Abstract
Hydroxychloroquine (HCQ) is an antimalarial drug also used in treating autoimmune diseases. Its antiviral activity was demonstrated in restricting HIV infection in vitro; however, the clinical implications remain controversial. Infection with dengue virus (DENV) is a global public health problem, and we lack an antiviral drug for DENV. Here, we evaluated the anti-DENV potential of treatment with HCQ. Immunofluorescence assays demonstrated that HCQ could inhibit DENV serotype 1–4 infection in vitro. RT-qPCR analysis of HCQ-treated cells showed induced expression of interferon (IFN)-related antiviral proteins and certain inflammatory cytokines. Mechanistic study suggested that HCQ activated the innate immune signaling pathways of IFN-β, AP-1, and NFκB. Knocking down mitochondrial antiviral signaling protein (MAVS), inhibiting TANK binding kinase 1 (TBK1)/inhibitor-κB kinase ɛ (IKKɛ), and blocking type I IFN receptor reduced the efficiency of HCQ against DENV-2 infection. Furthermore, HCQ significantly induced cellular production of reactive oxygen species (ROS), which was involved in the host defense system. Suppression of ROS production attenuated the innate immune activation and anti-DENV-2 effect of HCQ. In summary, HCQ triggers the host defense machinery by inducing ROS- and MAVS-mediated innate immune activation against DENV infection and may be a candidate drug for DENV infection.
Introduction
Flaviviruses include more than 70 enveloped RNA viruses that cause serious disease in humans. Several members of mosquito-born flaviviruses, such as dengue virus serotype 1–4 (DENV-1-4), yellow fever virus (YFV), West Nile virus (WNV), and Japanese encephalitis virus (JEV), are highly pathogenic to humans and these infections have become major international health problems (Seligman and Gould 2008).
Mosquitoes transmitting DENV in humans have been the main cause of dengue diseases globally, with 50 million people infected per year worldwide (Guzman and others 2010). DENV-infected people show typical syndromes of self-limited febrile dengue fever and dengue hemorrhagic fever (DHF). Life-threatening dengue shock syndrome (DSS) is more likely to occur after a second DENV infection (Kyle and Harris 2008; Martina and others 2009). A recent case surveillance revealed that in particular older adults are at increased risk of DHF/DSS and death (Lin and others 2012). Other than supportive treatments, no specific therapy or vaccine is available (Guzman and others 2010).
Hydroxychloroquine (HCQ) and chloroquine are safe and well-tolerated antimalarial drugs. Their modulation of inflammation also improves survival and reduces disease activity in autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, and Sjögren syndrome (Lee and others 2011). Several possible mechanisms of action of HCQ and chloroquine were revealed from in vitro or in vivo studies and included suppression of autoantigen presentation, reduced prostaglandin synthesis, antiproliferative effects, photoprotection, and decreased metalloproteinase activity and leukocyte activation (Lee and others 2011; Ben-Zvi and others 2012).
HCQ and chloroquine are cellular autophagy modulators that interfere with the pH-dependent steps of endosome-mediated viral entry and late stages of replication of enveloped viruses such as retroviruses, flaviviruses, and coronaviruses (Savarino and others 2003; Vincent and others 2005). The anti-HIV-1 effect of HCQ or chloroquine was demonstrated with the combination of antiretroviral drugs such as zidovudine, hydroxyurea, and didanosine in vitro or in patients (Savarino and others 2001b; Paton and Aboulhab 2005). However, HIV-infected patients receiving HCQ but not other antiretroviral therapy showed increased viral replication (Paton and others 2012). HCQ and chloroquine were reported to interfere in flavivirus infection at JEV internalization, YFV replication, and DENV maturation (Brandriss and Schlesinger 1984; Randolph and others 1990; Zhu and others 2012). However, in a randomized controlled trial, chloroquine failed to reduce viremia in patients with dengue disease (Tricou and others 2010), so despite the known antiviral mechanism of antimalarial drugs, other signaling pathways remain to be explored.
Innate immunity is a fast response of type I interferon (IFN) and inflammatory cytokine secretion that can be triggered by nucleic acids of virus replication products and toll-like receptor (TLR) signaling (Kumar and others 2009). During virus infection, double-stranded RNA can be detected by a group of cellular sensors such as melanoma differentiation-associated protein 5 (MDA5) and retinoic acid-inducible gene I (RIG-I). The signal is transferred to mitochondrial antiviral signaling protein (MAVS), then TANK binding kinase 1 (TBK1) and IKKɛ for activation of IFN regulatory factor 3 (IRF3), IRF7, and NFκB, thus leading to expression of type I IFN and various cytokines (Kumar and others 2009). These cytokines inhibit virus replication in infected cells and regulate the induction of adaptive immunity, for swift eradication of viruses.
Autophagy has been described as a regulatory mechanism of both innate and adaptive immunity (Deretic and Levine 2009; Levine and others 2011). Pharmacological study indicated that suppression of autophagy with inhibitors such as chloroquine and Bafilomycin A1 (Baf A1) activated innate immunity (Ke and Chen 2011a, 2011b).
In this study, we investigated the efficiency of the multipurpose drug HCQ against DENV infection and its ability to activate the host defense machinery.
Materials and Methods
Virus, cell lines, and chemicals
We used local Taiwanese strains of DENV-1 766733A and DENV-2 PL046 (Genbank accession no. AJ968413.1) isolated from patients with dengue fever and DENV-4 466088A isolated from a patient with DHF. DENV-3 H87 strain was kindly provided by D. J. Gubler (Lin and others 1998). These viruses were propagated in mosquito cell line C6/36 (ATCC: CRL-1660) grown in RPMI 1640 medium containing 5% fetal bovine serum (FBS). A549 human lung epithelial carcinoma cells (ATCC: CCL-185), Hepa1-6 hepatoma cells (BCRC: 60051), WS1 human fetal skin normal fibroblasts (BCRC: 60300), J774A.1 mouse macrophages (BCRC: 60140), and HEK-293T cells (ATCC: CRL-3216) were cultured in DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen). The reagents HCQ (Sigma-Aldrich; H0915), Baf A1 (Calbiochem; #196000), and recombinant human IFN-α 2a (Prospec; CYT-204) were used. TBK1/IKKɛ inhibitors BX795 (InvivoGen; tlrl-bx7) and Amlexanox (Sigma-Aldrich; SML0517) (Reilly and others 2013) and reactive oxygen species (ROS) inhibitor N-tert-Butyl-(-phenylnitrone (PBN) (Sigma-Aldrich; B7263) (Fidanboylu and others 2011) were used.
Plaque-forming assay
To determine virus titers, culture medium from DENV-2–infected cells was harvested for plaque-forming assays. Various virus dilutions were added to 80% confluent BHK-21 cells (BCRC: 60041) and incubated at 37°C for 2 h. After adsorption, cells were washed and overlaid with 1% agarose (SeaPlaque; FMC BioProducts) containing RPMI 1640 with 1% FBS for 7 days, then fixed with 10% formaldehyde, and stained with 0.5% crystal violet.
Cell proliferation and viability assay
Cells were assayed with 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT; Roche) at 1 mg/ml for 4 h, then the cell supernatant was replaced with DMSO to completely resolve the purple formazon crystals. The spectrophotometric absorbance of samples was measured by use of a microplate reader (Anthos). The net absorbance at OD570 indicated the enzymatic activity of mitochondria and cell viability.
Real-time quantitative PCR
TRIzol reagent (Invitrogen) was used for total RNA extraction, and cDNA was synthesized from 0.5 μg total RNA by use of Superscript III reverse transcriptase (Invitrogen). qPCR amplification involved 3 ng cDNA in 10 μL SYBR Green PCR master mix (Applied Biosystems) with 3 μM primers in ABI StepONE Plus Real-Time PCR system (Applied Biosystems). Transcript levels were normalized to that of hypoxanthine phosphoribosyltransferase. The primer sequences for gene detection are in Supplementary Tables S1 and S2 (Supplementary Data are available online at www.liebertpub.com/jir).
Luciferase reporter assay
TurboFect transfection reagent (Thermo Scientific) was used for transient transfection following the manufacturer's protocol. Cells cultured in 12-well plates were transfected with NFκB-, AP-1-, ISRE-, or IFN-β-Luc reporter plasmids (Chang and others 2009). pRL-TK (Promega), encoding Renilla luciferase under an herpes simplex virus thymidine kinase promoter, was an internal control. In some cases, pcDNA3.1 V5-tagged-MAVS was used (Yu and others 2010). Cell lysates were collected for dual-luciferase assay (Promega). Firefly luciferase activity was normalized relative to that of Renilla luciferase.
Immunofluorescence assay
Cells were fixed with 4% paraformaldehyde for 30 min, then permeabilized with 0.5% Triton X-100 for 10 min. After 2 washes with phosphate-buffered saline (PBS), cells were blocked with 10% skim milk in PBS. DENV-2 NS3 was detected by incubation with a monoclonal antibody against NS3 (#YH3304, 1:500 dilution; Yao-Hong Biotechnology) (Chang and others 2012), plus Alexa Fluor-488-conjugated goat anti-mouse IgG antibody (Invitrogen). Fluorescence signals were observed by fluorescence microscopy (ZEISS Observer. A1). The 50% inhibition concentration (IC50) of HCQ against DENV-2 in cells was estimated by immunofluorescence intensity measured with use of a microplate reader (Fluroskan Ascent FL; Thermo Scientific). Anti-E antibody (#YH3304, 1:100 dilution; Yao-Hong Biotechnology) was used for antibody-dependent enhancement (ADE) of DENV-2 infection in J774A.1 cells.
IRF3 and NFκB p65 nuclear translocation assay was described previously (Chang and others 2006). IRF3 and NFκB p65 location was detected on incubation with the primary antibody anti-IRF3 (#sc-9082) and anti-NFκB p65 (#sc-372; both Santa Cruz Biotechnology), respectively, then Alex 568-conjugated anti-rabbit IgG antibody (Invitrogen). Nuclei were stained with DAPI.
Immunoblot analysis
Cells were lyzed in RIPA buffer (150 mM NaCl, 0.5% sodium deoxycholate, 1% NP40, 0.1% SDS, and 50 mM Tris-HCl [pH 8.0]) containing protease inhibitor and phosphotase inhibitor cocktail (Roche). Harvested extracts were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes, which were incubated with primary antibody, then horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratory) and visualized by enhanced chemiluminescence (Thermo Scientific). Images were acquired by use of a digital image system, BioSpectrum Image System (UVP). The following primary antibodies were used: anti-phospho-c-Jun (Ser73) (#3270, D47G9), anti-c-Jun (#9165, 60A8), anti-MAVS (#3993), and anti-IκBα (#4814S; all Cell Signaling); anti-phospho-IRF3 (pS386) (#2562-1; Epitomics); anti-IRF3 (sc-9082) and anti-NFκB p65 (sc-372; both Santa Cruz Biotechnology); and anti-V5 (Invitrogen). Endoplasmic reticulum (ER) stress was measured by immunoblotting with use of an ER-stress antibody kit (#9956; Cell Signaling), which includes antibodies against immunoglobulin heavy chain-binding protein/glucose-regulated protein of molecular (BiP/GRP78), Calnexin, endoplasmic oxidoreductin1α (Ero1α), inositol-requiring protein 1α (IRE1α), C/EBP homologous protein (CHOP), PKR-like ER protein kinase (PERK), and protein disulfide isomerase (PDI).
MAVS knockdown and type I IFN receptor signaling blocking
A549 cells were transfected with MAVS knockdown siRNA (siMAVS) and negative control siRNA (siNC) (35 pmol, Dharmacon; Thermo Scientific) by use of DharmaFECT (Thermo Scientific) for 24 h, then treated with HCQ for 24 h. MAVS knockdown efficiency was analyzed by immunoblotting with anti-MAVS antibody. A549 cells with shMAVS or control shLacZ stable expression were previously established (Yu and others 2010). For blocking type I IFN receptor signaling, A549 cells were incubated with type I IFN receptor neutralization monoclonal antibody (Millipore; #MAB1155, MMHAR-2) at 1:100 dilution for 8 h before HCQ treatment and virus infection.
ROS detection
Cellular ROS was determined by dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay (Fluska-35845; Sigma-Aldrich). A549 cells were washed with PBS, then 10 μM DCFH-DA was added for 30 min. The dye was discarded and cells were washed with PBS; DCF fluorescence was observed under a fluorescence microscope and quantified by use of a microplate reader (Fluroskan Ascent FL; Thermo Scientific). DCF excitation and emission wavelength were 488 nm and 529 nm, respectively.
Statistical analysis
Quantitative data are presented as mean±SD. Student's t test was used to determine the significance between treatment groups. P<0.05 was considered statistically significant.
Results
HCQ inhibits DENV infection in vitro
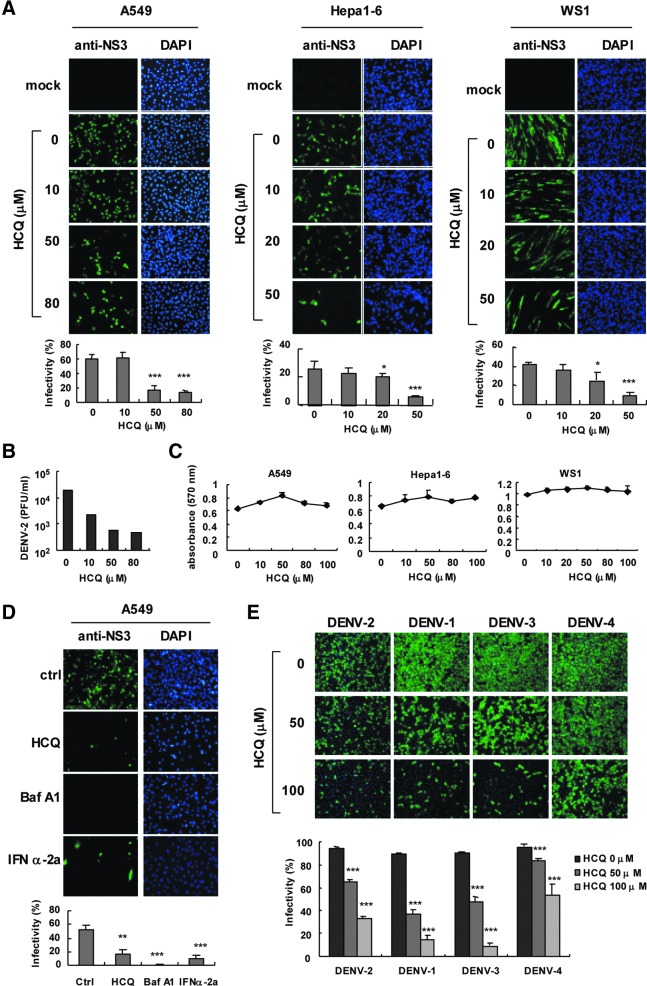
We evaluated the use of HCQ against DENV-2 infection by immunofluorescence assay (IFA) with anti-NS3 antibody in A549 lung carcinoma cells, murine hepatocellular carcinoma Hepa1-6 cells, and normal human fibroblast WS-1 cells. HCQ dose-dependently reduced DENV-2 infection (Fig. 1A). The 50% inhibition concentration (IC50) of HCQ against DENV-2 infection was estimated at 10.1±1.6 μM for A549 cells, 12.9±4.2 μM for Hepa1-6 cells, and 12.9±1.9 μM for WS-1 cells. Plaque assay indicated that 50–80 μM HCQ decreased DENV-2 titers by ∼100-fold in A549 cells (Fig. 1B). Because cell viability could be a factor in virus replication, we monitored the cytotoxicity of HCQ in A549, Hepa1-6, and WS-1 cells by MTT assay. The results indicated that equitable HCQ doses were used in our system (Fig. 1C). Thus, HCQ-restricted DENV2 replication was not due to impaired cell proliferation. Moreover, HCQ inhibited DENV-2 infection in J774A.1 macrophages (IC50=9.7±1.3 μM) (Supplementary Fig. S1A). MTT assay clarified that the dose of HCQ did not affect macrophage viability (Supplementary Fig. S1B). Consistent results were shown in HEK-293T cells, with DENV-2 infection, viral protein NS3 expression, 5′-UTR (untranslated region) viral gene expression, and virion production attenuated by HCQ (Supplementary Fig. S1C–F).
FIG. 1.
Hydroxychloroquine (HCQ) attenuates dengue virus infection in vitro. (A) A549, Hepa1-6 and WS-1 cells were pretreated with HCQ as indicated for 24 h before DENV-2 inoculation. Immunofluorescent assay (IFA) of DENV-2 infection with anti-DENV-2 NS3 antibody (left panels), and DAPI staining of nuclei (right panels) and quantification. (B) Plaque-forming assays of viral titers in DENV-2-infected A549 cells treated with HCQ as described in (A). (C) MTT assay of viability of A549, Hepa1-6, and WS-1 cells at 24 h after HCQ treatment. Data are mean±SD from 3 determinations. (D) A549 cells pretreated with 50 μM HCQ, 1 μM Bafilomycin A1 (Baf-A1), or 500 U type I IFN-α-2a for 24 h were infected with DENV-2 at multiplicity of infection (MOI)=1; IFA of infectivity with anti-DENV-2 NS3 antibody (left panels) and DAPI nuclear staining (right panels) and quantification. (E) A549 cells pretreated with 50 and 100 μM HCQ for 24 h were infected with DENV serotype 1–4 (MOI=5) for 24 h. Anti-NS3 (green fluorescence) and DAPI (blue florescence) stained images were merged to show DENV-infected cells, and quantification. *P≤0.05, **P≤0.01, ***P≤0.005 versus untreated control group.
Baf A1 is an autophagosome–lysosome fusion modulator with a similar pharmacological effect as HCQ (Rubinsztein and others 2012) and has been found to inhibit influenza A and B virus infection (Ochiai and others 1995). Here, we noted an anti-DENV-2 activity of Baf A1 similar to HCQ and the positive control IFN-α (Fig. 1D); so, DENV-2 infection requires cellular activity of autophagosome–lysosome fusion. Besides blocking DENV-2 infection, HCQ also had antiviral effects against DENV-1, -3, and -4 serotypes (Fig. 1E); however, a higher dose of HCQ might be required to suppress DENV-4 efficiently. Overall, our data show that HCQ interferes with DENV infection in vitro.
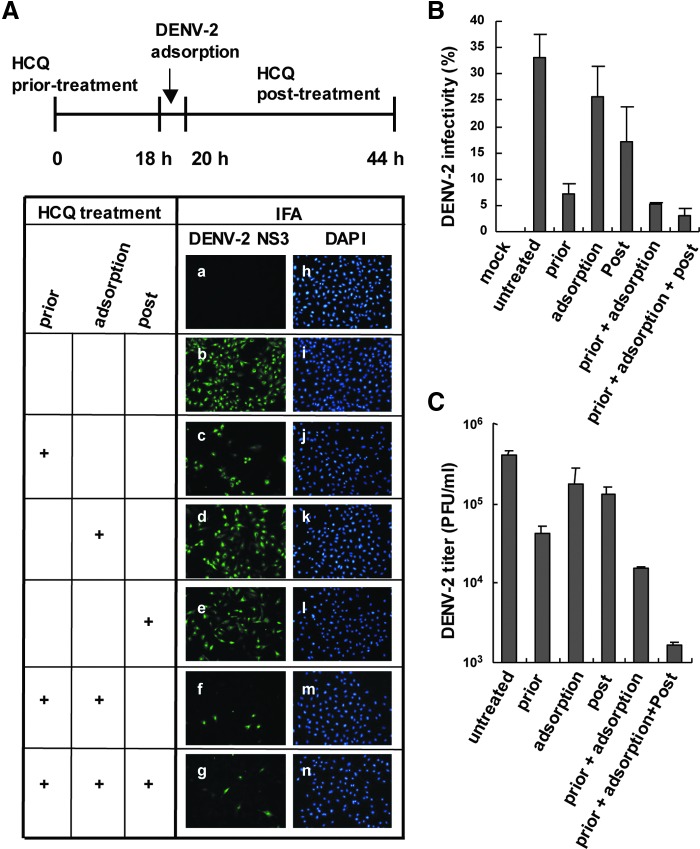
To address the antiviral mechanism of HCQ in DENV-2 infection, HCQ was added to cells before, during or after viral adsorption (Fig. 2A, upper panel). IFA of DENV-2 infectivity revealed that HCQ pretreatment suppressed DENV-2 infection, whereas DENV-2 replication was only partially reduced with HCQ added during virus adsorption or after viral adsorption (c–e in Fig. 2A). DENV-2 infection was greatly inhibited when cells were incubated with HCQ before and during viral adsorption or during all times (f, g in Fig. 2A). Thus, long-term HCQ treatment may have better inhibition effect on DENV-2 replication. Infectivity estimated by IFA gave similar results (Fig. 2B) and plaque-forming assay of DENV-2 titration confirmed the HCQ-mediated restriction of DENV-2 infection (Fig. 2C). Thus, HCQ pretreatment may change the host cell physical condition or trigger the host defense system against DENV infection.
FIG. 2.
HCQ pretreatment reduces DENV-2 infectivity. (A) A549 cells were treated with HCQ (50 μM) before, during and after viral adsorption (upper panel). A549 cells were mock-infected (a) or infected with DENV-2 for 24 h (b) or pretreated with HCQ for 18 h before DENV-2 infection (c), during DENV-2 adsorption for 2 h (d), or after DENV-2 adsorption for 24 h (e). IFA of DENV-2 infection with anti-DENV-2 NS3 antibody (left panels), and DAPI staining of nuclei (right panels, h–n) (combined HCQ treatment is shown: f and g) and (B) quantification of IFA infectivity results. Data are mean±SD of 3 observation fields. (C) Plaque-forming assay of DENV-2 titration with treatment as described in (A).
HCQ induces the expression of host antiviral genes
Type I IFN and associated antiviral proteins play crucial roles in host innate immunity. Because HCQ pretreatment efficiently inhibited DENV-2 infection, we investigated whether HCQ activates the host defense machinery. We found high induction of several innate immunity genes in human A549 cells treated with a high dose of HCQ (Table 1). The genes included IFN-β, IFN-induced protein with tetratricopeptide repeats 3 (IFIT3), C-X-C motif chemokine 10 (CXCL-10), MDA-5, Viperin, MAVS, and tumor necrosis factor receptor-associated factor 3 (TRAF3). In addition, several other genes, such as TNF receptor-associated factor 6 (TRAF6), IRF3, IRF7, RIG-I, ubiquitously expressed transcript V1 (Uxt-V1), tripartite motif-containing protein 21 (TRIM21), and major vault protein (MVP), were detected in HCQ-treated murine J774A.1 macrophages (Table 2). Furthermore, the mRNA expression of inflammatory cytokines such as IL-6, IL-23 p19, TNFα, and IL-12 p40 were induced by HCQ in J774A.1 macrophages (Table 2). Thus, HCQ-controlled DENV-2 infection may be associated with the activation of the host innate immune response. HCQ induced the antiviral genes IFN-β, IFIT3, Viperin, and TRAF3 in both A549 and J774A.1 cells, which suggests that these genes might be the major effectors induced by HCQ to attenuate DENV-2 replication.
Table 1.
Induction of Host Antiviral Genes in Hydroxychloroquine-Treated A549 Cells
| Relative fold induction with HCQ | |||
|---|---|---|---|
| Gene | Accession no. | HCQ, 50 μM | HCQ, 100 μM |
| IFIT3 | NM_001549.4 | 5.08±0.54 | 95.24±10.14 |
| IFN-β | NM_002176.2 | 1.86±1.23 | 28.33±6.06 |
| CXCL10 | NM_001565.3 | 0.64±0.28 | 22.11±3.16 |
| MDA-5 | AF095844 | 1.53±0.44 | 19.58±3.93 |
| Viperin | AF442151.1 | 1.44±0.22 | 14.42±1.71 |
| MAVS | NM_020746.4 | 1.63±0.24 | 4.11±0.46 |
| TRAF3 | NM_145725.2 | 1.42±0.21 | 3.60±0.37 |
Total RNA was harvested from A549 cells without or with hydroxychloroquine (HCQ) treatment (50 and 100 μM) for 24 h. RT-qPCR of mRNA levels normalized to that of the internal control hypoxanthine phosphoribosyltransferase (HPRT) and fold induction over the solvent control (mean±SD).
IFIT3, IFN-induced protein with tetratricopeptide repeats 3; IFN-β, interferon beta; CXCL-10, C-X-C motif chemokine 10; MDA-5, melanoma differentiation-associated protein 5, Viperin/cig5, cytomegalovirus-induced gene 5 protein; MAVS, mitochondrial antiviral signaling protein 5; TRAF3, TNF receptor-associated factor 3.
Table 2.
Induction of Host Antiviral and Inflammation Genes in HCQ-Treated Macrophages
| Gene | Accession no. | Relative fold induction with HCQ |
|---|---|---|
| Antiviral proteins | ||
| IFN-β | NM_010510.1 | 33.93±0.00 |
| Viperin | AK150069.1 | 18.01±0.36 |
| IFIT3 | NM_010501.2 | 8.06±0.66 |
| IRF3 | NM_016849.4 | 4.07±1.90 |
| TRAF3 | NM_011632. | 3.05±0.11 |
| IRF7 | NM_016850.3 | 2.91±0.02 |
| MAVS | NM_144888.2 | 2.87±0.16 |
| TRAF6 | NM_009424.2 | 2.77±0.27 |
| RIG-I | NM_172689.3 | 2.66±0.02 |
| MDA-5 | NM_027835.3 | 2.57±0.14 |
| UXT-V1 | NM_013840.3 | 2.57±0.15 |
| TRIM21 | NM_009277.3 | 2.25±0.39 |
| MVP | NM_080638.3 | 2.16±0.09 |
| Inflammatory cytokines | ||
| IL-6 | NM_031168.1 | 7.69±0.08 |
| IL-23 p19 | NM_031252.2 | 6.42±0.13 |
| TNFα | NM_013693.2 | 4.84±0.07 |
| IL-12 p40 | NM_008352.2 | 3.94±1.02 |
J774A.1 mouse macrophages were treated with 50 μM HCQ or solvent control for 24 h. RT-qPCR of mRNA levels normalized to that of the internal control HPRT and fold induction over the solvent control (mean±SD).
IFN-β, interferon beta; Viperin/cig5, cytomegalovirus-induced gene 5 protein; IFIT3, IFN-induced protein with tetratricopeptide repeats 3; IRF3 and IRF7, interferon regulatory factor 3 and 7; TRAF3 and 6, TNF receptor-associated factor 3 and 6; MAVS, mitochondrial antiviral signaling protein; RIG-I, retinoic acid-inducible gene I protein; MDA-5, melanoma differentiation-associated protein 5, MAVS, mitochondrial antiviral signaling protein 5; UXT-V1, ubiquitously expressed transcript-variant 1; TRIM21, tripartite motif-containing protein 21; MVP, major vault protein; IL-6, interleukin-6, IL-23 p19, interleukin-23 p19; TNFα, tumor necrosis factor alpha; IL-12 p40, interleukin-12 p40.
HCQ activates cellular signaling of innate immunity
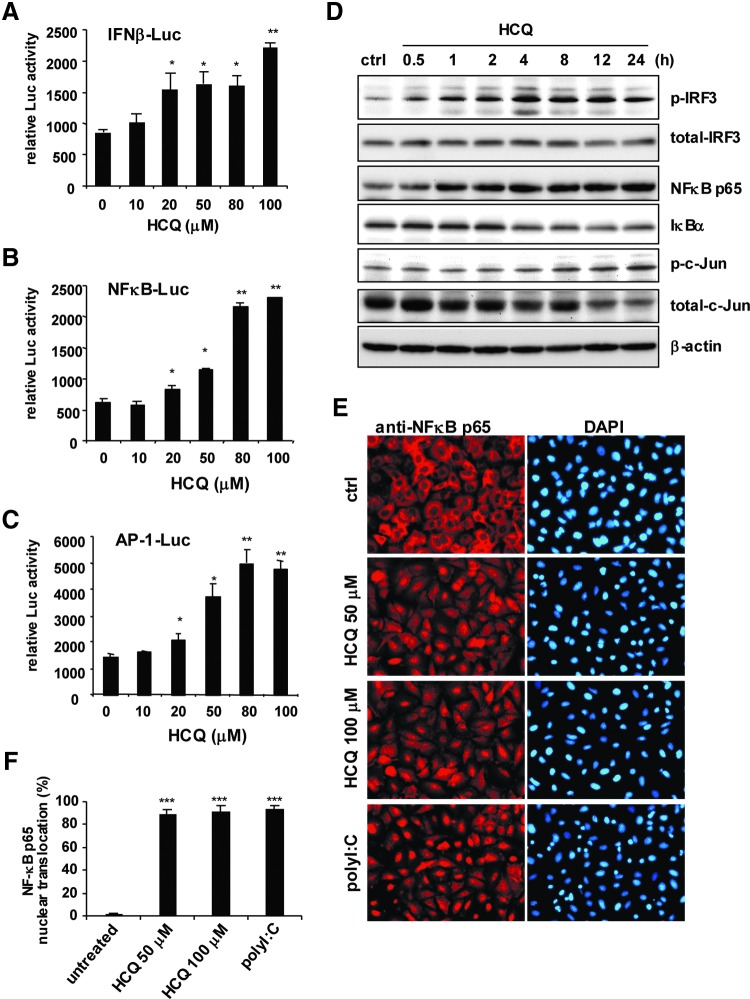
To address how HCQ induces the expression of these host defense genes, we measured the ability of HCQ to turn on the luciferase reporters driven by the IFN-β promoter, NFκB p65, and c-Jun/AP-1 binding sites (Doly and others 1998). HCQ dose-dependently increased IFN-β, NFκB and AP-1 reporter activity (Fig. 3A–C). Furthermore, stimulation with HCQ induced the phosphorylation of IRF3, which indicated IRF3 activation. Phospho-c-Jun, an indicator of AP-1 activation, was also induced after HCQ treatment. HCQ time-dependently activated NFκB by increasing NFκB p65 expression and degrading IκBα (Fig. 3D). HCQ significantly induced NFκB p65 translocation into the nucleus; the pattern was similar with the positive control of polyI:C stimulation (Fig. 3E, F). Thus, our reporter assays, immunoblot results, and NFκB p65 nuclear translocation analysis consistently show that HCQ can activate host antiviral signal transduction pathways.
FIG. 3.
HCQ induces the activities of IFN regulatory factor 3 (IRF3), NFκB, and c-Jun. (A–C) Dual luciferase assay of IFN-β, NFκB, and AP-1 luciferase reporters. A549 cells (3×105) transfected with Luc reporter (0.2 μg) and pRL-TK (0.02 μg) were treated with HCQ for 24 h at the indicated doses. Data are mean±SD from 3 independent tests. *P≤0.05, **P≤0.01 versus untreated group. (D) Immunoblot assay of levels of the indicated proteins in cell lysates from A549 cells treated or not with HCQ (50 μM). (E) IFA of NFκB p65 nuclear translocation with anti-NFκB p65 antibody (left panels), and DAPI staining of nuclei (right panels) in A549 cells (3×105) with HCQ (50 and 100 μM) or polyI:C (1 μg) stimulation for 24 h, and (F) quantification. Data are mean±SD of 5 observation fields. ***P≤0.005 versus untreated group.
MAVS is required for HCQ against DENV-2
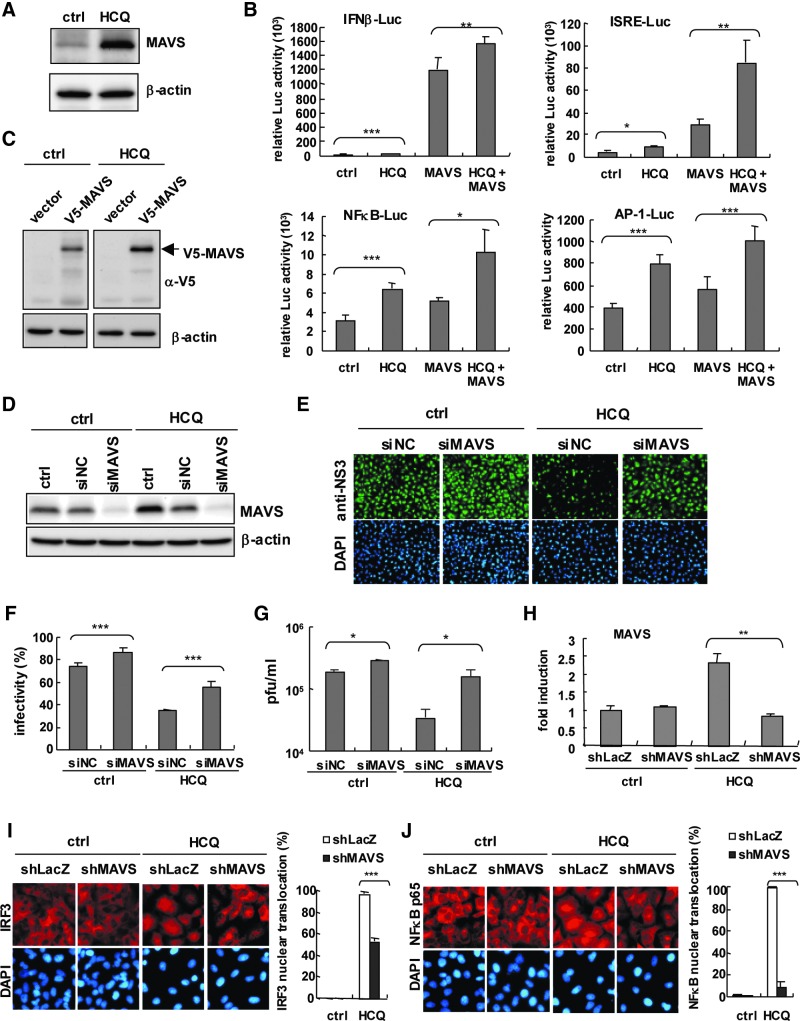
MAVS plays a pivotal role in the type I IFN activation pathway (Kumar and others 2009). Because MAVS is one of the antiviral genes induced by HCQ in A549 cells (Table 1), we aimed to confirm whether MAVS is involved in HCQ antiviral activity. Immunoblot results showed increased MAVS protein expression with HCQ (Fig. 4A), which is consistent with the mRNA induction of MAVS by HCQ. Reporter assay revealed that HCQ enhanced the MAVS-transactivated IFN-β-, ISRE-, NFκB-, and AP-1-driven luciferase activities (Fig. 4B). Exogenous MAVS expression was higher in HCQ-treated than untreated control A549 cells (Fig. 4C). These data implied that MAVS protein was stabilized or decreased by HCQ. We used siRNA transfection to knock down endogenous MAVS expression to further validate the role of MAVS in HCQ-restricted DENV-2. Immunoblot confirmed the efficiency of MAVS knockdown (Fig. 4D). MAVS knocked-down or negative control A549 cells were then infected with DENV-2. As compared with negative control siRNA-transfected cells, in MAVS knocked-down cells, HCQ failed to inhibit DENV-2 infection (Fig. 4E); the estimated data for DENV-2 infectivity on IFA and viral titers showed consistent results (Fig. 4E–G). Moreover, we examined the function of MAVS in the HCQ antiviral effect in our previously established A549 cells carrying shMAVS for MAVS knockdown or shLacZ for negative control (Yu and others 2010). RT-qPCR confirmed the MAVS knockdown efficiency by shRNA that HCQ-mediated MAVS mRNA expression was inhibited in shMAVS-A549 cells (Fig. 4H). HCQ-mediated nuclear translocation of IRF3 and NFκB p65 in control cells was downregulated in MAVS knocked-down cells (Fig. 4I, J). Therefore, MAVS is essential for HCQ-stimulated antiviral activity and activation of the innate immune pathway.
FIG. 4.
HCQ enhances mitochondrial antiviral signaling protein (MAVS) protein expression and its transactivity. (A) Immunoblot assay of endogenous MAVS level in A549 cells treated with 50 μM HCQ for 24 h. (B) Dual luciferase assay of IFN-β, ISRE, NFκB, and AP-1 luciferase reporters. A549 cells (1×105) transfected with Luc reporter (0.5 μg) and pRL-TK (0.04 μg), plus empty vector or pcDNA3.1-V5-MAVS (0.8 μg) treated with HCQ (50 μM) for 24 h. (C) Immunoblot assay with anti-V5 antibody of ectopic V5-MAVS protein level in luciferase assay in (B). (D) Immunoblot assay of MAVS protein level in A549 cells (2×105) transfected with MAVS siRNA (siMAVS) or negative control siRNA (siNC) for 48 h. β-actin was a internal loading control. (E) MAVS and control siRNA-transfected A549 cells were treated with HCQ for 24 h, then infected with DENV-2 at MOI=1. IFA of DENV-2-infected cells with anti-NS3 antibody (green color) and DAPI staining of nuclei (blue color). DENV-2 infectivity (F) and plaque assay of viral titration (G) of (E). (H) RT-qPCR analysis of MAVS mRNA expression in shLacZ or shMAVS stably expressed A549 cells (2×105) treated with HCQ (50 μM) for 24 h. (I and J) IFA of nuclear translocation of IRF3 and NFκB p65 in A549 cells described in (H). Right panels, estimated rate of IRF3 and NFκB p65 nuclear translocation. Data are mean±SD from at least 3 independent tests, *P≤0.05; **P≤0.01; ***P≤0.005.
Blocking type I IFN and TBK1/IKKɛ signaling reduces the anti-DENV-2 activity of HCQ
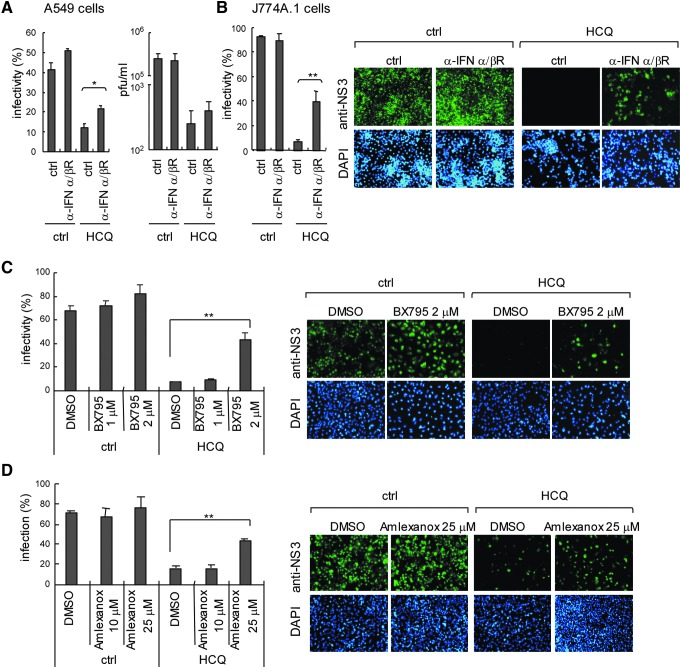
To clarify that the type I IFN antiviral system plays a critical role in DENV inhibition of HCQ, we blocked type I IFN signaling with an IFN-α/β receptor antagonist in A549 cells; cells were then treated with HCQ and infected with DENV-2. DENV-2 infectivity and titration data indicated reduced efficiency of HCQ against DENV-2 in cells with blocked type I IFN signaling (Fig. 5A). HCQ also inhibited ADE-mediated DENV-2 infection in J774A.1 macrophages; again, blocking type I IFN signaling reduced HCQ inhibition efficiency (Fig. 5B). MAVS downstream IκB kinase (IKK)-related kinase IKKɛ and TBK1 phosphorylate IRF3 and IRF7 in type I IFN induction pathway (Sharma and others 2003). Treatment with TBK1 and IKKɛ inhibitors, BX795 (2 μM; Clark and others 2009) and Amlexanox (25 μM; Reilly and others 2013) attenuated HCQ anti-DENV-2 activity (Fig. 5C, D). Again, these data support that HCQ activated the host type I IFN antiviral machinery against DENV infection.
FIG. 5.
Blocking type I IFN signaling reduces HCQ anti-DENV-2 activity. (A) A549 cells (2×105) were incubated with anti-type I IFN receptor antibody (1:100) for 8 h before HCQ treatment. After 24 h of 50 μM HCQ treatment, cells were infected with DENV-2 at MOI=1. Quantification of DENV-2 infectivity (left panel). Plaque-forming assay of DENV titers (right panel). (B) J774A.1 cells were incubated with anti-type I IFN receptor antibody (1:100) for 8 h before 30 μM HCQ incubation for 24 h, then infected with DENV-2 (MOI=10) plus anti-E antibody (1:100). Quantification of DENV-2 infectivity (left panel) and representative immunofluorescence images (right panels). TBK1/IKKɛ inhibitors BX795 (C) and Amlexanox (D) were co-incubated with 30 μM HCQ as indicated in A549 cells; then, cells were infected with DENV-2 (MOI=1) for 24 h. Quantification of DENV-2 infectivity (left panel) and immunofluorescence images (right panels) are shown. The data are mean±SD from 3 independent experiments, *P≤0.05; **P≤0.01.
HCQ-induced ROS contributes to innate immune activation
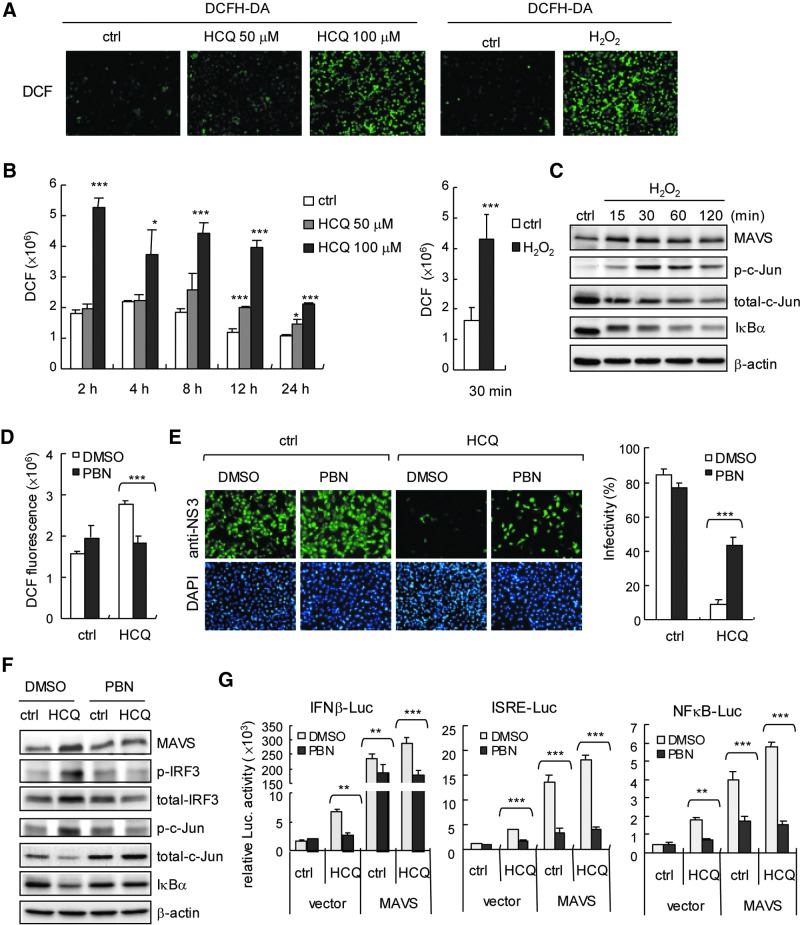
ROS play an important role in normal cell signaling and homeostasis; ROS can also activate the host defence system, for example, promoting a TLR4 signaling pathway to drive cJun/AP-1 and NFκB activation (Kohchi and others 2009; Morgan and Liu 2011) and proinflammatory cytokine production (Bulua and others 2011). In addition, drug-induced oxidative stress in cells has been described (Deavall and others 2012). Therefore, we investigated whether ROS contributes to HCQ-stimulated innate immunity against DENV-2 infection. DCFH-DA assay was used for assessment of cells (Aranda and others 2013). We found the ROS signal of dichlorofluorescein (DCF) in HCQ-treated cells (Fig. 6A) and confirmed that HCQ induced significant ROS production (Fig. 6B). H2O2 stimulation was a positive control (Fig. 6A, B, right panels). Similar to the immunoblot results of HCQ stimulation (Fig. 3D), H2O2 stimulation increased the cellular MAVS and phosphorylated c-Jun level, with IκB degradation (Fig. 6C); so, ROS was involved in the innate immune signaling pathway. To substantiate the role of ROS in the HCQ antiviral effect, we used a nonspecific ROS scavenger, PBN (Fidanboylu and others 2011) to globally decrease cellular ROS level. PBN significantly reduced the level of HCQ-induced ROS generation (Fig. 6D). HCQ-restricted DENV-2 infectivity was attenuated with PBN (Fig. 6E). Along with the PBN-reduced HCQ-mediated ROS generation, HCQ-enhanced expression of MAVS, phospho-IRF3, and phospho-c-Jun was downregulated in PBN-treated cells, and HCQ-mediated IκBα degradation was also inhibited by PBN. (Fig. 6F). Importantly, PBN reduced ROS-inhibited HCQ and HCQ+MAVS-stimulated luciferase reporter activity of IFN-β, ISRE, and NFκB (Fig. 6G). Our data provide novel insights into the antiviral activity of HCQ mediated by ROS and MAVS involved in the host defense machinery, which indicates a key role of ROS in drug-activated host antiviral innate immunity.
FIG. 6.
HCQ induces reactive oxygen species (ROS) generation. (A) ROS were detected by DCFH-DA assay in the A549 cells (2×105) with HCQ treatment or not for 24 h. DCF signal in A549 cells with H2O2 (3%) treatment for 5 min was a positive control. (B) Quantification of ROS production (DCF fluorescence) in HCQ- (left panel) or H2O2-treated A549 cells (2×104) (right panel). Data are mean±SD (N=6) *P≤0.05; ***P≤0.005. (C) Immunoblots of H2O2-treated A549 cells (1×106) with indicated antibodies. (D) A549 cells (2×104) were pretreated with 10 mM N-tert-Butyl-(-phenylnitrone (PBN) for 2 h, then HCQ (50 μM) for 24 h. ROS were detected by DCFH-DA assay. Data are mean±SD (N=6) ***P≤0.005. (E) Left panels, A549 cells (2×105) were pretreated with PBN (10 mM) for 6 h, then HCQ (50 μM) for 24 h. Cells were infected with DENV-2 at MOI=1. IFA of DENV-2 infectivity at 24 h with anti-NS3 antibody (green color) and DAPI staining of nuclei (blue color). Estimated DENV-2 infectivity in the right panel. Data are mean±SD (N=3) ***P≤0.005. (F) Immunoblots of MAVS, phospho-IRF3, and total IRF3 protein levels and loading control β-actin in A549 cells (5×105) pretreated with PBN (10 mM) for 6 h, then HCQ (50 μM) for 24 h. (G) Dual luciferase assay of IFN-β, NFκB, and ISRE luciferase reporters. A549 cells (1×105) cotransfected with Luc reporters (0.6 μg) and pRL-TK (0.06 μg), plus empty vector or pcDNA3.1-V5-MAVS (0.8 μg) were treated with PBN (10 mM) for 6 h, then HCQ (50 μM) for 24 h. Data are mean±SD from 3 independent experiments. **P≤0.001; ***P≤0.005.
Discussion
In this study, we validated HCQ as a potential DENV infection inhibitor that activates the innate immune signaling pathways of IRF3/ISRE, NFκB, and c-Jun/AP-1 and their downstream genes. HCQ induced the expression of antiviral factors such as IFN-β, IFIT3, CXCL-10, Viperin, MDA-5, MAVS, TRAF3, and TRIM21 and the cytokines IL-6, TNFα, IL-12 p40, and IL-23 p19. With MAVS knockdown, we showed the essential role of MAVS in HCQ-inhibited DENV-2 infection. Blocking the type I IFN pathway with an anti-IFN-α/β receptor antibody and TBK1/IKKɛ inhibitors reduced HCQ anti-DENV activity. In addition, HCQ-stimulated ROS generation was a key factor triggering host innate immune activation to restrict DENV-2. Our data reveal an emerging role of HCQ activating the host defence machinery against viral infection.
The role of ROS in inflammation and host defense has been established; mice lacking ROS activity have high susceptibility to bacterial infection (Shiloh and others 1999; Kohchi and others 2009; Bulua and others 2011). Similar to our finding, ROS-promoted host antiviral machinery was demonstrated in study of herpes simplex virus (HSV) infection in that natural-compound stilbenoids showed an anti-HSV effect by inducing ROS expression (Chen and others 2012). ROS might be a signaling messenger during HSV infection for activating NFκB and IRF3 pathways and producing type I IFN and inflammatory cytokines (Gonzalez-Dosal and others 2011; Hu and others 2011). A more sophisticated regulation mechanism was revealed in that ROS directly inducing the posttranslational modification of TRAF3 and TRAF6 with S-glutathionylation is important in activating the innate immune pathway (Gonzalez-Dosal and others 2011). Thus, elucidating whether HCQ promotes post-translational modification of signaling proteins to regulate innate immune responses would be of interest.
We revealed that ROS are critical in HCQ-activated innate immune signaling and the antiviral response and that ER stress was affected by HCQ treatment. HCQ enhanced the expression of IRE1α of the ER stress pathway but not immunoglobulin heavy chain-BiP/GRP78 or PERK. The level of chaperone proteins of ER, such as calnexin, Ero1α, CHOP, and PDI, in the ER was not changed after HCQ treatment. Along with the HCQ, dithiothreitol treatment was a positive control that could activate IRE1α, PERK, BiP, and Ero1α (Supplementary Fig. S2). (Nadanaka and others 2007). Thus, the IRE1α pathway of ER stress might be involved in HCQ-induced innate immunity activation. Because IRE1α has a role in the innate immune response, IRE1α is able to activate signaling of c-Jun/AP-1 and NFκB via TRAF2-JNK, -p38 MAPK and TRAF2-IKK pathways, for the expression of genes associated with host defense (Xu and others 2005). Also, the activation of IRE1α by ER stress is synergistic with TLR activation for cytokine production in macrophages against bacterial infection (Martinon and others 2010), so understanding the role of IRE1α in HCQ against DENV-2 infection would be important. Moreover, ROS production and ER stress activation show a crosstalk feature, which also supports our findings of HCQ (Malhotra and Kaufman 2007).
HCQ and CQ are weak basic drugs that accumulate in the acidic environment of cellular organelles to inhibit the replication of different viruses by interfering with endosome/lysosome trafficking or viral protein maturation during virion maturation. For example, CQ can change the structure of newly produced HIV gp120 glycoprotein (Savarino and others 2001a) and block the proteolytic processing of DENV prM to M protein in acidic post-Golgi vesicles (Randolph and others 1990). We also demonstrated a similar pneumonia of HCQ interrupting the endosome/lysosome pathway by monitoring the cellular expression pattern of transferrin receptor (TfnR), a marker for the early/recycling endosome (Shin and others 2004). Our data showed that TfnR-associated endosome vesicles were enlarged or accumulated and formed punctate structures in HCQ-treated cells (Supplementary Fig. S3). Because CQ was found to suppress JEV internalization (Zhu and others 2012), we do not exclude the possibility of HCQ blocking DENV-2 entry; however, this requires further investigation.
HCQ and CQ are well-known autophagy pathway inhibitors (Rubinsztein and others 2012). Double-stranded RNA stimulation was found to switch cellular activity from autophagy to innate immunity response with TBK1-dependent IRF3 activation (Simicek and others 2013), which suggests a mutually antagonistic regulation between autophagy and innate immunity, and ROS activity might be involved. For example, autophagy can modualte NACHT, LRR, and PYD domain-containing protein 3 (NALP3) inflammasome and ROS-mediated inflammtory cytokine expression (Nakahira and others 2011); autophagy also inhibits ROS-mediated MAVS aggregation to downregulate type I IFN production (Zhao and others 2012). Because autophagy can enhance JEV and DENV replication (Lee and others 2008; Heaton and Randall 2010; Li and others 2012), which might reflect flaviviruses downregulating the innate antiviral response by activating the autophagy pathway for replication. These findings may support our results. Nevertheless, the detailed role of autophagy in virus replication and host defence remains for further validation (Jounai and others 2007; Ke and Chen 2011a; Dong and Levine 2013).
We found that HCQ pretreatment induced the expression of multiple antiviral-associated proteins such as type I IFNs, IFIT3, Viperin, RIG-I, MDA5, and MAVS, demonstrated to suppress flavivirus infection (Shresta and others 2004; Diamond and others 2000; Perry and others 2009; Jiang and others 2010; Nasirudeen and others 2011; Szretter and others 2011; Teng and others 2012). However, the anti-DENV activity of other signaling proteins such as TRAF3, TRAF6, UXT-V1, and MVP remains to be validated (Konno and others 2009; Perez de Diego and others 2010; Huang and others 2012; Liu and others 2012). Because UXT-V1 is an integral component of the MAVS signalosome on mitochondria, which is critical in host antiviral activity (Huang and others 2012), understanding whether HCQ regulates UXT-V1-MAVS signalosome activity is critical.
In our study, HCQ greatly inhibited viral replication in cells treated with HCQ before but not after DENV-2 infection. Thus, the cellular environment change with HCQ treatment may be crucial to restrict DENV-2 infection, whereas the late stage of interference in virion maturation might not be the major mechanism of HCQ anti-DENV-2 activity. Thus, HCQ preventive treatment in the area or season of a dengue pandemic might be a feasible strategy to reduce the severity and spread of DENV outbreak. Moreover, results from clinical trials showed that patients receiving HCQ combined with other antiviral drugs, hydroyurea and didanosine, showed significantly reduced HIV viral load and increased CD4 count (Paton and Aboulhab 2005). Therefore, combined therapy with HCQ and other antiviral drugs could be considered in DENV infection control. For example, an anti-DENV-2 iminosugar derivative (Wu and others 2002) may be used with HCQ to prevent DENV infection.
In conclusion, we demonstrated that HCQ could restrict DENV infection by activating ROS and a MAVS-mediated host IFN antiviral pathway. HCQ is a marketed drug that may be developed for clinical therapy of DENV infection.
Supplementary Material
Acknowledgments
This work was supported by grants from Kaohsiung Veterans General Hospital (VGHKS102-004 and VGHKS103-107) and in part by the Zuoying Branch of Kaohsiung Armed Forces General Hospital, Kaohsiung, Taiwan (ZAFGH 102-16). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Author Disclosure Statement
No competing financial interests exist.
References
- Aranda A, Sequedo L, Tolosa L, Quintas G, Burello E, Castell JV, Gombau L. 2013. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: a quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol In Vitro 27(2):954–963 [DOI] [PubMed] [Google Scholar]
- Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. 2012. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol 42(2):145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandriss MW, Schlesinger JJ. 1984. Antibody-mediated infection of P388D1 cells with 17D yellow fever virus: effects of chloroquine and cytochalasin B. J Gen Virol 65 (Pt 4):791–794 [DOI] [PubMed] [Google Scholar]
- Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. 2011. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 208(3):519–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TH, Chen SR, Yu CY, Lin YS, Chen YS, Kubota T, Matsuoka M, Lin YL. 2012. Dengue virus serotype 2 blocks extracellular signal-regulated kinase and nuclear factor-kappaB activation to downregulate cytokine production. PLoS One 7(8):e41635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TH, Kubota T, Matsuoka M, Jones S, Bradfute SB, Bray M, Ozato K. 2009. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog 5(6):e1000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TH, Liao CL, Lin YL. 2006. Flavivirus induces interferon-beta gene expression through a pathway involving RIG-I-dependent IRF-3 and PI3K-dependent NF-kappaB activation. Microbes Infect 8(1):157–171 [DOI] [PubMed] [Google Scholar]
- Chen X, Qiao H, Liu T, Yang Z, Xu L, Xu Y, Ge HM, Tan RX, Li E. 2012. Inhibition of herpes simplex virus infection by oligomeric stilbenoids through ROS generation. Antiviral Res 95(1):30–36 [DOI] [PubMed] [Google Scholar]
- Clark K, Plater L, Peggie M, Cohen P. 2009. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: a distinct upstream kinase mediates Ser-172 phosphorylation and activation. J Biol Chem 284(21):14136–14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deavall DG, Martin EA, Horner JM, Roberts R. 2012. Drug-induced oxidative stress and toxicity. J Toxicol 2012:645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Levine B. 2009. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 5(6):527–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Roberts TG, Edgil D, Lu B, Ernst J, Harris E. 2000. Modulation of Dengue virus infection in human cells by alpha, beta, and gamma interferons. J Virol 74(11):4957–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doly J, Civas A, Navarro S, Uze G. 1998. Type I interferons: expression and signalization. Cell Mol Life Sci 54(10):1109–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Levine B. 2013. Autophagy and viruses: adversaries or allies? J Innate Immun 5(5):480–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidanboylu M, Griffiths LA, Flatters SJ. 2011. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS One 6(9):e25212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Dosal R, Horan KA, Rahbek SH, Ichijo H, Chen ZJ, Mieyal JJ, Hartmann R, Paludan SR. 2011. HSV infection induces production of ROS, which potentiate signaling from pattern recognition receptors: role for S-glutathionylation of TRAF3 and 6. PLoS Pathog 7(9):e1002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. 2010. Dengue: a continuing global threat. Nat Rev Microbiol 8(12 Suppl):S7–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton NS, Randall G. 2010. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 8(5):422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Schachtele SJ, Lokensgard JR. 2011. Reactive oxygen species drive herpes simplex virus (HSV)-1-induced proinflammatory cytokine production by murine microglia. J Neuroinflammation 8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Liu H, Ge R, Zhou Y, Lou X, Wang C. 2012. UXT-V1 facilitates the formation of MAVS antiviral signalosome on mitochondria. J Immunol 188(1):358–366 [DOI] [PubMed] [Google Scholar]
- Jiang D, Weidner JM, Qing M, Pan XB, Guo H, Xu C, Zhang X, Birk A, Chang J, Shi PY, Block TM, Guo JT. 2010. Identification of five interferon-induced cellular proteins that inhibit west nile virus and dengue virus infections. J Virol 84(16):8332–8341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, Ishii KJ, Kawai T, Akira S, Suzuki K, Okuda K. 2007. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci U S A 104(35):14050–14055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke PY, Chen SS. 2011a. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J Clin Invest 121(1):37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke PY, Chen SS. 2011b. Autophagy: a novel guardian of HCV against innate immune response. Autophagy 7(5):533–535 [DOI] [PubMed] [Google Scholar]
- Kohchi C, Inagawa H, Nishizawa T, Soma G. 2009. ROS and innate immunity. Anticancer Res 29(3):817–821 [PubMed] [Google Scholar]
- Konno H, Yamamoto T, Yamazaki K, Gohda J, Akiyama T, Semba K, Goto H, Kato A, Yujiri T, Imai T, Kawaguchi Y, Su B, Takeuchi O, Akira S, Tsunetsugu-Yokota Y, Inoue J. 2009. TRAF6 establishes innate immune responses by activating NF-kappaB and IRF7 upon sensing cytosolic viral RNA and DNA. PLoS One 4(5):e5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. 2009. Toll-like receptors and innate immunity. Biochem Biophys Res Commun 388(4):621–625 [DOI] [PubMed] [Google Scholar]
- Kyle JL, Harris E. 2008. Global spread and persistence of dengue. Annu Rev Microbiol 62:71–92 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Silverman E, Bargman JM. 2011. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat Rev Nephrol 7(12):718–729 [DOI] [PubMed] [Google Scholar]
- Lee YR, Lei HY, Liu MT, Wang JR, Chen SH, Jiang-Shieh YF, Lin YS, Yeh TM, Liu CC, Liu HS. 2008. Autophagic machinery activated by dengue virus enhances virus replication. Virology 374(2):240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. 2011. Autophagy in immunity and inflammation. Nature 469(7330):323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JK, Liang JJ, Liao CL, Lin YL. 2012. Autophagy is involved in the early step of Japanese encephalitis virus infection. Microbes Infect 14(2):159–168 [DOI] [PubMed] [Google Scholar]
- Lin CH, Schioler KL, Jepsen MR, Ho CK, Li SH, Konradsen F. 2012. Dengue outbreaks in high-income area, Kaohsiung City, Taiwan, 2003–2009. Emerg Infect Dis 18(10):1603–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YL, Liao CL, Chen LK, Yeh CT, Liu CI, Ma SH, Huang YY, Huang YL, Kao CL, King CC. 1998. Study of Dengue virus infection in SCID mice engrafted with human K562 cells. J Virol 72(12):9729–9737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Hao Q, Peng N, Yue X, Wang Y, Chen Y, Wu J, Zhu Y. 2012. Major vault protein: a virus-induced host factor against viral replication through the induction of type-I interferon. Hepatology 56(1):57–66 [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. 2007. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9(12):2277–2293 [DOI] [PubMed] [Google Scholar]
- Martina BE, Koraka P, Osterhaus AD. 2009. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev 22(4):564–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Chen X, Lee AH, Glimcher LH. 2010. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 11(5):411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Liu ZG. 2011. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21(1):103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadanaka S, Okada T, Yoshida H, Mori K. 2007. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol 27(3):1027–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. 2011. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12(3):222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasirudeen AM, Wong HH, Thien P, Xu S, Lam KP, Liu DX. 2011. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLoS Negl Trop Dis 5(1):e926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai H, Sakai S, Hirabayashi T, Shimizu Y, Terasawa K. 1995. Inhibitory effect of bafilomycin A1, a specific inhibitor of vacuolar-type proton pump, on the growth of influenza A and B viruses in MDCK cells. Antiviral Res 27(4):425–430 [DOI] [PubMed] [Google Scholar]
- Paton NI, Aboulhab J. 2005. Hydroxychloroquine, hydroxyurea and didanosine as initial therapy for HIV-infected patients with low viral load: safety, efficacy and resistance profile after 144 weeks. HIV Med 6(1):13–20 [DOI] [PubMed] [Google Scholar]
- Paton NI, Goodall RL, Dunn DT, Franzen S, Collaco-Moraes Y, Gazzard BG, Williams IG, Fisher MJ, Winston A, Fox J, Orkin C, Herieka EA, Ainsworth JG, Post FA, Wansbrough-Jones M, Kelleher P. 2012. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA 308(4):353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de Diego R, Sancho-Shimizu V, Lorenzo L, Puel A, Plancoulaine S, Picard C, Herman M, Cardon A, Durandy A, Bustamante J, Vallabhapurapu S, Bravo J, Warnatz K, Chaix Y, Cascarrigny F, Lebon P, Rozenberg F, Karin M, Tardieu M, Al-Muhsen S, Jouanguy E, Zhang SY, Abel L, Casanova JL. 2010. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity 33(3):400–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry ST, Prestwood TR, Lada SM, Benedict CA, Shresta S. 2009. Cardif-mediated signaling controls the initial innate response to dengue virus in vivo. J Virol 83(16):8276–8281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph VB, Winkler G, Stollar V. 1990. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology 174(2):450–458 [DOI] [PubMed] [Google Scholar]
- Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, Downes M, Yu RT, Liddle C, Evans RM, Oh D, Li P, Olefsky JM, Saltiel AR. 2013. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat Med 19(3):313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Codogno P, Levine B. 2012. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11(9):709–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. 2003. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis 3(11):722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A, Gennero L, Chen HC, Serrano D, Malavasi F, Boelaert JR, Sperber K. 2001a. Anti-HIV effects of chloroquine: mechanisms of inhibition and spectrum of activity. AIDS 15(17):2221–2229 [DOI] [PubMed] [Google Scholar]
- Savarino A, Gennero L, Sperber K, Boelaert JR. 2001b. The anti-HIV-1 activity of chloroquine. J Clin Virol 20(3):131–135 [DOI] [PubMed] [Google Scholar]
- Seligman SJ, Gould EA. 2008. Safety concerns with regard to live attenuated flavivirus vaccines. J Infect Dis 198(5):794–795 [DOI] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300(5622):1148–1151 [DOI] [PubMed] [Google Scholar]
- Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10(1):29–38 [DOI] [PubMed] [Google Scholar]
- Shin HW, Morinaga N, Noda M, Nakayama K. 2004. BIG2, a guanine nucleotide exchange factor for ADP-ribosylation factors: its localization to recycling endosomes and implication in the endosome integrity. Mol Biol Cell 15(12):5283–5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shresta S, Kyle JL, Snider HM, Basavapatna M, Beatty PR, Harris E. 2004. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J Virol 78(6):2701–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simicek M, Lievens S, Laga M, Guzenko D, Aushev VN, Kalev P, Baietti MF, Strelkov SV, Gevaert K, Tavernier J, Sablina AA. 2013. The deubiquitylase USP33 discriminates between RALB functions in autophagy and innate immune response. Nat Cell Biol 15(10):1220–1230 [DOI] [PubMed] [Google Scholar]
- Szretter KJ, Brien JD, Thackray LB, Virgin HW, Cresswell P, Diamond MS. 2011. The interferon-inducible gene viperin restricts West Nile virus pathogenesis. J Virol 85(22):11557–11566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng TS, Foo SS, Simamarta D, Lum FM, Teo TH, Lulla A, Yeo NK, Koh EG, Chow A, Leo YS, Merits A, Chin KC, Ng LF. 2012. Viperin restricts chikungunya virus replication and pathology. J Clin Invest 122(12):4447–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricou V, Minh NN, Van TP, Lee SJ, Farrar J, Wills B, Tran HT, Simmons CP. 2010. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis 4(8):e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. 2005. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SF, Lee CJ, Liao CL, Dwek RA, Zitzmann N, Lin YL. 2002. Antiviral effects of an iminosugar derivative on flavivirus infections. J Virol 76(8):3596–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC. 2005. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115(10):2656–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CY, Chiang RL, Chang TH, Liao CL, Lin YL. 2010. The interferon stimulator mitochondrial antiviral signaling protein facilitates cell death by disrupting the mitochondrial membrane potential and by activating caspases. J Virol 84(5):2421–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sun X, Nie X, Sun L, Tang TS, Chen D, Sun Q. 2012. COX5B regulates MAVS-mediated antiviral signaling through interaction with ATG5 and repressing ROS production. PLoS Pathog 8(12):e1003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YZ, Xu QQ, Wu DG, Ren H, Zhao P, Lao WG, Wang Y, Tao QY, Qian XJ, Wei YH, Cao MM, Qi ZT. 2012. Japanese encephalitis virus enters rat neuroblastoma cells via a pH-dependent, dynamin and caveola-mediated endocytosis pathway. J Virol 86(24):13407–13422 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.