Abstract
Background:
Anemia in pregnancy is a common cause of maternal morbidity and mortality in developing countries. Regular review of hematocrit (HCT) and anemia patterns in pregnancy is necessary in our environment.
Aim:
The aim was to determine the average HCT, prevalence, and pattern of anemia, as well the arm preferences for blood sample collection among pregnant women in Enugu, South East Nigeria.
Subjects and Methods:
HCT was determined using venous blood of 200 antenatal women at the University of Nigeria Teaching Hospital (UNTH) Enugu, Nigeria. Questionnaires were used to assess participants’ arm preference for blood sample collection for clinical investigations. Data analysis was descriptive and inferential at 95% confidence level.
Results:
Participants’ mean HCT was 33.3 (3.7%). The average HCT in second trimester 33.3% (3.76%) was significantly lower than that of third trimester (34.6 [3.4%], P = 0.01,). Prevalence of anemia was 28.0% (56/200), and a majority (94.6%, 53/56) of anemic women belong to the mild category. Only parity groups had a significant association with anemia in pregnancy (P = 0.04). None of the participants reported being asked about her arm preference during blood collection for routine antenatal investigations. One hundred and five (52.5%) women expressed preference for either left (34.5%, 69/200) or right arm (18.0%, 36/200) for blood sample collection.
Conclusion:
The average HCT among pregnant women at the UNTH, Enugu Nigeria was within normal range and the prevalence of anemia was relatively low. The majority of women expressed a preference for either right or left arm for blood sample collection for clinical investigations and would wish their choices sought for and respected.
Keywords: Anemia in pregnancy, Arm preference, Enugu, Hematocrit, Nigeria
Introduction
Pregnancy is a physiological condition characterized by systemic alterations for the maintenance of the growing fetus.[1] Both plasma volume and red cell mass increase in pregnancy at differential rates leading to a reduction of hematocrit (HCT) especially in the second trimester.[1,2] A lower cut-off of 33% or hemoglobin (Hb) concentration of 11 gram% was set by World Health Organization (WHO), below which pregnant women is considered to be anemic.[3] To ensure optimal perception of the magnitude of anemia in pregnancy as a global public health problem as well as for the standardization of study findings, the use of a lower cut-off (HCT of 30% or Hb concentration of 10 gram%), canvassed for developing countries, is discouraged.[4,5] Globally, the epidemiology of anemia in pregnancy is characterized by marked difference in prevalence between developed and developing nations thus, about 52% in developing countries versus 23% in the developed countries.[6] Furthermore, it has been observed that about one-half of pregnant women in Africa are anemic while the West African sub-region is the most affected.[3,6] Furthermore, varying prevalence of the condition has been reported from developing countries such as 85% in India,[7] 42.6 in Nepal,[8] 20% in Brazil,[9] 32% in Ghana,[10] 56.6% in Malawi,[11] 47.4% in Tanzania,[12] and 64% in Nigeria.[13] The most recent report from the study center showed a relatively high prevalence of 40.4% among antenatal clinic attendees.[14]
Iron deficiency is the most common cause of anemia in the general population and pregnancy state.[6,11] The predisposing factors during pregnancy include grand multiparity, low socioeconomic status, malaria infestation, late booking, human immunodeficiency virus (HIV) infection, and inadequate child spacing amongst others.[15,16,17,18] On the other hand, anemia in pregnancy is associated with increased rates of maternal and perinatal mortality, premature delivery, low birthweight, and other adverse outcomes[11,19,20] such that its management is one of the keys to optimal maternal and neonatal health especially in Sub-Saharan Africa. In Nigeria, the condition is responsible for up to 11% of maternal deaths.[21,22]
As a secondary preventive health strategy aimed at early detection and treatment of anemia in pregnancy, it is a standard practice to routinely screen pregnant women for anemia during antenatal care as well as institute prophylactic oral iron therapy. Despite this intervention, our clinical experiences suggest that the magnitude of anemia in pregnancy may not be reducing. This concern calls for a regular monitoring of the HCT and anemia patterns in our environment so as to guide the interventions targeted at reducing the burden of the disorder.
On the other hand, it is expected that patient's arm preference should be sought for and respected by a caregiver or phlebotomist during the collection of blood sample for any clinical investigation;[23] and this respect should even be more important during antenatal care since a majority of pregnant women are healthy and often need repeated HCT assessment. However, this consideration does not seem to be the practice in our environment. Therefore, in order to initiate and encourage the practice of seeking for and respecting arm preference during blood sample collection from pregnant women in our environment, it is important to determine whether this group of women does have varying arm preferences. Therefore, the objectives of this study were to determine the average HCT, prevalence, pattern, and predictors of anemia, as well as assess for arm preferences for blood specimen collection among pregnant women in Enugu, South East Nigeria.
Subjects and Methods
The study was a cross-sectional survey of pregnant women attending the antenatal clinic of the University of Nigeria Teaching Hospital (UNTH) Enugu, South-Eastern Nigeria, in the months of May and June 2012. The center is a teaching hospital owned by the Federal Government of Nigeria. The antenatal clinic holds every weekday and attends to an average of 795 women (first visit and revisits)/month.[24] As a routine, traditional antenatal care schedule is used at the clinic thus: Normal pregnant women are seen 4 weekly until 28 weeks of gestation, fortnightly until 36 weeks and then weekly until delivery;[25] blood and urine samples are collected during booking for laboratory investigation including HCT, hepatitis B surface antigen screening, counseling and testing for HIV, venereal disease research laboratory test, and urinalysis. Repeat blood samples are collected in each trimester for HCT estimation as a way of monitoring for development of anemia in pregnancy. All pregnant women receive hematics and intermittent preventive treatment for malaria (IPTp). The routine daily hematinics include 60 mg of elemental iron and 5 mg of folic acid, while sulfadoxine-pyrimethamine is used for the IPTp. The study was approved by the Ethics Committee of the UNTH Enugu.
The sample population was 200 consenting consecutive booked pregnant women on the follow-up visit at the antenatal clinic of the hospital within the study period. The minimum sample size required to determine our major variable of interest HCT was calculated using the formula (n = Z2 × S2/d2) where Z = Z-score (95% confidence level), S = population standard deviation, and d = error margin.[26] This sample population was adequate for the study based on an assumed population standard deviation of mean packed cell volume (PCV) of 1.41%,[27] at 95% confidence level (Z = 1.96), and error margin of 0.5%. All singletons booked pregnant women who were sure of their date as well as those who were unsure of their dates but had first trimester ultrasound dating, were eligible for the study. Exclusion criteria included medical illness in pregnancy such as diabetes mellitus and HIV infection, obstetrics complications including pre-eclampsia, antepartum hemorrhage, non-use of hematinics, sickle cell anemia, and prior sampling in the study. Semi-structured questionnaire was administered to each participant by trained assistants (medical doctors), after obtaining an informed consent. The information sought for included participant's age, educational status, marital status, parity, arm preference for blood specimen collection and the reason. Afterward, the cubital fossa of the preferred arm was cleaned with cotton wool soaked with 70% alcohol,[28] then; 0.5 ml of blood was collected from the antecubital vein using a sterile 2 ml syringe and transferred immediately into 2 plain heparinized capillary tubes. Clay sealant was used to seal the inferior ends of the capillary tubes before centrifuging for 5 min in a micro-HCT centrifuge (Hawksley HematoSpin 1400®). The HCT for each capillary tube was read immediately after centrifuging, with a Hawksley micro-HCT reader and the mean value for each participant was recorded on the result sheet. A duplicate copy of the result sheet was issued to each participant for presentation to her caregiver same day.
Statistical Package of the Social Sciences version 15 for windows (Chicago IL, USA) was used for data entry and analyses. Associations were compared using independent-samples t-test for continuous data, cross-tabulation, and multinomial logistic regression for categorical data. Results were presented using simple percentages, and tables as appropriate. Associations between variables were shown using P values, odd ratios (ORs) and confidence intervals (CI). A P < 0.05 was considered statistically significant.
The major variable of interest in the study was the mean HCT of pregnant women at the study center. The secondary measures included the effect of trimester on the women's HCT; prevalence and predictors of anemia in pregnancy; as well as pattern and reason for arm preference among the women.
For the purpose of this study, anemia in pregnancy was defined as HCT of < 33.0%;[3] and classified into mild (27.0–32.9), moderate (21.0–26.9), and severe (<21.0). Maternal obesity was defined as absolute weight of 90 kg and above, irrespective of trimester.[29,30]
Results
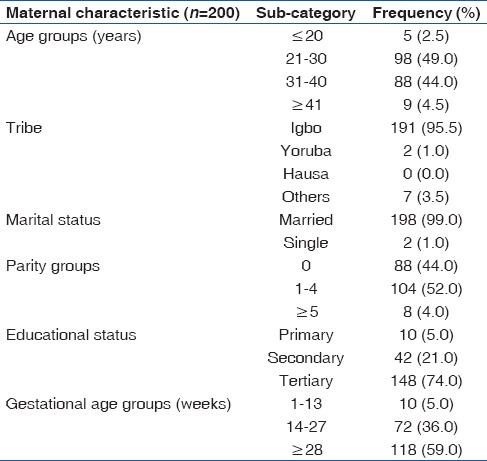
The mean age and parity of study participants were 30.1 (5.1) (range = 19–45) years and 1.3 (1.5) (range = 0–6), respectively. Most participants were married (99.0%, 198/200), had tertiary education (74.0%, 148/200) and were at gestational ages of 28 weeks and above (59.0%, 118/200). Details of participants’ characteristics are shown in Table 1.
Table 1.
Distribution of participants’ characteristics
For all participants, the mean HCT was 33.3 (3.7) (range = 21–44)%. The average HCT for women that were <28 weeks of gestation was 33.9 (4.1)% while that of those that had gestational ages of 28 and above was 34.6 (3.4)%. An independent-samples t-test indicated that the observed difference was not statistically significant (P = 0.16). However, when participants recruited in first trimester (5.0%, 10/200) were excluded, the difference became significant (33.3 [3.8] vs. 34.6 [3.4], P = 0.01).
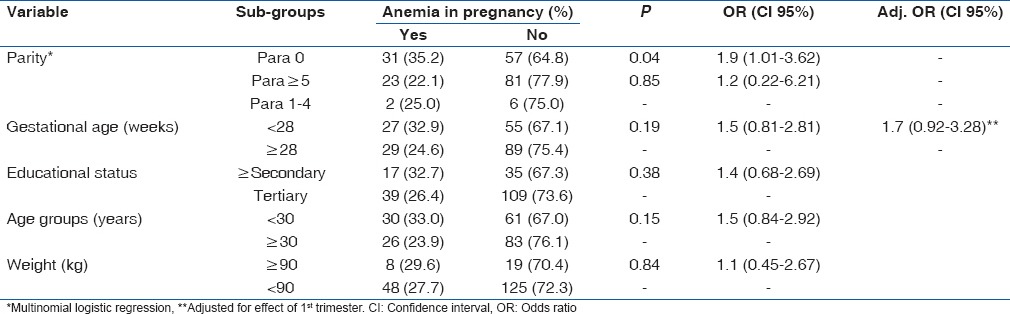
Of all participants, 56 women had HCT values below 33% which gave an anemia prevalence of 28.0%. A majority of the anemic women belong to the mild category (94.6%, 53/56) while the remainder had moderate anemia (5.4%, 3/56). There was no case of severe anemia. Only parity groups had a significant association with anemia in pregnancy thus: Linear-by-linear association showed an increasing prevalence of anemia across the parity sub-groups (P = 0.04) [Table 2]. Furthermore, when the odds of developing anemia were compared among the parity sub-groups using the multipara (para 1–4) as the baseline, a significant increase was only observed within the nulliparous group (OR = 1.9 [CI 95%: 1.03, 3.62], P = 0.04) [Table 3]. Gestational age of <28 weeks had no significant association with anemia in pregnancy (OR = 1.5 [CI 95%: 0.81, 2.81], P = 0.19), and this did not change after controlling for the effect of first trimester (OR = 1.7 [CI 95%: 0.92, 3.28], P = 0.09) [Table 3]. Furthermore, the age of mothers who were anemic (mean [M] =29.5 [5.2]) did not differ from those with normal HCT (M = 30.3 [5.0], t = −994, P = 0.32). Maternal age of <30 years (younger age), maternal obesity, and lower educational status (≤secondary) had no significant association with anemia in pregnancy. Details of the association between maternal variables and anemia in pregnancy are shown in Table 3.
Table 2.
Association between parity groups and anemia in pregnancy
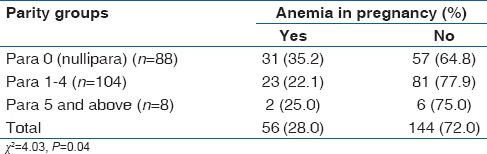
Table 3.
Association between maternal characteristics and anemia in pregnancy
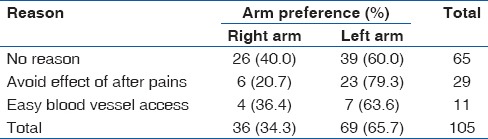
With respect to the participant's arm preference for blood specimen collection, none of the participants reported being asked about her arm preference during blood collection for routine antenatal investigations at booking and afterwards. Ninety-five (47.5%, 95/200) participants did not express any specific arm preference while 34.5% (69/200) and 18.0% (36/200) of the women preferred their left and right arms, respectively. Of the 105 participants who preferred either the right or left arm, a majority (61.9%, 65/105) had no reason, 29 (27.6%, 29/105) did not want the pain following the sample collection to affect the use of their dominant arms, and the remaining 11 (10.5%, 11/105) women felt that access to blood vessels was easier on their preferred arm. All participants that preferred their left arms were right handed while five (13.9%, 5/36) women that preferred their right arms were left handed. When participants were asked whether they would prefer their opinion (in terms of arm preference) to be sought and the choice respected during subsequent blood collection for any investigations, 91.5% (183/200) responded positively while 5% (10/200) responded negatively. The remaining 3.5% (7/200) were indifferent. Details of the participants’ responses in relation to their arm preferences are shown in Table 4.
Table 4.
Participants’ arm preferences and reasons
Discussion
The average HCT in this study is within the normal range for the pregnant population,[3,6] and this also holds for participants recruited in the second trimester–the period when hemodilution of pregnancy is most marked.[1] This encouraging finding may be related to the high social class of the majority of the participants as suggested by their higher educational status. Nevertheless, the average HCT in this study is lower than 37.1% reported from a faith-based specialist hospital in the study area.[27] The marked disparity is unexpected and difficult to explain, however, measurement bias cannot be ruled out from the earlier study which showed that the average HCT of its pregnant population was higher than that of nonpregnant control – an unusual finding because of the hemodilution associated with pregnancy that is responsible for a lower cut-off for anemia in pregnancy when compared to nonpregnant population.[1,2] On the other hand, the average HCT in this study is expectedly higher than 29.6% observed from rural communities in Enugu,[31] which may support the explanation above as regards women's social status and HCT. Furthermore, the average HCT found in this study is higher than 30.2%, and 33.0% reported from Lagos,[32] and Ibadan,[33] respectively. Furthermore, it is not surprising that the HCT observed in the second trimester was significantly lower than that in the third trimester because of the marked hemodilution that characterize this period of pregnancy.
This study showed an anemia prevalence of 28% which is almost a half of the figure reported in 2007 from the same center.[14] Baring any effect of sampling error, this study finding may reflect an improvement in maternal health care over the period preceding the current study and suggests a downward category shift in the public health burden of anemia in pregnancy.[6] It should be noted that participants in the previous study were recruited at booking and, therefore, were not on hematinics;[14] and following that study, antenatal education for pregnant women at the study center was reviewed and emphasis was placed on the importance of good nutrition and adherence to hematinics administered during pregnancy. It is possible that this intervention had contributed to the observed reduction in anemia prevalence. Furthermore, the anemia prevalence in this study is far lower than findings of recent studies from urban (64.1%),[13] and rural (69.3%)[31] areas of Enugu state, Nigeria. Nutritional anemia especially iron deficiency is the most common cause of anemia, and it is related to socioeconomic status.[6] Since the study population for these studies varied, it is likely that low socioeconomic status might have contributed to the observed disparity in anemia prevalence. A multi-center survey is, therefore, necessary to determine the actual epidemiology of the disorder in the state. We also suggest that the periodic HIV sentinel survey of Nigeria be expanded to include HCT or Hb concentration estimation so as to get a more precise prevalence of anemia in Nigeria and its constituent states. Furthermore, this study finding deviated markedly from WHO reports which showed that about one-half of pregnant women in Africa were anemic.[6] It is also lower than reports of several other studies from developing countries.[7,8,10,11,12,34]
A majority of anemia cases in this study were of the mild variety which conforms with the findings of related studies.[12,13] Also as in the preceding survey from the center, there was no case of severe anemia,[14] which suggests that severe form of anemia may be uncommon among registered pregnant women at the hospital.
Contrary to the findings of previous studies,[12,14] this study identified a relationship between women's parity and likelihood of anemia in pregnancy [Tables 2 and 3]. The odds of anemia in this study was the highest among women in their first ongoing pregnancy (nullipara) followed by grand multiparous women thus, a nulliparous woman was two times more likely to be anemic when compared to those who were para 1 to para 4. Though this finding may appear unusual, it however, supports the report from Oman which showed that nulliparous women had a higher risk of anemia when compared to women that were para 1–2.[35] Unfortunately, unlike the Oman study,[35] our study could not control for the effect of hemodilution phenomenon in the second trimester because of inadequate power for such analysis. The increased prevalence of anemia in nullipara may be related to poor nutrition as a result of nausea and vomiting that are more predominant in the group, while that of grandmultiparas may be associated with depleted iron stores as a result of repeated pregnancies. A recent study at the hospital showed that the average inter-birth interval was suboptimal,[36] which may support the depleted iron stores theory in grandmultiparous women. Furthermore, women in second trimester of pregnancy were about two times more likely to have anemia when compared to those in third trimester though, the relationship was not significant-a larger sample size would have increased the study's precision. This increased odds of anemia in the second trimester was also found in other studies,[14,34] and it is related to a significantly reduced average HCT in the second trimester as observed in the present study. On the other hand, maternal age, obesity, and educational status had no significant association with prevalence of anemia in this study. It is observed that the reports from the study area show varying levels of association between suspected maternal predictors and anemia in pregnancy;[13,14] therefore, a well-designed multi-center study will help determine the real predictors of the condition in our environment.
For varying reasons, over half of the study participants preferred one of either left or right arm for blood sample collection. Furthermore, over 90% of the respondents would want their choice of arm preference sought for and respected during blood collection. This study finding is interesting because it has been observed that most health providers and phlebotomist in our environment decide on the patient's arm to be used for blood sample collection without recourse to her preference. This study findings calls for more patients respect and exposes the need for training and retraining of health staff in the act of blood sample collection. It is hoped that this form of patients’ respect would be adopted as a routine practice in all health centers in Nigeria including the study center.
The study was based on one hospital which limits its generalization to the study area. However, a rigorous quality control measures employed during the study ensured its internal validity. Outside the measures noted in the study's methods, others quality control measures employed were availability of written standard operating protocol including eligibility and exclusion criteria as well as study protocol for PCV estimation and data recording, intermittent certification of centrifuge efficiency. The study was further strengthened by the fact that it explored participant's arm preference for blood sample collection–an important aspect of patients’ autonomy that is often violated by caregivers in our environment.
Conclusion
The average HCT among pregnant women at the UNTH, Enugu Nigeria was within normal range, and the prevalence of anemia was lower than the preceding report. The majority of women expressed a preference for either right or left arm for blood collection for clinical investigations and would wish their choices are sought for and respected. It is important that phlebotomist and caregivers enquire about, and respect the arm preference of pregnant women during blood sample collection for any investigation.
Acknowledgments
The study was funded by authors.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Flick AA, Kahn DA. Maternal Physiology during pregnancy and Fetal and Early Neonatal Physiology. In: DeCherney AH, Nathan L, Laufer N, Roman AS, editors. Current Obstetrics and Gynecologic Diagnosis and Treatment. 11th ed. New York: McGraw-Hill; 2013. pp. 163–79. [Google Scholar]
- 2.Marya RK. 3rd ed. New Delhi: CBS Publishers and Distributors Ltd; 2010. Medical Physiology. [Google Scholar]
- 3.Geneva: World Health Organization; 1992. World Health Organization. The Prevalence of Anaemia in Women: A Tabulation of Available Information. WHO/MCH/MSM/92.2. [Google Scholar]
- 4.Ogunbode O. Anaemia in pregnancy. In: Okonofua F, Odunsi K, editors. Contemporary Obstetrics and Gynaecology for Developing Countries. Benin City: Women's Health and Action Research Center; 2003. pp. 514–29. [Google Scholar]
- 5.Agboola A. Severe anaemia in pregnancy. In: Agboola K, editor. Textbook of Obstetrics and Gynaecology for Medical Students. 2nd ed. Ibadan: Heinemann Educational Books (Nig.) Plc; 2006. pp. 336–9. [Google Scholar]
- 6.Geneva: World Health Organization; 2001. World Health Organization. Iron Deficiency Anaemia Assessment, Prevention and Control: A Guide for Programme Managers. WHO/NHD/01.3. [Google Scholar]
- 7.Amel Ivan E, Mangaiarkkarasi A. Evaluation of anaemia in booked antenatal mothers during the last trimester. J Clin Diagn Res. 2013;7:2487–90. doi: 10.7860/JCDR/2013/6370.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marahatta R. Study of anaemia in pregnancy and its outcome in Nepal Medical College Teaching Hospital, Kathmandu, Nepal. Nepal Med Coll J. 2007;9:270–4. [PubMed] [Google Scholar]
- 9.Fujimori E, Sato AP, Szarfarc SC, Veiga GV, Oliveira VA, Colli C, et al. Anemia in Brazilian pregnant women before and after flour fortification with iron. Rev Saude Publica. 2011;45:1027–35. doi: 10.1590/s0034-89102011005000078. [DOI] [PubMed] [Google Scholar]
- 10.Stephens JK, Ofori MF, Quakyi IA, Wilson ML, Akanmori BD. Prevalence of peripheral blood parasitaemia, anaemia and low birthweight among pregnant women in a suburban area in coastal Ghana. Pan Afr Med J. 2014;17(Suppl 1):3. doi: 10.11694/pamj.supp.2014.17.1.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Broek NR, Letsky EA. Etiology of anemia in pregnancy in south Malawi. Am J Clin Nutr. 2000;72:247S–56. doi: 10.1093/ajcn/72.1.247S. [DOI] [PubMed] [Google Scholar]
- 12.Msuya SE, Hussein TH, Uriyo J, Sam NE, Stray-Pedersen B. Anaemia among pregnant women in northern Tanzania: Prevalence, risk factors and effect on perinatal outcomes. Tanzan J Health Res. 2011;13:33–9. doi: 10.4314/thrb.v13i1.60881. [DOI] [PubMed] [Google Scholar]
- 13.Ezugwu EC, Mbah BO, Chigbu CO, Onah HE. Anaemia in pregnancy: A public health problem in Enugu, southeast Nigeria. J Obstet Gynaecol. 2013;33:451–4. doi: 10.3109/01443615.2013.771158. [DOI] [PubMed] [Google Scholar]
- 14.Dim CC, Onah HE. The prevalence of anemia among pregnant women at booking in Enugu, South Eastern Nigeria. MedGenMed. 2007;9:11. [PMC free article] [PubMed] [Google Scholar]
- 15.Amadi AN, Onwere S, Kamanu CI, Njoku OO, Aluka C. Study on the association between maternal malaria infection and anaemia. J Med Invest Pract. 2000;1:23–5. [Google Scholar]
- 16.Aluka C, Amadi AN, Kamanu CI, Feyi-Waboso PA. Anaemia in pregnancy in Abia State University teaching hospital Aba. J Med Invest Pract. 2001;2:58–61. [Google Scholar]
- 17.Adinma JI, Ikechebelu JI, Onyejimbe UN, Amilo G, Adinma E. Influence of antenatal care on the haematocrit value of pregnant Nigerian Igbo women. Trop J Obstet Gynaecol. 2002;19:68–70. [Google Scholar]
- 18.Aimakhu CO, Olayemi O. Maternal haematocrit and pregnancy outcome in Nigerian women. West Afr J Med. 2003;22:18–21. doi: 10.4314/wajm.v22i1.27972. [DOI] [PubMed] [Google Scholar]
- 19.Mahomed K. The Reproductive Health Library CD-ROM, Issue 8, 2005. Oxford, Chichester, UK: Update Software Ltd. (Reprinted from The Cochrane Library, Issue 4), John Wiley and Sons, Ltd; 2004. Iron and folate supplementation in pregnancy (Cochrane Review) [Google Scholar]
- 20.van den Broek NR, Jean-Baptiste R, Neilson JP. Factors associated with preterm, early preterm and late preterm birth in Malawi. PLoS One. 2014;9:e90128. doi: 10.1371/journal.pone.0090128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nigeria, Abuja: Federal Ministry of Health; 2007. Federal Ministry of Health (FMOH). Integrated maternal, newborn and child health strategy. [Google Scholar]
- 22.Nwagha UI, Nwachukwu D, Dim C, Ibekwe PC, Onyebuchi A. Maternal mortality trend in South East Nigeria: Less than a decade to the millennium developmental goals. J Womens Health (Larchmt) 2010;19:323–7. doi: 10.1089/jwh.2008.1028. [DOI] [PubMed] [Google Scholar]
- 23.University of Virginia Health System. Phlebotomy Procedure. 2011. [Last accessed on 2009 Aug 06]. Available from: http://www.healthsystem.virginia.edu/pub/medlabs/lab-handbook-test-directory/appendices/phlebotomyprocedure.html .
- 24.Obiora CC, Dim CC, Ezegwui HU, Nwogu-Ikojo EE, Okeudo C. Asymptomatic bacteriuria among pregnant women with sickle cell trait in Enugu, South Eastern Nigeria. Niger J Clin Pract. 2014;17:95–9. doi: 10.4103/1119-3077.122856. [DOI] [PubMed] [Google Scholar]
- 25.Aniebue UU, Aniebue PN. Women's perception as a barrier to focused antenatal care in Nigeria: The issue of fewer antenatal visits. Health Policy Plan. 2011;26:423–8. doi: 10.1093/heapol/czq073. [DOI] [PubMed] [Google Scholar]
- 26.Daniel WW. Biostatistics: Basic Concepts and Methodology for the Health Sciences. 9th ed. New Jersey: John Wiley and Sons, Inc; 2010. Using sample data to make estimates about population parameters; pp. 162–214. [Google Scholar]
- 27.Nneli RO, Egene J. Packed cell volume of HIV positive women in Enugu, Nigeria. Res J Med Sci. 2007;1:135–7. [Google Scholar]
- 28.USAID. Anemia Detection Methods in Low-Resource Settings: A Manual for Health Workers. 1997. [Last accessed on 2009 Mar 02]. Available from: http://www.path.org .
- 29.Klufio CA. Obesity in pregnancy. In: Kwawukume EY, Emuveyan EE, editors. Comprehensive Obstetrics in the Tropics. Dansoman: Asante and Hittscher Printing Press Ltd; 2002. pp. 219–25. [Google Scholar]
- 30.Olayemi OO, Umuerri CO, Aimakhu CO. Obstetric performance of Nigerian obese parturients. Trop J Obstet Gynaecol. 2002;19:17–20. [Google Scholar]
- 31.Ugwu EO, Dim CC, Uzochukwu BS, Iloghalu EI, Ugwu AO. Malaria and anaemia in pregnancy: A cross-sectional study of pregnant women in rural communities of Southeastern Nigeria. Int Health. 2014;6:130–7. doi: 10.1093/inthealth/ihu009. [DOI] [PubMed] [Google Scholar]
- 32.Akinbami AA, Ajibola SO, Rabiu KA, Adewunmi AA, Dosunmu AO, Adediran A, et al. Hematological profile of normal pregnant women in Lagos, Nigeria. Int J Womens Health. 2013;5:227–32. doi: 10.2147/IJWH.S42110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olubukola A, Odunayo A, Adesina O. Anemia in pregnancy at two levels of health care in Ibadan, south west Nigeria. Ann Afr Med. 2011;10:272–7. doi: 10.4103/1596-3519.87042. [DOI] [PubMed] [Google Scholar]
- 34.Idowu OA, Mafiana CF, Dapo S. Anaemia in pregnancy: A survey of pregnant women in Abeokuta, Nigeria. Afr Health Sci. 2005;5:295–9. doi: 10.5555/afhs.2005.5.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Farsi YM, Brooks DR, Werler MM, Cabral HJ, Al-Shafei MA, Wallenburg HC. Effect of high parity on occurrence of anemia in pregnancy: A cohort study. BMC Pregnancy Childbirth. 2011;11:7. doi: 10.1186/1471-2393-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dim CC, Ugwu EO, Iloghalu EI. Duration and determinants of inter-birth interval among women in Enugu, south-eastern Nigeria. J Obstet Gynaecol. 2013;33:175–9. doi: 10.3109/01443615.2012.747494. [DOI] [PubMed] [Google Scholar]


