Abstract
Contemporary cnidarian-algae symbioses are challenged by increasing CO2 concentrations (ocean warming and acidification) affecting organisms' biological performance. We examined the natural variability of carbon and nitrogen isotopes in the symbiotic sea anemone Anemonia viridis to investigate dietary shifts (autotrophy/heterotrophy) along a natural pCO2 gradient at the island of Vulcano, Italy. δ13C values for both algal symbionts (Symbiodinium) and host tissue of A. viridis became significantly lighter with increasing seawater pCO2. Together with a decrease in the difference between δ13C values of both fractions at the higher pCO2 sites, these results indicate there is a greater net autotrophic input to the A. viridis carbon budget under high pCO2 conditions. δ15N values and C/N ratios did not change in Symbiodinium and host tissue along the pCO2 gradient. Additional physiological parameters revealed anemone protein and Symbiodinium chlorophyll a remained unaltered among sites. Symbiodinium density was similar among sites yet their mitotic index increased in anemones under elevated pCO2. Overall, our findings show that A. viridis is characterized by a higher autotrophic/heterotrophic ratio as pCO2 increases. The unique trophic flexibility of this species may give it a competitive advantage and enable its potential acclimation and ecological success in the future under increased ocean acidification.
Increasing carbon dioxide (CO2) emissions drive ongoing ocean acidification (OA) and place marine ecosystems in a vulnerable state1. Predictions warn of a further decrease of 0.3–0.5 pH units in oceanic surface water by the end of this century2. Natural CO2 vents at sub-tropical coastal areas3,4,5 and tropical reefs6 serve as natural laboratory locations to study long-term effects of elevated pCO2 (pH) across many biological and spatial scales. Such a location has been reported in the Levante Bay of Vulcano Island (Italy) in the Mediterranean Sea where many studies have examined physiological adaptations of biota to OA, including seagrass7, benthic micro- and macroalgaes8,9, sea urchins10, and sea anemones11,12. The distinctive characteristics of this location render it a unique environmental setting where the seawater chemistry varies along a pCO2 gradient of several hundred meters moving away from the venting source. The submarine gas emissions in Levante Bay are characterized by high CO2 content volume (>90%) and variable low H2S (ranging 0.8 to 2.5% volume)13.
A large body of research has focused on the potential impact of OA on reef organisms, particularly scleractinian corals. However, non-calcifying cnidarians such as sea anemones have received less attention14. Like many cnidarians, they are mixotrophic organisms, which derive their energy from both photoassimilates translocated from the dinoflagellate symbionts (Symbiodinium) and from a variety of external food sources15. Symbiodinium utilize bicarbonate (HCO3−), rather than CO2(aq), as the primary source for photosynthesis16. Extrinsic sources of carbon for the host include zooplankton and particulate organic carbon (POC)17. The two partners that make up the holobiont interact at the basic metabolic level, which includes reciprocal fluxes of energy and nutrient-rich compounds18. Anemonia viridis Forskål (Cnidaria: Anthozoa), the temperate Mediterranean species chosen for this study, occurs naturally at high densities throughout Levante Bay and harbors the dinoflagellate Symbiodinium muscatinei LaJeunesse and Trench (Dinomastigota: Dinophyceae)12. Hence it is a powerful comparative model to assess the effects of the changing seawater environment along a natural pCO2 gradient. Other reports on the response of A. viridis near CO2 vents discovered changes in their associated microbial communities19, reduced dimethylsulfoniopropionate (DMSP) production12 and enhanced productivity3,11.
The purpose of this paper is to investigate dietary changes of A. viridis using isotopic compositions, particularly carbon source shifts in the anemone metabolism, in response to high pCO2/low pH conditions in situ. We measured how the natural variability of carbon and nitrogen isotopes in Symbiodinium and host tissues of A. viridis varies along a natural pCO2 gradient. This was compared with other key physiological parameters (i.e. total protein concentration; Symbiodinium density, mitotic index, and chlorophyll concentration) which were used in the present and in previous studies11. Since the δ13C and δ15N signatures of an organism are related to those of its diet20,21,22,23,24, our main objective was to estimate the relative contribution of photosynthetic compounds versus heterotrophically derived food to the anemone energetic budget (autotrophic/heterotrophic ratio) with increasing seawater pCO2. This may facilitate better understanding of the environmental fate of cnidarians in a high CO2 world.
Results
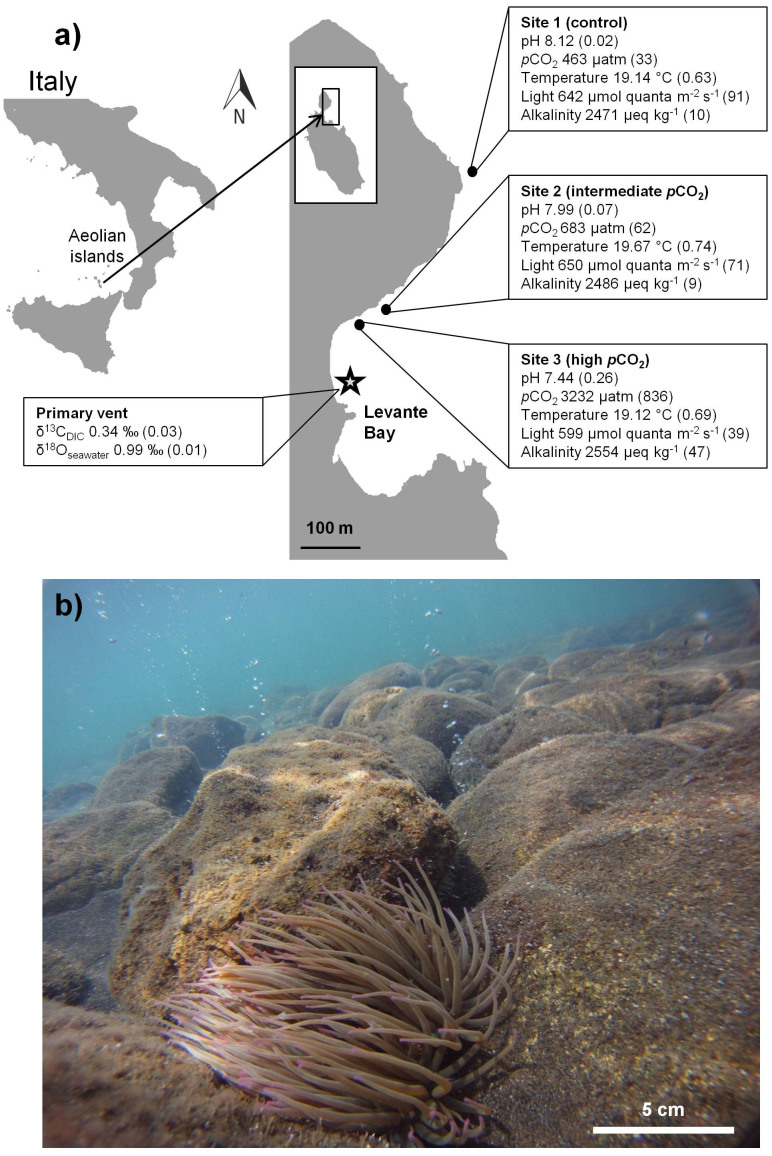
Visual observations made during the course of sampling found anemones at all sampling sites attached to hard substratum at high abundances (of ca. 10–40 anemones m−2), consistent with previous findings11. Anemones appeared to be healthy with their tentacles fully extended and no visible excess amounts of mucus at the high pCO2 site (Fig. 1b). Data for seawater pH, pCO2, TA, temperature and light intensity at all anemone sampling sites is summarized in Figure 1a.
Figure 1. General information on the study sites and the studied organism.
(a) Map of the study area with sampling sites 1 (control), 2 (intermediate pCO2) and 3 (high pCO2). Boxes show mean values (±SD) of each site for: pH, pCO2, temperature, light and alkalinity. δ13CDIC and δ18Oseawater (‰) are presented for the primary vent site. The map was created in Adobe Illustrator CS3 (Adobe Systems Inc., San Jose, USA). (b) Image showing A. viridis at sampling site 3 (high pCO2). Photo credit: M. F. (b).
Total protein, Symbiodinium density, mitotic index and chlorophyll concentration
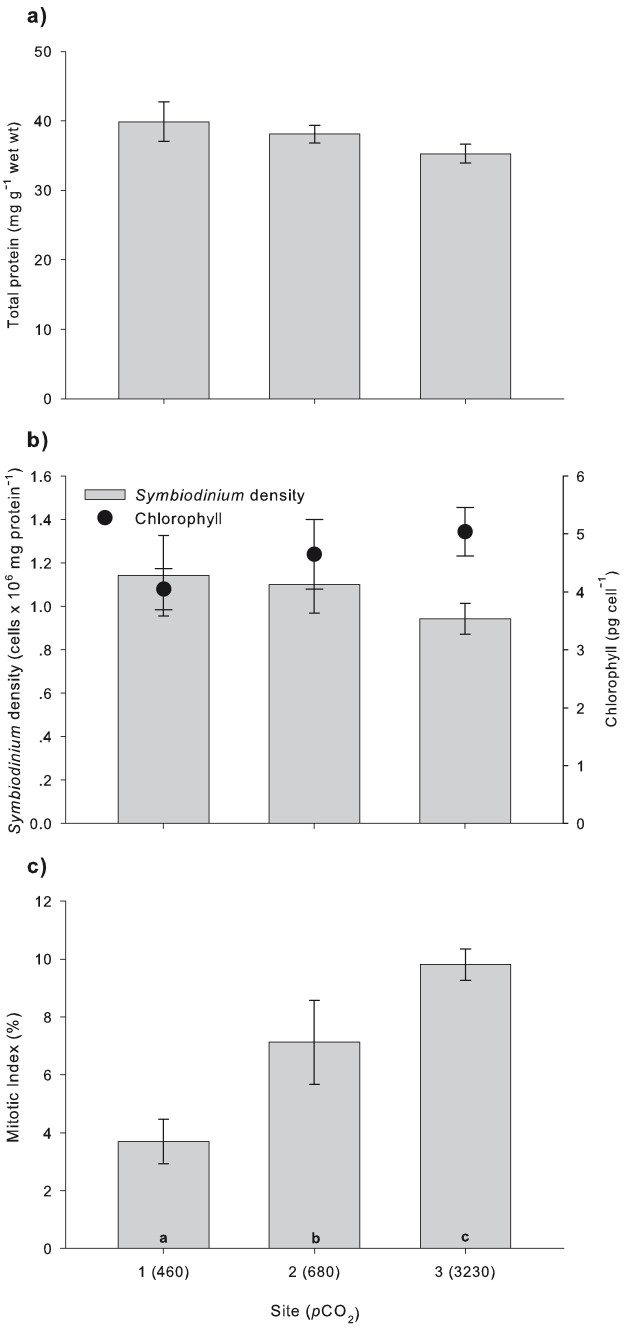
There was no significant difference in anemone protein concentration [1-way ANOVA: F (2, 45) = 1.438, P = 0.248] (Fig. 2a), Symbiodinium density [1-way ANOVA: F (2, 45) = 0.583, P = 0.562] and cell chlorophyll a concentration [1-way ANOVA: F (2, 45) = 1.125, P = 0.334] between sites (Fig. 2b). Mean protein concentration (mg protein g−1 wet wt ± SE) between sites was 37.65 ± 1.12. Symbiodinium density (cells mg protein−1 ± SE) between sites averaged to 1.06 ± 0.07 × 106 and mean chlorophyll a content (pg cell−1 ± SE) was 4.57 ± 0.27. The number of dividing Symbiodinium cells (MI) was progressively greater in anemones inhabiting the higher pCO2 sites [1-way ANOVA: F (2, 21) = 3.722, P = 0.041], increasing from 3.69 ± 0.76% at the control site to 7.12 ± 1.44% and 9.8 ± 0.54% at the intermediate and high pCO2 sites, respectively (Fig. 2c).
Figure 2. Physiological parameter measurements of A. viridis from sites 1 (control), 2 (intermediate pCO2) and 3 (high pCO2).
(a) Protein concentration (n = 16). (b) Symbiodinium density (bars) and chlorophyll concentration (circles) (n = 16). (c) Mitotic index (n = 8). Note that the mean pCO2 (μatm; Table 1) is given in parentheses for each site. All data represent the mean ± SEM. Letters indicate significant differences between sites (Tukey, P < 0.05).
Seawater isotopic signature
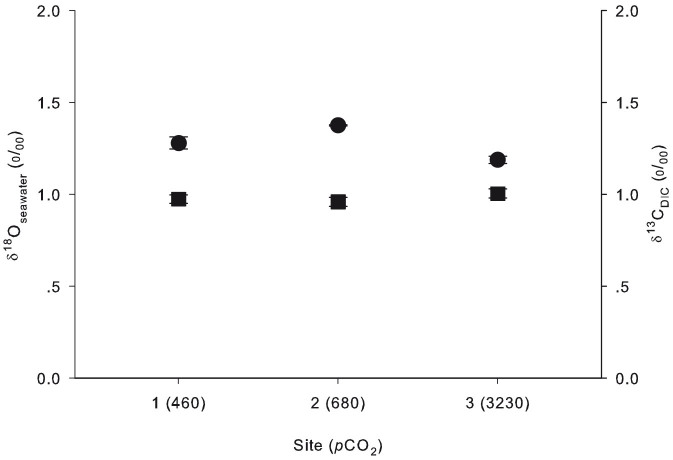
Stable isotope analysis showed constant δ18Oseawater between all sites, including the primary vent (Kruskal-Wallis ANOVA: df = 3, P = 0.361), with an average of 0.98 ± 0.01‰ (Fig. 3; vent site value not shown). δ13CDIC values were similar between sites 1–3, with an average of 1.28 ± 0.05‰ (Fig. 3), although all were significantly heavier compared to the primary vent site (0.34 ± 0.03‰) (Fig. 1a) (Kruskal-Wallis ANOVA: df = 3, P = 0.016).
Figure 3. Isotopic measurements of seawater at the sampling sites.
δ13CDIC (circles) and δ18Oseawater (squares) (‰) at sites 1 (control), 2 (intermediate pCO2) and 3 (high pCO2). Note that the mean pCO2 (μatm; Table 1) is given in parentheses for each site. All values represent the mean ± SEM (n = 3).
δ13C variability
δ13C values of both animal tissue (δ13CT) and Symbiodinium (δ13CS) decreased under high pCO2 conditions (Fig. 4a, b). One-way ANOVA revealed a significant difference in δ13CT between all sampling sites [F (2, 12) = 42.901, P = 0.000003], with a decrease from −16.66 ± 0.2‰ at the control site to −17.62 ± 0.19‰ and −19.12 ± 0.16‰ at the intermediate and high pCO2 sites, respectively. δ13CS also differed significantly between all sampling sites [1-way ANOVA: F (2, 12) = 25.606, P = 0.000047], decreasing from −15.1 ± 0.28‰ at the control site to −16.65 ± 0.37‰ and −18.21 ± 0.24‰ at the intermediate and high pCO2 sites, respectively. The difference in δ13C between the anemone tissue (δ13CT) and Symbiodinium (δ13CS) at each site was calculated as δ13CS- δ13CT to evaluate changes in autotrophic/heterotrophic ratios. δ13CT was considerably lighter than δ13CS at all sampling sites with δ13CS- δ13CT reduced with increasing pCO2 (Fig. 4a). In ambient seawater (control) this difference was relatively large (1.56 ± 0.21‰), while it decreased significantly at the intermediate and high pCO2 sites (0.96 ± 0.31‰ and 0.9 ± 0.17‰, respectively) [1-way ANOVA: F (2, 12) = 5.036, P = 0.026].
Figure 4. δ13C in A. viridis from sites 1 (control), 2 (intermediate pCO2) and 3 (high pCO2).
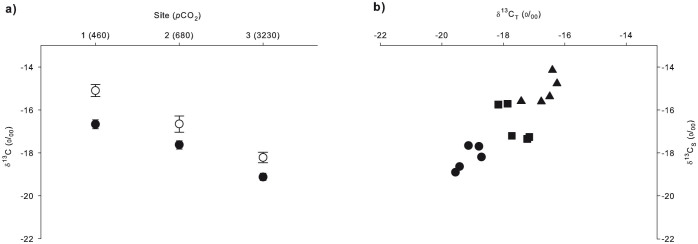
(a) Mean δ13C (‰) values (±SEM; n = 5) of Symbiodinium (white circles) and animal tissue (black circles). (b) δ13CT vs. δ13CS (‰) for individual A. viridis specimens from sites 1 (triangles), 2 (squares) and 3 (circles). Note that the mean pCO2 (μatm; Table 1) is given in parentheses for each sampling site.
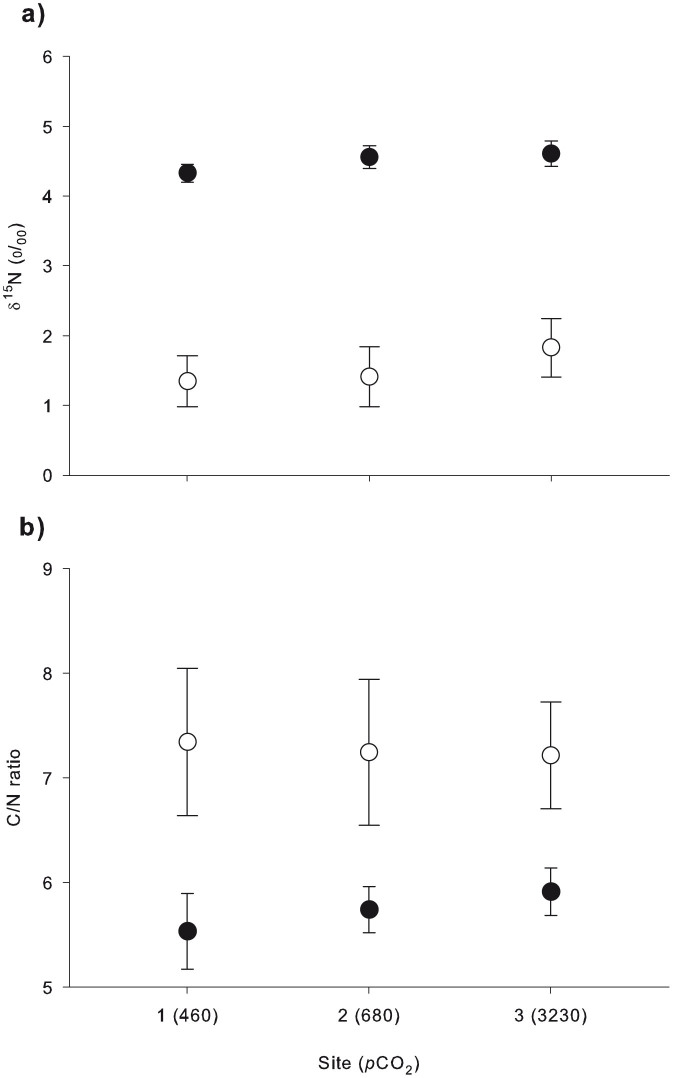
δ15N variability and C/N ratios
There was no significant difference in δ15N values of anemone tissue (δ15NT) [1-way ANOVA: F (2, 12) = 0.848, P = 0.452] and Symbiodinium (δ15NS) [1-way ANOVA: F (2, 12) = 0.266, P = 0.771] with increasing pCO2 (Fig. 5a). δ15NT was lowest at the control site (4.32 ± 0.12‰) and increased to 4.55 ± 0.16‰ and 4.6 ± 0.18‰ at the intermediate and high pCO2 sites, respectively. δ15NS averaged to 1.34 ± 0.36 at the control site and increased to 1.41 ± 0.42 and 1.82 ± 0.41 at the intermediate and high pCO2 sites, respectively. δ15NS was substantially lighter compared to δ15NT at all sampling sites, with an average difference of 2.5 ± 0.23‰ (Fig. 5a). The carbon to nitrogen ratios (C/N) of anemone tissue and Symbiodinium did not have any significant differences along the pCO2 gradient (1-way ANOVAs; F (2, 12) = 0.301, P = 0.745 for anemone tissue; F (2, 12) = 0.069, P = 0.934 for Symbiodinium) (Fig. 5b). The C/N ratio of anemone tissue at the control site was 5.53 ± 0.36 and increased to 5.73 ± 0.21 and 5.91 ± 0.22 at the intermediate and high pCO2 sites, respectively. The C/N ratio of Symbiodinium ranged from 7.34 ± 0.7 at the control site to 7.21 ± 0.51 at the high pCO2 site.
Figure 5. δ15N and C/N ratios in A. viridis from sites 1 (control), 2 (intermediate pCO2) and 3 (high pCO2).
Measurements in Symbiodinium (white circles) and animal tissue (black circles) of: (a) δ15N (‰), and (b) C/N ratio. Note that the mean pCO2 (μatm; Table 1) is given in parentheses for each site. All values represent the mean ± SEM (n = 5).
Discussion
A. viridis collected at all pCO2 sites lacked any apparent signs of stress (i.e. no mucus, tentacles fully extended; see Fig. 1b). Their general health was further supported by our results for physiological and algal characteristics. Protein concentrations, which are widely accepted as a sensitive indicator for the health of an organism25, showed no difference between sampling sites, indicating A. viridis was in fact well acclimated to the high seawater pCO2 (Fig. 2a). In addition, there were no changes in Symbiodinium densities and their chlorophyll a concentrations along the pCO2 gradient (Fig. 2b). This is in agreement with observations of the anemone Anthopleura elegantissima, following exposure to elevated pCO2 conditions in a laboratory setting, using the standard algal cell normalization to mg of protein methodology as in the present study14. However, Symbiodinium densities in A. viridis under high pCO2 conditions nearby the vent at Vulcano have been reported to increase relative to algal densities in anemones at the control site11. This discrepancy may be the result of a different methodology (using surface area as a normalization index in the same study11) in determining algal cell densities. The handling of anemones greatly influences tentacle contraction, which may have led to inaccuracy in surface area measurement, thereby making the comparison of results difficult.
The substantial increase in dividing algal cells under elevated pCO2 (MI; Fig. 2c) is in accordance with previous studies reporting high MIs in anemones under high pCO211,14. It is important to note that there was no variation in algal genotype as the anemones from all three sites were found to harbor Symbiodinium type A1912, excluding the possibility that genetic makeup of the Symbiodinium is responsible for the difference. The marked increase in algal division is most likely a direct result of massive CO2 input, as Symbiodinium in anemones remain carbon limited under normal conditions11,14,26,27. Since cnidarians are required to maintain cell-specific densities of their algal symbionts to avoid toxicity from excess oxidative products28, the host may initiate either active expulsion of symbionts and/or chemically-signaled arrest of algal reproduction29. Here, the high MIs but same algal densities, relative to algal densities at the control site, suggest that the anemones were unable to regulate algal reproduction under the elevated pCO2 conditions and therefore densities were likely maintained through Symbiodinium expulsion. Considering that in addition iron (Fe) is the most important trace element for algal growth30, Fe enrichment in the seawater near the vent site13,31 may have also affected algal proliferation to some extent.
The acidification of seawater close to the venting source arises from the constant gas emissions13. In addition to total DIC increasing by 17% at the high pCO2 site as compared to the control, CO2(aq) increased near the venting source (7-fold increase at the high pCO2 site; see Table 1). Although the carbonate system still consists mostly of bicarbonate (94%), CO2(aq) increased from less than 1% at the control site to 4% at the high pCO2 site (Table 1). Nonetheless, the isotopic composition of the inorganic carbon source in this area for the anemones appears to be constant as data shows that δ13CDIC does not change between sites (Fig. 3). Consequently, the pronounced and persistent depletion in 13C in the tissues of A. viridis and its Symbiodinium close to the vent cannot be explained by the assimilation of a 13C-depleted carbon source. The large increase in pCO2 in the seawater (Table 1; Fig. 1a) and its availability for A. viridis most likely account for the decrease in A. viridis δ13C values in both Symbiodinium and host tissue. The values near the vent (Fig. 4a, b) were well below the lower limit of the range reported previously for both tropical and subtropical sea anemones and Symbiodinium32,33.
Table 1. Carbonate chemistry of seawater at sampling sites 1 (control), 2 (intermediate pCO2) and 3 (high pCO2). Parameters were calculated from pHNBS, total alkalinity (TA), ambient seawater temperature, and salinity (38‰) using the program CO2SYS54. All data shown are the mean (±SD). Dissolved inorganic carbon (DIC).
| Site | pHNBS | TA (μeq kg−1) | pCO2 (μatm) | DIC (μmol kg−1) | HC03− (μmol kg−1) | CO32− (μmol kg−1) | CO2(aq) (μmol kg−1) |
|---|---|---|---|---|---|---|---|
| 1. Control | 8.12 (0.02) | 2554 (47) | 463 (33) | 2206 (22) | 1998 (29) | 193 (8) | 15 (1) |
| 2. Intermediate pCO2 | 7.99 (0.07) | 2486 (9) | 683 (62) | 2287 (46) | 2113 (65) | 152 (23) | 22 (4) |
| 3. High pCO2 | 7.44 (0.26) | 2501 (20) | 3232 (836) | 2585 (123) | 2430 (93) | 50 (25) | 105 (53) |
δ13CT values decreased at the intermediate and high pCO2 sites to −17.62 ± 0.19‰ and −19.12 ± 0.16‰, respectively, as compared to the control site (−16.66 ± 0.2‰) (Fig. 4a), suggesting an increase in photosynthetically fixed carbon relative to heterotrophically acquired carbon in the host20,34,35. Taking seasonal and regional variability into account, average zooplankton and particulate organic carbon (POC) δ13C values reported in the area for surface waters range between −21 and −22‰36. We assumed that the availability of these extrinsic carbon sources was constant across all sampling sites in our study, as the relatively short distance between sampling sites (<500 m) and their orientation in Levante Bay towards the open sea renders differences in food availability most unlikely as a factor. Based on mass balance estimation, our calculations show about 5% heterotrophic input to δ13CT at the control site (using δ13CT = −16.66‰ and δ13CS = −15.1‰, assuming δ13Czooplankton/POC = −22‰). This is typical of cnidarian-algae symbioses, in which Symbiodinium may contribute up to 95% of their photosynthetically-produced carbon to the host37. Based on the same assumptions, at the high pCO2 site the heterotrophic input to δ13CT reduced to about 2.5% (using δ13CT = −19.12‰ and δ13CS = −18.21‰, assuming δ13Czooplankton/POC = −22‰), leading to a greater autotrophic input. This observation is also supported by the difference in δ13C values between host tissue and Symbiodinium, which reflects the relative contribution of heterotrophy and photosynthesis to fixed carbon20,38. Cnidarian host tissue and Symbiodinium stable carbon isotopic values are usually within 2‰ of each other20,39,40. There was a significant reduction in δ13CS- δ13CT with increasing pCO2 from 1.56 ± 0.21‰ at the control site to 0.96 ± 0.31‰ and 0.9 ± 0.17‰ at the intermediate pCO2 and high pCO2 sites, respectively (Fig. 4a). This further indicates an increase in the autotrophic/heterotrophic ratio via translocated autotrophic carbon to the host.
Our results suggest that elevated pCO2 near the vent promotes carbon isotope fractionation by Symbiodinium during photosynthesis, leading to lighter δ13CS values. δ13CS showed a substantial decrease from −15.1 ± 0.28‰ at the control site to −16.65 ± 0.37‰ and −18.21 ± 0.24‰ at the intermediate and high pCO2 sites, respectively (Fig. 4a). Many studies have shown that δ13C is depleted in marine photosynthetic organisms under elevated pCO241,42,43,44. Under normal conditions, the majority of Symbiodinium carbon requirements (~85%) are met via energy-demanding carbon-concentrating mechanisms (CCMs), whilst the remainder diffuses passively from seawater to the Symbiodinium cells28. When pCO2 is elevated, CO2(aq) can replace HCO3− as the main carbon source for photosynthesis while energy-consuming CCMs become less important43,45. Form II ribulose 1,5-bisphosphate carboxylase/oxygenase (form II Rubisco), which is the carboxylating enzyme in Symbiodinium46, discriminates against 13C47. Enhanced levels of pCO2 in the proximity of the vent diffuse to the Rubisco, which favors 12C for carbon fixation and ultimately results in a lightning trend of δ13CS values. Krief et al. (2010) reported the same trend in two species of scleractinian corals after experimental exposure to high pCO2 in a controlled pCO2 system. While Krief et al. (2010) kept corals under elevated pCO2 for a period of 14 months, our in situ study at the CO2 vent site lends insight into a long-term exposure scenario48.
δ15NT and δ15NS values did not change along the pCO2 gradient, suggesting that the anemones' function and performance reside within normal bounds close to the vent after long-term exposure to acidification conditions (Fig. 5a). Further supporting this concept is the lack of change in C/N ratio between sites (Fig. 5b). The C/N ratio is considered a good proxy for an organism's condition since it reflects the ratio of lipids and carbohydrates to proteins49. The apparent absence of preferential accumulation/loss of lipids, carbohydrates or proteins in A. viridis in high pCO2/low pH surroundings indicates therefore that the anemones were well acclimated.
Generally, animals exposed to high pCO2/low pH have to compensate for acid-base imbalance in intra- and extracellular spaces thereby imposing elevated metabolic costs50. A recent study by Laurent et al. (2014) demonstrated the high capacity of A. viridis to regulate against decreases in internal and external pH, thereby maintaining normal cellular metabolism and physiology51. Our results indicate the adaptation and potential resilience of A. viridis to acidification conditions, as physiological data (i.e. protein content, Symbiodinium density and chlorophyll a concentration; Fig. 2a, b), along with δ15N values and C/N ratios (Fig. 5a, b), remained unaffected among sites along the pCO2 gradient. Moreover, the high pCO2 environment probably stimulated cell division of algal symbionts (Fig. 2c).
We have shown that the anemone host relies more on photosynthetically derived carbon under elevated pCO2. We propose that A. viridis optimizes energy utilization under elevated pCO2 through an increased autotrophic input, although isotopic data show that heterotrophy is maintained as an additional source of energy/nutrients. These factors may contribute, at least in part, to the increased size and abundance of the A. viridis population proximate to the vent site as reported in a previous study11. In conclusion, increased autotrophic/heterotrophic ratio may enhance the competitive advantage of symbiotic anemones over other invertebrates and improve their ecological success in benthic communities. These are valuable findings that merit further study for predicting the performance of non-calcifying symbiotic cnidarians in future high-CO2 oceans.
Methods
Study sites
This study was conducted along the sublittoral in Levante Bay, Vulcano Island (38° 25′ N, 14° 57′ E), part of the Aeolian Island chain, NE Sicily (Fig. 1a) in May 2012. Shallow-water CO2 vents create a natural pCO2/pH gradient along the north-easterly side of the bay, ranging from pH 6.05 to 8.29 at >350 m from the vent site8,13.
Three sites were selected for animal sampling in accordance with previous studies (see Fig. 1a)7,8,11,13. Site 1 (control) was an ambient seawater reference station, located outside the vent area (>400 m); Site 2 (intermediate pCO2) was ~300 m away from the CO2 vents; Site 3 (high pCO2) was in the proximity of the CO2 vents (~260 m). Sampling at the primary vent site (indicated by the star symbol in Fig. 1a) was for collection of seawater samples only.
Carbonate chemistry and physical measurements
Seawater pH (NBS scale) and temperature were measured at all sites several times a day for 4 days using a pH meter (YSI Professional Plus, Handheld Multiparameter Instrument, USA). Water samples for total alkalinity (TA) analysis were collected from each site, cooled and stored in the dark until analysis. TA was quantified with a Metrohm 862 compact titrosampler52. The pCO2 levels were calculated from salinity ( = 38‰, as reported by Johnson, 201253) and TA and pHNBS measurements using the program CO2SYS [Pierrot, D. E., Lewis, E. & Wallace, D. W. R. MS Excel program developed for CO2 system calculations. Carbon dioxide information analysis center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN, USA (2006)], selecting the constants of Mehrbach et al. (1973)54. Carbonate chemistry parameters are shown in Table 1. Light intensity at each site was measured hourly for 3 consecutive days close to the seabed (1–2 m depth) with HOBO Pendant® Temperature/Light data loggers (Onset, Pocasset, MA, USA). The logged light data were converted from lux to μmol quanta m2 s−1 (Fig. 1a)55.
Sample collection in the field
Anemones
A. viridis, a dominant benthic organism in Levante Bay, was prevalent throughout the study area. Sixteen anemones were collected randomly from each site at a depth of 1–2 m and immediately frozen until further analyses. To minimize any confounding responses due to age and/or size all samples were of similar size (oral disc diameter of 2.5–3.5 cm)56. Between 5 and 10 tentacles were clipped from each anemone at every site (n = 16). Tentacles were processed for total protein and algal characteristics (i.e., Symbiodinium density, chlorophyll a concentration and mitotic index) at the sampling site. Samples were weighed (CT 1202, Citizen, accuracy 0.01 g) and homogenized in 0.2 μm sterile filtered seawater (FSW) with an electric homogenizer (DIAX 100 homogenizer Heidolph Instruments GmbH & Co. KG, Schwabach, Germany). The homogenate and all anemones were immediately frozen and then transported on dry ice to the Interuniversity Institute for Marine Sciences (IUI), Israel, where they were stored at −80 °C pending analyses.
Seawater
Seawater samples were collected from the four sites for carbon isotopes of dissolved inorganic carbon (DIC; δ13CDIC) and oxygen isotopic analysis (δ18Oseawater). Triplicate samples for δ13CDIC analysis were immediately poisoned upon collection with 60 μl saturated solution of mercuric chloride and stored in 60 ml brown bottles at room temperature until analysis. Triplicate samples for δ18Oseawater analysis were collected in 50 ml test tubes (Stardest) and stored at room temperature until analysis.
Total protein, Symbiodinium density, mitotic index and chlorophyll concentration
The tissue homogenate of each anemone (n = 16) was further processed and analyzed for measurements of physiological parameters. Total protein analysis was performed by removing 100 μl of the tissue homogenate and sonication on ice with a Branson Sonifier B12 (Branson Sonic Power Co., Danbury, Connecticut, USA) for 20 s. Quantification was done after Bradford (1976) using the Quick Start Bradford Protein Assay Kit and Quick Start Bovine Serum Albumin Standard Set (Bio-Rad Laboratories, Hercules, CA, USA)57. Optical density was read at 595 nm using an ELISA reader (Multiskan spectrum, Thermo Fisher Scientific Inc., USA).
For measurement of algal characteristics, 2 ml of homogenate of each sample (n = 16) were centrifuged (5000 rpm at 4°C; 4K15 centrifuge, Sigma) and re-suspended four times in FSW. Re-suspended Symbiodinium were used for chlorophyll a extraction in acetone (100%) at 4°C in the dark for 24 hours. Concentrations of chlorophyll a were measured using spectrophotometry (Ultrospec 2100 pro, GE Bioscience, USA) and calculated using standard equations58. Chlorophyll concentration was calculated per Symbiodinium cell. Symbiodinium densities were quantified from 4 replicate counts using a Neubauer haemocytometer and normalized to protein concentration. Mitotic index (MI) was measured as an indicator of Symbiodinium growth and was calculated as a percentage of doublets with a complete cleavage furrow observed per 1000 cells (n = 8 per sampling station)59.
Separation of anemone tissue and Symbiodinium for isotope analysis
Sub-samples of 250 mg were excised from the tentacles of each anemone (n = 5 per site) and placed in sterile 15 ml falcon tubes (Stardest). After adding 1 ml 0.2 μm filtered seawater (FSW), an electric homogenizer (DIAX 100 homogenizer Heidolph Instruments GmbH & Co. KG, Schwabach, Germany) was used to homogenize the tissue extract for 2 min. Separation of anemone tissue and Symbiodinium was done by the following protocol. The homogenate was centrifuged for 5 min at 5000 rpm (4K15 centrifuge, Sigma) to separate the algae (pellet) and the host tissue (supernatant). Visual inspections revealed no crossover of material between these components, but both were washed carefully.
The host supernatant was homogenized and centrifuged for 10 min at 13,500 rpm (4K15 centrifuge, Sigma, USA), resulting in pelleted host material for analysis. The Symbiodinium pellet was then re-suspended in 1 ml FSW, homogenized, and centrifuged for 5 min at 5000 rpm (4K15 centrifuge, Sigma, USA). The procedure was repeated twice in order to remove remaining tissue. All samples were washed with double-distilled water (DDW) to remove any remaining salts. Both the host tissue and Symbiodinium samples were dried with a lyophilizer (VirTis, Sentry 2.0, SP Scientific, USA) for 24 h for further isotopic analysis.
Stable isotope analyses
The isotopic measurements were made at the stable isotopes laboratory in the Department of Earth and Planetary Sciences, the Weizmann Institute of Science, Israel. The oxygen, carbon and nitrogen isotope measurements are reported in the conventional δ-notation.
Anemone tissue and Symbiodinium samples
δ13C and δ15N of 240–270 μg of dried tissue and algae were analyzed using an elemental analyzer (CE 1110) interfaced to the MAT 252 mass spectrometer. Long term precision of working standards for δ13C is 0.05‰ and for δ15N is 0.1‰ relative to V-PDB and Air respectively (±1σ SD). The carbon to nitrogen ratios (C/N) of anemone tissue and Symbiodinium were calculated from simultaneous %C and %N.
Seawater samples
δ18Oseawater was analyzed by equilibrating 0.5 ml of samples with a mixture of 0.5% CO2 in He at 25 °C for 24 h. The samples were analyzed on a Gas Bench II connected in-line to a Finigan MAT 252 mass spectrometer. The results are reported relative to VSMOW with 0.08‰ (±1σ SD) long-term precision of the laboratory working standards.
For δ13CDIC analysis, 1 ml sea water was injected into vials, flushed with He gas, acidified with 0.15 ml orthophosphoric acid (H3PO4) and left to react for 24 h at 25 °C. The samples were analyzed on a Gas Bench II and Finigan MAT 252. The results are reported relative to VPDB with 0.08‰ long-term precision (±1σ SD) of the NaHCO3 laboratory standard.
Data analyses
All data was checked for normality using the Kolmogorov-Smirnov test and for homogeneity of variance using Cochran's test. In cases in which homogeneity of variance was achieved, we used one-way ANOVA and a multiple comparison test (Tukey). If homogeneity of variance or normality was not achieved, we used a non-parametric Kruskal-Wallis ANOVA and post-hoc Mann-Whitney U-tests for separation of significant factors. Differences between factors were considered significant for a P value < 0.05. Unless otherwise specified, mean values are presented ± standard error of mean (SEM). All data were analyzed using SPSS version 20 (SPSS IBM, New York, USA).
Author Contributions
R.H., E.M.B. and M.F. conceived the overall project. R.H. and E.M.B. conducted the field and laboratory work and analysed data. R.Y. and A.S. carried out stable isotope analyses. All authors reviewed and edited the manuscript.
Acknowledgments
Thanks to Marco Milazzo (University of Palermo) for essential academic and logistical support. We are grateful to Gabriela Perna for help with the physiological parameter analyses. This study was funded in part by the FP7 ASSEMBLE project no. 227799, the EU MedSeA project, and an Israel Science Foundation grant to M.F.. E.M.B. was funded by the Minerva fellowship program.
References
- Hoegh-Guldberg O. The adaptation of coral reefs to climate change: is the red 549 queen being outpaced? Sci. Mar. 76, 403–408 (2012). [Google Scholar]
- Intergovernmental Panel on Climate Change. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [eds Stocker T. F. et al.] (Cambridge Univ. Press, Cambridge, 2013). [Google Scholar]
- Hall-Spencer J. M. et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99 (2008). [DOI] [PubMed] [Google Scholar]
- Kroeker K. J., Micheli F., Gambi M. C. & Martz T. R. Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc. Natl. Acad. Sci. USA 108, 14515–14520 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meron D. et al. Changes in coral microbial communities in response to a natural pH gradient. ISME J. 6, 1775–1785 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K. E. et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Change 1, 165–169 (2011). [Google Scholar]
- Arnold T. et al. Ocean acidification and the loss of phenolic substances in marine plants. PLoS ONE 7, e35107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. R. et al. Responses of marine benthic microalgae to elevated CO2. Mar. Biol. 160, 1813–1824 (2011). [Google Scholar]
- Johnson V. R., Russell B. D., Fabricius K. E., Brownlee C. & Hall-Spencer J. M. Temperate and tropical brown macroalgae thrive, despite decalcification, along natural CO2 gradients. Glob. Change Biol. 18, 2792–2803 (2012). [DOI] [PubMed] [Google Scholar]
- Calosi P. et al. Distribution of sea urchins living near shallow water CO2 vents is dependent upon species acid–base and ion-regulatory abilities. Mar. Poll. Bull. 73, 470–484 (2013). [DOI] [PubMed] [Google Scholar]
- Suggett D. J. et al. Sea anemones may thrive in a high CO2 world.Glob. Change Biol. 18, 3015–3025 (2012). [DOI] [PubMed] [Google Scholar]
- Borell E. M., Steinke M., Horwitz R. & Fine M. Increasing pCO2 correlates with low concentrations of intracellular dimethylsulfoniopropionate in the sea anemone Anemonia viridis. Ecol. Evol. 10.1002/ece3.946 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatta F. et al. Geochemical survey of Levante Bay, Vulcano Island (Italy), a natural laboratory for the study of ocean acidification. Mar. Poll. Bull. 73, 485–494 (2013). [DOI] [PubMed] [Google Scholar]
- Towanda T. & Thuesen E. V. Prolonged exposure to elevated CO2 promotes growth of the algal symbiont Symbiodinium muscatinei in the intertidal sea anemone Anthopleura elegantissima. Biology Open 1, 615–621 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachar A., Achituv Y., Pasternak Z. & Dubinsky Z. Autotrophy versus heterotrophy: the origin of carbon determines its fate in a symbiotic sea anemone. J. Exp. Mar. Biol. Ecol. 349, 295–298 (2007). [Google Scholar]
- Brading P., Warner M. E., Smith D. J. & Suggett D. J. Contrasting modes of inorganic carbon acquisition amongst Symbiodinium (Dinophyceae) phylotypes. New Phytol. 200, 432–442 (2013). [DOI] [PubMed] [Google Scholar]
- Dubinsky Z. & Jokiel P. L. Ratio of energy and nutrient fluxes regulates symbiosis between zooxanthellae and corals. Pac. Sci. 48, 313–324 (1994). [Google Scholar]
- Muscatine L. et al. Stable isotopes (δ13C and δ15N) of organic matrix from coral skeleton. Proc. Natl. Acad. Sci. USA 102, 1525–1530 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meron D., Buia M. C., Fine M. & Banin E. Changes in microbial communities associated with the sea anemone Anemonia viridis in a natural pH gradient. Microbial Ecol. 65, 269–276 (2013). [DOI] [PubMed] [Google Scholar]
- Muscatine L., Porter J. W. & Kaplan I. R. Resource partitioning by reef corals as determined from stable isotope composition. I. δ13C of zooxanthellae and animal tissue vs depth. Mar. Biol. 100, 185–193 (1989). [Google Scholar]
- Rodrigues L. J. & Grottoli A. G. Calcification rate and the stable carbon, oxygen, and nitrogen isotopes in the skeleton, host tissue, and zooxanthellae of bleached and recovering Hawaiian corals. Geochim. Cosmochim. Ac. 70, 2781–2789 (2006). [Google Scholar]
- Reynaud S. et al. Effect of light and feeding on the nitrogen isotopic composition of a zooxanthellate coral: role of nitrogen recycling. Mar. Ecol. Prog. Ser. 392, 103–110 (2009). [Google Scholar]
- Alamaru A., Yam R., Shemesh A. & Loya Y. Carbon and nitrogen utilization in two species of Red Sea corals along a depth gradient: Insights from stable isotope analysis of total organic material and lipids. Geochim. Cosmochim. Ac. 73, 5333–5342 (2009). [Google Scholar]
- Leal M. C. et al. Trophic ecology of the facultative symbiotic coral Oculina arbuscula. MEPS 504, 171–179 (2014). [Google Scholar]
- Houlbrèque F., Tambutté E., Allemand D. & Ferrier-Pagès C. Interactions between zooplankton feeding, photosynthesis and skeletal growth in the scleractinian coral Stylophora pistillata. J. Exp. Biol. 207, 1461–1469 (2004). [DOI] [PubMed] [Google Scholar]
- Jarrold M. D. et al. Physiological plasticity preserves the metabolic relationship of the intertidal non-calcifying anthozoan–Symbiodinium symbiosis under ocean acidification. J. Exp. Mar. Biol. Ecol. 449, 200–206 (2013). [Google Scholar]
- Gibbin E. M. & Davy S. K. The photo-physiological response of a model cnidarian–dinoflagellate symbiosis to CO2-induced acidification at the cellular level. J. Exp. Mar. Biol. Ecol. 457, 1–7 (2014). [Google Scholar]
- Furla P. et al. The symbiotic anthozoan: a physiological chimera between alga and animal. Integr. Comp. Biol. 45, 595–604 (2005). [DOI] [PubMed] [Google Scholar]
- Baghdasarian G. & Muscatine L. Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis. Biol. Bull. 199, 278–286 (2000). [DOI] [PubMed] [Google Scholar]
- Falkowski P. G., Barber R. T. & Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science 281, 200–206 (1998). [DOI] [PubMed] [Google Scholar]
- Horwitz R., Borell E. M., Fine M. & Shaked Y. Trace element profiles of the sea anemone Anemonia viridis living nearby a natural CO2 vent. Peer J 2, e538 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergschneider H. & Muller-Parker G. Nutritional role of two algal symbionts in the temperate sea anemone Anthopleura elegantissima Brandt. Biol. Bull. 215, 73–88 (2008). [DOI] [PubMed] [Google Scholar]
- Cleveland A., Verde E. A. & Lee R. W. Nutritional exchange in a tropical tripartite symbiosis: direct evidence for the transfer of nutrients from anemonefish to host anemone and zooxanthellae. Mar. Biol. 158, 589–602 (2011). [Google Scholar]
- Swart P. K., Leder J. J., Szmant A. & Dodge R. E. The origin of variations in the isotopic record of scleractinian corals: II. Carbon. Geochim. Cosmochim. Ac. 60, 2871–2886 (1996). [Google Scholar]
- McConnaughey T. A., Burdett J., Whelan J. F. & Paull C. K. Carbon isotopes in biological carbonates: respiration and photosynthesis. Geochim. Cosmochim. Ac. 61, 611–622 (1997). [Google Scholar]
- Mazzola A. et al. Origin and distribution of suspended organic matter as inferred from carbon isotope composition in a Mediterranean semi-enclosed marine system. Chem. Ecol. 16, 215–238 (1999). [Google Scholar]
- Muscatine L. Productivity of zooxanthellae. In: Primary Production in the Sea [Falkowski P. G. (ed.)] [649–658] (Plenum, New York, USA, 1980). [Google Scholar]
- Swart P. K. et al. The isotopic composition of respired carbon dioxide in scleractinian corals: implications for cycling of organic carbon in corals. Geochim. Cosmochim. Ac. 69, 1495–1509 (2005). [Google Scholar]
- Reynaud S. et al. Effect of feeding on the carbon and oxygen isotopic composition in the tissues and skeleton of the zooxanthellate coral Stylophora pistillata. Mar. Ecol. Prog. Ser. 238, 81–89 (2002). [Google Scholar]
- Grottoli A. G., Rodrigues L. J. & Juarez C. Lipids and stable carbon isotopes in two species of Hawaiian corals, Porites compressa and Montipora verrucosa, following a bleaching event. Mar. Biol. 145, 621–631 (2004). [Google Scholar]
- Rau G. H., Takahashi T., Desmarais D. J., Repeta D. J. & Martin J. H. The relationship between δ13C of organic matter and [CO2](aq) in ocean surface water – data from a JGOFS site in the northeast Atlantic Ocean and a model. Geochim. Cosmochim. Ac. 56, 1413–1419 (1992). [DOI] [PubMed] [Google Scholar]
- Laws E. A., Popp B. N., Bidigare R. R., Kennicutt M. C. & Macko S. A. Dependence of phytoplankton carbon isotopic composition on growth rate and [CO2](aq) – theoretical considerations and experimental results. Geochim. Cosmochim. Ac. 59, 1131–1138 (1995). [Google Scholar]
- Erez J., Bouevitch A. & Kaplan A. Carbon isotope fractionation by photosynthetic aquatic microorganisms: experiments with Synechococcus PCC7942, and a simple carbon flux model. Can. J. Bot. 76, 1109–1118 (1997). [Google Scholar]
- Vizzini S. et al. Effect of explosive shallow hydrothermal vents on δ13C and growth performance in the seagrass Posidonia oceanica. J. Ecol. 98, 1284–1291 (2010). [Google Scholar]
- Goericke R. & Fry B. Variations of marine plankton δ13C with latitude, temperature, and dissolved CO2 in the world ocean. Global Biogeochem. Cy. 8, 85–90 (1994). [Google Scholar]
- Mayfield A. B., Hsiao Y. Y., Chen H. K. & Chen C. S. Rubisco expression in the dinoflagellate Symbiodinium sp. is influenced by both photoperiod and endosymbiotic lifestyle. Mar. Biotechnol. 16, 371–384 (2014). [DOI] [PubMed] [Google Scholar]
- Raven J. A. Inorganic carbon acquisition by marine autotrophs. Adv. Bot. Res. 27, 85–209 (1997). [Google Scholar]
- Krief S. et al. Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochim. Cosmochim. Ac. 74, 4988–5001 (2010). [Google Scholar]
- Bodin N., Le Loc'h F. & Hily C. Effect of lipid removal on carbon and nitrogen stable isotope ratios in crustacean tissues. J. Exp. Mar. Biol. Ecol. 341, 168–175 (2007). [Google Scholar]
- Fabry V. J., Seibel B. A., Feely R. A. & Orr J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432 (2008). [Google Scholar]
- Laurent J. et al. Regulation of intracellular pH in cnidarians: response to acidosis in Anemonia viridis. FEBS J. 281, 683–695 (2014). [DOI] [PubMed] [Google Scholar]
- Cohen S. Measuring gross and net calcification of a reef coral under ocean acidification conditions: methodological considerations. MSc Thesis. Bar-Ilan University, Israel (2011).
- Johnson V. R. A study of marine benthic algae along a natural carbon dioxide gradient. PhD Thesis. University of Plymouth, UK (2012).
- Mehrbach C., Culberson C. H., Hawley J. E. & Pytkowicz R. M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973). [Google Scholar]
- Thimijan R. W. & Heins R. D. Photometric, radiometric, and quantum light units of measure: a review of procedures for interconversion. HortScience 18, 818–822 (1983). [Google Scholar]
- Perez S. F., Cook C. B. & Brooks W. R. The role of symbiotic dinoflagellates in the temperature-induced bleaching response of the subtropical sea anemone Aiptasia pallida. J. Exp. Mar. Biol. Ecol. 256, 1–14 (2001). [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- Jeffrey S. W. & Humphrey G. F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pfl. 167, S191–S194 (1975). [Google Scholar]
- Wilkerson F. P., Muller-Parker G. & Muscatine L. Temporal patterns of cell division in natural populations of endosymbiotic algae. Limnol. Oceanogr. 28, 1009–1014 (1983). [Google Scholar]