruption of BCAT2, the enzyme responsible for the initial transamination step, in skeletal muscle leads to decreased exercise capacity (She et al., 2010). Based on our data, changes in the capacity to oxidize FA and BCAA could be due to differential expression and post-translational modification of enzymes in FA and BCAA metabolism and oxidative phosphorylation (Figure 7). Intrinsic genetic differences could be amplified by mitochondrial acetylation in the setting of inactivity and overnutrition (Hirschey et al., 2011). Thus, phenotypes associated with metabolic disease, such as increased circulating BCAA and metabolic inflexibility, may reflect differences in the capacity to utilize BCAA and FA.
Beyond implications to metabolic health, these data suggest a possible mechanistic link between exercise capacity and longevity. Like HCR, a number of longevity models have elevated FA and BCAA oxidation, including Ames dwarf and growth hormone receptor knock-out mice (Westbrook et al., 2009) and caloric restriction in drosophila (Katewa et al., 2012), mice (Bruss et al., 2010) and humans (Huffman et al., 2012). In addition to enhanced FA and BCAA oxidation, HCR have reduced weight gain, lower percent body fat, and small elevations in non-resting energy expenditure (Gavini et al., 2014), and yet HCR have greater food consumption (Thyfault et al., 2009), implying energy wasting. Thus, the enhanced efficiently of FA and BCAA utilization is not accompanied by enhanced efficiency of energy production and suggests that HCR have mitochondrial uncoupling. This uncoupling may be in part due to expression of uncoupling protein 3 (UCP3) (Table S4A) (Gavini et al., 2014), in addition to the lower ATP yield per mole of oxygen consumed during of FA oxidation (compared to carbohydrate) and the intrinsic ability of lipids to uncouple mitochondria (Skulachev, 1998). Indeed, it has been shown that exercise training results in increased substrate utilization humans, accompanied by mitochondrial uncoupling (Befroy et al., 2008). Uncoupling in individuals with increased VO2max appears to be associated specifically with FA oxidation (Jacobs and Lundby, 2013). Despite the uncoupling there is reduced oxidative damage in HCR vs. LCR (Tweedie et al., 2011), similar to caloric restriction (Lanza et al., 2012). Coincidently, long-lived organisms with mitochondrial mutations have elevated FA utilization and display upregulation of energy producing pathways (Martin et al., 2011; Munkacsy and Rea, 2014). Like these models of longevity, HCR and LCR show pathway specific differences in mitochondrial protein abundance (Figure 4B–C). It has been suggested that metabolites mediate the response of the organism to mitochondrial dysfunction (Butler et al., 2013). Ongoing studies directed towards understanding the genetic basis of maximal exercise capacity (Ren et al., 2013) and the commonalities of metabolites in long-lived animals, may provide important insights into the integration of signals in modulating the whole-animal response to exercise and aging.
Methods
Animal exercise protocol
Animals were housed at the University of Michigan and the University Committee on Use and Care of Animals (Ann Arbor, Michigan) approved the study. HCR and LCR rats were used for indirect calorimetry and metabolomics analysis (male, generation 26 at 4.5 months of age), proteomic analysis (male, generation 31 at 3 months of age), and the isotope tracer study (female, generation 31 at 3 months of age). Exercise capacity was determined using an increasing intensity exercise test (Koch and Britton, 2001) on a motorized treadmill (Columbus Instruments, Columbus, OH), and this protocol was used for all subsequent tests.
For metabolomic profiling, rats were run for 0 min, 10 min, 45 min (HCR only). For the proteomics analysis, rats were run for 0 min and 10 min (n=5). For the isotope tracer study, HCR and LCR were intraperitoneally injected with U-13C15N valine (100 mg/kg) and placed on a stationary treadmill for 10 min. Between 10 and 20 min post injection, the Run groups underwent the treadmill running protocol and the Rest groups remained on the stationary treadmill (n=4–6).
Immediately after removal from the treadmill, animals were euthanized by decapitation. Trunk blood was used for immediate glucose and lactate quantitation (Accu-Chek Aviva meter and Nova Biomedical Lactate Plus meter) and to prepare plasma and/or serum. Tissues were harvested and frozen in liquid nitrogen. For subsequent assays, frozen tissues were pulverized with a mortar and pestle that had been pre-chilled with liquid nitrogen.
Indirect Calorimetry
Volume of oxygen consumed (VO2) and carbon dioxide produced (VCO2) were recorded using a Comprehensive Laboratory Animal Monitoring System (CLAMS, Columbus Instruments), with a 5-second sampling period every 2 minutes. VO2 and VCO2 were scaled to lean mass determined using an NMR-based analyzer (Minispec LF90II, Bruker Optics, Billerica, MA). Whole body carbohydrate and fat oxidation were estimated from non-protein RQ (Peronnet and Massicotte, 1991). We adjusted the carbohydrate oxidation as reported previously (Jeukendrup and Wallis, 2005), see supplemental methods.
Muscle glycogen
Gastrocnemius muscle was digested for 15 min at 37°C in 30% KOH (m/v). Ethanol and Na2SO4 (64% and 0.32% final) were added to samples to precipitate glycogen overnight at −20°C. Sample pellets were washed twice with 10% KOH and 66% ethanol. Pellets were dissolved with 4 N H2SO4 at 100°C for 2 hr and then neutralized with 4 N NaOH. Glucose concentrations was determined using Sigma’s glucose assay kit (HK; St. Louis, MO) and was normalized to starting tissue mass.
Plasma lipid analysis
Total plasma non-esterified FA was measured with a kit from Wako (Richmond, VA). For lipid species analysis, lipids were extracted from plasma (Bligh and Dyer, 1959). Methyl esters were purified by thin layer chromatography and analyzed by gas chromatography (Sattler et al., 1991).
Muscle and plasma Metabolites
For detailed methods, see supplemental methods. Briefly, metabolites from frozen tissue and serum/plasma were extracted with a solvent mixture consisting of 8:1:1 HPLC grade methanol:chloroform:water. Polar metabolites were analyzed by hydrophilic interaction chromatography – electrospray time of flight mass spectrometry (HILIC-ESI-TOF)(Lorenz et al., 2011). Acylcarnitines were analyzed by reversed phase liquid chromatography – tandem quadrupole mass spectrometry (RPLC-ESI-QQQ). Amino acids and other polar metabolites were derivatized and analyzed by gas chromatography – electron ionization mass spectrometry (GC-EI-MS) (Badawy et al., 2007; Fiehn et al., 2000). Keto acids were analyzed by RPLC-ESI-TOF in negative ion mode (Evans et al., 2013). Targeted metabolite quantitation was performed by peak area using Agilent Masshunter Quantitative Analysis software, and natural isotope abundance correction was performed using MATLAB (2012a, The MathWorks, Natick, MA).
Mitochondrial isolation and proteomic analysis
For detailed methods, see supplemental methods. Intact mitochondria were isolated from homogenized extensor digitorum longus muscle. Proteins were reduced, alkylated, and digested with LysC followed by trypsin. The resulting peptides were labeled with TMT isobaric labels, mixed in equal amounts by mass, and fractionated by strong cation exchange chromatography. Phospho peptides were enriched with immobilized metal affinity chromatography (IMAC) with magnetic beads. Acetylated peptides were enriched with pan-acetyl lysine antibody-agarose conjugate. Enriched and non-enriched fractions were analyzed by nano-RPLC coupled to an Orbitrap Fusion (Thermo). Spectra were searched using the open mass spectrometry search algorithm and results were filtered to 1% FDR at the unique peptide level using the COMPASS software suite. TMT quantification and protein grouping were performed according to previously reported rules (Phanstiel et al., 2011). Proteins were identified as mitochondrial by mapping rat genes to a previously compiled MitoCarta compendium of mitochondrial mouse proteins (Pagliarini et al., 2008).
Statistics and Figures
Metabolite data are presented as mean ± standard error of the mean (SEM). Proteomic data was expressed as log2 fold change between rest and run and HCR and LCR. Statistical analysis and figures were made with the R statistical and graphing environment (R Core Team, 2013). P-values were determined by permutation t-test using R perm package (Fay, 2010).
Supplementary Material
Acknowledgments
This work was supported by R24OD010950 (LK and SB), R01GM104194 (SB), K25DK092558 (CE), R01GM080148 (J. Coon), R01DK098672 (DP) and R01DK077200, R01DK099034 and R24DK097153 (CB), as well as an American Heart Association Predoctoral Fellowship (J. Carson) and an NLM training grant (NLM T15LM007359) to the Computation and Informatics in Biology and Medicine Training Program (CM). This work utilized Metabolomics Core Services supported by U24DK097153 to the University of Michigan. We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Heckenkamp and technical assistance by Mary Kay Treutelaar. Finally, we thank David Lombard for advice and critical comments on the manuscript.
Footnotes
Author Contributions:
Conceived and designed the experiments: KO, CE, NQ, J. Coon, CB, LK, SB. Performed the experiments: KO, CE, CM, J. Carson, CCS, DP, NQ. Analyzed the data: KO, CE, CM, CCS, CB. Wrote the paper: KO, CE, CB.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badawy AAB, Morgan CJ, Turner JA. Application of the Phenomenex EZ:faast™ amino acid analysis kit for rapid gas-chromatographic determination of concentrations of plasma tryptophan and its brain uptake competitors. Amino Acids. 2007;34:587–596. doi: 10.1007/s00726-007-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia GM, Zheng D, Hickner RC, Houmard JA. Effect of exercise training on metabolic flexibility in response to a high-fat diet in obese individuals. American Journal of Physiology - Endocrinology and Metabolism. 2012;303:E1440–E1445. doi: 10.1152/ajpendo.00355.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befroy D, Petersen K, Dufour S, Mason G, Rothman D, Shulman G. Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16701–16706. doi: 10.1073/pnas.0808889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab. 2010;298:E108–116. doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burniston JG, Kenyani J, Wastling JM, Burant CF, Qi NR, Koch LG, Britton SL. Proteomic analysis reveals perturbed energy metabolism and elevated oxidative stress in hearts of rats with inborn low aerobic capacity. Proteomics. 2011;11:3369–3379. doi: 10.1002/pmic.201000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JA, Mishur RJ, Bhaskaran S, Rea SL. A metabolic signature for long life in the Caenorhabditis elegans Mit mutants. Aging cell. 2013;12:130–138. doi: 10.1111/acel.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nature reviews Molecular cell biology. 2014;15:536–550. doi: 10.1038/nrm3841. [DOI] [PubMed] [Google Scholar]
- Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27:83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Carlin JI, Cederblad G, Harris RC, Hultman E. Acetyl group accumulation and pyruvate dehydrogenase activity in human muscle during incremental exercise. Acta physiologica Scandinavica. 1991;143:367–372. doi: 10.1111/j.1748-1716.1991.tb09247.x. [DOI] [PubMed] [Google Scholar]
- Dvorak RV, Tchernof A, Starling RD, Ades PA, DiPietro L, Poehlman ET. Respiratory fitness, free living physical activity, and cardiovascular disease risk in older individuals: a doubly labeled water study. J Clin Endocrinol Metab. 2000;85:957–963. doi: 10.1210/jcem.85.3.6432. [DOI] [PubMed] [Google Scholar]
- Evans CR, Karnovsky A, Kovach MA, Standiford TJ, Burant CF, Stringer KA. Untargeted LC–MS Metabolomics of Bronchoalveolar Lavage Fluid Differentiates Acute Respiratory Distress Syndrome from Health. J Proteome Res. 2013;13:640–649. doi: 10.1021/pr4007624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard R, Bielen E, Amery A. Heritability of aerobic power and anaerobic energy generation during exercise. J Appl Physiol. 1991;70:357–362. doi: 10.1152/jappl.1991.70.1.357. [DOI] [PubMed] [Google Scholar]
- Fay MPS, AP Exact and Asymptotic Weighted Logrank Tests for Interval Censored Data: The interval R Package. Journal of Statistical Software. 2010;36:1–34. doi: 10.18637/jss.v036.i02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn O, Kopka J, Trethewey RN, Willmitzer L. Identification of Uncommon Plant Metabolites Based on Calculation of Elemental Compositions Using Gas Chromatography and Quadrupole Mass Spectrometry. Analytical Chemistry. 2000;72:3573–3580. doi: 10.1021/ac991142i. [DOI] [PubMed] [Google Scholar]
- Gavini CK, Mukherjee S, Shukla C, Britton SL, Koch LG, Shi H, Novak CM. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am J Physiol Endocrinol Metab. 2014;306:E635–647. doi: 10.1152/ajpendo.00555.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974;241:45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LML, Moran C, Milne G, Wilson J, MacFarlane N, Forouhi N, Hariharan N, Salt I, Sattar N, Gill JMR. Fat oxidation, fitness and skeletal muscle expression of oxidative/lipid metabolism genes in South Asians: implications for insulin resistance? PLoS ONE. 2010;5:e14197–e14197. doi: 10.1371/journal.pone.0014197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM, et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Molecular cell. 2011;41:139–149. doi: 10.1016/j.molcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Molecular cell. 2013;49:186–199. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt WR, Regensteiner JG, Wolfel EE, Ruff L, Brass EP. Carnitine and acylcarnitine metabolism during exercise in humans. Dependence on skeletal muscle metabolic state. The Journal of clinical investigation. 1989;84:1167–1173. doi: 10.1172/JCI114281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Molecular cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Kohrt WM, Hansen PA. The regulation of carbohydrate and fat metabolism during and after exercise. Front Biosci. 1998;3:D1011–1027. doi: 10.2741/a342. [DOI] [PubMed] [Google Scholar]
- Horton TJ, Pagliassotti MJ, Hobbs K, Hill JO. Fuel metabolism in men and women during and after long-duration exercise. Journal of applied physiology (Bethesda, Md: 1985) 1998;85:1823–1832. doi: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- Howarth KR, Burgomaster KA, Phillips SM, Gibala MJ. Exercise training increases branched-chain oxoacid dehydrogenase kinase content in human skeletal muscle. American journal of physiology Regulatory, integrative and comparative physiology. 2007;293:R1335–1341. doi: 10.1152/ajpregu.00115.2007. [DOI] [PubMed] [Google Scholar]
- Huffman KM, Redman LM, Landerman LR, Pieper CF, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Kraus VB, Newgard CB, et al. Caloric restriction alters the metabolic response to a mixed-meal: results from a randomized, controlled trial. PLoS One. 2012;7:e28190. doi: 10.1371/journal.pone.0028190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RA, Lundby C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. Journal of applied physiology (Bethesda, Md : 1985) 2013;114:344–350. doi: 10.1152/japplphysiol.01081.2012. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Wallis GA. Measurement of substrate oxidation during exercise by means of gas exchange measurements. International journal of sports medicine. 2005;26(Suppl 1):S28–37. doi: 10.1055/s-2004-830512. [DOI] [PubMed] [Google Scholar]
- Katewa SD, Demontis F, Kolipinski M, Hubbard A, Gill MS, Perrimon N, Melov S, Kapahi P. Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell metabolism. 2012;16:97–103. doi: 10.1016/j.cmet.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiological reviews. 2006;86:205–243. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivela R, Silvennoinen M, Lehti M, Rinnankoski-Tuikka R, Purhonen T, Ketola T, Pullinen K, Vuento M, Mutanen N, Sartor MA, et al. Gene expression centroids that link with low intrinsic aerobic exercise capacity and complex disease risk. FASEB J. 2010;24:4565–4574. doi: 10.1096/fj.10-157313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisloff H, Hoydal MA, Rolim N, et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circulation research. 2011;109:1162–1172. doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA (Chicago, Ill) 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Zabielski P, Klaus KA, Morse DM, Heppelmann CJ, Bergen HR, 3rd, Dasari S, Walrand S, Short KR, Johnson ML, et al. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell metabolism. 2012;16:777–788. doi: 10.1016/j.cmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Molecular and cellular biology. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MA, Burant CF, Kennedy RT. Reducing Time and Increasing Sensitivity in Sample Preparation for Adherent Mammalian Cell Metabolomics. Analytical Chemistry. 2011;83:3406–3414. doi: 10.1021/ac103313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nature reviews Endocrinology. 2014;10:723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FP, Spanier B, Collino S, Montoliu I, Kolmeder C, Giesbertz P, Affolter M, Kussmann M, Daniel H, Kochhar S, et al. Metabotyping of Caenorhabditis elegans and their culture media revealed unique metabolic phenotypes associated to amino acid deficiency and insulin-like signaling. J Proteome Res. 2011;10:990–1003. doi: 10.1021/pr100703a. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Klein S. Effect of aging on glucose and lipid metabolism during endurance exercise. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S86–91. doi: 10.1123/ijsnem.11.s1.s86. [DOI] [PubMed] [Google Scholar]
- Morris C, Grada CO, Ryan M, Roche HM, De Vito G, Gibney MJ, Gibney ER, Brennan L. The relationship between aerobic fitness level and metabolic profiles in healthy adults. Molecular nutrition & food research. 2013;57:1246–1254. doi: 10.1002/mnfr.201200629. [DOI] [PubMed] [Google Scholar]
- Munkacsy E, Rea SL. The paradox of mitochondrial dysfunction and extended longevity. Experimental gerontology. 2014;56:221–233. doi: 10.1016/j.exger.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naples SP, Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Mikus CR, Koch LG, Britton SL, Ibdah JA, Thyfault JP. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Appl Physiol Nutr Metab. 2010;35:151–162. doi: 10.1139/h09-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, Lust JA, Britton SL, Koch LG, Dudek RW, et al. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2007;293:E31–41. doi: 10.1152/ajpendo.00500.2006. [DOI] [PubMed] [Google Scholar]
- Nordby P, Saltin B, Helge JW. Whole-body fat oxidation determined by graded exercise and indirect calorimetry: a role for muscle oxidative capacity? Scand J Med Sci Sports. 2006;16:209–214. doi: 10.1111/j.1600-0838.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, Britton SL, Koch LG, Akil H, Levine JA. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58:355–367. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Canadian journal of sport sciences = Journal canadien des sciences du sport. 1991;16:23–29. [PubMed] [Google Scholar]
- Phanstiel DH, Brumbaugh J, Wenger CD, Tian S, Probasco MD, Bailey DJ, Swaney DL, Tervo MA, Bolin JM, Ruotti V, et al. Proteomic and phosphoproteomic comparison of human ES and iPS cells. Nature methods. 2011;8:821–827. doi: 10.1038/nmeth.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, Schilling B, Mooney SD, Kahn CR, Verdin E, et al. Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6601–6606. doi: 10.1073/pnas.1302961110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren YY, Overmyer KA, Qi NR, Treutelaar MK, Heckenkamp L, Kalahar M, Koch LG, Britton SL, Burant CF, Li JZ. Genetic analysis of a rat model of aerobic capacity and metabolic fitness. PLoS One. 2013;8:e77588. doi: 10.1371/journal.pone.0077588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas DA, Lessard SJ, Saito M, Friedhuber AM, Koch LG, Britton SL, Yaspelkis BB, 3rd, Hawley JA. Low intrinsic running capacity is associated with reduced skeletal muscle substrate oxidation and lower mitochondrial content in white skeletal muscle. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300:R835–843. doi: 10.1152/ajpregu.00659.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K. Muscle carnitine metabolism during incremental dynamic exercise in humans. Acta physiologica Scandinavica. 1990;138:259–262. doi: 10.1111/j.1748-1716.1990.tb08845.x. [DOI] [PubMed] [Google Scholar]
- Sattler W, Puhl H, Hayn M, Kostner GM, Esterbauer H. Determination of fatty acids in the main lipoprotein classes by capillary gas chromatography: BF3/methanol transesterification of lyophilized samples instead of Folch extraction gives higher yields. Analytical biochemistry. 1991;198:184–190. doi: 10.1016/0003-2697(91)90526-y. [DOI] [PubMed] [Google Scholar]
- She P, Zhou Y, Zhang Z, Griffin K, Gowda K, Lynch CJ. Disruption of BCAA metabolism in mice impairs exercise metabolism and endurance. Journal of Applied Physiology. 2010;108:941–949. doi: 10.1152/japplphysiol.01248.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis LS, Gastaldelli A, Klein S, Wolfe RR. Regulation of plasma fatty acid oxidation during low- and high-intensity exercise. Am J Physiol. 1997;272:E1065–1070. doi: 10.1152/ajpendo.1997.272.6.E1065. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochimica et biophysica acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- Still AJ, Floyd BJ, Hebert AS, Bingman CA, Carson JJ, Gunderson DR, Dolan BK, Grimsrud PA, Dittenhafer-Reed KE, Stapleton DS, et al. Quantification of mitochondrial acetylation dynamics highlights prominent sites of metabolic regulation. The Journal of biological chemistry. 2013;288:26209–26219. doi: 10.1074/jbc.M113.483396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, et al. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587:1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie C, Romestaing C, Burelle Y, Safdar A, Tarnopolsky MA, Seadon S, Britton SL, Koch LG, Hepple RT. Lower oxidative DNA damage despite greater ROS production in muscles from rats selectively bred for high running capacity. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300:R544–553. doi: 10.1152/ajpregu.00250.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables MC, Achten J, Jeukendrup AE. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. J Appl Physiol. 2005;98:160–167. doi: 10.1152/japplphysiol.00662.2003. [DOI] [PubMed] [Google Scholar]
- Wagenmakers AJ, Beckers EJ, Brouns F, Kuipers H, Soeters PB, van der Vusse GJ, Saris WH. Carbohydrate supplementation, glycogen depletion, and amino acid metabolism during exercise. Am J Physiol. 1991;260:E883–890. doi: 10.1152/ajpendo.1991.260.6.E883. [DOI] [PubMed] [Google Scholar]
- Wagner GR, Payne RM. Widespread and enzyme-independent Nepsilon-acetylation and Nepsilon-succinylation of proteins in the chemical conditions of the mitochondrial matrix. The Journal of biological chemistry. 2013;288:29036–29045. doi: 10.1074/jbc.M113.486753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64:443–451. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AT, Schenk S. NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab. 2012;303:E308–321. doi: 10.1152/ajpendo.00054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.