Abstract
Retinoic acid (RA), an active metabolite converted from vitamin A, plays an active role in immune function, such as defending against infections and immune regulation. Although RA affects various types of immune cells, including antigen-presenting cells, B lymphocytes, and T lymphocytes, whether it affects natural killer T (NKT) cells remain unknown. In this study, we found that RA decreased interferon (IFN)-γ production by activated NKT cells through T-cell receptor (TCR) and CD28. We also found that RA reduced extracellular signal-regulated kinase (ERK) phosphorylation, but increased phosphatase 2A (PP2A) activity in TCR/CD28-stimulated NKT cells. The increased PP2A activity, at least partly, contributed to the reduction of ERK phosphorylation. Since inhibition of ERK activation decreases IFN-γ production by TCR/CD28-stimulated NKT cells, RA may downregulate IFN-γ production by TCR/CD28-stimulated NKT cells through the PP2A-ERK pathway. Our results demonstrated a novel function of RA in modulating the IFN-γ expression by activated NKT cells.
Introduction
Natural killer T (NKT) cells, a subset of lymphocytes, possess distinct invariant T-cell receptors (TCRs) and an NK cell marker, NK1.1 (Bendelac and others 2007). Similar to conventional T cells, NKT cells develop from double positive thymocytes by passing through 3 stages that are defined by the expression of CD44 and NK1.1 (Matsuda and Gapin 2005; Bendelac and others 2007). NKT cells are distributed among several organs, including the spleen, bone marrow, lymph nodes, and liver. Uniquely, ∼10%–20% of liver mononuclear cells (MNCs) are NKT cells (Bendelac and others 2007). NKT cell activation is initiated by the engagement of TCRs with glycolipid antigens presented in the major histocompatibility complex class I-like molecule, CD1d (Kawano and others 1997). On stimulation, NKT cells are rapidly activated and produce multiple cytokines that regulate B, NK, or dendritic cells (DCs) (Brennan and others 2013). Furthermore, NKT cells exhibit other regulatory functions, such as tumor rejection, clearance of pathogens, and regulation in autoimmune diseases (Godfrey and Kronenberg 2004).
Several studies have proved that cytokines secreted by NKT cells under different stimulations are not identical. Although a well-known antigen, α-galactosylceramide (αGC) induces the production of Th1 and Th2 cytokines from NKT cells and enhances immune responses to infection, autoimmune diseases, and tumor rejection; the structural variants of αGC induce a systemic polarization of the cytokine production initiated by NKT cells (Miyamoto and others 2001; Kronenberg 2005). For example, OCH, a truncated derivative of αGC, has been reported to polarize NKT cells, resulting in Th2 responses and suppressing Th1-caused autoimmune diseases (Miyamoto and others 2001; Chiba and others 2004). However, the C-glycoside analog of αGC was reported to induce a Th1 polarizing effect and enhance the responses against pathogens and tumors (Fujii and others 2006). A few studies have indicated that this bias of cytokine production might result from the binding stability between lipids and CD1d, which alters TCR affinity, TCR signaling, and duration or localization of Ag presentation (Stanic and others 2003; Oki and others 2004; Im and others 2009; Sullivan and others 2010). In addition, although studies have showed that the biased cytokine secretion from NKT cells is regulated by prostaglandin D2 and Tim-1, the mechanisms underlying this cytokine bias remain unclear (Torres and others 2008; Kim and others 2010).
Retinoic acid (RA), the most active metabolite of vitamin A, is essential in the regulation of innate and adaptive immunity (Pino-Lagos and others 2008; Kim 2011). RA activity is mediated by RA receptors (RARs) and retinoid X receptors (RXRs) that form hetero dimers to bind to specific hormone response elements in the promoter/enhancer region of target genes (Blomhoff and Blomhoff 2006). Through the formation of RAR-RXR dimers, RA regulates the expression of genes associated with cell proliferation, differentiation, and homeostasis (Blomhoff and Blomhoff 2006). With regard to immune cells, studies have reported the participation of RA in the differentiation of Th1/Th2 and regulatory T (Treg) cells, plasma cell formation, and T-cell imprinting for gut homing (Iwata and others 2003, 2004; Mucida and others 2007; Ertesvag and others 2009). These findings suggest a crucial regulatory role of RA in the immune system. In the human body, most vitamin A is stored in the liver, which also contains condensed amounts of NKT cells. However, the relationship between RA and NKT cells is obscure. Therefore, we analyzed αGC-induced responses of hepatic NKT cells in the presence or absence of RA. The results indicated that RA played a modulating role in NKT cell activation and interferon (IFN)-γ production by phosphatase 2A (PP2A) via extracellular signal-regulated kinase (ERK) pathway. Thus, RA might play a role in regulating NKT cell activity and cytokine production.
Materials and Methods
Animals
C57BL/6 mice were purchased from the National Laboratory Animal Center (Taipei, Taiwan) and inbred in an experimental animal facility at the Institute of Cellular and Organism Biology in Academia Sinica (Taipei, Taiwan). All of the experiments conformed to the principles of laboratory animal research outlined by Academia Sinica and were approved by the Academia Sinica Institutional Animal Care and Utilization Committee (IACUC) (Permit No. RMiGRCHW2007033).
Reagents
αGC was purchased from Funakoshi (Tokyo, Japan) and dissolved in phosphate-buffered saline (PBS) containing 0.5% Tween-20 (Sigma-Aldrich, St. Louis, MO). RA (all-trans) and okadaic acid (OA) were purchased from Enzo Life Sciences (Farmingdale, NY), LE540 from Wako (Osaka, Japan), PD98059 from Calbiochem (Darmstadt, Germany), and corn oil from Sigma-Aldrich. All the chemicals were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich). For phenotypic analyses, antibodies (Abs) for TCR-β, NK1.1, CD69, CD122, CD25, CD40L, CD27, and PD-L1 were purchased from eBioscience (San Diego, CA), and mouse CD1d dimer was purchased from BD Biosciences (San Diego, CA).
Analyses of phenotypes and cytokine production from NKT cells by using flow cytometry
The C57BL/6 mice were treated with αGC (4 μg/mice) and RA (60 mg/kg) through an intraperitoneal injection. After 1.5 h, liver MNCs were harvested and stained with the Abs mentioned earlier. The phenotypes of the NKT cells gated by the expression of TCR-β and NK1.1 or CD1d dimer were analyzed using a fluorescence-activated cell sorting (FACS) canto II flow cytometer (BD Biosciences). For evaluating cytokine production from the NKT cells, the liver MNCs from treated the mice or the cells stimulated with αGC and RA were treated with 10 μg/mL of Brefeldin A (Sigma-Aldrich) at 37°C for 6 h. After being washed with PBS, the cells were fixed using a Fix/Perm buffer at 4°C for 30 min and permeabilized with a Perm/Wash buffer according to the manufacturer's instructions (eBioscience). IFN-γ or IL-4 expression was detected using phycoerythrin (PE)-conjugated Abs against IFN-γ or IL-4 (eBioscience). The stained cells were analyzed using the FACS canto II cytometer (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR).
Preparation of liver MNCs and expansion of primary NKT cells
The mice were euthanized at the age of 6–10 weeks. After perfusion with PBS, their livers were collected and dissected using nylon meshes and syringes. Debris was removed using 37% percol (GE Healthcare Life Sciences, Pittsburgh, PA) with continuous spinning at 500 g for 10 min. After RBC depletion with an ammonium-chloride-potassium (ACK) buffer, liver MNCs were cultured in RPMI 1640 medium (Life Technologies, Grand Island, NY) containing 10% fetal bovine serum, 1% penicillin–streptomycin, 1 mM pyruvate, 1% nonessential amino acid, 50 μM 2-mercaptoethanol, and 20 mM HEPES and were stimulated with αGC (100 ng/mL) in the presence or absence of RA or TTNPB (Sigma-Aldrich) at the indicated concentrations. For in vitro expansion of the NKT cells, these cells were enriched with liver MNCs using the NK1.1 iNKT cell isolation kit (Miltenyi Biotech, Bergisch Gladbach, Germany) following the manufacturer's instructions. After enrichment, at least 85% of the cells were labeled with CD1d-dimer pulsed with αGC (BD Biosciences). Subsequently, the enriched NKT cells were expanded as previously described (Watarai and others 2008). Briefly, the enriched NKT cells were stimulated with plate-bound anti-CD3 (3 μg/mL) Abs and soluble anti-CD28 Abs (1 μg/mL) in RPMI-1640 medium containing 10 μg/mL murine IL-2 (Peprotech, Rocky Hill, NJ) and 1 μg/mL murine IL-12 (R&D Systems, Minneapolis, MN). After culture for 2 days, the cells were placed in a medium containing murine IL-7 (5 μg/mL; R&D Systems), and the medium was replaced every 2 days. The expanded NKT cells were re-stimulated on day 8 with plate-bound anti-CD3 Abs for 3 days and then maintained in the medium containing IL-7. On day 15, the cells were stimulated through TCR/CD28 by using plate-bound anti-CD3 Ab (10 μg/mL) and soluble anti-CD28 Ab (1 μg/mL) or αGC-pulsed DCs for cytokine production, or by using Western blotting for the mitogen-activated protein kinase (MAPK) pathway assays.
Cytokine enzyme-linked immunosorbent assay or multiplex assay
The IFN-γ and IL-4 levels in the supernatant or serum were analyzed using the enzyme-linked immunosrobent assay (ELISA; eBioscience). For cytokine profiling, the NKT cells (2×104) enriched with liver MNCs were pretreated with LE540 or DMSO and stimulated through TCR/CD28 by using plate-bound anti-CD3 Abs (10 μg/mL; BD Biosciences) and soluble anti-CD28 Abs (1 μg/mL; BD Biosciences) in the presence or absence of RA (1 μM). After culture for 2 days, the cytokine levels in the supernatants were determined using Luminex (Merck Millipore, Billerica, MA) for IL-2, IL-3, IL-5, IL-6, IL-9, IL-10, and IL-13 or ELISA for IFN-γ and IL-4.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from the NKT cells stimulated with Abs for 24 h by using Trizol (Life Technologies) and converted to cDNA by using superscript III (Life Technologies) based on the manufacturer's instructions. The mRNA levels for IFN-γ, IL-4, CD69, or GAPDH were detected using the Taqman probe (Applied Biosystems, Foster City, CA) for IFN-γ, IL-4, and GAPDH or the SYBR Green (ThermoFisher Scientific, Waltham, MA) for CD69 and determined using the ABI-7300 real-time polymerase chain reaction (PCR) System (Applied Biosystems). The primers for CD69 included Forward-TGGAACATTGGATTGGGCTGA and Reversed-CTCACAGTCCACAGCGGTAA, those for c-Fos included Forward-GGAGAATCCGAAGGGAACGG and Reversed-GCAATCTCAGTCTGCAACGC, those for Bcl-2 included Forward-ACCCCTGGTGGACAACATC and Reversed-AGAAATCAAACAGAGGTCGCA, those for Bcl-XL included Forward-TGCGTGGAAAGCGTAGACAA and Reversed-GCTGCATTGTTCCCGTAGAG, those for Mcl-1 included Forward-TTTCTTTCGGTGCCTTTGTGG and Reversed-TCTGATGCCGCCTTCTAGGT, those for CCNE1 included Forward-GGCGAGGATGAGAGCAGTTC and Reversed-CGATCAAAGAAGTCCTGTGCC, those for CDK2 included Forward-CTCACGGGCATTCCTCTTCC and Reversed-CAGCTTGATGGACCCCTCTG, and those for CDK4 included Forward-GGAGCGTTGGCTGTATCTTT and Reversed-GTCGCTTTCCTCCTTGTGC.
Analyses for the MAPK pathways by using intracellular staining and Western blotting and the modification on PP2AC by using Western blotting
For intracellular staining, the NKT cells were expanded for 14 days and pretreated using DMSO or RA. After incubation at 37°C for 16 h, the cells were washed and stimulated with plated-bound anti-CD3 (10 μg/mL) Abs and soluble anti-CD28 (1 μg/mL) Abs. The cells were then fixed with 2% paraformaldehyde (Sigma-Aldrich) and incubated at 37°C for 10 min. After permeabilization with Fix/Perm buffer (eBioscience) at 4°C for 30 min, the cells were washed with Perm/Wash buffer (eBioscience) and stained with PE-conjugated Abs against phospho-ERK (p-ERK), phospho-JNK(p-JNK), and phosoh-p38 (p-p38) (Cell Signaling Technology, Danvers, MA). The mean of fluorescence (MFI) of each cell sample was determined using flow cytometry. The difference was determined according to the MFI of the Abs-stimulated cells/MFI of the nonstimulated cells. For Western blotting, the expanded NKT cells were pretreated with RA and stimulated with the Abs mentioned earlier. After stimulation for 30 min, proteins were extracted from the NKT cells using radioimmunoprecipitation assay buffer containing both protease (Sigma-Aldrich) and phosphatase inhibitors (Roche Applied Science, Indianapolis, IN). The proteins were separated using 10% sodium dodecyl sulfate-polyacrylamide gels and then transferred to PVDF membranes (GE Healthcare Life Sciences). After blocking with TBS-0.05% Tween-20 containing 5% no-fat milk, the blots were incubated in the presence of the primary Abs specific for p-ERK, ERK, p-JNK, JNK, p-p38, p38, p-IκBα, IκBα (Cell Signaling Technology), and GAPDH (Genetex, Irvin, CA), followed by horseradish peroxidase -conjugated anti-rabbit or anti-mouse Abs (GE Healthcare Life Sciences). The membranes were developed using ECL reagent (GE Healthcare Life Sciences), and protein signals were detected using the BioSpectrum image system (UVP LLC, Upland, CA). The density of p-ERK in the RA-treated cells compared with that in the vehicle-treated cells was quantified and normalized to total ERK by using the VisionWorksLS software (UVP LLC). For modification on PP2AC, the proteins on the membrane were observed with Abs for p-PP2AC (Abcam, Cambridge, United Kingdom) and PP2AC (Cell Signaling Technology) as described earlier and detected by using BioSpectrum image system (UVP LLC).
Measurement of PP2A activity in NKT cells
The NKT cells were pretreated with RA for 16 h. After stimulation with plate-bound anti-CD3 and soluble anti-CD28 Abs for 30 min, the cells were lysed in lysis buffer containing 50 mM HEPES (pH=7.5), 0.1 mM EGTA, 0.1 mM EDTA, 120 mM NaCl, and 0.5% NP-40 (Sigma-Aldrich). The PP2A activity was determined using the PP2A activity assay kit (R&D Systems). Briefly, cell lysates were added to the ELISA plate coated with anti-PP2A Abs and incubated at 4°C for 3 h. After the washing process, the phosphate-labeled substrates for PP2A were added into wells and incubated at 37°C for 30 min. The PP2A activity was determined by the amounts of free phosphate detected through the dye-binding assay using malachite green and molybdic acid.
Statistical analysis
The results were expressed by means±standard error of the mean. For statistical analysis, various experimental groups were compared using the Student's t-test with Prism 5 (GraphPad Software, La Jolla, CA). P<0.05 was considered statistically significant.
Results
Cytokine production and phenotypes of NKT cells in vivo RA treatment
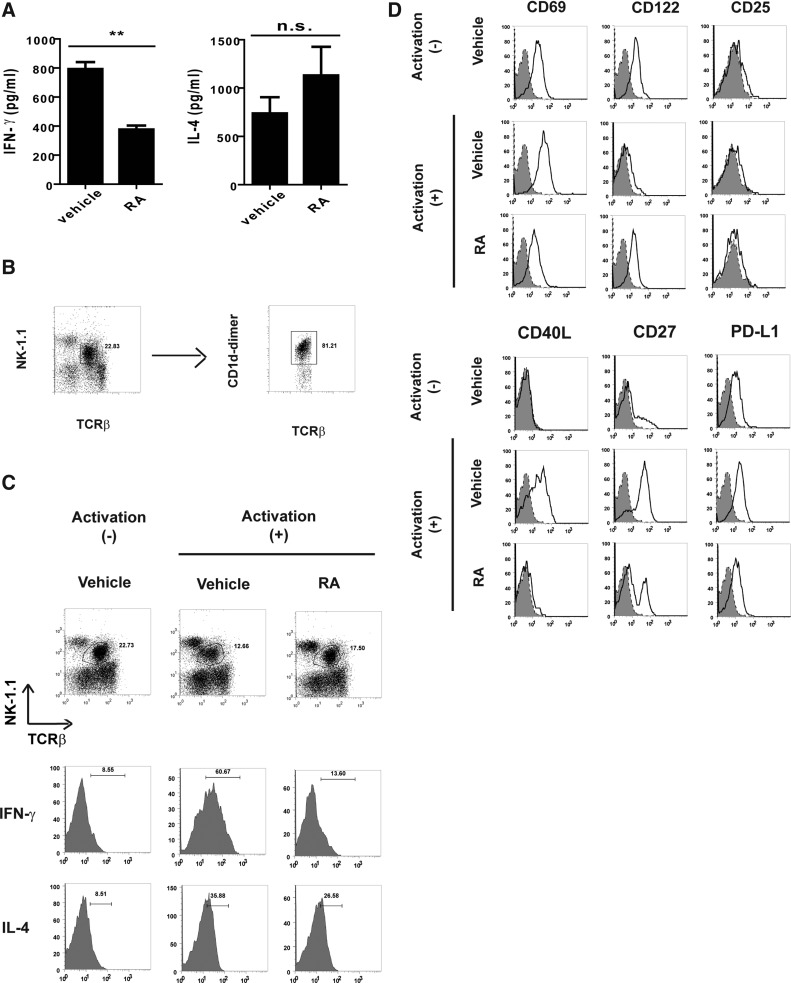
To determine the modulating effect of RA on hepatic NKT cell responses, the serum samples derived from the mice treated with αGC and a vehicle (corn oil) or RA were analyzed for 2 major NKT cell cytokines, IFN-γ and IL-4. The mice treated using αGC and RA (αGC/RA) exhibited decreased serum IFN-γ levels compared with those exhibited by the αGC-treated mice (αGC) (Fig. 1A). Although the RA-treated mice exhibited slightly increased serum IL-4 levels, no significant difference was observed in the IL-4 levels between the αGC-treated and the αGC/RA-treated mice (Fig. 1A). In addition, we analyzed cellular IFN-γ and IL-4 in the NKT cells expressing TCR-β and NK1.1 from αGC- or αGC/RA-treated mice. Most TCR-β- and NK1.1-expressing cells in the liver MNCs were CD1d-dimer positive cells (Fig. 1B). Moreover, the results demonstrated that the IFN-γ levels in the NKT cells from the αGC/RA-treated mice were lower than those in the NKT cells from the αGC-treated mice. However, no significant difference was observed between the IL-4 levels in the NKT cells from the αGC-treated or the αGC/RA-treated mice (Fig. 1C). These results indicated the in vivo modulating effects of RA on the IFN-γ production, but not the IL-4 production, in the NKT cells. To identify any additional effects of RA on the NKT cells, we further analyzed the phenotypes of the hepatic NKT cells from the αGC- or αGC/RA-treated mice. Although no CD25 expression was detected in the hepatic NKT cells, enhanced CD69 expression was observed in the NKT cells from αGC-treated mice. However, the NKT cells from αGC/RA-treated mice exhibited a decrease in this αGC-induced CD69 (Fig. 1D). In addition, the NKT cells from the RA-treated mice exhibited a decrease in the enhanced αGC-induced expressions of the other activation markers CD27, CD40L, and PD-L1 (Fig. 1D). These results revealed that the treatment reduced the NKT cells activation, indicating that RA altered the in vivo NKT cell responses induced by the αGC antigen.
FIG. 1.
The effect of retinoic acid (RA) on cytokine production and natural killer T (NKT) cells in α-galactosylceramide (αGC)-treated mice. Mice (n=3 for each group) received one intra-peritoneal injection of αGC (4 μg/mouse) with a vehicle (corn oil) or RA (60 mg/kg), and were analyzed at the indicated time. (A) Cytokines in serum. Serum samples were collected and analyzed for interferon (IFN)-γ (17 h) or IL-4 (1.5 h) by using ELISA. (B) Analyses of NKT cells in liver mononuclear cells (MNCs). Liver MNCs were isolated and stained with antibodies (Abs) for T-cell receptor β (TCRβ), NK1.1, and CD1d dimer loaded with αGC (CD1d-dimer). The proportion of NKT cells (CD1d-dimer+ cells) was assessed in TCR-β+ NK1.1+ T cells. (C) Cytokine production in NKT cells. Liver MNCs were isolated, treated with Brefeldin A for 6 h, and analyzed for IFN-γ or IL-4 in the NKT cells (TCR-β+ and NK1.1+) by intracellular staining. (D) NKT cell phenotypes. The mice were treated for 1.5 h. Liver MNCs were isolated and stained with the Abs mentioned earlier (solid line) or the isotype control Ab (shadow). The surface markers on the TCR-β+ NK1.1+ cells were analyzed by flow cytometry. The data are representative of 2 (A) or 3 (C, D) independent experiments. The data are presented as mean±SEM. **P<0.01; n.s., no significance.
Modulating effects of RA on hepatic NKT cell responses
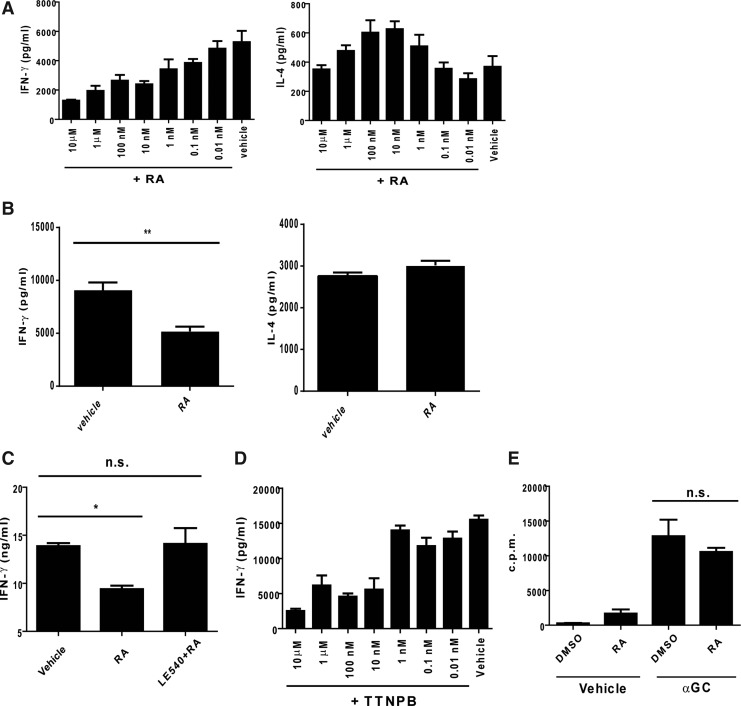
To further understand the modulating effects of RA on the hepatic NKT cell responses, we isolated the liver MNCs and analyzed the cytokine production in the presence or absence of RA at different concentrations. Compared with the cells in the absence of RA, the RA concentrations and the IFN-γ production revealed an inverse correlation in the presence of RA (Fig. 2A). Furthermore, with regard to the IL-4 production, the IL-4 expression was slightly enhanced in the presence of RA at concentrations of 10 and 100 nM but was absent at other RA concentrations (Fig. 2A). To exclude the possibility of IFN-γ production from the NK cells that were trans-activated during αGC treatment, the NKT cells were enriched with MACs and stimulated with splenic DCs pulsed with αGC. The IFN-γ levels from NKT cells consistently decreased under the RA treatment, but the IL-4 expression from the NKT cells was not altered by this treatment (Fig. 2B). This modulating effect of RA on IFN-γ was interfered with by LE540, an antagonist of the RA receptor, suggesting the specificity of the effects of RA on the NKT cells (Fig. 2C). Furthermore, the IFN-γ expression reduced in the presence of TTNPB, an agonist of the RA receptor, thus mimicking the effects of RA (Fig. 2D). Although RA was reported to regulate cell proliferation (Blomhoff and Blomhoff 2006), the activation-induced proliferation of the NKT cells was not affected by the RA treatment in this study (Fig. 2E). Thus, these results indicated that RA modulated only the IFN-γ production from the NKT cells.
FIG. 2.
RA modulated IFN-γ production by αGC-stimulated NKT cells in vitro. (A) Correlation between cytokine production from NKT cells and RA. The liver MNCs were stimulated with αGC in the presence of RA at the indicated concentrations for 2 days. The level of cytokines in the supernatants was assessed by ELISA. (B) Cytokine production from NKT cells. The NKT cells (1×105) were sorted from the liver MNCs and cultured with splenic dendritic cells (DCs) (1×105) pulsed with αGC. The IFN-γ and IL-4 levels in the supernatants were assessed using ELISA (n=4 for each group). (C) Confirming effect of RA on IFN-γ. The liver MNCs were pretreated with a vehicle [dimethyl sulfoxide (DMSO)] or LE540 (3.75 μM) for 1 h and stimulated with αGC in the presence or absence of RA (1 μM). The IFN-γ levels were measured using ELISA (n=3 for each group). (D) Effect of TTNPB on IFN-γ production. The liver MNCs were stimulated with αGC in the presence of TTNPB at the indicated concentrations. The IFN-γ levels were measured using ELISA after 2 days of culture (n=3 for each group). (E) Effect of RA on proliferation. The liver MNCs were stimulated with the vehicle or αGC in the presence or absence of RA. After culture for 1 day, the cells were labeled as H3-thymidine (1 μCi/well) and cultured for an additional 18 h. Proliferation was determined by the intensity of incorporated radioactivity (n=3 for each group). The data are representative of 2 (B–D) or 4 independent experiments (A, E). All data are presented as mean±SEM.*P<0.05, **P<0.01; n.s., no significance.
RA altered cytokine profiles of NKT cells and downregulated IFN-γ transcription in a TCR-dependent manner
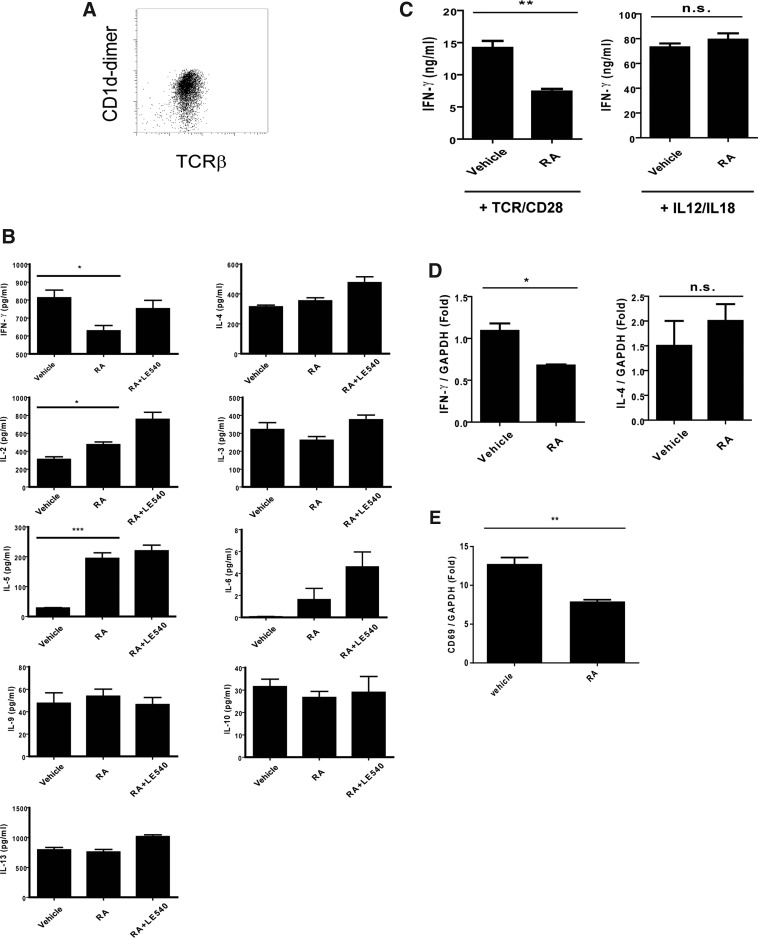
As previously reported, in addition to IFN-γ and IL-4, activated NKT cells produced multiple cytokines, including Th1- and Th2-related cytokines (Kronenberg 2005). Therefore, in this study, to realize the cytokine profiles of the NKT cells under the RA treatment, the NKT cells from the liver tissues were enriched with MACS and stimulated with plate-bound anti-CD3 and soluble anti-CD28 Abs in the presence or absence of RA. The cytokine profiles of the experimental NKT cells were assessed using multiplex assay and ELISA. The enriched NKT cells were confirmed using CD1d-dimer and TCR-β double positive staining (Fig. 3A). The results showed that the RA treatment not only decreased IFN-γ production but also enhanced the IL-2 and IL-5 expression (Fig. 3B). However, the expression of other cytokines, including IL-3, IL-4, IL-6, IL-9, IL-10, and IL-13, was not altered by the RA treatment. This effect of RA on the cytokines modulated the NKT cells with Th2-like cytokine profile. Unexpectedly, LE540 treatment only reduced the effect of RA on IFN-γ but not on other cytokines that were altered by RA (Fig. 3B). Considering that the β subunit of RARs was the major target for LE540, different RARs subunits may have contributed to the modulating effect of RA on IFN-γ and on other cytokines (Li and others 1999). Studies have reported that IFN-γ production from NKT cells can be induced in a TCR-dependent or TCR-independent manner (Baxevanis and others 2003). Nevertheless, the exact mechanism of how RA modulates through NKT cell activation remains unknown. To resolve this problem, we enriched the NKT cells and analyzed the effects of RA on IFN-γ production induced through TCR/CD28 by plate-bound CD3 Abs and soluble CD28 Abs or IL-12 and IL-18, respectively. The former stimulation was used to determine whether RA affected the IFN-γ production through TCR signaling, and the latter one was used to assess the non-TCR pathway. Compared with cells in the absence of RA, the TCR/CD28-stimulated NKT cells, but not the IL-12/IL-18-stimulated cells, exhibited a decrease in the IFN-γ production in the presence of RA (Fig. 3C). Moreover, the RA treatment resulted in decreased mRNA levels of IFN-γ from the activated NKT cells but not the IL-4 levels in these cells (Fig. 3D). These results indicated that the decreased IFN-γ gene expression by RA was pertinent to the TCR-dependent activation. Consistently, the decreased expression of CD69 in the activated NKT cells by the RA treatment was similar to the in vivo results observed earlier (Figs. 3E and 1D). Therefore, these findings suggest that RA not only modulated the NKT cells with an altered cytokine profile but also reduced the cell activation and IFN-γ expression in a TCR-dependent manner.
FIG. 3.
Modulating effect of RA on cytokine profile of NKT cells and IFN-γ production induced by different stimulations. (A) Enrichment of NKT cells with MACS. The NKT cells were enriched with MACS and stained with Abs for TCR-β and CD1d-dimer. (B) Cytokine profile of NKT cells. The NKT cells (2×104) isolated from the liver MNCs were pretreated with a vehicle (DMSO) or LE540 (3.75 μM) for 1 h and stimulated through TCR/CD28 by using plate-bound anti-CD3 Abs (10 μg/mL) plus soluble anti-CD28 Abs (1 μg/mL) in the presence of or the vehicle (DMSO) or RA (1 μM). The level of cytokine in the supernatants was determined using multiplex assay or ELISA (n=3 for each group). (C) The NKT cells were stimulated through TCR/CD28 using plate-bound anti-CD3 plus soluble anti-CD28 Abs (TCR/CD28, left) or IL-12 (10 ng/mL) and IL-18 (100 ng/mL) (IL-12/IL-18, right) in the presence of the vehicle or RA. The IFN-γ level was measured using ELISA (n=3 for each group). (D) Liver NKT cells were stimulated through TCR/CD28 using plate-bound anti-CD3 and soluble anti-CD28 Abs in the presence of the vehicle or RA. After culture for 24 h, total RNA was extracted and the mRNA level of IFN-γ and IL-4 were determined using quantitative PCR (qPCR) and normalized to GAPDH (n=3 for each group). (E) mRNA level of CD69 in NKT cells were treated with RA. The mRNA level (normalized to GAPDH) of CD69 in NKT cells were determined using qPCR after stimulation with Abs in the presence of the vehicle or RA for 24 h. The data are representative of 2 (B–E) or 3 independent experiments (D) and are presented as mean±SEM. *P<0.05, **P<0.01, ***P<0.001; n.s., no significance.
RA modulated the IFN-γ production from NKT cells via the ERK pathway
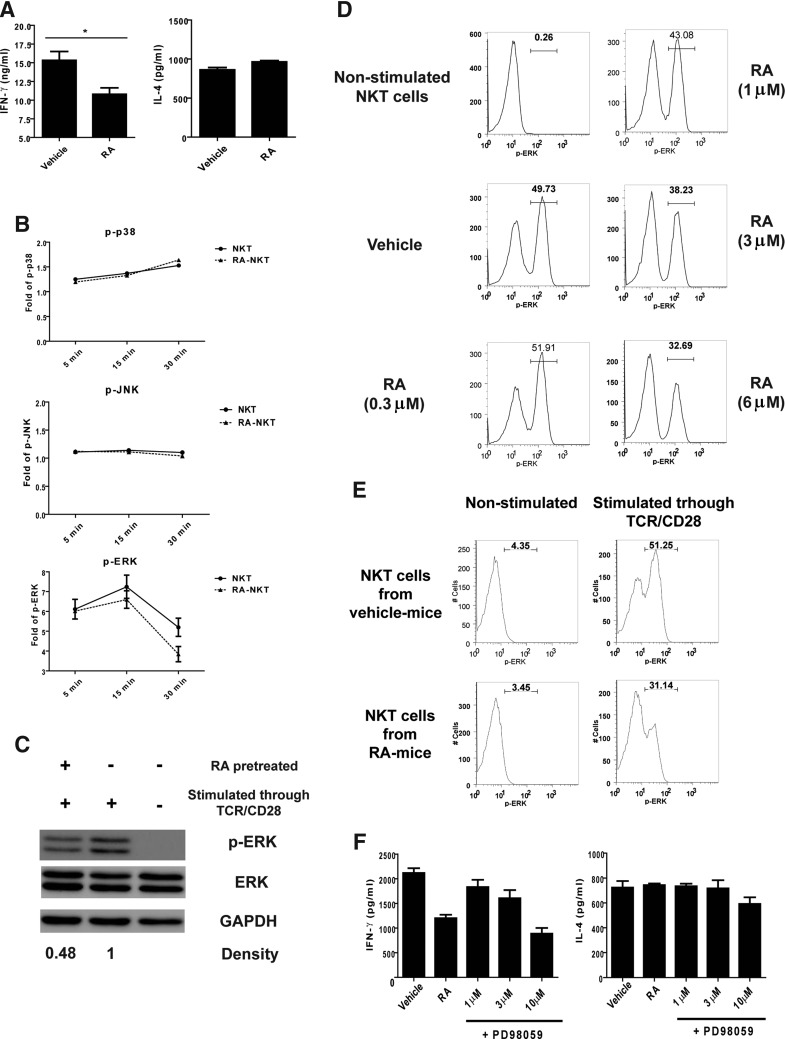
To determine how RA regulated IFN-γ production, the primary hepatic NKT cells were expanded as described in the Materials and Methods section and activated in the presence of RA. The results revealed that the RA treatment reduced the IFN-γ production, but not the IL-4 production, by the expanded NKT cells (Fig. 4A). Considering that the MAPK pathways participate in cytokine production of NKT cells (Wang and others 2008, 2012; Nagaleekar and others 2011), we examined the effect of RA on the activation of p38, JNK, and ERK. We found that RA reduced the level of p-ERK, but not p-p38 and p-JNK, in activated NKT cells (Fig. 4B, C and Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/jir). RA reduced the percentage of p-ERK+ NKT cells in a dose-dependent manner in vitro (Fig. 4D). The percentage of p-ERK+ NKT cells also reduced in mice treated with RA (Fig. 4E). These in vitro and in vivo results indicated that RA treatment interfered with the activation of ERK pathway in TCR/CD28-stimulated NKT cells. Since RA did not affect IFN-γ production by NKT cells stimulated with IL-12/IL-18 (Fig. 3D), we compared the effect of TCR/CD28 and IL-12/IL-18 stimulation on ERK activation in NKT cells. We found that TCR/CD28, but not via IL-12/IL-18, stimulation induced ERK phosphorylation in NKT cells (Supplementary Fig. S1B). To further investigate the relationship between the ERK pathway and the cytokine production, we used an ERK pathway inhibitor PD98059. We found that, similar to RA, PD98059 decreased IFN-γ production but not IL-4 production by the TCR/CD28-activated NKT cells (Fig. 4F). Moreover, we examined the expression of genes regulated by ERK signaling, including death-related and cell cycle-related genes (Boucher and others 2000; McCubrey and others 2007). These results showed that the expression of ERK signaling targets, including Bcl-2, Bcl-XL, Mcl-1, CCNE1, CDK2, and CDK4, was not altered by RA treatment (Supplementary Fig. S2C). These results indicated that RA reduced IFN-γ production by TCR/CD28-stimulated NKT cells via the ERK pathway.
FIG. 4.
Modulating effect of RA on NKT cells via the extracellular signal-regulated kinase (ERK) pathway. (A) The expanded NKT cells were stimulated with αGC-pulsed DCs (αGC-DCs) in the presence of a vehicle (DMSO) or RA (1 μM). After culture for 24 h, the IFN-γ and IL-4 levels in the supernatants were determined using ELISA (n=3 for each group). (B) The NKT cells that were pretreated with the vehicle (DMSO) or RA (1 (M) (RA-NKT) were stimulated through TCR/CD28 by using specific Abs. Phosphorylation of JNK (p-JNK), p38 (p-p38), and ERK (p-ERK) was analyzed using intracellular staining at indicated times. (C) The amounts of p-ERK, ERK, and GPAHD in the RA- or vehicle-treated TCR/CD28-stimulated NKT cells were determined using Western blotting. (D) The NKT cells were pretreated with the vehicle (DMSO) or RA at the indicated concentrations. After incubation for 16 h, the cells were stimulated through TCR and CD28 with specific Abs for 30 min and analyzed for p-ERK. The proportion of NKT cells containing p-ERK was determined using intracellular staining. (E) Proportion of p-ERK in TCR/CD28-activated NKT cells from vehicle- or RA-treated mice. (F) The NKT cells from the liver were enriched and stimulated with the anti-CD3 and anti-CD28 Abs in the presence of the vehicle (DMSO), RA (1 μM), or PD98059 at the indicated concentrations. After incubation for 24 h, the IFN-γ and IL-4 productions from the NKT cells were determined by ELISA (n=3 for each group). The data (A–D, F) are representative of 2–3 independent experiments and are presented as mean±SEM. *P<0.05.
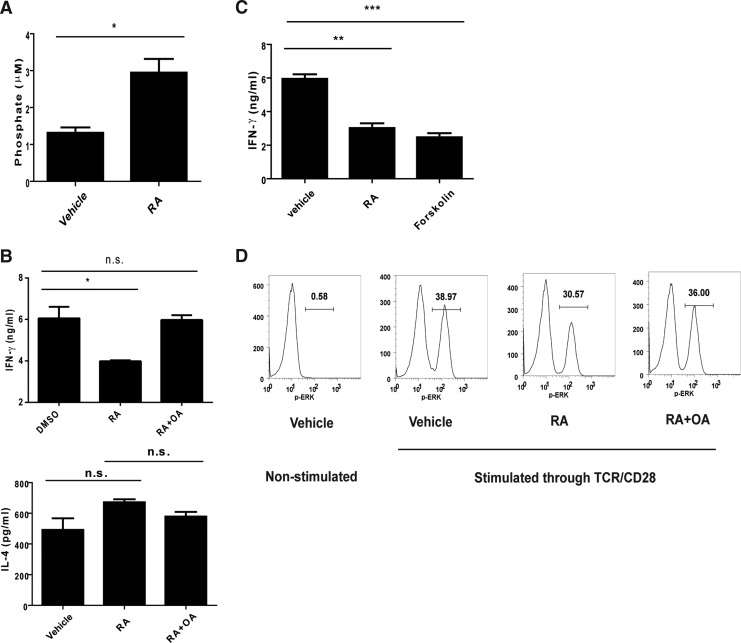
RA reduced ERK activation via enhancing PP2A activity
Several mechanisms have been proposed with regard to the modulating effects of RA on the ERK pathway. A study reported that PP2A played a regulatory role in the ERK pathway (Letourneux and others 2006). To determine whether PP2A contributed to the modulating effects of RA on IFN-γ, we examined the effect of RA on PP2A activity in activated NKT cells. The results revealed that the RA treatment enhanced PP2A activity in the NKT cells (Fig. 5A). To investigate how RA regulated PP2A activity, we first examined the expression of PP2A subunits and endogenous PP2A inhibitors. We found no difference in the expression of PP2A subunits (Ppp2r5b, Ppp2r5d, Ppp2r5e, Ppp2ca, and Ppp2cb) and endogenous inhibitors (Set and Cip2a) between vehicle-treated and RA-treated NKT cells (Supplementary Fig. S2A). We then examined the level of phosphorylation of the catalytic subunit (PP2AC) that correlates to PP2A activity (Chen and others 1992). The result showed that the level of phospho-PP2AC reduced in RA-treated NKT cells compared with that in vehicle-treated cells (Supplementary Fig. S2B). Next, PP2A inhibitor and activator were used to assess the contribution of PP2A in the modulating effect of RA. We found that the PP2A inhibitor OA did not affect IL-4 production, but reversed the RA-induced IFN-γ reduction (Fig. 5B). Consistently, similar to RA, PP2A activator forskolin reduced IFN-γ production (Fig. 5C). We also used another PP2A activator FTY720, which binds to SET to promote dissociation from PP2A to promote PP2A activity (Saddoughi and others 2013), to investigate the participation of PP2A in regulating IFN-γ production. We found that FTY720, similar to RA, reduced IFN-γ production by TCR/CD28-stimulated NKT cells (Supplementary Fig. S2C). In addition, FTY720 reduced the level of p-ERK in TCR/CD28-stimulated NKT cells in a dose-dependent manner (Supplementary Fig. S2D). We also found that IL-12/IL-18-induced IFN-γ production in NKT cells was not altered by RA or OA treatment (Supplementary Fig. S2E). These results implied that PP2A reduced IFN-γ production via ERK signaling. To determine whether RA reduced IFN-γ production via the PP2A-ERK-IFN-γ pathway, NKT cells were treated with RA and stimulated through TCR/CD28 in the presence of OA. The results showed that RA-induced p-ERK reduction was interfered with by OA treatment, indicating that RA reduced IFN-γ production via the ERK pathway by PP2A (Fig. 5D).
FIG. 5.
Participation of phosphatase 2A (PP2A) in the modulating effect of RA on IFN-γ production by NKT cells through the ERK pathway. (A) The expanded NKT cells were pretreated with a vehicle (DMSO) or RA (6 μM) for 16 h, then stimulated with anti-CD3 and anti-CD28 Abs for 30 min, and examined for the PP2A activity as described in the Materials and Methods section (n=3). (B) The NKT cells were pretreated with the vehicle (DMSO) or okadaic acid (OA) (1 nM) and stimulated with anti-CD3 and anti-CD28 Abs in the presence of the vehicle (DMSO) or RA (1 μM) for 24 h. The IFN-γ and IL-4 productions by the NKT cells were determined using ELISA (n=3). (C) The NKT cells were stimulated through TCR/CD28 in the presence of the vehicle, RA (1 μM), or forskolin (40 μM). The IFN-γ production by the NKT cells was analyzed using ELISA (n=3). (D) The expanded NKT cells were treated with the vehicle or RA (6 μM) for 16 h, then treated with the vehicle or OA (3 nM) for 1 hr, and activated through TCR and CD28. ERK phosphorylation in the NKT cells was determined using intracellular staining after TCR/CD28 activation for 30 min. The data are representative of 2 (A, C and D) or 3 (B) independent experiments and presented as mean±SEM. *P<0.05, **P<0.01, and ***P<0.001; n.s., no significance.
Discussion
Vitamin A is essential for several physiological activities, including the development of the neural system and vision (Blomhoff and Blomhoff 2006). With regard to the immune system, vitamin A is not only crucial for immune responses against pathogens, but also participates in myeloid cell differentiation, plasma cell formation, and Ab production (Blomhoff and Blomhoff 2006; Mora and others 2008; Ertesvag and others 2009). With regard to T lymphocytes, vitamin A is pivotal for functional T-cell responses, Th2 differentiation, and Treg cell formation, and induces the expression of CCR9 and α4β7 integrin on T lymphocytes for gut homing (Iwata and others 2003, 2004; Mucida and others 2007; Hall and others 2011). Furthermore, RA has been beneficial for autoimmune disease alleviation in experimental allergic encephalomyelitis (EAE) or systemic lupus erythematosus models (Gershwin and others 1984; Racke and others 1995). These studies demonstrated an indispensible role of vitamin A in the immune system.
However, the impact of vitamin A on NKT cells remains unknown. In this study, we demonstrated that RA, a functional metabolite of vitamin A, could modulate NKT cell activation at an early stage; moreover, RA affected the cytokine production by decreasing the IFN-γ production but increasing IL-5 production, which primed the reaction toward Th2 responses. With regard to the significant IFN-γ reduction by RA, the modulating effect of RA directly decreased ERK phosphorylation by activating PP2A, resulting in the downregulation of the IFN-γ production. These results unequivocally proved RA as a promising modulating molecule for cytokine generation in NKT cells. Recently, a study reported that RA ameliorated concanavalin A-induced hepatitis and regulated NKT cell cytokine production, including IFN-γ and IL-4 (Lee and others 2012). However, in this study, no downregulating effect of RA was observed in the IL-4 production from the αGC-activated NKT cells. The discrepancy in these results might be attributed to the various methods used for NKT cell activation. Additional experiments will be required in the future to delineate the modulating effect of RA on NKT cells in different experimental settings.
NKT cells play a regulatory role in immune responses. On activation, they express prodigious amounts of cytokines to regulate the immune responses (Brennan and others 2013). A few studies have reported that cytokines produced from NKT cells by αGC analogs polarized Th1 or Th2 (Oki and others 2005; Fujii and others 2006). Since different αGC analogs can induce promoting or reducing effects in tumor rejection or autoimmune disease alleviation, these cytokines could be appropriately manipulated to direct the immune responses for therapeutic applications (Miyamoto and others 2001; Chiba and others 2004; Fujii and others 2006). This study determined the modulating effect of RA on IFN-γ production from NKT cells. Although IFN-γ is critical in Th1 cell differentiation, inflammation, and clearance of infection, it plays contradictory roles in promoting or repressing inflammation diseases (Billiau and Matthys 2009). Reportedly, in αGC-induced hepatitis, IFN-γ deficiency aggravated the hepatitis, which indicated the protective effects of IFN-γ from NKT cells in this hepatitis model (Biburger and Tiegs 2005). IFN-γ is also a potent cytokine that induces the expression of chemokine IP-10/CXCL-10 which recruits immune cells into tissues. A study demonstrated that NKT cell activation resulted in the recruitment of Treg cells into the liver through the expression of the chemokine receptor CXCR-3, the receptor for CXCL-10 (Santodomingo-Garzon and others 2009). Furthermore, another study also showed that IFN-γ and IL-4 production from NKT cells played opposite roles in a αGC-induced murine hepatitis model (Wang and others 2013). These reports indicated that the dual effects of NKT cells and the interaction between NKT cells and neutrophils are essential in both healthy and diseased states. Another study reported that IFN-γ production from NKT cells was responsible for allergic airway disease, indicating the importance of IFN-γ production from NKT cells in immune regulation (Matsuda and others 2010). In this study, RA enhanced the IL-5 expression from the NKT cells. IL-5 production from NKT cells was reported to recruit eosinophils in liver tissues and promote hepatitis (Louis and others 2002). Therefore, in future, different disease models could be used to explore the distinctive roles of IL-5 and other pro-inflammatory cytokines that are regulated by RA signaling.
The mechanisms through which RA modulates IFN-γ expression have not been completely understood (Carman and Hayes 1991). Although a study reported that T-bet and other members of NF-κB participated in IFN-γ expression in NKT cells (Oki and others 2004; Matsuda and others 2006), the expression of these transcriptional factors was not affected under RA treatment (Supplementary Fig. S3A). Furthermore, no elements for RAR were detected in the promoter region of IFN-γ, suggesting that the modulating effect of RA on IFN-γ expression was mediated through other pathways (Cippitelli and others 1996). Based on our findings, the IFN-γ production was modulated by RA via the ERK pathway. The MAPK has been reported to participate not only in activation of transcription factor but also in positive regulation of RNA stability (Tiedje and others 2014). To investigate how RA reduces IFN-γ production, we examined the expression of c-Fos, a downstream transcription factor of ERK signaling and a component of activation protein-1 (AP-1) that activates IFN-γ gene transcription (Merlin and others 1997; Sweetser and others 1998; Lee and others 2004). We found that the expression of c-Fos was reduced by RA treatment in TCR/CD28-stimulated NKT cells (Supplementary Fig. S3B). Considering the relationship between MAPK and RNA stability, we also examine the kinetic of IFN-γ RNA decay in TCR/CD28-stimulated NKT cells using actinomycin D to suppress transcription. We found that RA-treated NKT cells had less IFN-γ RNA than the vehicle-treated NKT cells before actinomycin D treatment (0 h), and did not show a higher RNA decay rate compared with the vehicle-treated cells (Supplementary Fig. S3C). This result showed that the reduction of IFN-γ by RA did not involve RNA stability. Moreover, a study reported the involvement of the ERK pathway in cytokine production from NKT cells under autoreactive activation (Wang and others 2008). Although ERK pathway activation was necessary in the IFN-γ expression through AP-1 (Cippitelli and others 1995), IFN-γ transcription could be regulated by the ERK pathway through H4 histone acetylation in NKT cells (Wang and others 2012). These lines of evidence indicate that at least 2 factors aid the ERK pathway in modulating the IFN-γ expression in NKT cells. In addition, we found that the effect of RA on the ERK pathway was mediated by PP2A. PP2A is a major serine/threonine phosphatases (Roskoski 2012). A previous study showed that the overexpression of PP2A increased the susceptibility to autoimmune disease, thereby suggesting a role of PP2A in immune regulation (Crispin and others 2012). Another study reported that the expression of SET, a PP2A inhibitor, enhanced IFN-γ expression, indicating a downregulatory role of PP2A in IFN-γ expression (Trotta and others 2007). Based on our findings and other previous reports, PP2A may be considered a candidate that modulates NKT cell function in immune regulation and, thus, requires future investigation.
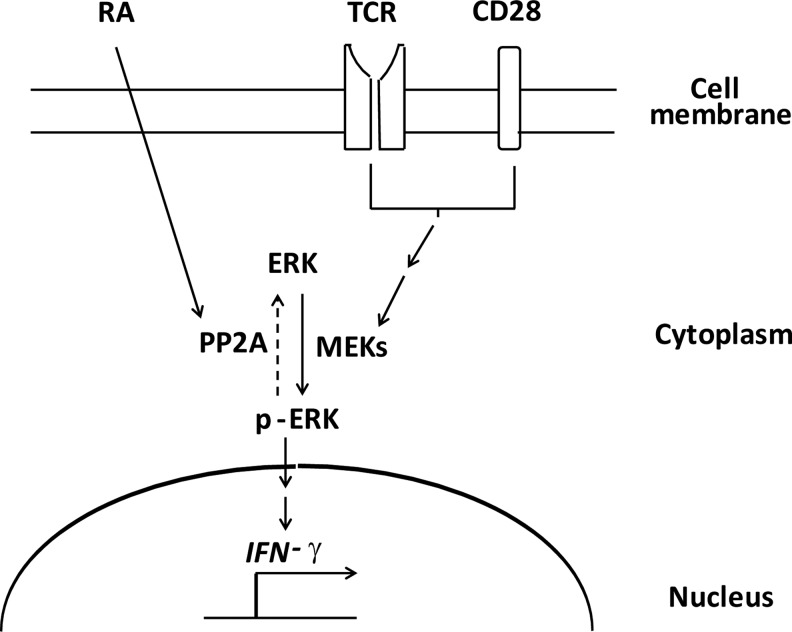
In conclusion, the data presented here demonstrated that RA recruited PP2A to decrease the INF-γ expression in the NKT cells through the ERK pathway, suggesting a potential pathway for directing the immune responses through NKT cell modulation (Fig. 6). Notably, since cytokines produced by NKT cells regulate cellular activities and participate in disease pathogenesis, the impact of RA-modulated NKT cells in healthy or diseased states should be explored in future studies.
FIG. 6.
Model of modulating effect of RA on IFN-γ production by NKT cells via the PP2A-ERK pathway. The activity of MEKs (also known as MAPKKs) in the NKT cells was induced by the signaling cascade triggered via TCR and CD28, which promotes ERK phosphorylation and IFN-γ expression (solid arrow). RA increased the PP2A activity to de-phosphorylate p-ERK and, subsequently, reduced the IFN-γ expression (dot arrow).
Supplementary Material
Acknowledgments
The authors thank the animal facility at the Institute of Cellular and Organism Biology in Academia Sinica for animal management, and Academia Sinica for financial support.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Baxevanis CN, Gritzapis AD, Papamichail M. 2003. In vivo antitumor activity of NKT cells activated by the combination of IL-12 and IL-18. J Immunol 171(6):2953–2959 [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. 2007. The biology of NKT cells. Annu Rev Immunol 25:297–336 [DOI] [PubMed] [Google Scholar]
- Biburger M, Tiegs G. 2005. Alpha-galactosylceramide-induced liver injury in mice is mediated by TNF-alpha but independent of Kupffer cells. J Immunol 175(3):1540–1550 [DOI] [PubMed] [Google Scholar]
- Billiau A, Matthys P. 2009. Interferon-gamma: a historical perspective. Cytokine Growth Factor Rev 20(2):97–113 [DOI] [PubMed] [Google Scholar]
- Blomhoff R, Blomhoff HK. 2006. Overview of retinoid metabolism and function. J Neurobiol 66(7):606–630 [DOI] [PubMed] [Google Scholar]
- Boucher MJ, Morisset J, Vachon PH, Reed JC, Laine J, Rivard N. 2000. MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J Cell Biochem 79(3):355–369 [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, Brenner MB. 2013. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 13(2):101–117 [DOI] [PubMed] [Google Scholar]
- Carman JA, Hayes CE. 1991. Abnormal regulation of IFN-gamma secretion in vitamin A deficiency. J Immunol 147(4):1247–1252 [PubMed] [Google Scholar]
- Chen J, Martin BL, Brautigan DL. 1992. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science 257(5074):1261–1264 [DOI] [PubMed] [Google Scholar]
- Chiba A, Oki S, Miyamoto K, Hashimoto H, Yamamura T, Miyake S. 2004. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of alpha-galactosylceramide. Arthritis Rheum 50(1):305–313 [DOI] [PubMed] [Google Scholar]
- Cippitelli M, Sica A, Viggiano V, Ye J, Ghosh P, Birrer MJ, Young HA. 1995. Negative transcriptional regulation of the interferon-gamma promoter by glucocorticoids and dominant negative mutants of c-Jun. J Biol Chem 270(21):12548–12556 [DOI] [PubMed] [Google Scholar]
- Cippitelli M, Ye J, Viggiano V, Sica A, Ghosh P, Gulino A, Santoni A, Young HA. 1996. Retinoic acid-induced transcriptional modulation of the human interferon-gamma promoter. J Biol Chem 271(43):26783–26793 [DOI] [PubMed] [Google Scholar]
- Crispin JC, Apostolidis SA, Rosetti F, Keszei M, Wang N, Terhorst C, Mayadas TN, Tsokos GC. 2012. Cutting edge: protein phosphatase 2A confers susceptibility to autoimmune disease through an IL-17-dependent mechanism. J Immunol 188(8):3567–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertesvag A, Naderi S, Blomhoff HK. 2009. Regulation of B cell proliferation and differentiation by retinoic acid. Semin Immunol 21(1):36–41 [DOI] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, Franck RW, Tsuji M, Steinman RM. 2006. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci U S A 103(30):11252–11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershwin ME, Lentz DR, Beach RS, Hurley LS. 1984. Nutritional factors and autoimmunity. IV. Dietary vitamin A deprivation induces a selective increase in IgM autoantibodies and hypergammaglobulinemia in New Zealand Black mice. J Immunol 133(1):222–226 [PubMed] [Google Scholar]
- Godfrey DI, Kronenberg M. 2004. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest 114(10):1379–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. 2011. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity 34(3):435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, Jerud ES, Goldberg MF, Baena A, Yu KO, Ndonye RM, Howell AR, Yuan W, Cresswell P, Chang YT, Illarionov PA, Besra GS, Porcelli SA. 2009. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity 30(6):888–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Eshima Y, Kagechika H. 2003. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol 15(8):1017–1025 [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21(4):527–538 [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278(5343):1626–1629 [DOI] [PubMed] [Google Scholar]
- Kim CH. 2011. Retinoic acid, immunity, and inflammation. Vitam Horm 86:83–101 [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim HS, Lee CW, Chung DH. 2010. T cell Ig domain and mucin domain 1 engagement on invariant NKT cells in the presence of TCR stimulation enhances IL-4 production but inhibits IFN-gamma production. J Immunol 184(8):4095–4106 [DOI] [PubMed] [Google Scholar]
- Kronenberg M. 2005. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 23:877–900 [DOI] [PubMed] [Google Scholar]
- Lee DU, Avni O, Chen L, Rao A. 2004. A distal enhancer in the interferon-gamma (IFN-gamma) locus revealed by genome sequence comparison. J Biol Chem 279(6):4802–4810 [DOI] [PubMed] [Google Scholar]
- Lee KA, Song YC, Kim GY, Choi G, Lee YS, Lee JM, Kang CY. 2012. Retinoic acid alleviates Con A-induced hepatitis and differentially regulates effector production in NKT cells. Eur J Immunol 42(7):1685–1694 [DOI] [PubMed] [Google Scholar]
- Letourneux C, Rocher G, Porteu F. 2006. B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK. EMBO J 25(4):727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hashimoto Y, Agadir A, Kagechika H, Zhang X. 1999. Identification of a novel class of retinoic acid receptor beta-selective retinoid antagonists and their inhibitory effects on AP-1 activity and retinoic acid-induced apoptosis in human breast cancer cells. J Biol Chem 274(22):15360–15366 [DOI] [PubMed] [Google Scholar]
- Louis H, Le Moine A, Flamand V, Nagy N, Quertinmont E, Paulart F, Abramowicz D, Le Moine O, Goldman M, Deviere J. 2002. Critical role of interleukin 5 and eosinophils in concanavalin A-induced hepatitis in mice. Gastroenterology 122(7):2001–2010 [DOI] [PubMed] [Google Scholar]
- Matsuda H, Takeda K, Koya T, Okamoto M, Shiraishi Y, Miyahara N, Dakhama A, Matsuda JL, Gapin L, Gelfand EW. 2010. Plasticity of invariant NKT cell regulation of allergic airway disease is dependent on IFN-gamma production. J Immunol 185(1):253–262 [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Gapin L. 2005. Developmental program of mouse Valpha14i NKT cells. Curr Opin Immunol 17(2):122–130 [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Zhang Q, Ndonye R, Richardson SK, Howell AR, Gapin L. 2006. T-bet concomitantly controls migration, survival, and effector functions during the development of Va14i NKT cells. Blood 107:2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. 2007. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773(8):1263–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin G, van der Leede BJ, McKune K, Knezevic N, Bannwarth W, Romquin N, Viegas-Pequignot E, Kiefer H, Aguet M, Dembic Z. 1997. The gene for the ligand binding chain of the human interferon gamma receptor. Immunogenetics 45(6):413–421 [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyake S, Yamamura T. 2001. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413(6855):531–534 [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, von Andrian UH. 2008. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 8(9):685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317(5835):256–260 [DOI] [PubMed] [Google Scholar]
- Nagaleekar VK, Sabio G, Aktan I, Chant A, Howe IW, Thornton TM, Benoit PJ, Davis RJ, Rincon M, Boyson JE. 2011. Translational control of NKT cell cytokine production by p38 MAPK. J Immunol 186(7):4140–4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki S, Chiba A, Yamamura T, Miyake S. 2004. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J Clin Invest 113(11):1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki S, Tomi C, Yamamura T, Miyake S. 2005. Preferential T(h)2 polarization by OCH is supported by incompetent NKT cell induction of CD40L and following production of inflammatory cytokines by bystander cells in vivo. Int Immunol 17(12):1619–1629 [DOI] [PubMed] [Google Scholar]
- Pino-Lagos K, Benson MJ, Noelle RJ. 2008. Retinoic acid in the immune system. Ann N Y Acad Sci 1143:170–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke MK, Burnett D, Pak SH, Albert PS, Cannella B, Raine CS, McFarlin DE, Scott DE. 1995. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J Immunol 154(1):450–458 [PubMed] [Google Scholar]
- Roskoski R., Jr.2012. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res 66(2):105–143 [DOI] [PubMed] [Google Scholar]
- Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, Bielawski J, Szulc ZM, Thomas RJ, Selvam SP, Senkal CE, Garrett-Mayer E, De Palma RM, Fedarovich D, Liu A, Habib AA, Stahelin RV, Perrotti D, Ogretmen B. 2013. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med 5(1):105–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santodomingo-Garzon T, Han J, Le T, Yang Y, Swain MG. 2009. Natural killer T cells regulate the homing of chemokine CXC receptor 3-positive regulatory T cells to the liver in mice. Hepatology 49(4):1267–1276 [DOI] [PubMed] [Google Scholar]
- Stanic AK, Shashidharamurthy R, Bezbradica JS, Matsuki N, Yoshimura Y, Miyake S, Choi EY, Schell TD, Van Kaer L, Tevethia SS, Roopenian DC, Yamamura T, Joyce S. 2003. Another view of T cell antigen recognition: cooperative engagement of glycolipid antigens by Va14Ja18 natural T(iNKT) cell receptor [corrected]. J Immunol 171(9):4539–4551 [DOI] [PubMed] [Google Scholar]
- Sullivan BA, Nagarajan NA, Wingender G, Wang J, Scott I, Tsuji M, Franck RW, Porcelli SA, Zajonc DM, Kronenberg M. 2010. Mechanisms for glycolipid antigen-driven cytokine polarization by Valpha14i NKT cells. J Immunol 184(1):141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser MT, Hoey T, Sun YL, Weaver WM, Price GA, Wilson CB. 1998. The roles of nuclear factor of activated T cells and ying-yang 1 in activation-induced expression of the interferon-gamma promoter in T cells. J Biol Chem 273(52):34775–34783 [DOI] [PubMed] [Google Scholar]
- Tiedje C, Holtmann H, Gaestel M. 2014. The role of mammalian MAPK signaling in regulation of cytokine mRNA stability and translation. J Interferon Cytokine Res 34(4):220–232 [DOI] [PubMed] [Google Scholar]
- Torres D, Paget C, Fontaine J, Mallevaey T, Matsuoka T, Maruyama T, Narumiya S, Capron M, Gosset P, Faveeuw C, Trottein F. 2008. Prostaglandin D2 inhibits the production of IFN-gamma by invariant NK T cells: consequences in the control of B16 melanoma. J Immunol 180(2):783–792 [DOI] [PubMed] [Google Scholar]
- Trotta R, Ciarlariello D, Dal Col J, Allard J, 2nd, Neviani P, Santhanam R, Mao H, Becknell B, Yu J, Ferketich AK, Thomas B, Modi A, Blaser BW, Perrotti D, Caligiuri MA. 2007. The PP2A inhibitor SET regulates natural killer cell IFN-gamma production. J Exp Med 204(10):2397–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Feng D, Park O, Yin S, Gao B. 2013. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: opposite regulation by IL-4 and IFN-gamma. Hepatology 58(4):1474–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bishop KA, Hegde S, Rodenkirch LA, Pike JW, Gumperz JE. 2012. Human invariant natural killer T cells acquire transient innate responsiveness via histone H4 acetylation induced by weak TCR stimulation. J Exp Med 209(5):987–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen X, Rodenkirch L, Simonson W, Wernimont S, Ndonye RM, Veerapen N, Gibson D, Howell AR, Besra GS, Painter GF, Huttenlocher A, Gumperz JE. 2008. Natural killer T-cell autoreactivity leads to a specialized activation state. Blood 112(10):4128–4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M. 2008. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protoc 3(1):70–78 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.