Abstract
Diffuse axonal injury is the predominant mechanism of injuries in patients with traumatic brain injury. Neither conventional brain computed tomography nor magnetic resonance imaging has shown sufficient sensitivity in the diagnosis of diffuse axonal injury. In the current study, we attempted to demonstrate the usefulness of diffusion tensor imaging in the detection of lesion sites of diffuse axonal injury in a patient with head trauma who had been misdiagnosed as having a stroke. A 44-year-old man fell from a height of about 2 m. Brain magnetic resonance imaging (32 months after onset) showed leukomalactic lesions in the isthmus of the corpus callosum and the left temporal lobe. He presented with mild quadriparesis, intentional tremor of both hands, and trunkal ataxia. From diffusion tensor imaging results of 33 months after traumatic brain injury onset, we found diffuse axonal injury in the right corticospinal tract (centrum semiovale, pons), both fornices (columns and crus), and both inferior cerebellar peduncles (cerebellar portions). We think that diffusion tensor imaging could be a useful tool in the detection of lesion sites of diffuse axonal injury in patients with head trauma.
Keywords: traumatic brain injury, diffuse axonal injury, diffusion tensor imaging, head trauma
INTRODUCTION
Accurate elucidation of lesions in patients with traumatic brain injury (TBI) is essential for guiding treatment strategies, for providing information regarding final outcomes. TBI can accompany various types of injuries simultaneously. Diffuse axonal injury (DAI) is the predominant mechanism of TBI, and its sequelae are the most frequent cause of poor clinical outcomes[1]. DAI is characterized by widespread axonal damage due to shearing forces by acceleration, deceleration, or rotation of the brain[1,2]. Neither conventional brain computed tomography (CT) nor magnetic resonance imaging (MRI) has shown sufficient sensitivity in diagnosis of DAI; therefore, it is difficult in the diagnosis of DAI and there is a possibility of underestimation of DAI in TBI[3].
By contrast, recent advances in diffusion tensor imaging (DTI) have enabled both morphologic and quantitative estimation of the state of neural tracts at the subcortical level[4,5,6,7]. Therefore, usefulness of DTI in the detection of DAI has been reported in various neural tracts, including fornix, corticospinal tract (CST), cerebellar peduncles, and the medial cholinergic pathway[8,9,10,11,12,13,14,15,16,17,18].
In the current study, we attempted to demonstrate the usefulness of DTI in the detection of DAI in a patient with head trauma who had been misdiagnosed as having a stroke.
CASE REPORT
A 44-year-old right-handed patient and 10 age- and sex-matched healthy control subjects (10 males, mean age: 43.9 years, range: 33–52 years) with no history of neurological diseases were recruited. The patient fell from a height of about 2 m while working at a factory. The patient lost consciousness for about 4 hours from the time of onset. Brain CT at onset showed subarachnoid hemorrhage and petechial hemorrhage on the left temporal lobe, and the patient underwent conservative management at a local hospital (Figure 1A). After 32 months following head trauma, the patient was admitted to the rehabilitation department of a university hospital for evaluation and rehabilitation. He complained of hand tremor, gait disturbance, cognitive dysfunction, and urinary difficulty with urgency. He showed mild quadriparesis (more severe in the right lower extremity), intentional tremor of both hands, and trunkal ataxia. The patient was unable to walk on stairs or uneven surfaces, and the fine motor function of his right hand had deteriorated to such an extent that the Purdue Pegboard score was 12[19]. Brain MRI (32 months after onset) showed leukomalactic lesions in the isthmus of the corpus callosum and the left temporal lobe (Figure 1A). Cognitive function was evaluated using the Wechsler Intelligence Scale and Memory Assessment Scale[20,21]. The patient showed a normal intelligence quotient (IQ) (104): verbal IQ (101) and performance IQ (108). However, Memory Assessment Scale score for visual memory (86:18%ile) was decreased, compared with that of verbal memory (99:47%ile). On the other hand, the patient showed hyperreflexic neurogenic bladder findings on urodynamic study, indicating coincident occurrence of spinal cord injury. All subjects provided written informed consent.
Figure 1.
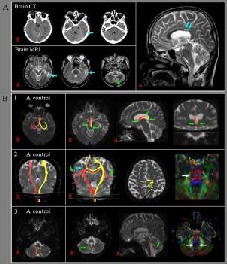
CT, brain MRI images, and diffusion tensor tractography (DTT) results of a 44-year-old male patient with traumatic diffuse axonal injury. CT: Computed tomography; MRI: magnetic resonance imaging.
(A) Brain CT images taken at onset showed hemorrhage in the left temporal lobe (blue arrow). T2-weighted brain MRI (32 months after onset) showed leukomalactic lesions (blue arrows) in the left temporal lobe and isthmus of the corpus callosum, and signal change in the left cerebellum (green arrow).
(B) Results of DTT for controls and the patient.
1: The fornix. Both fornices of the patient showed disruptions at both columns and crura (green arrows).
2: The corticospinal tract. The right corticospinal tract of the patient showed a disruption at the centrum semiovale (blue arrow) and the abnormal pathways, including transcallosal fibers (green arrows). The color map of diffusion tensor imaging showed an injury at the right pons (white arrow).
3: The inferior cerebellar peduncles. The right inferior cerebellar peduncle of the patient showed a disruption (green arrow) at the cerebellar portion and the color map showed injury to both inferior cerebellar peduncles in the cerebellar portions (white arrows).
DTI acquisition
A 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Hoffmann LaRoche, Ltd, Best, the Netherlands) with single-shot echo-planar imaging was used for acquisition of DTI data at an average of 33 months after TBI onset. For each of the 32 non-collinear diffusion sensitizing gradients, we acquired 67 contiguous slices parallel to the anterior commissure-posterior commissure line. Imaging parameters are as follows: acquisition matrix = 96 × 96; reconstructed matrix = 128 × 128; field of view = 221 mm × 221 mm; repetition time/echo time = 10 726 ms/76 ms; sensitivity encoding factor = 2; echo planar imaging factor = 49; b =1 000 s/mm2; number of excitations = 1; and a slice thickness of 2.3 mm (acquired isotropic voxel size 2.3 mm × 2.3 mm × 2.3 mm).
DTI analysis
Affine multi-scale two-dimensional registration at the Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl) was used for removal of eddy current-induced image distortions[22]. DTI-Studio software (CMRM, Johns Hopkins Medical Institute, Baltimore, MD, USA) was used for evaluation of the fornix, CST, and three cerebellar peduncles (superior cerebellar peduncle, middle cerebellar peduncle, and inferior cerebellar peduncle)[18,23,24,25]. For the fornix, two regions of interest (ROIs) were placed on the isolated area (ROI 1: junction between the column and body, ROI 2: junction between the body and crus). For the CST, ROI 1 was the upper pons (anterior blue color on the axial view), ROI 2 was the lower pons (anterior blue color on the axial view). For the superior cerebellar peduncle, ROI 1 was the junction of the superior cerebellar peduncle between the upper pons and cerebellum (blue color on the coronal view), ROI 2 was between the lateral wall of the fourth ventricle and the inferior cerebellar peduncle at the fourth ventricle level (green color on the axial view). For the middle cerebellar peduncle, ROI 1 was the anterior junction of the middle cerebellar peduncle between the pons and cerebellum, ROI 2 was the posterior junction of the middle cerebellar peduncle between the pons and cerebellum (green color on the coronal view). For the inferior cerebellar peduncle, ROI 1 was the restiform body (blue color on the axial view), ROI 2 was the caudal part of the superior cerebellar peduncle on the axial view at the upper pons level (green color on the axial view). For the fornix, fiber tracking was initiated at the center of a seed voxel with a fractional anisotropy > 0.15 and ended at a voxel with a fiber assignment of < 0.15 and a tract turning-angle of < 60°. For the CST and three cerebellar peduncles, fiber tracking was initiated at the center of a seed voxel with a fractional anisotropy > 0.2 and ended at a voxel with a fiber assignment of < 0.2 and a tract turning-angle of < 60°. We measured fractional anisotropy and apparent diffusion coefficient values of the neural tracts and all regions along the DTTs. DTI parameter value showing deviation of more than two standard deviations that of normal control was defined as abnormal.
In terms of configuration of DTTs, the patient showed lesions of both fornices, right CST, and both inferior cerebellar peduncles, compared with those of control subjects. For the fornix, the patient revealed a disruption of both columns and crura. The right CST showed a disruption at the centrum semiovale, with abnormal branches, including transcallosal fibers, and the right inferior cerebellar peduncle showed a disruption at the cerebellar portion (Figure 1B). A summary of DTI parameters for tracts and injury regions of the patient and normal control subjects is shown in Table 1.
Table 1.
Diffusion tensor image parameter values of the patient and control subjects
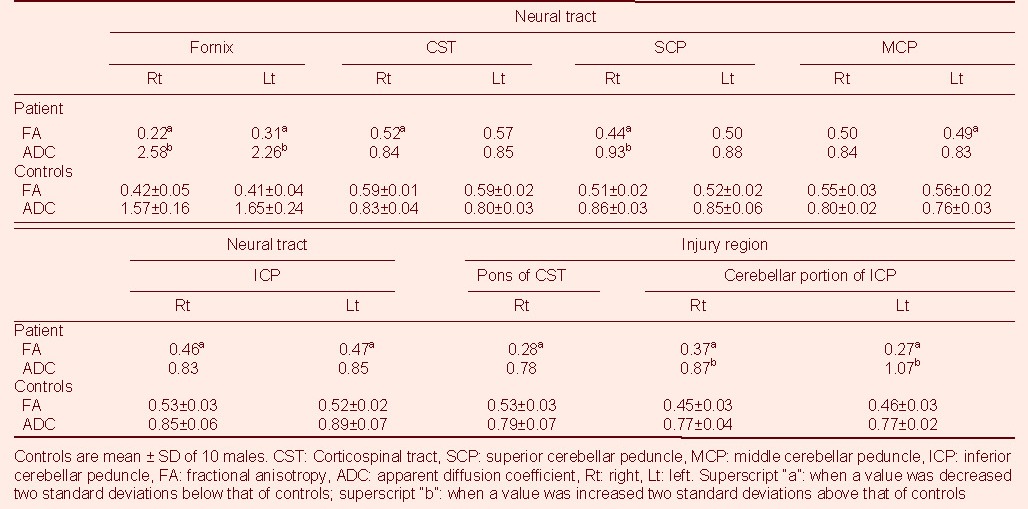
Fractional anisotropy and apparent diffusion coefficient values of some tracts (both fornices, right superior cerebellar peduncle) and injury regions (cerebellar portion of both inferior cerebellar peduncles) showed a decrease and increase of more than two standard deviations, compared with those of normal controls, respectively. By contrast, only fractional anisotropy values showed a decrease of more than two standard deviations, compared with normal controls, in DTTs for the right CST, left middle cerebellar peduncle, and both inferior cerebellar peduncles, and the injured region of the right pons.
DISCUSSION
In the current study, we attempted to find lesions of DAI in patients with head trauma. This patient applied for compensation insurance for an industrial accident after 3 years since the onset of head trauma. However, his request was rejected because three medial doctors (one neuroradiologist, one neurosurgeon, and one medical doctor: speciality unknown) diagnosed his brain lesions as a stroke. However, we think that this patient had TBI for the following reasons. First, in terms of configuration and DTI parameters, this patient had DAI in the fornix, CST, and inferior cerebellar peduncle. On the DTT, the patient showed lesions in both the fornix (columns and crura), the right CST (centrum semiovale), and the right inferior cerebellar peduncle (cerebellar portion). As for DTI parameters, fractional anisotropy and apparent diffusion coefficient values of some tracts (both fornices and right superior cerebellar peduncle) and regions (cerebellar portions of both inferior cerebellar peduncles) were decreased and increased, respectively; in contrast, only fractional anisotropy values were decreased in DTTs for the right CST, left middle cerebellar peduncle, and both inferior cerebellar peduncles, and the region of the right pons. Fractional anisotropy value is the most widely used DTI parameter and represents the degree of directionality of microstructures (e.g., axons, myelin, and microtubules)[4,5,6]. The apparent diffusion coefficient value indicates the magnitude of water diffusion in tissue, which can increase with some forms of pathology, particularly vasogenic edema or accumulation of cellular debris from neuronal damage[4,5,6]. Therefore, a decrease of fractional anisotropy with no change or increase of apparent diffusion coefficient value of the DTT or regions is suggestive of neuronal injury. As a result, we suspect neuronal injury of both fornices, right CST, right superior cerebellar peduncle, left middle cerebellar peduncle, and both inferior cerebellar peduncle. Considering the focal lesions, which were detected at DTT configuration and DTI parameters, it seems that there were DAIs in the right CST (centrum semiovale, pons), both fornices (columns and crus), and both inferior cerebellar peduncle (cerebellar portions). Neurological signs of this patient (ataxia, quadriparesis, memory dysfunction) appeared to be well correlated with these DAI findings. The ataxia of the patient, which is a typical sign of cerebellar peduncle injury in patients with TBI, was well correlated with cerebellar peduncle injury[26,27], the memory dysfunction with fornix injury, and quadriparesis with CST injury; however, coincidently, due to the accident, this patient appeared to have a spinal cord injury. Second, the patient's condition was compatible with the diagnostic criteria of DAI. He fell from a height of about 2 m while working at a factory. Considering that the height for DAI diagnostic criteria is 1.8 m[2,28], this accident had the potential to cause an acceleration/deceleration head trauma in this patient. In addition, the patient showed a loss of consciousness for 4 hours at the time of injury, without a lucid interval[2,28,29], and a corpus callosum lesion, which meets the criteria of grade II of DAI[28]. In addition, hemorrhages were observed on brain CT, which was taken at onset of head trauma.
In the current study, we demonstrated the usefulness of DTI in the detection of DAI lesions in a patient with head trauma. We think that the three medial doctors who had diagnosed this patient as a stroke could have made a diagnosis of TBI if they had been careful, because this patient showed significant evidence of TBI in terms of accident history, initial CT evidence, and conventional MRI evidence of corpus callosum lesions, except for DTI findings. Therefore, DTI evidence of DAI appears to be additional evidence for diagnosis as a TBI. We think that DTI could be a useful tool in the detection of DAI in patients with head trauma. Therefore, we recommend evaluation of neural tracts using DTI for patients with head trauma. This study is limited by the number of case reports. Therefore, further complementary studies involving larger case numbers are warranted.
Footnotes
Conflicts of interest: None declared.
Funding: This work was supported by Daegu Metropolitan City R&D Project.
(Edited by Borgwardt S, NG WH/Wang L)
REFERENCES
- [1].Meythaler JM, Peduzzi JD, Eleftheriou E, et al. Current concepts: Diffuse axonal injury-associated traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- [2].Adams JH, Graham DI, Murray LS, et al. Diffuse axonal injury due to nonmissile head injury in humans: An analysis of 45 cases. Ann Neurol. 1982;12:557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- [3].Gentry LR, Godersky JC, Thompson B, et al. Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. AJR Am J Roentgenol. 1988;150:673–682. doi: 10.2214/ajr.150.3.673. [DOI] [PubMed] [Google Scholar]
- [4].Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [5].Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008;27:1–7. doi: 10.1002/jmri.21087. [DOI] [PubMed] [Google Scholar]
- [6].Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- [7].Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- [8].Chang MC, Jang SH. Corpus callosum injury in patients with diffuse axonal injury: A diffusion tensor imaging study. NeuroRehabilitation. 2010;26:339–345. doi: 10.3233/NRE-2010-0571. [DOI] [PubMed] [Google Scholar]
- [9].Chang MC, Kim SH, Kim OL, et al. The relation between fornix injury and memory impairment in patients with diffuse axonal injury: A diffusion tensor imaging study. NeuroRehabilitation. 2010;26:347–353. doi: 10.3233/NRE-2010-0572. [DOI] [PubMed] [Google Scholar]
- [10].Hong JH, Jang SH. Left fornical crus injury and verbal memory impairment in a patient with head trauma. Eur Neurol. 2010;63:252. doi: 10.1159/000277477. [DOI] [PubMed] [Google Scholar]
- [11].Jang SH, Kim SH, Kim OL. Fornix injury in a patient with diffuse axonal injury. Arch Neurol. 2009;66:1424–1425. doi: 10.1001/archneurol.2009.242. [DOI] [PubMed] [Google Scholar]
- [12].Nakayama N, Okumura A, Shinoda J, et al. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry. 2006;77:850–855. doi: 10.1136/jnnp.2005.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sugiyama K, Kondo T, Higano S, et al. Diffusion tensor imaging fiber tractography for evaluating diffuse axonal injury. Brain Inj. 2007;21:413–419. doi: 10.1080/02699050701311042. [DOI] [PubMed] [Google Scholar]
- [14].Wang JY, Bakhadirov K, Devous MD, Sr, et al. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch Neurol. 2008;65:619–626. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- [15].Jang SH, Kim SH, Kim OL, et al. Corticospinal tract injury in patients with diffuse axonal injury: A diffusion tensor imaging study. NeuroRehabilitation. 2009;25:229–233. doi: 10.3233/NRE-2009-0519. [DOI] [PubMed] [Google Scholar]
- [16].Ahn YH, Kim SH, Han BS, et al. Focal lesions of the corticospinal tract demonstrated by diffusion tensor imaging in patients with diffuse axonal injury. NeuroRehabilitation. 2006;21:239–243. [PubMed] [Google Scholar]
- [17].Han BS, Kim SH, Kim OL, et al. Recovery of corticospinal tract with diffuse axonal injury: A diffusion tensor image study. NeuroRehabilitation. 2007;22:151–155. [PubMed] [Google Scholar]
- [18].Hong JH, Kim OL, Kim SH, et al. Cerebellar peduncle injury in patients with ataxia following diffuse axonal injury. Brain Res Bull. 2009;80:30–35. doi: 10.1016/j.brainresbull.2009.05.021. [DOI] [PubMed] [Google Scholar]
- [19].Kim YT, Kang SY, Kim HS, et al. Hand strength and dexterity evaluation with age. J Korean Acad Rehabil Med. 1994;18:780–788. [Google Scholar]
- [20].Williams JM. Odessa, FL: Psychological Assessment Resources; 1991. MAS: Memory Assessment Scales. Professional manual. [Google Scholar]
- [21].Wechsler D. New York: The Psychological Corporation; 1981. Manual for the Wechsler Adult Intelligence Scale-Revised. [Google Scholar]
- [22].Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- [23].Jang SH, Kim DS, Son SM, et al. Clinical application of diffusion tensor tractography for elucidation of the causes of motor weakness in patients with traumatic brain injury. NeuroRehabilitation. 2009;24:273–278. doi: 10.3233/NRE-2009-0478. [DOI] [PubMed] [Google Scholar]
- [24].Jiang H, van Zijl PC, Kim J, et al. Dtistudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- [25].Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol. 2005;26:2267–2274. [PMC free article] [PubMed] [Google Scholar]
- [26].Thomsen IV. Late outcome of very severe blunt head trauma: A 10-15 year second follow-up. J Neurol Neurosurg Psychiatry. 1984;47:260–268. doi: 10.1136/jnnp.47.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].DeLisa JA, Gans BM. Philadelphia: Lippincott; 1993. Rehabilitation Medicine: Principles and Practice. [Google Scholar]
- [28].Adams JH, Doyle D, Ford I, et al. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- [29].Parizel PM, Ozsarlak, Van Goethem JW, et al. Imaging findings in diffuse axonal injury after closed head trauma. Eur Radiol. 1998;8:960–965. doi: 10.1007/s003300050496. [DOI] [PubMed] [Google Scholar]


