Abstract
Background:
Proficient laboratory service is the cornerstone of modern healthcare systems and has an impact on over 70% of medical decisions on admission, discharge, and medications. In recent years, there is an increasing awareness of the importance of errors in laboratory practice and their possible negative impact on patient outcomes.
Aim:
We retrospectively analyzed data spanning a period of 3 years on analytical errors observed in our laboratory. The data covered errors over the whole testing cycle including pre-, intra-, and post-analytical phases and discussed strategies pertinent to our settings to minimize their occurrence.
Materials and Methods:
We described the occurrence of pre-analytical, analytical and post-analytical errors observed at the Komfo Anokye Teaching Hospital clinical biochemistry laboratory during a 3-year period from January, 2010 to December, 2012. Data were analyzed with Graph Pad Prism 5(GraphPad Software Inc. CA USA).
Results:
A total of 589,510 tests was performed on 188,503 outpatients and hospitalized patients. The overall error rate for the 3 years was 4.7% (27,520/58,950). Pre-analytical, analytical and post-analytical errors contributed 3.7% (2210/58,950), 0.1% (108/58,950), and 0.9% (512/58,950), respectively. The number of tests reduced significantly over the 3-year period, but this did not correspond with a reduction in the overall error rate (P = 0.90) along with the years.
Conclusion:
Analytical errors are embedded within our total process setup especially pre-analytical and post-analytical phases. Strategic measures including quality assessment programs for staff involved in pre-analytical processes should be intensified.
Keywords: Errors, Post-analytical, Pre-analytical Quality control
Introduction
Unlike many components of the health care system that are still besieged with the issue of patient quality outcomes, laboratories have always been forerunners in pursuing quality in their analytical processes.[1] The concepts and practices of quality assessment programs have been a routine in laboratory diagnostics. Proficient laboratory service is the cornerstone of modern health care systems and contributes about 70% towards medical diagnoses and treatments.[2] Automated innovations have also contributed to a significant improvement in the field of laboratory science, but errors still prevail.[3] These errors are classified as pre-analytical, analytical, and post-analytical. Clinical laboratories have long focused their concentration on quality control (QC) materials and quality assessment programs. In recent years, however, there is an increasing awareness of the importance of errors in laboratory practice and their possible negative impact on patient outcomes. Many strategies are used to reduce laboratory errors, including certification/accreditation by professional bodies, internal QC procedures, external quality assessment programs, and certification of education programs. Course of action analysis has demonstrated that laboratory errors occur primarily in the pre-analytic phase, influencing patient outcomes and costs.[4] Literature also indicates that pre-analytical and post-analytical errors account for 93% of the total errors encountered in the laboratory.[5] With the advent of evidence based medicine, it is imperative for physicians to confirm their diagnosis through laboratory data than presumptive clinical presentations alone. Reviews on available literature on laboratory error indicate great heterogeneity in the studies where data collection method is the strongest factor that influences the prevalence and type of errors. There is also a concomitant increase in the types and the number of laboratory requests leading to an increased work load.
We retrospectively evaluated data covering a 3 year period of analytical errors observed in our laboratory over the whole testing cycle including pre-, intra-, and post-analytical phases and discussed strategies pertinent to our settings to minimize their occurrence.
Materials and Methods
Study settings
The Komfo Anokye Teaching Hospital (KATH) is a 1000 bed facility, offering tertiary services to the Ashanti region and northern parts of Ghana and beyond. The facility has a well equipped and well resourced Diagnostic Directorate of which the Clinical Biochemistry Department is part. Our well-equipped biochemistry laboratory is manned by Biomedical Scientists who have undergone mandatory training courses in laboratory science. Collection of blood samples for biochemical analysis is done by doctors and nurses in the individual wards and phlebotomist at the OPD.
We retrospectively collected data covering the period from January, 2010 to December, 2012 from both hospitalized and outpatients. This evaluation was exempted from ethical consideration because it was based on quality assurance.
Collection of data
We documented the occurrence of pre-analytical, analytical, and post-analytical errors observed at the KATH's clinical biochemistry laboratory. Samples with their accompanying request slips were received by Biomedical Scientists from Nurses, Doctors and Health Care Assistants from various wards of the hospital. Trained phlebotomists at a collection center also took all outpatient samples and sent them to the laboratory. Upon receiving the samples, the biomedical scientists examined the samples with their corresponding request slips and any errors observed were entered in the problem notification log book.
Standard operating procedures for phlebotomy techniques, patient preparation, sample handling, instrument handling and maintenance, and other aspects of sample processing were documented. Sample analysis was performed using two fully automated auto-analyzers – COBAS INTEGRA 400 PLUS (Roche Diagnostics, Switzerland). Quality procedures such as changing of expired calibrators, reagents lot number, and troubleshooting are done as required. Equipment inbuilt calibration traceability and internal QC was monitored from time to time. In addition, weekly calibrations were performed under the protocol developed by the QC team in our department. Any analyte observed to be out of range was then recalibrated.
Statistical analysis
All data capture was performed using Microsoft Excel (Microsoft, Redmond, WA) and analyzed with Graph Pad Prism 5 (GraphPad Software Inc. CA, USA).
Errors that we encountered as pre-analytical, analytical, and post-analytical are tabulated below
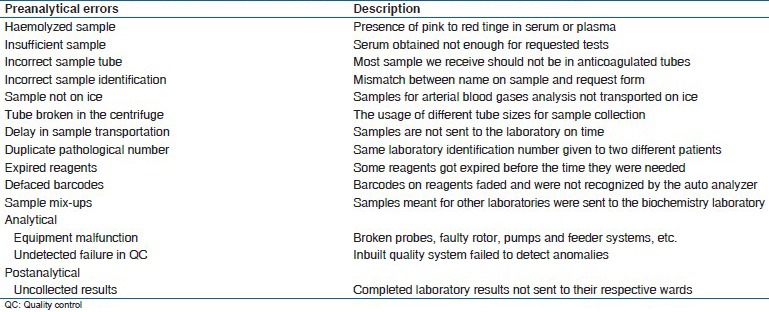
Results
Table 1 shows the common errors reported in the KATH clinical biochemistry laboratory.
Table 1.
Frequency of analytical errors
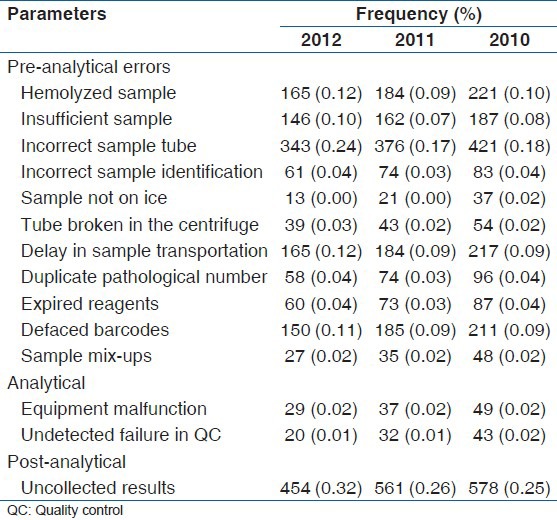
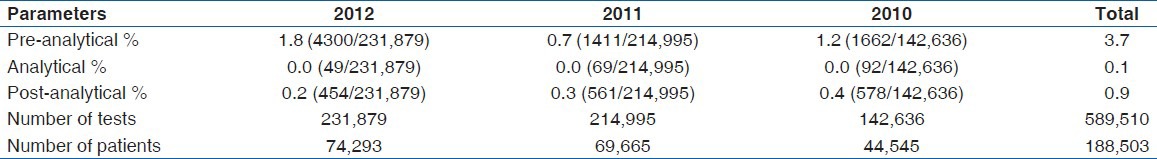
A total of 589,510 tests was done during the period under study by 188,503 patients. The overall analytical errors observed was 4.7%, with pre-analytical errors contributing the highest with 3.7% followed by post analytical error with 0.9% [Table 2].
Table 2.
Percentage distribution of total analytical errors
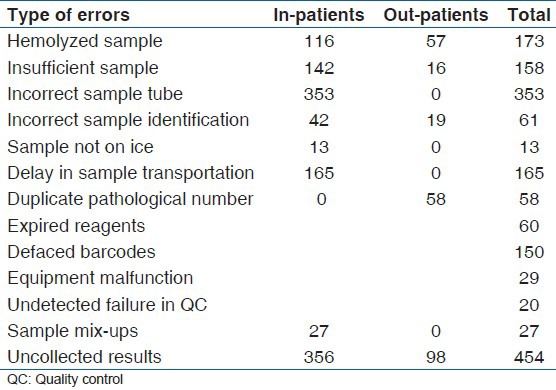
Equipment malfunction was a major cause of analytical error and non-postage of or uncollected results were the main causes of post-analytical error. Incorrect sample tubes, delay in sample transportation from ward to the laboratory were identified as peculiar to samples from the various wards. Samples with duplicate pathological numbers were all from outpatients sources [Table 3].
Table 3.
Distribution of error frequencies for 2012 between in-patients and out-patients
There was no significant increase (P = 0.90) in the overall analytical errors during the three year study period even though there was a significant (P = 0.01) decrease in the total number of patients and hence samples over the period [Figure 1].
Figure 1.
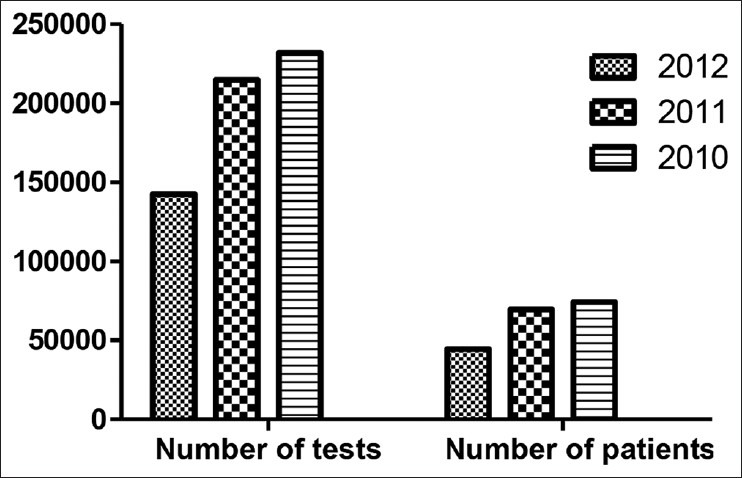
Comparison of number of tests with patients
Discussion
Modern innovations have transformed laboratory diagnostics from labor-intensive service to almost fully automated steps or processes that have required complementary reduction in staff. Despite all the automation, findings from this study clearly showed that the laboratory continues to be a source of errors which can translate to inappropriate patients care decisions. Even though many studies have been done to improve analytical quality, errors in the laboratory testing process still prevail.[4,5,6]
In this study, we evaluated a 3 year period of total error rate observed in our laboratory and discussed strategies pertinent to our settings to minimize it re-occurrence. We observed that the overall error rate for the 3 year period was 4.7% with pre-analytical, analytical and post-analytical contributing 3.7%, 0.1% and 0.9% respectively. Again, even though the number of tests reduced significantly (P = 0.01) over the period (2010-2012) there was no corresponding reduction in the total error rate it did not correspond with a reduction in the overall error rate (P = 0.90) among the years.
The total error rate of 4.7% observed in this study is within the range of 0.1% to 9.3% reported by Carraro and Plebani.[7] The pre-analytical error rate of 3.7% observed in this study was mainly due to hemolyzed samples, incorrect sample tubes and delays in transporting samples from wards to the laboratory for analysis. This observation is similar to 3-5% pre-analytical errors observed by Hawkins[3] in his review. Increased hemolysis observed from this study was mainly due to the increased pressure with which blood was dispensed from syringes into sample tubes in most wards by nurses. Frequent changes of health care assistants, nurses and periodic influx of students from various training institutions was found to be the cause of use of wrong sample tubes and delay in sample transportation because of a lack of education about ideal phlebotomy procedures. To reduce these challenges, vacuum tubes along with the closed system collection of blood were been introduced to make blood collection efficient and easy. However, in-spite of these interventions most clinicians at the wards do not use the vacutainer tubes or the closed system of blood collection sometimes the vacutainer tubes are not readily available for use on the wards.
We observed an analytical error rate of 0.1% in this study. This is much better than 3.8% systemic analytical errors observed by Goswani et al.[1] This difference is due to increase in the number of errors they classified under the analytical errors, notably pipetting difficulties, contamination of reagents, and malfunctioning probes and photo lamps. From this study, equipment malfunction and undetected failure in internal QC were identified mainly as analytical errors. In our settings automation, training of laboratory staff and espousal of internal and external QC programs contributed immensely to the remarkable decline in our analytical errors and also the good condition of our state-of-the artanalyzer. Many studies have emphasized that these activities impact positively in reducing analytical errors.[8,9,10] In our quest to further increase analytical precision and accuracy, we enrolled our laboratory in External Quality Assurance Programs. This demands that results are analyzed periodically during the course of work and any observed shortcomings promptly addressed. Even though there is no LIS in our hospital, the automated equipment print out final results thereby removing manual transcription of numerical data which is prone to error.
Recently, the Center for Disease Control in-conjunction with the Ministry of Health is in the process of enrolling our laboratory on the program: Strengthening Laboratory Management Systems towards Accreditation for ISO 15189. It is envisaged that upon completion it will improve our laboratory information management system.
In the post-analytical phase, the frequency of errors was 0.83% which is better than the 3.2% observed by Goswani et al[1]. Even though, we recorded a low percentage uncollected results could be blamed for this. The lack of LIS in our hospital compels us to deposit completed results in pigeon holes created for the respective wards for collection and onward submission to the wards. Only a few of the wards were punctual with the collection of results from the laboratory.
It is obvious from the above discussion that pre-analytical and post-analytical errors constitute majority of the errors. The reason, for incorrect phlebotomy practice includes lack of attentiveness or possibly a heavy workload. For this reason phlebotomy has been considered a separate area of specialization in developed countries. Developing nations, must therefore, adopt an analogous approach toward phlebotomy and initiate steps to inculcate ideal practices among health care workers.
Errors still prevail within the laboratory setup. Conscious efforts must be made to achieve 100% precision all and accuracy in the whole testing cycle. Strategies to reduce all laboratory errors, such as internal QC procedures, external quality assessment programs, certification of educational programs, licensing of laboratory professionals, accreditation of clinical laboratories, and the regulation of laboratory services should be adopted and enforced. Moreover, total quality management, which encompasses all the steps involved in sample processing, beginning from test ordering to the final interpretation of results by the clinicians, must be evaluated periodically to reduce or eliminate the errors that may arise during the various steps. We must adopt the practice of keeping a record of the errors at all stages of analysis and then devising corrective strategies for their prevention. This can gradually free a laboratory from such errors. To this end, we would like to state as laboratory scientists we need to adopt a holistic approach toward laboratory diagnosis and function in concert with the clinicians to provide effective services to the patients.
Acknowledgement
The authors acknowledge the immense contribution of the entire staff of the clinical laboratory departments of the KATH, especially staff of the Clinical Biochemistry department, nurses and clinicians in making this work a success.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Goswami B, Singh B, Chawla R, Mallika V. Evaluation of errors in a clinical laboratory: A one-year experience. Clin Chem Lab Med. 2010;48:63–6. doi: 10.1515/CCLM.2010.006. [DOI] [PubMed] [Google Scholar]
- 2.Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V, et al. Causes, consequences, detection, and prevention of identification errors in laboratory diagnostics. Clin Chem Lab Med. 2009;47:143–53. doi: 10.1515/CCLM.2009.045. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins R. Managing the pre- and post-analytical phases of the total testing process. Ann Lab Med. 2012;32:5–16. doi: 10.3343/alm.2012.32.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem. 2002;48:691–8. [PubMed] [Google Scholar]
- 5.Howanitz PJ. Errors in laboratory medicine: Practical lessons to improve patient safety. Arch Pathol Lab Med. 2005;129:1252–61. doi: 10.5858/2005-129-1252-EILMPL. [DOI] [PubMed] [Google Scholar]
- 6.Astion ML, Shojania KG, Hamill TR, Kim S, Ng VL. Classifying laboratory incident reports to identify problems that jeopardize patient safety. Am J Clin Pathol. 2003;120:18–26. doi: 10.1309/8EXC-CM6Y-R1TH-UBAF. [DOI] [PubMed] [Google Scholar]
- 7.Carraro P, Plebani M. Errors in a stat laboratory: Types and frequencies 10 years later. Clin Chem. 2007;53:1338–42. doi: 10.1373/clinchem.2007.088344. [DOI] [PubMed] [Google Scholar]
- 8.Witte DL, VanNess SA, Angstadt DS, Pennell BJ. Errors, mistakes, blunders, outliers, or unacceptable results: How many? Clin Chem. 1997;43:1352–6. [PubMed] [Google Scholar]
- 9.Hurst J, Nickel K, Hilborne LH. Are physicians’ office laboratory results of comparable quality to those produced in other laboratory settings? JAMA. 1998;279:468–71. doi: 10.1001/jama.279.6.468. [DOI] [PubMed] [Google Scholar]
- 10.Stull TM, Hearn TL, Hancock JS, Handsfield JH, Collins CL. Variation in proficiency testing performance by testing site. JAMA. 1998;279:463–7. doi: 10.1001/jama.279.6.463. [DOI] [PubMed] [Google Scholar]


