Abstract
Patients with mild cognitive impairment after a transient ischemic attack were included in this study. They were treated with Yizhi Xingnao prescription, ergoloid mesylates or aspirin for 60 days. Evaluation using the Montreal Cognitive Assessment Scale showed that cognitive function was significantly improved in all patients, especially after the combined treatment of Yizhi Xingnao and aspirin. The scores from the Montreal Cognitive Assessment Scale were improved overall and the effective treatment rate was as high as 79%, which was higher than patients treated with a combination of ergoloid mesylates and aspirin, or aspirin alone. Our experimental findings indicate that Yizhi Xingnao prescription can improve mild cognitive impairment after a transient ischemic attack, and that it is more effective than ergoloid mesylates.
Keywords: Yizhi Xingnao prescription, transient ischemic attack, mild cognitive impairment, Montreal Cognitive Assessment Scale, ergoloid mesylates, aspirin
INTRODUCTION
Transient ischemic attack (TIA)-caused cerebral ischemic impairment may lead to pathological damage in cognitive-related regions such as the hippocampal CA1 region, thalamus and temporal cortex, thus triggering cognitive dysfunction[1,2,3]. A number of existing studies has shown that an early TIA is characteristics of mild cognitive impairment (MCI)[4,5], which is scarcely detected clinically at the early stage and gradually aggravates until dementia occurs as a result of a decline in intelligence. A TIA is an important risk factor for vascular dementia and Alzheimer's dementia, and it can exacerbate the degeneration of the brain and the decline of cognitive function[6]. Therefore, intervention in cognitive impairment is important after a TIA. Numerous studies suggest that spatial cognitive impairment at an early stage of cerebral ischemia may be associated with a defect in the hippocampal cholinergic pathway and neuropeptide dysfunction[7,8,9]. Thus, improving blood flow to the ischemic area, reducing the frequency of TIA recurrence, and restoring neuronal signal transduction pathways may be effective ways of improving cognitive impairment following a TIA.
Clinical studies have found Yizhi Xingnao prescription has an apparent effect on the treatment of vascular dementia, but the Mini-Mental State Examination and other scales displayed a limited effect of this prescription for the improvement of cognitive function[10]. Meta analysis regarding the treatment for vascular dementia confirmed that the traditional Chinese medicine cannot improve the Mini-Mental State Examination score in vascular dementia patients[11].
In this study, patients associated with MCI after a TIA were treated with Yizhi Xingnao prescription and their cognitive function was determined using the Montreal Cognitive Assessment (MoCA) scale. While ergoloid mesylates, a western drug used for the clinical treatment of vascular cognitive impairment, and aspirin, both of which have been shown to inhibit platelet aggregation and prevent a TIA[12], served as controls.
RESULTS
Quantitative analysis of subjects
In total, 88 patients with MCI after a TIA were recruited from the Third Affiliated Hospital of Nantong University, China from 2007 to 2010. Patients were divided into three groups according to the random digital number table (orders of admission numbers). After 60 days of the corresponding treatments, the results from 81 patients were included in the study (seven patients were excluded due to cerebral infarction or absence of scale assessment). The screening and grouping management is shown in Figure 1.
Figure 1.
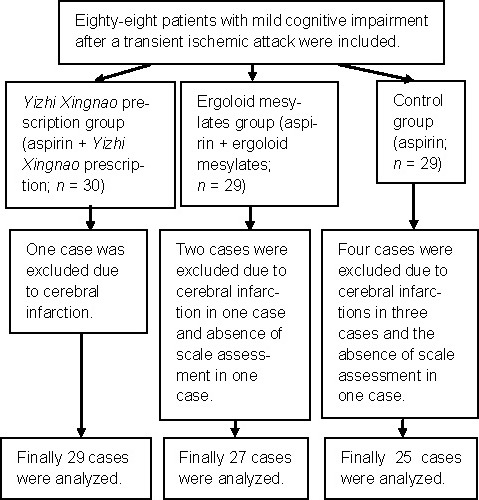
Screening flowchart for involved patients.
Baseline information of subjects
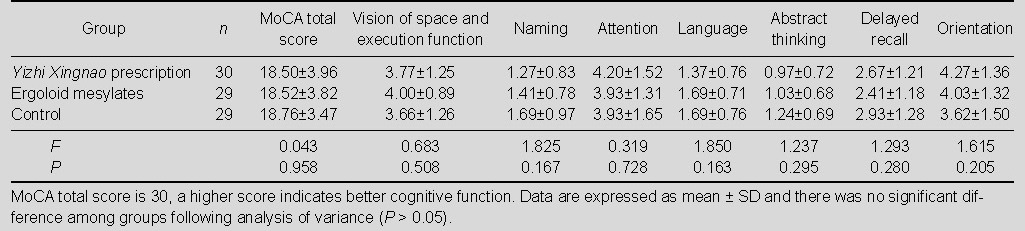
Baseline information of the 88 patients is shown in Table 1. There was no significant difference among the three groups in terms of age, gender, educational level, TIA frequency and cognitive function (P > 0.05). The total score and sub-scale score of MoCA showed no statistically significant difference among the three groups before treatment (P > 0.05; Table 2).
Table 1.
Comparison of baseline information in the three groups
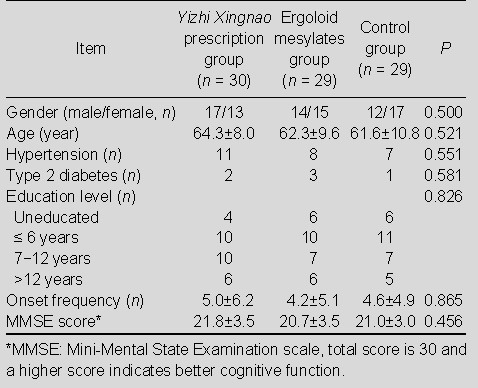
Table 2.
Total score and sub-scale scores of Montreal Cognitive Assessment (MoCA) in the three groups before treatment
Yizhi Xingnao prescription increased MoCA scores in MCI patients after TIA
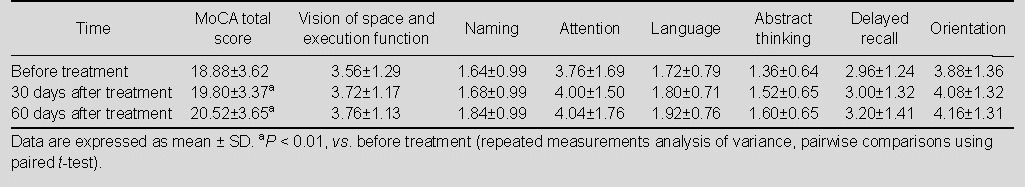
The cognitive function of patients was assessed at 30 and 60 days after drug treatments. Results showed that the MoCA score was significantly improved in the three groups of patients compared with before treatment (P < 0.01). For each sub-scale of MoCA, scores of vision of space, execution function, naming, attention, language, abstract thinking, delayed recall and orientation were significantly increased after Yizhi Xingnao prescription treatment, compared with before treatment (P < 0.01; Table 3); the scores of naming, attention, language, abstract thinking and delayed recall were significantly increased after ergoloid mesylates treatment (P <0.01; Table 4). There was no statistically significant difference of sub-scale scores in the control group before and after treatment (P > 0.05; Table 5).
Table 3.
Total score and sub-scale scores of Montreal Cognitive Assessment (MoCA) in Yizhi Xingnao prescription group (n = 29) before and after treatment
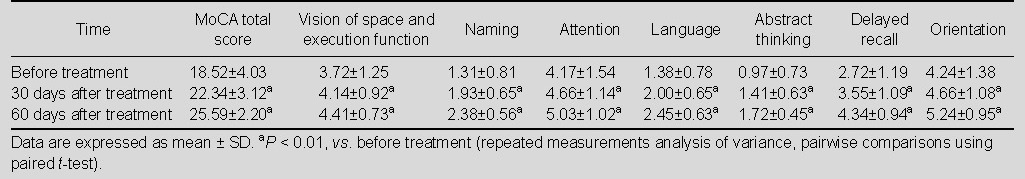
Table 4.
Total score and sub-scale scores of Montreal Cognitive Assessment (MoCA) in ergoloid mesylates group (n = 27) before and after treatment
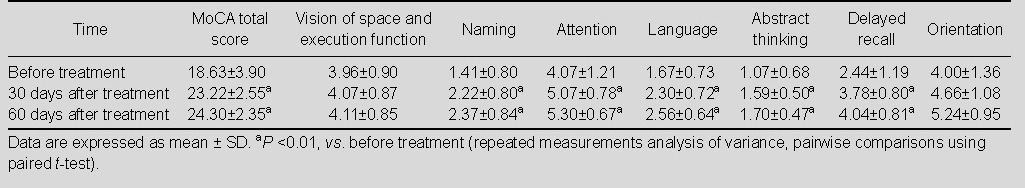
Table 5.
Total score and sub-scale scores of Montreal Cognitive Assessment (MoCA) in control group (n = 25) before and after treatment
After treatment for 30 days, the MoCA total scores after Yizhi Xingnao prescription or ergoloid mesylates treatment were significantly increased compared with the control group (P < 0.01), but there was no significant difference between the two groups (P > 0.05). After 60 days of treatment, the MoCA total score was higher in the Yizhi Xingnao prescription group than that in the ergoloid mesylates group and control group (P < 0.05 or P < 0.01; Figure 2).
Figure 2.
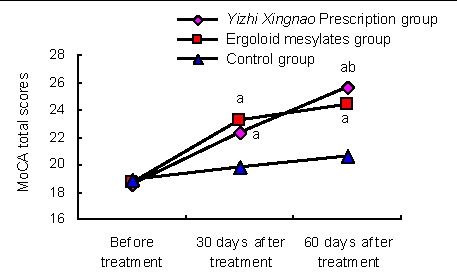
Comparison of Montreal Cognitive Assessment (MoCA) total scores in the three groups.
Data are expressed as mean ± SD. There were 29, 27, 25 patients in Yizhi Xingnao prescription group, ergoloid mesylates group and control group, respectively.
aP < 0.01, vs. control group; bP < 0.05, vs. ergoloid mesylates group (one-way analysis of variance, two groups were compared using the independent two-sample t-test).
Yizhi Xingnao prescription treatment of patients with MCI
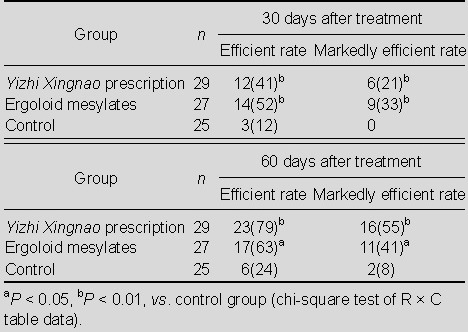
Analysis of the efficacy index showed that the effective rate and markedly effective rate were significantly increased after Yizhi Xingnao prescription or ergoloid mesylates treatment compared with the control group (P < 0.01 or P < 0.05). These two indices were lower in the Yizhi Xingnao prescription group at 30 days after treatment, but higher at 60 days, compared with the ergoloid mesylates group. There was no statistically significant difference between the two groups at 30 and 60 days (P > 0.05; Table 6).
Table 6.
Comparison of the efficient rate and markedly efficient rate [n (%)] in the three groups
Adverse reactions
Three patients had loss of appetite and five patients appeared to have mild nausea after treatment with Yizhi Xingnao prescription. Four cases in each of the ergoloid mesylates group and control group showed mild nausea, which was then remitted by itself or by symptomatic treatment.
DISCUSSION
TIA patients are associated with MCI regarding memory, language, attention, spatial perception, calculation, abstract thinking and reasoning, which are the preliminary symptoms of dementia[13,14,15,16]. A timely and effective clinical intervention is important for preventing or delaying the occurrence of dementia. The MoCA scale has been widely applied to screen MCI in clinical practice[17,18], is recognized as a reliable and valid means to assess many aspects of cognitive domains[19,20,21,22], and is sensitive and specific for the determination of cognitive impairment after a TIA[23,24]. Therefore, the present study utilized the MoCA scale as an assessment tool for drug efficacy. Bakker et al[9] conducted a one-year follow-up on patients with cognitive impairment after a TIA suggesting that reducing the frequency of a recurrent TIA would help improve cognitive impairment in patients. Accordingly, the reduced frequency of a TIA is considered as a basic principle of treatment in this study. In accordance with the Guidelines for the Management of Transient Ischemic Attack, formulated by the U.S. National Stroke Association[12], aspirin served as a basic treatment. In addition, Yizhi Xingnao prescription and ergoloid mesylates[25,26,27] were also administered, to compare and search for an optimal therapeutic scheme.
Many Chinese herbs can be used as therapeutic ingredients, including Radix Polygoni Multiflori, Salvia miltiorrhiza, Fructus Ligustri lucidi, fructus amomi amari, Rhizoma Acori Talarinowii, Radix Polygalae, Fructus Schisandrae, honey-fried Radix Astragali, Fructus Lycii, Semen Astragali complanati, Semen Cuscutae, Radix Angelicae Sinensis, Rhizoma Chuanxiong, Allolobophora caliginosa trapezoides, and Arisaema Cum Bile. Radix Polygoni Multiflori can enhance hematogenesis, raise immunity and improve cognitive performance[28]. Salvia miltiorrhiza promotes tissue repair and regeneration[29]. Fructus Ligustri lucidi has a protective effect against chromosomal damage[30]. Fructus amomi amari, Rhizoma Acori Talarinowii, Radix Polygalae, and Fructus Schisandrae can activate the nervous system, restore intelligence, protect against cerebral injury and show anti-dementia effects[31,32,33]. Meanwhile, honey-fried Radix Astragali, Fructus Lycii, Semen Cuscutae, and Radix Angelicae Sinensis have anti-anoxic and anti-fatigue effects, and also improve one's ability to cope with stress[34,35,36]. Salvia miltiorrhiza, Semen Astragali complanati and Allolobophora caliginosa trapezoides have been shown to ameliorate the rate of hemorheology[37,38]. Rhizoma Chuanxiong, Rhizoma Acori Talarinowii, Radix Polygalae, and Arisaema Cum Bile have sedative and anti-convulsant effects[39,40]. Radix Polygoni Multiflori, Radix Polygalae, Fructus Schisandrae, honey-fried Radix Astragali, and Fructus Lycii contribute to promote metabolism and delay aging[41,42]. Moreover, combinations of the various above-mentioned herbs can improve cognitive impairment in TIA patients.
Results from this study found that total scores from MoCA were significantly improved in all patients after treatment, indicating that any treatment using Yizhi Xingnao prescription, ergoloid mesylates and aspirin can improve cognitive function in patients after a TIA. As for cognitive domains (vision of space and execution function, naming, attention, language, abstract thinking, delayed recall and orientation), the impairments were significantly improved after treatment with Yizhi Xingnao prescription, partially improved after treatment with ergoloid mesylates and no improvement was observed in the control group. This result is evidence that Yizhi Xingnao prescription has a broader range of improvement on cognitive functions than ergoloid mesylates. After 30 days of treatment, the MoCA total scores in the Yizhi Xingnao prescription and ergoloid mesylates groups were significantly higher than that in the control group, but there was no significant difference observed between the Yizhi Xingnao prescription group and the ergoloid mesylates group. At 60 days, the Yizhi Xingnao prescription group showed a significantly higher total score than the ergoloid mesylates group, indicating that Yizhi Xingnao prescription is not effective in early stages of treatment and gradually becomes superior to ergoloid mesylates. Analysis of the efficacy index showed that Yizhi Xingnao prescription and ergoloid mesylates were more effective than the control group, although there was no significant difference between the two. In summary, Yizhi Xingnao prescription is more effective than ergoloid mesylates or aspirin alone in the treatment of cognitive dysfunction after TIA, and no apparent adverse reactions are found. Thus it is a safe and effective decoction of traditional Chinese medicine that prevents or delays the occurrence of dementia.
SUBJECTS AND METHODS
Design
A randomized, controlled, clinical trial.
Time and setting
Experiments were performed from January 2007 to February 2011 at the Third Affiliated Hospital of Nantong University, China.
Subjects
Patients with MCI after a TIA were selected from the Third Affiliated Hospital of Nantong University, China between 2007 and 2010.
Diagnosis and inclusive criteria
All patients met the diagnosis criteria revised by the Fourth Academic Conference of National Cerebrovascular Diseases[43] and the evaluative standards reported by Petersen et al[44]: complaints of loss of memory; memory impairment in verbal memory test; a clinical dementia rating of 0.5[45]; general cognitive function is normal and the Mini-Mental State Examination total score is more than the threshold value of dementia; ability to maintain normal daily life within 72 hours after the onset of a TIA; the duration from the first TIA attack to enrollment in the study ranged from 24 hours to 4 years; patients were normal by computed tomography or magnetic resonance imaging examination, with good visual acuity and hearing.
Exclusion criteria
Patients with cerebral infarction, cerebral hemorrhage, central nervous system infections, carbon monoxide or alcoholism encephalopathy, dementia, and history of mental illness were excluded.
All patients were informed of the whole research procedure before experimentation, and signed the informed consent form. In total, 88 patients that met the inclusion criteria were included in the study.
Methods
Drugs
Yizhi Xingnao prescription was produced by Jiangyin Tianjiang Pharmaceutical Co., Ltd. (Jiangyin City, China; lot No. 070928). Crude drugs in each pouch included Radix Polygoni Multiflori 10 g, honey-fried Radix Astragali 6 g, and 5 g of each Salvia miltiorrhiza, Fructus Ligustri lucidi, fructus amomi amari, Rhizoma Acori Talarinowii, Fructus Lycii, Semen Astragali complanati, Semen Cuscutae, Radix Angelicae Sinensis, Rhizoma Chuanxiong, Allolobophora caliginosa trapezoides, and Arisaema Cum Bile, as well as 3 g of each Radix Polygalae and Fructus Schisandrae.
Administering drugs
All patients were orally administered with aspirin (100 mg × 30 tablets; Bayer, Leverkusen, Germany) anticoagulant therapy, 100 mg a day, for 60 consecutive days. Previous treatment can continue to treat underlying diseases (such as diabetes and hypertension), except some traditional Chinese medicines that can activate blood circulation and dissipate blood stasis. Yizhi Xingnao prescription group: patients were orally administered with Yizhi Xingnao prescription dissolved in warm water, twice per day for 60 consecutive days. Ergoloid mesylates group: patients were orally administered with ergoloid mesylates (1 mg; 50 tablets; Tianjin Huajin Pharmaceutical Ltd., Tianjin, China), twice per day for 60 successive days. The control group received no other drugs.
Cognitive function in patients assessed by the MoCA scale
The cognitive function in patients was evaluated with the MoCA scale at 30 and 60 days after treatment. The MoCA scale comprised the visual space and executive function (cube, watch), naming, memory, attention, repeat sentences, verbal fluency, abstract thinking, delayed recall and orientation, conducted in an alternative connectivity test pattern. The total score of this scale was 30 and a higher total score indicated better cognitive function[18]. Patients who received less than 12 years of education were graded one more point, to correct for bias in education. All patients were assessed by two trained assessment staff by face-to-face measurement, and their consistency coefficient was 0.912.
Evaluation of clinical efficacy
The clinical efficacy of the treatment was evaluated according to the Assessing Criteria for the Diagnosis, Dialectic and Curative Effect of Vascular Dementia, issued by Tian et al[46]. Marked efficacy: efficacy index ≥ 50%; advance (efficacy): efficacy index ≥ 20%; ineffective: efficacy index < 20%. Efficacy index = (MoCA scores after treatment - MoCA scores before treatment)/MoCA scores before treatment × 100%.
Statistical analysis
Quantitative data were expressed as mean ± SD and compared using SPSS 16.0 software (SPSS, Chicago, IL, USA). The average number of samples among multiple groups were compared by one-way analysis of variance, and comparisons between the two groups was done using the independent two-sample t-test; the average number of samples in groups at different times before and after treatment was compared with repeated measurements analysis of variance, and pairwise comparisons were conducted using the paired t-test; the rate difference of groups was compared with the R × C table data chi-square test. All testing was assumed as α = 0.05, and a level of P < 0.05 was considered statistically significant difference.
Acknowledgments:
We would like to thank Professor Lizhong Guo from Nanjing University of Chinese Medicine for providing guidance in screening and validation.
Footnotes
Conflicts of interest: None declared.
Funding: This study was sponsored by Jiangsu Provincial Administration of Traditional Chinese Medicine, No. LB09090.
Ethical approval: The pilot study was given approval from the Ethics Committee at the Third Affiliated Hospital of Nantong University, China.
(Edited by Chen X, Zhang L/Yang Y/Wang L)
REFERENCES
- [1].Dodge HH, Chang CC, Kamboh IM, et al. Risk of Alzheimer's disease incidence attributable to vascular disease in the population. Alzheimers Dement. 2011;7(3):356–360. doi: 10.1016/j.jalz.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Walters RJ, Fox NC, Schott JM, et al. Transient ischemic attacks are associated with increased rates of global cerebral atrophy. J Neurol Neurosurg Psychiatry. 2003;74(2):213–216. doi: 10.1136/jnnp.74.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sachdev PS, Chen X, Joscelyne A, et al. Amygdala in stroke/transient ischemic attack patients and its relationship to cognitive impairment and psychopathology: the Sydney Stroke Study. Am J Geriatr Psychiatry. 2007;15(6):487–496. doi: 10.1097/JGP.0b013e3180581fe6. [DOI] [PubMed] [Google Scholar]
- [4].Popović IM, Serić V, Demarin V. Mild cognitive impairment in symptomatic and asymptomatic cerebrovascular disease. J Neurol Sci. 2007;257(1-2):185–193. doi: 10.1016/j.jns.2007.01.029. [DOI] [PubMed] [Google Scholar]
- [5].Takahashi PY, Dyrbye LN, Thomas KG, et al. The association of transient ischemic attack symptoms with memory impairment among elderly participants of the Third US National Health and Nutrition Examination Survey. J Geriatr Psychiatry Neurol. 2009;22(1):46–51. doi: 10.1177/0891988708328218. [DOI] [PubMed] [Google Scholar]
- [6].Meyer JS, Rauch GM, Rauch RA, et al. Cardiovascular and other risk factors for Alzheimer's disease and vascular dementia. Ann N Y Acad Sci. 2000;903:411–423. doi: 10.1111/j.1749-6632.2000.tb06393.x. [DOI] [PubMed] [Google Scholar]
- [7].You DL, Shieh FY, Tzen KY, et al. Cerebral perfusion SPECT intransient ischemic attack. Eur J Radiol. 2000;34(1):48–51. doi: 10.1016/s0720-048x(99)00099-6. [DOI] [PubMed] [Google Scholar]
- [8].Bakker FC, Klijn CJ, van der Grond J, et al. Cognition and quality of life in patients with carotid artery occlusion: a follow-up study. Neurology. 2004;62(12):2230–2235. doi: 10.1212/wnl.62.12.2230. [DOI] [PubMed] [Google Scholar]
- [9].Nichelli P, Bonito V, Candelise L, et al. Three-year neuro-psycholgical follow-up of patients with reversible ischemic attacks. Ital J Neurol Sci. 1986;7(4):443–446. doi: 10.1007/BF02283023. [DOI] [PubMed] [Google Scholar]
- [10].Niu JX, Li YM. Impact of Yizhi Xingnao decoction on the cognitive dysfunction for patients with vascular dementia. Zhongxiyi Jiehe Xin Xueguan Bing Zazhi. 2008;6(11):1288–1289. [Google Scholar]
- [11].Niu YL, Liang WX. Systemic evaluation of treatment for vascular dementia by Chinese traditional medicine. Tianjin Zhongyiyao Zazhi. 2007;24(3):190–193. [Google Scholar]
- [12].Johnston SC, Nguyen-Huynh MN, Schwarz ME, et al. National Stroke Association guidelines for the management of transient ischemic attacks. Ann Neurol. 2006;60(3):301–313. doi: 10.1002/ana.20942. [DOI] [PubMed] [Google Scholar]
- [13].Grau-Slevin M, Arboix A, Gaffney J, et al. The role of small vessel disease in development of Alzheimer's disease. Neural Regen Res. 2010;5(4):310–320. [Google Scholar]
- [14].Pendlebury ST, Rothwell PM. Risk of recurrent stroke, other vascular events and dementia after transient ischaemic attack and stroke. Cerebrovasc Dis. 2009;27(Suppl 3):1–11. doi: 10.1159/000209260. [DOI] [PubMed] [Google Scholar]
- [15].Bakker FC, Klijn CJ, Jennekens-Schinkel A, et al. Cognitive impairment in patients with carotid artery occlusion and ipsilateral transient ischemic attacks. J Neurol. 2003;250(11):1340–1347. doi: 10.1007/s00415-003-0222-1. [DOI] [PubMed] [Google Scholar]
- [16].Dufouil C, Godin O, Chalmers J, et al. Severe cerebral white matter hyperintensities predict severe cognitive decline in patients with cerebrovascular disease history. Stroke. 2009;40(6):2219–2221. doi: 10.1161/STROKEAHA.108.540633. [DOI] [PubMed] [Google Scholar]
- [17].Wang W, Wang RN. The application of Montreal Cognitive Assessment in patients with mild cognitive impairment. Chin J Intern Med. 2007;46(5):414–416. [Google Scholar]
- [18].Pendlebury ST, Cuthbertson FC, Welch SJ, et al. Underestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke. 2010;41(6):1290–1293. doi: 10.1161/STROKEAHA.110.579888. [DOI] [PubMed] [Google Scholar]
- [19].Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment (MoCA): A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- [20].Wong A, Xiong YY, Kwan PW, et al. The validity, reliability and clinical utility of the Hong Kong Montreal Cognitive Assessment (HK-MoCA) in patients with cerebral small vessel disease. Dement Geriatr Cogn Disord. 2009;28(1):81–87. doi: 10.1159/000232589. [DOI] [PubMed] [Google Scholar]
- [21].Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009;24(2):197–201. doi: 10.1002/gps.2101. [DOI] [PubMed] [Google Scholar]
- [22].Nazem S, Siderowf AD, Duda JE, et al. Montreal cognitive assessment performance in patients with Parkinson's disease with “normal” global cognition according to mini-mental state examination score. J Am Geriatr Soc. 2009;57(2):304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chu X, Hu LL, Jiang DL, et al. The clinical application of Montreal cognitive assessment in patients with transient ischemic attack. Zhonghua Xingwei Yixue yu Nao Kexue Zazhi. 2010;19(3):203–205. [Google Scholar]
- [24].Jiang DL, Chu X, Hu LL, et al. Relationship between the cognitive function and score of Montreal cognitive assessment scale, event related potential and serum neuron specific enolase level in patients with transient ischemic attack. Linchuang Shenjingbing Xue Zazhi. 2010;23(3):168–170. [Google Scholar]
- [25].Bicalho B, Giolo JM, Lilla S, et al. Identification and human pharmacokinetics of dihydroergotoxine metabolites in man: preliminary results. Biopharm Drug Dispos. 2008;29(1):17–28. doi: 10.1002/bdd.585. [DOI] [PubMed] [Google Scholar]
- [26].Arrigo A, Casale R, Giorgi I, et al. Effects of intravenous high dose co-dergocrine mesylate (‘Hydergine’) in elderly patients with severe multi-infarct dementia: a double-blind, placebo-controlled trial. Curr Med Res Opin. 1989;11(8):491–500. doi: 10.1185/03007998909110460. [DOI] [PubMed] [Google Scholar]
- [27].Yu C, Meng J, Chen J, et al. Preparation of ergoloid mesylate submicron emulsions for enhancing nasal absorption and reducing nasal ciliotoxicity. Int J Pharm. 2009;375(1-2):16–21. doi: 10.1016/j.ijpharm.2009.03.006. [DOI] [PubMed] [Google Scholar]
- [28].Chan YC, Wang MF, Chang HC. Polygonum multiflorum extracts improve cognitive performance in senescence accelerated mice. Am J Chin Med. 2003;31(2):171–179. doi: 10.1142/S0192415X03000862. [DOI] [PubMed] [Google Scholar]
- [29].Ling S, Luo R, Dai A, et al. A pharmaceutical preparation of Salvia miltiorrhiza protects cardiac myocytes from tumor necrosis factor-induced apoptosis and reduces angiotensin II-stimulated collagen synthesis in fibroblasts. Phytomedicine. 2009;16(1):56–64. doi: 10.1016/j.phymed.2008.09.008. [DOI] [PubMed] [Google Scholar]
- [30].Cai J, Zheng T, Zhang L, et al. Effects of Herba Epimedii and Fructus Ligustri lucidi on the transcription factors in hypothalamus of aged rats. Chin J Integr Med. doi: 10.1007/s11655-011-0636-z. In press. [DOI] [PubMed] [Google Scholar]
- [31].Szopa A, Ekiert H. Lignans in Schisandra chinensis in vitro cultures. Pharmazie. 2011;66(8):633–634. [PubMed] [Google Scholar]
- [32].Guan S, Bao YM, Jiang B, et al. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur J Pharmacol. 2006;538(1-3):73–79. doi: 10.1016/j.ejphar.2006.03.065. [DOI] [PubMed] [Google Scholar]
- [33].Jesky R, Hailong C. Are herbal compounds the next frontier for alleviating learning and memory impairments. An integrative look at memory, dementia and the promising therapeutics of traditional chinese medicines? Phytother Res. 2011;25(8):1105–1118. doi: 10.1002/ptr.3388. [DOI] [PubMed] [Google Scholar]
- [34].Chan E, Wong CY, Wan CW, et al. Evaluation of anti-oxidant capacity of root of Scutellaria baicalensis Georgi, in comparison with roots of Polygonum multiflorum Thunb and Panax ginseng CA Meyer. Am J Chin Med. 2010;38(4):815–827. doi: 10.1142/S0192415X10008263. [DOI] [PubMed] [Google Scholar]
- [35].Chen D, Tang J, Khatibi NH, et al. Treatment with Z-ligustilide, a component of Angelica sinensis, reduces brain injury after a subarachnoid hemorrhage in rats. J Pharmacol Exp Ther. 2011;337(3):663–672. doi: 10.1124/jpet.110.177055. [DOI] [PubMed] [Google Scholar]
- [36].Hu R, Yin CL, Wu N, et al. Traditional Chinese herb Dihuang Yinzi (DY) plays neuroprotective and anti-dementia role in rats of ischemic brain injury. J Ethnopharmacol. 2009;121(3):444–450. doi: 10.1016/j.jep.2008.09.035. [DOI] [PubMed] [Google Scholar]
- [37].Xue B, Li J, Chai Q, et al. Effect of total flavonoid fraction of Astragalus complanatus R. Brown on angiotensin II-induced portal-vein contraction in hypertensive rats. Phytomedicine. 2008;15(9):759–762. doi: 10.1016/j.phymed.2007.11.030. [DOI] [PubMed] [Google Scholar]
- [38].Chiu PY, Wong SM, Leung HY, et al. Acute treatment with Danshen-Gegen decoction protects the myocardium against ischemia/reperfusion injury via the redox-sensitive PKCε/mK(ATP) pathway in rats. Phytomedicine. 2011;18(11):916–925. doi: 10.1016/j.phymed.2011.03.006. [DOI] [PubMed] [Google Scholar]
- [39].Zhou XZ, Zhang RS, Shah J, et al. Patterns of herbal combination for the treatment of insomnia commonly employed by highly experienced Chinese medicine physicians. Chin J Integr Med. 2011;17(9):655–662. doi: 10.1007/s11655-011-0841-9. [DOI] [PubMed] [Google Scholar]
- [40].Lee TM, Guo LG, Shi HZ, et al. Neural correlates of traditional Chinese medicine induced advantageous risk-taking decision making. Brain Cogn. 2009;71(3):354–361. doi: 10.1016/j.bandc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- [41].Um MY, Choi WH, Aan JY, et al. Protective effect of Polygonum multiflorum Thunb on amyloid beta-peptide 25-35 induced cognitive deficits in mice. J Ethnopharmacol. 2006;104(1-2):144–148. doi: 10.1016/j.jep.2005.08.054. [DOI] [PubMed] [Google Scholar]
- [42].Liu C, Zhang S, Wu H. Non-thermal extraction of effective ingredients from Schisandra chinensis Baill and the antioxidant activity of its extract. Nat Prod Res. 2009;23(15):1390–1401. doi: 10.1080/14786410902726100. [DOI] [PubMed] [Google Scholar]
- [43].Society for Chinese Neuroscience. Diagnosis essentials of different cranial vascular diseases. Zhonghua Shenjingke Zazhi. 1996;29(6):379–380. [Google Scholar]
- [44].Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- [45].Lim WS, Choug MS, Sahadevan S. Utility of the clinical dementia rating in Asian populations. Clin Med Res. 2007;5(1):61–70. doi: 10.3121/cmr.2007.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tian XZ, Han MX, Tu JW, et al. Assessing criteria for the diagnosis, dialectic and curative effect of vascular dementia. Beijing Zhongyiyao Daxue Xuebao. 2000;23(5):16–23. [Google Scholar]


