Abstract
The present study showed that the latency of rats moving on a vertical grid was significantly prolonged, and the number of rats sliding down from the declined plane was increased remarkably, in rotenone-induced Parkinson's disease model rats compared with control rats. The moving latency recovered to normal levels, but the number of slides was significantly increased at 28 days after model establishment. The slope test is a meaningful approach to evaluate the symptoms of Parkinson's disease model rats treated with rotenone. In addition, loss of substantia nigral dopaminergic neurons in model rats was observed at 1 day after the model was established, and continued gradually at 14 and 28 days. The expression of tyrosine hydroxylase-positive cells was significantly increased in gastrodin-treated rats at 14 days. Significant numbers of activated microglia cells were observed in model rats at 14 and 28 days; treatment of rats with Madopar at 28 days suppressed microglial activation. Treatment of rats with gastrodin or Madopar at 28 days significantly reduced interleukin-1β expression. The loss of substantia nigral dopaminergic neurons paralleled the microglial activation in Parkinson's disease model rats treated with rotenone. The inflammatory factors tumor necrosis factor-α and interleukin-1β are involved in the substantia nigral damage. Gastrodin could protect dopaminergic neurons via inhibition of interleukin-1β expression and neuroinflammation in the substantia nigra.
Keywords: gastrodin, rotenone, neuroinflammation, dopamine, microglia cells, interleukin-1β, Parkinson's disease
INTRODUCTION
Neuroinflammation processes have been shown to be associated with the pathogenesis of Parkinson's disease (PD)[1,2,3]. Both postmortem analysis and various animal models of PD[4] have detected massive amounts of activated microglia, as well as reactive astrocytes around degenerated neurons. Microglial activation might participate in the development of PD, as suggested by the results of an epidemiological investigation[5]. Activated microglia and astrocytes produce a wide array of prostanoids, free radicals and cytokines, which work in concert to induce neurodegeneration[6,7].
Rotenone-induced models reproduce effectively the pathological features of PD[8,9,10,11,12]. The loss of substantia nigral dopaminergic neurons and microglial activation in rotenone-induced rats were observed in our previous study. However, little has been reported about the changes to the numerous cytokines in the brains of model rats and the relationship between neuroinflammation and loss of dopaminergic neurons.
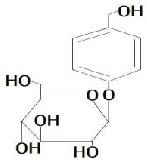
Complex prescription of Chinese drugs, with Rhizoma Gastrodiae as a monarch or ministerial drug, has been widely used in the treatment of PD[13,14,15,16,17]. However, the effects of these drugs on PD animals and treatment-related mechanism are still unknown[18]. Gastrodin is the major active component of Rhizoma Gastrodiae, with a molecular weight of 286 Da, a molecular formula of C13H18O7, and the following chemical structural formula:
The present study sought to elucidate the effects of gastrodin on substantia nigra dopaminergic neurons in rotenone-induced PD rats, as well as its effects on microglial activity and cytokine expression in rat models of PD, using Madopar as a control. Moreover, this study also demonstrates the relationships among microglial activation, inflammatory cytokines and loss of dopaminergic neurons in the substantia nigra of model rats.
RESULTS
Quantitative analysis of experimental animals
A total of 90 Wistar rats were used in this study; 11 of them were selected as controls, while the remaining rats were used to make PD models induced by rotenone. A total of 49 successful model rats were treated with Madopar (Madopar group, n = 15), gastrodin (gastrodin group, n = 15) or saline (model group, n = 19) by gavage. In total, 60 rats were included in the final analysis.
Effect of gastrodin on the neurobehavior of rotenone-induced PD rats
Grid tests showed that the moving latency in rats at 1 day after model establishment was lengthened remarkably compared with that in normal rats (P < 0.01), but that it recovered to the normal latency by 28 days after model establishment (P > 0.05). There was no significant difference between model rats and rats treated with gastrodin or Madopar (Table 1).
Table 1.
Effect of gastrodin on behavioral changes in rotenone-treated Parkinson's disease rats
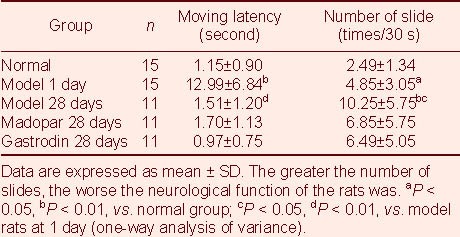
Slope tests showed that the number of sliding model rats at 1 day after model establishment was significantly higher than the number of sliding normal rats (P < 0.05), and that it was higher still at 28 days (P < 0.05). There was a trend toward fewer sliding gastrodin- or Madopar-treated rats, but there was no significant difference between the models and drug-treated rats (Table 1).
Gastrodin increases tyrosine hydroxylase (TH) expression in the substantia nigra of PD rats
TH-positive expression was significantly decreased in the model rats at 4 days after model establishment compared with normal rats (P < 0.05; Table 2, Figures 1A, B), and this decrease was even greater at 14 and 28 days (P < 0.01; Figures 1C, D). TH-positive expression at 14 days after model establishment was significantly increased in gastrodin-treated rats compared with model rats (P < 0.05; Figures 1C, E, F), but the level of TH-positive expression in Madopar-treated rats was not significantly greater than that in model rats, despite a trend toward an increase.
Table 2.
Area of tyrosine hydroxylase-positive cells (×103 μm2) in the substantia nigra of rats
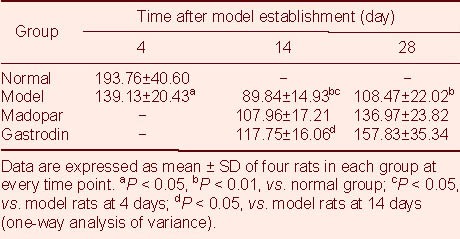
Figure 1.
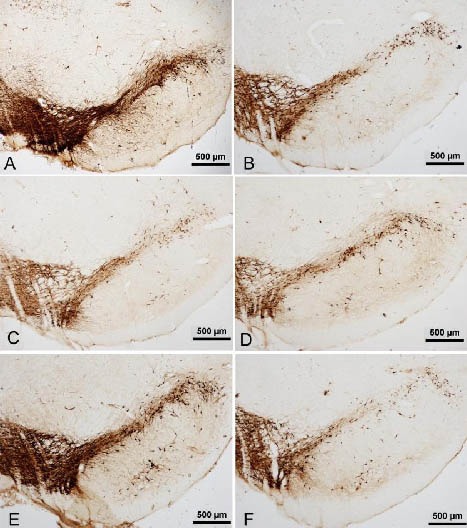
Tyrosine hydroxylase (TH) expression in the substantia nigra of rats (immunohistochemical staining, light microscope, scale bars: 500 μm).
A large number of TH-positive cells with deeply colored cell bodies and abundant processes were observed in the substantia nigra of normal rats (A).
Compared with normal rats (A), the numbers of TH-positive cells and processes in model rats at 4 days (B) were clearly decreased, and lighter colored cell bodies were seen.
The loss of TH-positive cells was aggravated in model rats at 14 days (C) and 28 days (D).
Gastrodin at 14 days increased the numbers of TH-positive cells and processes (E) compared with the model group at 14 days (C).
The numbers of TH-positive cells and processes of Madopar-treated rats also tended towards a decrease at 14 days (F).
Effect of gastrodin on complement receptor (OX42) expression in the substantia nigra of PD rats
Microglial activation was observed in model rats at 14 and 28 days, and the area of OX42-positive cells was significantly lower in model rats than in controls (P < 0.05; Table 3, Figures 2A–D). Microglial activation in rats treated with gastrodin and Madopar at 28 days showed decreased microglial activation, with significant differences between the Madopar group and the model group (P < 0.05; Figures 2E, F).
Table 3.
Area of OX42-positive cells (×103 μm2) in the substantia nigra of rats
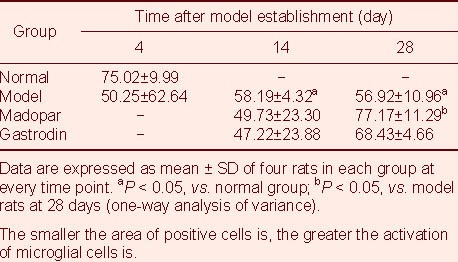
Figure 2.
Complement receptor (OX42) staining in the midbrains of rats (immunohistochemical staining, light microscope, scale bars: 50 μm).
Resting microglia had branches in the substantia nigra of normal controls that stained for OX42 (A).
Microglia cells showed an amoeba-like morphology in the model at 4 days (B), 14 days (C) and 28 days (D). Microglial activation was reduced in gastrodin-(E) and Madopar-treated rats (F).
Arrows represent OX42 positive expression.
Gastrodin inhibits tumor necrosis factor (TNF)-α expression in the substantia nigra of PD rats
TNF-α expression decreased sharply in model rats at 4 days after model establishment compared with the normal group (P < 0.05; Table 4, Figures 3A, B). It showed a tendency toward an increase at 14 and 28 days, but was not significantly different from the expression levels in normal control rats. Treatment of rats with gastrodin and Madopar at 14 days significantly downregulated TNF-α expression (P < 0.05; Figures 3C, E). TNF-α expression in the Madopar group at 28 days was higher than that at 14 days (P < 0.05; Figures 3E, F), but the expression level continued to decrease in the gastrodin-treated group.
Table 4.
Area of tumor necrosis factor-α-positive cells (×103 μm2) in the substantia nigra of rats
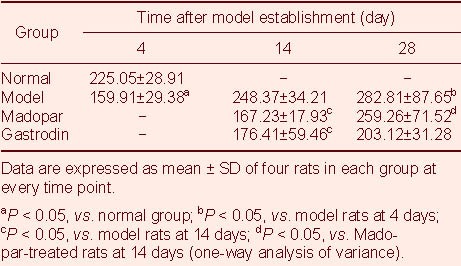
Figure 3.
Tumor necrosis factor-α staining in the midbrains of rats (immunohistochemical staining, light microscope, scale bars: 100 μm).
Many dark brown-colored cells can be observed in the control rats (A).
The number of positive cells was reduced in the model rats at 4 days (B) and 14 days (C). This was also the case in the gastrodin (D) and Madopar (E) groups at 14 days.
The number of positive cells in the Madopar group at 28 days (F) was greater than that at 14 days (E).
Gastrodin inhibits interleukin (IL)-1β expression in the substantia nigra of PD rats
IL-1β expression showed a tendency to increase continuously in the substantia nigra of model rats. IL-1β expression was clearly increased in the model group at 14 days (P < 0.05; Table 5, Figures 4A, B), and it was significantly higher at 28 days (P < 0.01; Figure 4C). There was a tendency for IL-1β expression in rats treated with gastrodin and Madopar to be slightly lower than that in model rats at 14 days, and this difference was significant at 28 days (P < 0.05; Figures 4D, E).
Table 5.
Area of interleukin-1β-positive cells (×103 μm2) in the substantia nigra of rats
Figure 4.
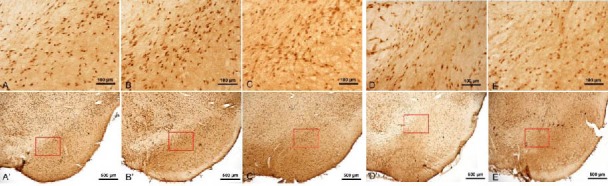
Interleukin-1β staining in the midbrains of rats (immunohistochemical staining, light microscopy; scale bars in A–E: 100 μm; scale bars in A’–E’: 500 μm). (A, A’) Normal group; (B, B’): Model group at 14 days; (C, C’): Model group at 28 days; (D, D’): Gastrodin group at 28 days; (E, E’): Madopar group at 28 days. Dark brown-colored cells were observed in the control (A), and the number of these was increased in the model at 14 days (B) and 28 days (C). The number of positive cells was reduced in the gastrodin (D) and Madopar groups at 28 days (E).
Effect of gastrodin on IL-6 expression in the substantia nigra of PD rats
IL-6 expression in the substantia nigra of model rats tended to be up-regulated at first and then down-regulated; there was no significant difference in the level of IL-6 expression in model rats compared with the normal group. Treatment of rats with gastrodin or Madopar produced a trend towards a decrease in the level of IL-6 expression, but no significant difference was observed compared with the expression level in the normal group (Table 6; supplementary Figure 1 online).
Table 6.
Area of interleukin-6-positive cells (×103 μm2) in the substantia nigra of rats
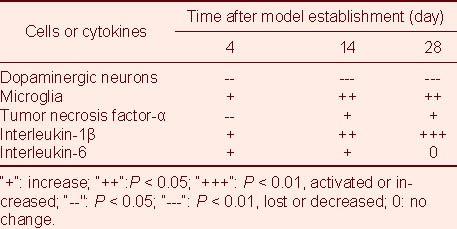
Temporal correlation between the loss of TH cells, microglial activation, and expression of TNF-α, IL-1β and IL-6
As shown in Table 7, the loss of dopaminergic neurons was associated with microglial activation and expression of the inflammatory factor IL-1β in PD model rats.
Table 7.
Relationship between substantia nigra dopaminergic neuronal loss and neuroinflammation in rote-none-treated Parkinson's disease rats
DISCUSSION
In the present study, moving latency was significantly prolonged in model rats with slow movement, and the number of slides increased significantly; combined with behavioral findings, these findings indicated successful establishment of the model. The sluggish symptoms improved greatly in model rats by 28 days, which suggested that it would be difficult to assess the effects of drugs on neurobehaviour based on sluggish symptoms that only occurred at an early stage. By contrast, the number of slides in model rats at 28 days was higher than that at the initial stage of model establishment, and the rats’ muscular strength was clearly weakened, suggesting that the inclined slope plane test could be applied to evaluate the effects of interventions. Additionally, the loss of dopaminergic neurons was aggravated in model rats at 28 days. Both behavioral and immunohistochemical analyses indicated that damage to dopaminergic neurons developed following model establishment, consistent with previous findings. However, the degree of damage and the time when it occurred varied among individuals (data not shown); thus, model animals with the same scores should be chosen for studies on interferences.
Microglia are the brain's resident immune cells. As an important line of defense in the brain, microglia become readily activated in response to injuries to the brain or to immunologic stimuli[19,20]. Activated microglia undergo dramatic morphologic changes, metamorphosing from a ramified morphology into an amoeboid morphology[21]. They exhibit functionally increased expression of surface molecules for antigen recognition and presentation, such as major histocompatibility complex molecules and complement receptor 3, and show up-regulated secretion of a large number of cytokines, such as TNF-α, IL-1β and IL-6, which magnify and perpetuate inflammation and immunological reactions[22]. At the same time, activated microglia also produce large amounts of free radicals like superoxide, eicosanoids and nitric oxide, which mediate neural damage[23].
The present study showed that microglia continue to be activated in model rats from 14 days to 28 days after model establishment, and massive loss of dopaminergic neurons was observed at 14 days. These findings suggest that microglial activation likely parallels dopaminergic neural damage, similar to previously described findings. Activated microglial cells are extensively found in 1-Methy-4-phenyl-1, 2, 3, 6-tetrahydropyridine-[1], 6-hydroxydopamine-[2] and rotenone-induced[3] PD models, and microglial inhibitors could relieve dopaminergic neuronal degeneration. All of these results suggest that loss of dopaminergic neurons is closely associated with microglial activation, even though the temporal relations between the two vary in different studies, probably being related to the type of nerve agents used and observation points.
TNF-α expression was reduced in conjunction with distinct loss of TH cells in model rats at 4 days, suggesting that TNF-α might not be the key factor involved in the initial dopaminergic loss. However, there was a significant difference between minimum (in the model at 4 days) and maximum (at 28 days) TNF-α expression levels, which indicated that TNF-α underwent a bigger change after the model was successfully established. IL-1β expression increased consistently from 14 days to 28 days, in parallel with continuous microglial activation and further loss of TH cells, suggesting that microglial activation and upregulation of IL-1β expression are major components in concert with dopaminergic neural inflammatory injury. IL-6, a secondary inflammatory factor was not a key player in the development of dopaminergic neural damage at this stage. These results have not previously been reported in PD models induced by rotenone. The rotenone model of PD with the features of non-invasion and chronic formation reveal a likely connection between dopaminergic neuronal loss and neuroinflammation during the development of PD.
Gastrodin possess the following activities: anti-inflammation[18], antioxidation[24], antagonistic neurotoxicity of excitatory amino acid[24,25], immunomodulation[26,27] and anti-epilepsy[28]. Research has shown that gastrodin has a therapeutic effect in 6-OHDA-induced PD rats, and that it can improve behavioral abnormalities, decrease the loss of nigral TH-positive cells, and inhibit nigral TNF-α expression[18]. In the present study, it was observed for the first time that gastrodin was able to reduce the loss of TH-positive cells in a rotenone-induced model of PD: it had a protective action on dopaminergic neurons, down-regulated nigral IL-1β expression resulting in a restraining of neuroinflammation during the damage, and improved muscle rigidity and endurance in PD rats. These activities might provide a pharmacological basis for improving the clinical symptoms of PD patients. Similar to gastrodin, Madopar could also ameliorate muscle rigidity and endurance loss, depress IL-1β expression, and inhibit microglial activation. However, it could not reduce the loss of TH cells, and had no protective effect on dopaminergic neurons. Collectively, the results presented here show that gastrodin is superior to Madopar in improving clinical symptoms and protecting dopaminergic neurons in rotenone-induced PD rats.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment pertaining to the neuropathology.
Time and setting
This experiment was performed at the School of Traditional Chinese Medicine, Capital Medical University, China, from April to July in 2009.
Materials
A total of 90 male Wistar rats, aged 3 months, weighing 220–250 g, of clean grade, were purchased from the Vital River Laboratory Animal Technology Co., Ltd., China (Certification number SCXK (Jing) 2002-0003). Experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[29].
Methods
Rotenone-induced PD model establishment and drug treatment
Rotenone oily emulsion (2 mg/kg per day; Sigma, St. Louis, MO, USA) was injected into the backs of the rats for 3–5 weeks, and rats with behavioral scores ≥ 2 (see the Behavioral scoring system section below for details) were regarded as successful PD models[10]. Rats that scored 2 were chosen for the present study, and divided into three groups: a Madopar group (intragastric injection of Madopar, 7.8 mg/kg per day; F. Hoffmann-La Roche Ltd., Basel, Switzerland); a gastrodin group[18,30] (intragastric injection of gastrodin, 0.2 g/kg per day; Youcare Pharmaceutical Group Co., Ltd., Beijing, China); and a model group (intragastric injection of saline).
Behavioral scoring system
Behavioral scores were measured using a scoring system with seven behavioral features scored. (1) Resistance was suppressed, piloerection, hair dirty, roachback, bradykinesia, score 1. (2) Score 1 plus clear bradykinesia, tremor, instability of gait, score 2. (3) Score 2 plus instability of gait, or cannot go straight, or rotation, score 3. (4) The body inclines towards one side, forelimb or posterior limb of unilateral paralysis, walk and taking food difficulty, score 4. (5) Forelimb and/or posterior limb of unilateral paralysis entirely, muscular constriction, weight loss, decreased food intake, score 8. (6) Impending death or died, score 10. (7) No abnormal behavior, score 0[10].
Neurobehavioral test
Neurobehavioral experiments were carried out on days 1 and 28 after model establishment[31].
Grid test: The experimental installation was an independent metallic meshes upright to the horizon (50 cm × 40 cm, distance between meshes: 0.9 cm × 1.7 cm). The rats were placed upright on the center of the meshes, and grasped the meshes with four paws. Moving latency, that is, the time from a resting state to the moment that facultative paw moved, was noted.
Inclined plane test: Rats were placed head upward at the top of an inclined plane (60 cm × 48 cm) situated at 50° to the ground. It was ensured that the rats could not grasp the edge of the plane or any other objects. The number of slides in a 30-second period was recorded three times.
Sampling
Four rats were selected from the model group on day 4 for scoring, and a total of four rats were selected from each group on days 14 and 28, followed by anesthetization, sacrifice and perfusion of paraformaldehyde. Brains were removed and stored in 70% paraformaldehyde containing 30% sucrose overnight. Frozen sections (50 μm) through the substantia nigra[32] were prepared using a freezing microtome and used for immunohistochemistry.
Immunohistochemical detection of TNF-α, TH, OX42, IL-1β and IL-6 expression in the substantia nigra of rats
TNF-α immunohistochemical staining: Frozen sections containing the substantia nigra were blocked with 0.1% trypsin solution for 30 minutes for antigen retrieval. The sections were washed three times in distilled water three times (for 5 minutes each time), treated with 3% hydrogen peroxide for 10 minutes to block endogenous peroxidase, washed in distilled water and 0.01 M PBS, incubated with 5% normal goat serum for 30 minutes to inhibit non-specific binding, and incubated in primary rabbit anti-rat TNF-α monoclonal antibody (1: 50; Wuhan Boster Bio-engineering Co., Ltd, Wuhan, China) for 48 hours at 4°C. They were then washed, incubated in biotinylated-goat anti-rabbit IgG (1: 300; Beijing Zhongshan Goldenbridge Biological Technology, Beijing, China) for 2 hours, washed, incubated in horseradish peroxidase-avidin (1: 300; Beijing Zhongshan Goldenbridge Biological Technology) for 2 hours, and colorized with 3, 3-diaminobenzidine for 10–30 minutes.
TH immunohistochemical staining: The primary antibody was mouse anti-rat TH monoclonal antibody (1: 10 000; Sigma), in which sections were incubated at room temperature for 24 hours. Then, the antibody solution was changed to the biotin-labeled goat anti-mouse immunohistochemical staining kit (Beijing Zhongshan Goldenbridge Biological Technology). Instead of 0.1% trypsin solution, frozen sections were blocked with 1 N hydrochloric acid for 30 minutes for antigen retrieval, and other treatments were the same as for TNF-α immunohistochemical staining.
OX42 immunohistochemical staining: The primary antibody was mouse anti-rat OX42 monoclonal antibody (1: 2 000; BD), in which sections were incubated at room temperature for 24 hours. Then, the antibody solution was changed to the biotin-labeled goat anti-mouse immunohistochemical staining kit (Beijing Zhongshan Goldenbridge Biological Technology). No antigen retrieval was required; other treatments were the same as for TNF-α immunohistochemical staining.
IL-1β and IL-6 immunohistochemical staining: The primary antibodies were rabbit anti-rat IL-1β monoclonal antibody (1: 50; Wuhan Boster Bio-engineering Co., Ltd.) or rabbit anti-rat IL-6 monoclonal antibody (1: 50; Wuhan Boster Bio-engineering Co., Ltd.). All other treatments were the same as for TNF-α immunohistochemical staining.
Image analysis
The area of positive cells (×103 μm2) in midbrain sections at one location (three slices from each rat) was quantified using the NIS-Elements pathological image analysis system (NIS-Elements Ver.2.1; Nikon Shanghai Company, Shanghai, China) with a Nikon optical microscope (ECLIPSE80i; Nikon, Tokyo, Japan).
Statistical analysis
SPSS 10.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. Data are expressed as mean ± SD and evaluated using one-way analysis of variance followed by the Student-Newman-Keuls (SNK-q) test. A value of P < 0.05 was considered statistically significant.
Footnotes
Conflicts of interest: None declared.
Funding: This study was supported by grants from the Scientific Research Common Program of Beijing Municipal Commission of Education (Protective effect of Baicalin in rats of Parkinson's disease), No. KM200610025008.
Ethical approval: The project received full ethical approval from the Animal Ethics Committee of Capital Medical University of China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol. 7, No. 5, 2012 after selecting the “NRR Current Issue” button on the page.
(Edited by Luo XG/Yang Y/Song LP)
REFERENCES
- [1].Sugama S, Yang L, Cho BP, et al. Age-related microglial activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Res. 2003;964(2):288–294. doi: 10.1016/s0006-8993(02)04085-4. [DOI] [PubMed] [Google Scholar]
- [2].He Y, Appel S, Le W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain Res. 2001;909(1-2):187–193. doi: 10.1016/s0006-8993(01)02681-6. [DOI] [PubMed] [Google Scholar]
- [3].Sherer TB, Betarbet R, Kim JH, et al. Selective microglial activation in the rat rotenone model of Parkinson's disease. Neurosci Lett. 2003;341(2):87–90. doi: 10.1016/s0304-3940(03)00172-1. [DOI] [PubMed] [Google Scholar]
- [4].Teismann P, Schulz JB. Cellular pathology of Parkinson's disease: astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318(1):149–161. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- [5].Ling Z, Gayle DA, Ma SY, et al. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov Disord. 2002;17(1):116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- [6].Wang QR, Shen HP. Microglia regulation neuroprotective for PD. Zhongxiyi Jiehe Xinxueguan Bing Zazhi. 2008;6(9):1079–1080. [Google Scholar]
- [7].Venters HD, Broussard SR, Zhou JH, et al. Tumor necrosis factor(alpha) and insulin-like growth factor-I in the brain: is the whole greater than the sum of its parts? J Neuroimmunol. 2001;119(2):151–165. doi: 10.1016/s0165-5728(01)00388-5. [DOI] [PubMed] [Google Scholar]
- [8].Heikkila RE, Nicklas WJ, Vyas I, et al. Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neurosci Lett. 1985;62(3):389–394. doi: 10.1016/0304-3940(85)90580-4. [DOI] [PubMed] [Google Scholar]
- [9].Zhao XL, Gu ZL, Qin ZH. Chronic subcutaneous injection of rotenone produces rodent Parkinsonian models. Zhongguo Yaolixue Tongbao. 2005;21(10):1274–1277. [Google Scholar]
- [10].Chen X, Zhang N, Zhao H, et al. The relations of behavioral features and loss of Nigrostriatum on the model of Parkinson's disease induced by rotenone on rats. Zhongguo Shenjing Jingshen Jibing Zazhi. 2008;34(4):232–234. [Google Scholar]
- [11].Chen X, Zhang N, Zhao H, et al. The protect effect of Baicalin on the substantial nigra dopaminergic neuron in Parkinson's rats induced by rotenone. Zhongfeng yu Shenjing Jibing Zazhi. 2008;25(2):174–177. [Google Scholar]
- [12].Li C, Chen X, Zhang N, et al. Changes of expression of apoptosis-related proteins Bcl-2 and Bax in Parkinson's disease rat induced by rotenone. Zhongguo Shiyan Dongwu Xuebao. 2009;17(1):50–52. [Google Scholar]
- [13].Qiao SZ, Wang J, Bai CE. Treating 42 cases of PD with Dingchan decoction. Shaanxi Zhongyi. 2003;24(2):138. [Google Scholar]
- [14].Zhang YQ, Tan WL, Lu H, et al. Treating 62 cases of PD with Tianmagouteng decoction add-on to levodopa. Shaanxi Zhongyi. 2008;29(6):666–667. [Google Scholar]
- [15].Ma YZ, Li SF, Shen XM, et al. Clinical observation of PD 30 cases treated with traditional Chinese medicine and western medicine. Xin Zhongyi. 2005;37(10):55–56. [Google Scholar]
- [16].Wang HM, Wu DH, Liu XH, et al. Treating 30 cases of PD with nourishing kidney and liver, calming endogenous wind, dredging therapy add-on levodopa. Shaanxi Zhongyi. 2007;28(6):668–669. [Google Scholar]
- [17].Su QZ, Sun YZ, Zeng L, et al. The current state of traditional Chinese medicine in treating Parkinson's disease. Guangming Zhongyi. 2008;23(4):542–543. [Google Scholar]
- [18].Xu GL. Beijing: Beijing University of Chinese Medicine; 2007. Study on Protecting Neurons and Mechanism of PD Treated with Tianma. [Google Scholar]
- [19].McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- [20].Liu B, Gao HM, Hong JS. Parkinson's disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect. 2003;111(8):1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Depino AM, Earl C, Kaczmarczyk E, et al. Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson's disease. Eur J Neurosci. 2003;18(10):2731–2742. doi: 10.1111/j.1460-9568.2003.03014.x. [DOI] [PubMed] [Google Scholar]
- [22].He Y, Appel S, Le W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain Res. 2001;909(1-2):187–193. doi: 10.1016/s0006-8993(01)02681-6. [DOI] [PubMed] [Google Scholar]
- [23].Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304(1):1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- [24].Li YM, Chen FP, Liu GQ. Studies on inhibitive effect of Gastrodin on PC12 cell damage induced by glutamate and H2O2. Zhongguo Yaoke Daxue Xuebao. 2003;34(5):456–460. [Google Scholar]
- [25].Zhang LD, Gong XJ, Hu MM, et al. Anti-vascular dementia effect of Gastrodin and its mechanism of action. Zhongguo Tianran Yaowu. 2008;6(2):130–134. [Google Scholar]
- [26].Meng QY. Effects of Tianma injection on transformation of the splenic lymphocyte in mice. Guangxi Zhongyiyao. 2003;26(2):52–54. [Google Scholar]
- [27].Li J, Wu S. Therapeutical effect of Salvia miltiorrhiza and Gastrodin to rats after cerebral hemorrhage. Guiyang Yixueyuan Xuebao. 2007;32(2):118–122. [Google Scholar]
- [28].Cao YQ, Su YF, Chen H, et al. Effects of gastrodinon Cx43 expression in temporal lobe cortex and hippocampus of pentylenetetrazole-induced epileptic immature rats. Lanzhou Daxue Xuebao: Yixue Ban. 2008;34(3):54–46. [Google Scholar]
- [29].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [30].Hao JD, Wang MK, Xia HJ, et al. Effect of gastrodin on the contents of DA, DOPAC/HVA in Parkinson's disease rats’ striatum. Beijing Zhongyiyao. 2009;28(6):463–465. [Google Scholar]
- [31].He DF, Zhang B, Wang J, et al. Neurotoxic effects of chronic rotenone and pyridaben exposure on rat. Zhongguo Laonianxue Zazhi. 2005;25:1099–1101. [Google Scholar]
- [32].Paxinos G, Watson C. Beijing: People's Medical Publishing House; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


