Abstract
In this study, a rat vascular dementia model was established by permanent bilateral common carotid arterial occlusion. Rats were intraperitoneally injected with puerarin 3 days before modeling, for 45 successive days. Results demonstrated that in treated animals hippocampal structures were clear, nerve cells arranged neatly, and cytoplasm was rich in Nissl bodies. The number of cells positive for hypoxia inducible factor-1 alpha, erythropoietin and endothelial nitric oxide synthase was reduced; and the learning and memory abilities of rats were significantly improved. Our experimental findings indicate that puerarin can significantly improve learning and memory in a vascular dementia model, and that the underlying mechanism may be associated with the regulation of the expression of hypoxia inducible factor-1 alpha.
Keywords: puerarin, vascular dementia, hypoxia-inducible factor-1 alpha, erythropoietin, endothelial nitric-oxide synthase
INTRODUCTION
Donepezil, Huperzine and other cholinesterase inhibitors are the drugs presently approved and widely used for treatment of vascular dementia. However, these drugs do not always produce satisfactory results and have side effects[1,2]. Puerarin, a major active ingredient of the traditional Chinese medicine pueraria radix, is classified as one of the isoflavones. It can dilate vessels and improve microcirculation[3]. It is widely prescribed for patients with cardiovascular and cerebrovascular diseases[4,5,6,7,8]. Clinical application of puerarin for vascular dementia patients has obtained good curative effects[9], obviously improving the patient's prognosis.
Erythropoietin (EPO) and endothelial nitric oxide synthase (eNOS) are critical downstream factors of the hypoxia-inducible factor-1 alpha (HIF-1α) pathway[10,11,12] involved in the regulation of ischemic tolerance. Brain-derived EPO antagonizes toxicity from excitatory amino acids and free radicals, has anti-inflammatory and anti-apoptotic effects, enhances synaptic transmission, and has neurotrophic factor-like effects[13,14,15]. When brain tissue undergoes ischemic injury, eNOS in nerve cells and endothelial cells generates protective nitric oxide, which induces angiogenesis, enhances the function of ischemic tissue revascularization and promotes recovery of brain function[16,17]. Through observing the effects of puerarin on HIF-1α and the related factors EPO and eNOS in the hippocampus of vascular dementia rats, this study sought to explore the mechanism underlying puerarin in the treatment of vascular dementia.
RESULTS
Quantitative analysis of experimental animals
Thirty-five rats were used in the experiment. The rats were randomly divided into three groups: sham-operated (n = 10), dementia (n = 13) and puerarin (n = 12). Rats in the puerarin and dementia groups were treated with a permanent bilateral common carotid arterial occlusion. Rats in the puerarin group were injected intraperitoneally with puerarin for 45 days, beginning 3 days before surgery. In the dementia group, two rats died during modeling and one died during the intervention. In the puerarin group, two rats died during the modeling. Thirty rats were involved in the final analysis of results, with 10 rats in each group.
Effects of puerarin on behaviors of vascular dementia rats
After ligation, rats in the dementia group showed blepharoptosis, excitation, and ataxia. They quickly recovered without dyskinesia, but spontaneous movement was reduced and bradykinesia remained. The symptoms of rats in the puerarin group were much improved compared with those in the dementia group. There was no significant change in the sham-operated group before and after ligation.
Puerarin enhanced the learning and memory abilities of vascular dementia rats
Compared with the sham-operated group, the learning-memory scores of both the dementia and puerarin groups were significantly decreased 45 days after ligation of the common carotid arteries (P < 0.01). However, the puerarin group showed improved learning-memory scores compared with the dementia group (P < 0.05; Table 1).
Table 1.
Effect of puerarin on the learning and memory performances in different groups of rats
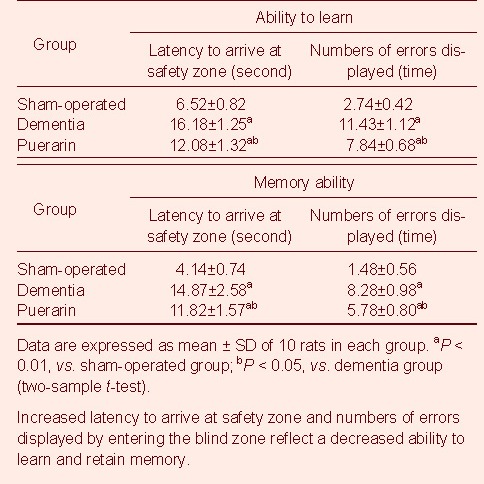
Puerarin ameliorated the hippocampal morphological changes in vascular dementia rats
In the sham-operated group, light microscopy showed that hippocampal structures were clear, nerve cells were arranged regularly, and cytoplasm was rich in Nissl bodies. In contrast, in the dementia group, hippocampal structures were chaotic, nerve cells were enlarged and sparsely distributed, and cytoplasmic Nissl bodies were decreased or absent. In the puerarin group hippocampal structures were clearer than in the dementia group, nerve cells were regularly arranged and cytoplasm was rich in Nissl bodies (Figure 1).
Figure 1.
Hippocampal morphology in rats from different groups (Nissl staining, × 400).
(A) Sham-operated group: Hippocampal structures were clear, nerve cells arranged neatly, cytoplasm was rich in Nissl bodies.
(B) Dementia group: Hippocampal structures were chaotic, nerve cells were enlarged and sparsely arranged, and Nissl bodies decreased or disappeared.
(C) Puerarin group: Hippocampal structures were clearer than in the dementia group. Neurons were neatly arranged and rich in cytoplasmic Nissl bodies.
Puerarin inhibited the expression of HIF-1α, EPO and eNOS in the hippocampus of vascular dementia rats
Immunohistochemical staining showed that hippocampal tissues of all three groups expressed HIF-1α, EPO and eNOS. The immunoreactive products were stained brown. The positive cells were mainly distributed in the pyramidal cell layer in the CA1 region of the hippocampus, consistent with the characteristics of pyramidal cells (Figure 2).
Figure 2.
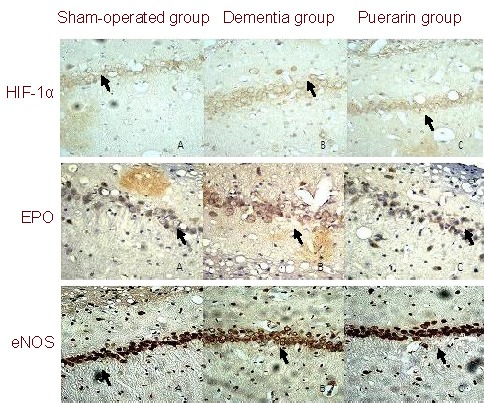
Expression of hypoxia-inducible factor-1 alpha (HIF-1α), erythropoietin (EPO) and endothelial nitric-oxide synthase (eNOS) in the hippocampus of rats from different groups (immunohistochemical staining, × 400).
In the sham-operated group few HIF-1α, EPO and eNOS positive cells were present in the CA1 region of the hippocampus.
HIF-1α, EPO and eNOS positive cells were markedly increased in both the dementia and puerarin groups compared with the sham-operated group.
A decrease in immunopositive cells was observed in the puerarin treated group compared with the control group. Arrows show HIF-1α, EPO or eNOS positive cells.
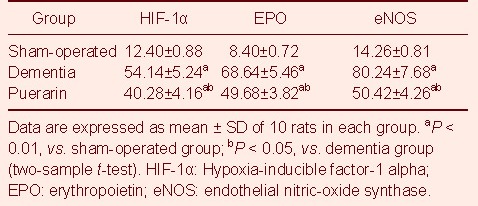
HIF-1α, EPO and eNOS positive cells in the CA1 region of the hippocampus were markedly increased in both the dementia and puerarin groups compared with the sham-operated group. Significant decreases in HIF-1α, EPO and eNOS positive cells were observed in the puerarin treated group compared with the control group (Table 2).
Table 2.
HIF-1α, EPO and eNOS positive cells (numbers of positive cells/mm2) in rats from different groups (immu-nohistochemical staining)
DISCUSSION
In this study, a cerebral ischemia model was produced by occlusion of bilateral common carotid arteries. After ligation, rats in the dementia group showed blepharoptosis, excitation, and ataxia. They quickly recovered without dyskinesia, but spontaneous movement was reduced and bradykinesia remained. The learning-memory scores of the dementia group rats were markedly decreased in the Y-shaped water maze test. In the dementia group, hippocampal structures were chaotic; nerve cells were enlarged and arranged sparsely; and Nissl bodies in the cytoplasm were decreased or absent. Thus, this animal model, which is simple to prepare and shows features in line with clinical symptoms of vascular dementia, is an ideal model for studying vascular dementia.
Puerarin is widely prescribed for patients with ischemic cardiovascular and cerebrovascular diseases. The therapeutic dose is 200–400 mg per day according to the description. There is no definite conclusion in the literature about the drug dose of puerarin for use in animal experiments. According to previously described findings[18,19] and our previous studies and preliminary experiments, we found that an intraperitoneal injection of 100 mg/kg was the optimal dose for treatment of vascular dementia rats. In the present study, the puerarin group remained healthy and demonstrated a better learning and memory performance than the dementia group. Histological examination showed a significant increase in the number of Nissl bodies, clear hippocampal structures, and significant changes in expression of HIF-1α, EPO and eNOS in the hippocampal CA1 region. Such structural and functional changes suggested that an intraperitoneal injection of 100 mg/kg puerarin is appropriate for vascular dementia in rats.
Previous studies investigating the effects of puerarin on HIF-1α, EPO and eNOS of hypoxic-ischemic animals showed varied results. In the cerebral ischemia-reperfusion model, Luo et al[18] found that the expression of EPO in cortex and basal ganglia in the cerebral ischemic-reperfusion rats was significantly increased after puerarin treatment. In contrast, results from Gu et al[19] showed that expression of eNOS in the brain tissue of rats with cerebral ischemia was decreased after puerarin treatment, but was still higher compared with the sham-operated group. In the rat model of myocardial infarction, Zhang et al[20] discovered that the expression of HIF-1 mRNA and eNOS mRNA in myocardial tissue in the puerarin group was significantly decreased. As reported by Wen et al[21], puerarin can elevate the level of eNOS protein and mRNA in myocardial cells. In this study, puerarin improved learning and memory, improved the hippocampal formation, and increased the intraneuronal Nissl bodies in vascular dementia rats, showing that it has protective effects against vascular dementia. Furthermore, in the hippocampal CA1 region, we observed the expression of HIF-1α, EPO and eNOS. Significantly more positive cells were present in the dementia group compared with the sham-operated group. The results indicated that HIF-1α, EPO and eNOS were closely related to vascular dementia. Following induction of vascular dementia, hippocampal neurons showed hypoxic-ischemic injury. HIF-1α was increased and played a role in self-protection by up-regulating the expression of EPO and eNOS which have neuroprotective effects. In this study, there were fewer positive cells in the puerarin group than in the dementia group, suggesting that puerarin down-regulated the expression of HIF-1α, EPO and eNOS in ischemic hippocampal CA1. This result is different from previous studies. We speculate that the possible causes are as follows: (1) Through increasing blood flow and improving cerebral microcirculation, puerarin improves hippocampal perfusion and increases oxygen concentration in nerve cells[7], thus negatively regulating the expression of HIF-1α and downstream protective factors EPO and eNOS. (2) Puerarin possesses an estrogen substitution effect[3,22]. It is likely to increase tolerance of cells for hypoxia by acting at estrogen receptors, therefore down-regulating the expression of HIF-1α and the downstream protective factors EPO and eNOS. Indeed, in this study puerarin down-regulated the expression of the protective HIF-1α pathway and downstream factors EPO and eNOS. Additionally, the learning and memory abilities of puerarin treated rats were significantly improved compared with the dementia group, but were still poorer than the sham-operated group. At the same time, there were fewer positive cells in the puerarin group than in the dementia group, but more than in the sham-operated group. This shows that puerarin can improve clinical symptoms of vascular dementia but cannot completely reverse the process of vascular dementia. Therefore, the most important thing is to prevent the occurrence of vascular dementia in clinic.
Our study confirms that puerarin can improve clinical symptoms of vascular dementia and demonstrates that this action is associated with down-regulation of HIF-1α and its downstream factors, EPO and eNOS.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
The study was performed at the Medical College of Xi’an Jiaotong University, China from December 2009 to September 2010.
Materials
Animals
A total of 35 male, specific pathogen free Sprague-Dawley rats, weighing 280 ± 20 g, aged 2-3 months, were provided by the Experimental Animal Center of Medical School of Xi’an Jiaotong University, China (certificate No. SCXK (Shaan) 2007-001). Rats were housed on a 12-hour light/dark cycle, at 23.0 ± 0.5°C and a relative humidity of 50.0 ± 0.5%, with free access to food and water. Animal procedures were in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[23].
Drugs
Puerarin injection (4, 7-dihydroxy-8-β-D-glucosyl-isoflavone; Ankang Pharmaceutical Factory, Shaanxi, China; lot No. H20053144, 100 mg: 2 mL/bottle). The structure of puerarin is shown in Figure 3.
Figure 3.
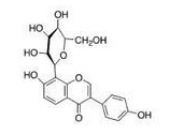
Structure of puerarin.
Methods
Establishment of vascular dementia rat models
According to the methods described by de la Torre et al[24], rats in the puerarin group and dementia group were treated with a permanent bilateral common carotid arterial occlusion under anesthesia with peritoneal injection of 10% hydral chlorate (300 mg/kg). A longitudinal incision was made in the ventral neck to expose the common carotid arteries which were carefully dissected free from the vagus nerve and adjacent tissues. Then the common carotid artery was ligated with a silk suture. Animals assigned to the sham-operated group underwent the same surgical procedure without vessel occlusion.
Puerarin intervention
From 3 days before surgery, animals in the puerarin group were injected intraperitoneally with puerarin at a dose of 100 mg/kg per day for 45 days. Animals were injected 1 hour before ligation in the day of surgery. At the same time, animals in the sham-operated group and dementia group were injected intraperitoneally with 0.9% sodium chloride solution at a dose of 100 mg/kg.
Y-type water maze test for detection of rat behavior
The learning-memory abilities of rats were assessed in the Y-type water maze test[25]. The water maze consisted of a long arm (50 cm × 30 cm × 45 cm) and two short arms (40 cm × 30 cm × 45 cm). The long arm was the initiation region. One short arm was the blind zone (on the top of which there was an opaque plastic board, thereby decreasing the visibility of the canal); the other short arm was the safety zone with a platform of 30 cm × 10 cm and lamplight. Rats were trained over ten-trials. Their latency to arrive at safety zone from initiation region and the numbers of errors displayed by entering the blind zone were recorded as learning performances. After a 24-hour interval, the rats were tested again to obtain their memory performances. Before the experiment, the rats whose latency to arrive at safety zone were longer than 25 seconds were eliminated. After 45 days of intervention, the learning and memory abilities of the rats were once again evaluated with the Y-type water maze test.
Collection of hippocampal samples
After 45 days of intervention, the rats were anesthetized, the chest was opened, the animals were perfused through the heart and the whole brain was removed. Brain tissues including hippocampus were then taken from coronal sections located from 3 mm after the bregma to the superior colliculus[26], and were paraformaldehyde fixed and paraffin-embedded.
Nissl staining for cell morphology in the rat hippocampus
The hippocampal tissues were cut into 3-μm thick paraffin sections. They were deparaffinized with two changes of xylene for 15 minutes each, followed by 95% alcohol and 80% alcohol for 5 minutes each, then washed for 5 minutes with running water. The sections were next incubated in 1% toluidin blue (Tianyuan Biotechnology Co., Ltd., Shanghai, China) at 55°C for 30 minutes, and rinsed for 5 minutes. The sections were then rinsed with 80% alcohol, 90% alcohol, and 95% alcohol for 5 minutes each, then with three changes of xylene for 15 minutes each[27]. Last, the sections were mounted with neutral gum. The sections were observed under an optical microscope (B-type biomicroscope; Global Motic Group, Xiamen, China).
Immunohistochemical staining for HIF-1α, EPO and eNOs expression
The streptavidin-peroxidase immunohistochemical staining was executed in hippocampal tissues sections according to the kit instructions (Boster Biological Engineering Company, Wuhan, China). In brief, hippocampal tissue sections underwent dehydration through the ethanol gradient. The sections were treated with 3% H2O2 for 40 minutes at room temperature, microwave treated for 10 minutes for antigen retrieval, and cooled for 2 hours at room temperature. After incubation for 40 minutes in normal rabbit serum, they were incubated with primary antibodies, including rabbit anti-HIF-1α polyclonal antibody (1: 200; Boster Biological Engineering Company, Wuhan, China)[28], sheep anti-EPO multiclonal antibody (1: 500; Santa Cruz Biotechnology, Delaware Santa Cruz, CA, USA)[29] and rabbit anti-eNOS polyclonal antibody (1: 100; Boster Biological Engineering Company)[19]. Subsequently, they were kept at 4°C overnight, treated with the biotinylated secondary antibodies, sheep anti rabbit IgG (HIF-1α, eNOS, 1: 200) or horse anti sheep IgG (EPO, 1: 500) for 40 minutes at room temperature, and incubated for 30 minutes in streptavidin with horseradish peroxidase (1: 200) at room temperature. This was followed by DAB for 5 minutes, counterstaining with hematoxylin, dehydration, and coverslipping. Negative control sections were treated with TBS instead of primary antibody. Six sections from each group were observed under an optical microscope. The positive cells were nerve cells in hippocampus showing buffy granules. The data were expressed as the mean of number of positive cells/mm2 in five random high-power fields (400 × magnification) from the CA1 region of the hippocampus.
Statistical analysis
Data were analyzed using SPSS 12.0 software (SPSS, Chicago, IL, USA) and a level of P < 0.05 was assessed as a statistically significant difference. All values in the figures of present study indicate mean ± SD. Group comparisons were analyzed with analysis of two-sample t-test.
Acknowledgments:
We would like to thank Ming Li, from Department of Anatomy, Medical College of Xi’an Jiaotong University, China for technical support.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study had the approval of the Animals Ethics Committee of Medical College of Xi’an Jiaotong University in China.
(Edited by Wang XD, Shi ZH/Yang Y/Wang L)
REFERENCES
- [1].Román GC, Salloway S, Black SE, et al. Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke. 2010;41(6):1213–1221. doi: 10.1161/STROKEAHA.109.570077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wilkinson D, Róman G, Salloway S, et al. The long-term efficacy and tolerability of donepezil in patients with vascular dementia. Int J Geriatr Psychiatry. 2010;25(3):305–313. doi: 10.1002/gps.2340. [DOI] [PubMed] [Google Scholar]
- [3].Yin LH, Li YF, Meng FL. Puerarin's chemical composition pharmacological effects and clinical application. Heilongjiang Yiyao. 2010;23(3):371–373. [Google Scholar]
- [4].Sun GH. A review on the clinical application of Puerarin. Zhongyi Linchuang Yanjiu. 2010;2(6):100–101. [Google Scholar]
- [5].Ji AL, Zhang XJ. Effect of puerarin injection on inflammatory factors in patients with acute cerebral infarction. Nanjing Zhongyiyao Daxue Xuebao: Ziran Kexue Ban. 2009;25(2):145–147. [Google Scholar]
- [6].Yao PF, Fan HX, Qian YL. Study of puerarin injection on acute ischemic brain infarction in the aged. Xiandai Zhongxiyi Jiehe Zazhi. 2009;18(14):1587–1590. [Google Scholar]
- [7].Yu WH, Chu GL. Researching development of the protective effects of puerarin in hypoxic ischemic brain damage. Yixue Zongshu. 2009;15(20):3163–3165. [Google Scholar]
- [8].Zhang R, Cheng HX, Wu HQ, et al. Effect of Puerarin on nNOS expression in hippocampal neurons after focal cerebral ischemia in rats. Xi’an Jiaotong Daxue Xuebao: Yixue Ban. 2010;31(6):669–672. [Google Scholar]
- [9].Zhang T, Zhang XQ, Zhu RH. The report of puerarin and citicoline in 36 cases with vascular dementia. Shandong Zhongyiyao Daxue Xuebao. 2000;24(1):37–38. [Google Scholar]
- [10].Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20(1):51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Corzo CA, Condamine T, Lu L, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7(3):391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- [13].Noguchi CT, Asavaritikrai P, Teng R, et al. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64(2):159–171. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang F, Xing J, Liou AK, et al. Enhanced delivery of erythropoietin across the blood-brain barrier for neuroprotection against ischemic neuronal Injury. Transl Stroke Res. 2010;1(2):113–121. doi: 10.1007/s12975-010-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Genc K, Egrilmez MY, Genc S. Erythropoietin induces nuclear translocation of Nrf2 and heme oxygenase-1 expression in SH-SY5Y cells. Cell Biochem Funct. 2010;28(3):197–201. doi: 10.1002/cbf.1639. [DOI] [PubMed] [Google Scholar]
- [16].Ito Y, Ohkubo T, Asano Y, et al. Nitric oxide production during cerebral ischemia and reperfusion in eNOS- and nNOS-knockout mice. Curr Neurovasc Res. 2010;7(1):23–31. doi: 10.2174/156720210790820190. [DOI] [PubMed] [Google Scholar]
- [17].Aoki T, Nishimura M, Kataoka H, et al. Complementary inhibition of cerebral aneurysm formation by eNOS and nNOS. Lab Invest. 2011;91(4):619–626. doi: 10.1038/labinvest.2010.204. [DOI] [PubMed] [Google Scholar]
- [18].Luo YM, Gao L, Ji XM, et al. Effects of puerarin on EPO expressions in ischemic brain tissues of rats following MCAO. Zhongguo Laonian Xue Zazhi. 2008;28(12):1057–1059. [Google Scholar]
- [19].Gu CZ, Lu HL, Wu QH, et al. The effects of puerarin on the epression of eNOS in brain tissues after cerebral ischemia reperfusion in rats. Zuzhong yu Shenjing Jibing. 2010;17(6):336–338. [Google Scholar]
- [20].Zhang SY, Shen YJ, Chen SL, et al. The effects of puerarin on HIF-1, eNOS, VEGF genes expressions in myocardium and NO in serum of rats with myocardial infarction. Zhongyi Yaoli yu Linchuang. 2007;23(5):54–57. [Google Scholar]
- [21].Wen J, Chen SL, Filly C, et al. Effect of puerarin on nitric oxide production in H9C2 cells. Zhongguo Xinxueguan Bing Zazhi. 2006;4(10):777–779. [Google Scholar]
- [22].Huang T, Jin BQ, Sun GJ, et al. Effects of puerarin on the bone metabolism in ovariectomized rats. Zhongguo Laonian Xue Zazhi. 2009;29(19):2482–2484. [Google Scholar]
- [23].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [24].de la Torre JC, Fortin T, Park GA, et al. Chronic cerebrovascular insufficiency induces dementia-like deficits in aged rats. Brain Res. 1992;582(2):186–195. doi: 10.1016/0006-8993(92)90132-s. [DOI] [PubMed] [Google Scholar]
- [25].Wang CF, Li DQ, Xue HY, et al. Oral supplementation of catalpol ameliorates diabetic encephalopathy in rats. Brain Res. 2010;1307:158–165. doi: 10.1016/j.brainres.2009.10.034. [DOI] [PubMed] [Google Scholar]
- [26].Paxinos G, Watson C. 5th ed. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- [27].Yu SS, Zhao J, Zheng WP, et al. Neuroprotective effect of 4-hydroxybenzyl alcohol against transient focal cerebral ischemia via anti-apoptosis in rats. Brain Res. 2010;1308:167–175. doi: 10.1016/j.brainres.2009.10.037. [DOI] [PubMed] [Google Scholar]
- [28].Peng Z, Ren P, Kang Z, et al. Up-regulated HIF-1alpha is involved in the hypoxic tolerance induced by hyperbaric oxygen preconditioning. Brain Res. 2008;1212:71–78. doi: 10.1016/j.brainres.2008.03.027. [DOI] [PubMed] [Google Scholar]
- [29].Wu H, Wang H, Sha J, et al. Expression of hypoxia inducible factor-1alpha and erythropoietin in the hippocampus of aging rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34(9):856–860. [PubMed] [Google Scholar]


