Abstract
Turnera diffusa Willd, commonly known as Damiana, is employed in traditional medicine as a stimulant, aphrodisiac, and diuretic. Its leaves and stems are used for flavoring and infusion. Damiana is considered to be safe for medicinal use by the FDA. Pharmacological studies have established the hypoglycemic, antiaromatase, prosexual, estrogenic, antibacterial, and antioxidant activity of T. diffusa. The aim of the present study was to evaluate the possible cytotoxic effect of extracts and organic fractions of this plant on five tumor cell lines (SiHa, C-33, Hep G2, MDA-MB-231, and T-47D) and normal human fibroblasts. The results show that the methanolic extract (TdM) displayed greater activity on MDA-MB-231 breast cancer cells (with an IC50 of 30.67 μg/mL) than on the other cancer cell lines. Four organic fractions of this extract exhibited activity on this cancer cell line. In the most active fraction (F4), two active compounds were isolated, arbutin (1) and apigenin (2). This is the first report of a cytotoxic effect by T. diffusa on cancer cells. The IC50 values suggest that the methanolic extract of T. diffusa has potential as an anticancer therapy.
Key Words: : apigenin, breast cancer, cytotoxic activity, Damiana, fibroblast, MDA-MB-231 cells, T-47D cells
Introduction
Cancer is one of the main causes of death worldwide, claiming over 6 million lives each year.1 Breast cancer is the leading cause of cancer-related death among women in developed countries.2 Treatments are currently very expensive and their effectiveness is limited by side effects and the capacity of tumors to develop resistance to drugs used in chemotherapy. Thus, there is a need to develop new anticancer drugs.
Plants have long been a source of compounds used to treat cancer.3 Plant-derived anticancer drugs in clinical use today include the so-called vinca alkaloids, vinblastine and vincristine, isolated from the Catharanthus roseus4,5 as well as two semisynthetic derivatives, etoposide and teniposide, of the natural product epipodophyllotoxin.6,7 Moreover, paclitaxel (Taxol®) and several key precursors (the baccatins) are found in the leaves of various Taxus species.8–10 Recent additions to plant-derived anticancer chemotherapeutic agents include the semisynthetic derivatives of camptothecin, isolated from the Chinese ornamental tree Camptotheca acuminate. Among such derivatives, topotecan, irinotecan (CPT-11), and belotecan are in clinical use, while 9-amino-camptothecin and 9-nitro-camptothecin11 have not yet been approved.
Turnera diffusa Willd (Turneraceae), a small tropical and subtropical shrub commonly known as Damiana, is employed in traditional medicine as a stimulant, aphrodisiac, diuretic, and nerve tonic, and to treat menstrual and pregnancy disorders.12 Damiana products have been sold in the United States as invigorant and aphrodisiac agents13 since 1874. Scientific reports have established the hypoglycemic,14,15 antiaromatase,16 prosexual,17,18 estrogenic,19 antibacterial,20 and antioxidant21,22 activity of T. diffusa. Phytochemical studies of this plant have demonstrated the presence of tetraphyllin B,23 gonzalitozin I, damianin, tricosan-2-one, hexaconazole, squalene, p-arbutin, α-pinene, β-pinene, p-cymene, 1,8-cineole, β-sytosterol, acacetin, pinocembrin, teuhetenone A, eremophyllane, echinaticin, apigenin, and simple sugars.24
Although studies have shown that natural phytochemical-containing compounds have antitumor and antimetastatic properties,25 to our knowledge there are no reports on the cytotoxic or antitumoral activity of T. diffusa. The aim of the present study was to evaluate the activity of the methanol extract of T. diffusa on the viability of five tumor cell lines to evaluate the chemopreventive properties of this plant.
Materials and Methods
Plant material
A whole plant of T. diffusa Willd was collected in Mexico State, Mexico. The plant was identified by biologist Alfredo Patiño, comparing it with a specimen deposited in the herbarium of the Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional (IPN). The register number 2247 was assigned to T. diffusa.
Extraction
Leaves and stems (300 g) of T. diffusa were dried and ground into a powder, which was successively extracted with 2 L each of hexane, methylene chloride, and methanol at room temperature by maceration for 48 h. All solvents were of analytical grade. The evaporation of the solutions under reduced pressure at 40°C yielded the hexane (TdH), dichloromethane (TdD), and methanol (TdM) extracts. After concentration, the residue was lyophilized to yield 5.1, 15.6, and 29.7 g, respectively, and stored at 4°C.26 Only TdM was used for the cytotoxicity assays.
Cell lines and cell culture
The five tumor cell lines employed in the present study were human cervical carcinoma HPV-16 positive (SiHa), human cervical carcinoma HPV negative (C-33), human breast carcinoma (MDA-MB-231 and T-47D), and human liver carcinoma (Hep G-2). Normal human fibroblasts were used as the control. SiHa, C-33, MDA-MB-231, Hep G-2, and normal fibroblasts were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and streptomycin-penicillin. T-47D cells were maintained in a RPMI medium. All were cultivated at 37°C in a 5% CO2 atmosphere.
Cytotoxicity assay
Cells were cultivated to 100% of confluence, harvested, and counted with trypan blue (Sigma-Aldrich) in a hemocytometer. 104 cells were deposited in each well of a 96-well culture microplate and then preincubated for 24 h before being treated for 72 h with different concentrations of the extract. The extract was dissolved in DMSO (DMSO final concentration: 0.1%) and prepared in a complete medium at the following concentrations to calculate the IC50: 0, 6.25, 12.5, 25, 50, 75, and 100 μg/mL.26–28 Cellular viability was measured with an MTT ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; Sigma-Aldrich) assay. After 72 h of treatment, the medium was removed and cultures were washed twice with a phosphate-buffered solution (PBS). MTT (5 mg/mL) was added at 10% concentration in PBS supplemented with 10% FBS. Subsequently, cells were incubated for 2 h, the medium was removed again, and DMSO was added. The microplates were shaken for 15 min and read at 550 nm with a reference wavelength of 620 nm on an ELISA plate reader.29,30 The treatment used for normal cells was the same. Doxorubicin (Sigma-Aldrich) was applied at the same extract concentrations as the positive control, and DMSO in the culture medium at 0.1% concentration as the negative control. Each assay was carried out in triplicate. The IC50 was calculated from a dose–response curve obtained with the v.2.0 KyPlot program.
Bioassay-guided fractionation
The crude methanolic extract (5 g) was chromatographed over a silica gel column (230–400 mesh; Merck) and eluted with gradients of increasing polarity of hexane–dichloromethane and dichloromethane–methanol to obtain 20 fractions. The most representative fractions (F1–F10) were selected based on thin-layer chromatography analysis, which was carried out on silica gel GF60254 with hexane–ethyl acetate and dichloromethane–methanol mixtures (80:20), and observed with UV light at 254 and 365 nm. F1–F10 were used for the cytotoxicity assays on MDA-MB-231 cell cultures at concentrations of 0, 2, 10, 25, 50, and 100 μg/mL, employing the same protocol as that used for the crude extract. The most active fractions, F3 and F4, were subjected to column chromatography over silica gel by using a gradient of dichloromethane–methanol (9:1 F3; 8:2 F4). Selected subfractions were submitted to subsequent column chromatography over silica gel to afford two major compounds, the structures of which were determined by using spectroscopic techniques, including 1H- and 13C-NMR. NMR spectra were compared to published data.31
Annexin assay
For evaluation of the type of death (apoptosis or necrosis) induced by the crude extract, 4×104 MDA-MB-231 cells were cultivated and exposed to the TdM extract and doxorubicin at the IC50 concentration in 24-well culture microplates. Cells were exposed for 12 and 24 h, at which time the culture medium was removed and cells were washed with PBS, detached, and incubated with Guava Nexin reactive for 20 min (protocol kit). Cells were double stained with Annexin V and 7-aminoactinomycin D (7AAD). Necrotic cells are positive to 7AAD, the early stages of apoptotic cells to Annexin V, and the later stages of apoptosis to both these fluorochromes. Fluorescence was read in a Guava PCA cytometer (Millipore) with the program specifically designed for this assay.32,33
Statistical analysis
All results are expressed as the mean±SD. Statistical comparisons were performed using the Tukey HSD test after ANOVA. The results were considered significant with P<.02.
Results
Cytotoxicity of T. diffusa
With the MTT assay, the methanolic extract of T. diffusa (TdM) showed similar cytotoxicity for all cancer cell lines, except for those of MDA-MB-231 cells. Whereas the best IC50 value was obtained for the latter cell line (30.67 μg/mL; Table 1), TdM was least cytotoxic for normal fibroblasts.
Table 1.
Cytotoxic Effect of Methanolic Extract of Turnera diffusa on Tumor and Normal Cell Lines
| Cell line | IC50 (μg/mL)±SD |
|---|---|
| SiHa | 50.14±2.88 |
| C-33 A | 45.10±6.20 |
| Hep G-2 | 43.87±7.90 |
| MDA-MB-231* | 30.67±3.50 |
| T-47D | 54.02±2.14 |
| Fibroblasts | 63.24±3.26 |
The effects of the methanolic extract on cells were examined by an MTT assay. The cells were treated with various concentrations for 72 h. Data from three independent experiments are expressed as the mean±SD.
Significant difference p<0.03.
The morphology associated with the effect of TdM at 25 and 50 μg/mL on MDA-MB-231 breast cancer cells can be appreciated in Figure 1. Compared with cells treated with the negative control, it can be seen that those exposed to TdM are less in number and have greater cellular detritus. Several cells are rounded and detached, while others are swollen. Contrarily, the effect on normal fibroblasts produced by TdM at 50 μg/mL was similar to that found with the negative control (Fig. 2). Compared to TdM, doxorubicin (the positive control) showed greater cytotoxic activity on all cell lines, with IC50 values of 0.02–0.3 μg/mL.
FIG. 1.
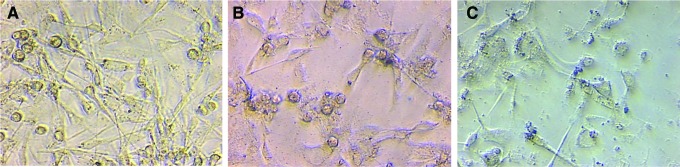
Morphology of MDA-MB-231 cells exposed to methanolic extract of T. diffusa (TdM) at different concentrations 0 (A), 25 (B), and 50 (C) μg/mL (40×, confocal microscope). It shows a dose-dependent effect in the cells. Color images available online at www.liebertpub.com/jmf
FIG. 2.
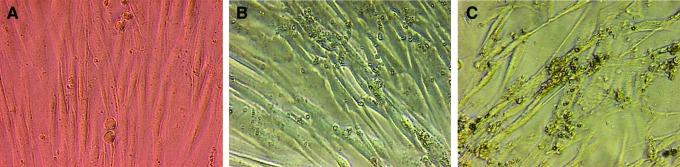
Morphology of normal human fibroblasts exposed to 0 (A), 50 (B), and 100 (C) μg/mL of the methanolic extract of T. diffusa (TdM). Cells exposed at 50 μg/mL do not show a serious damage (40×, confocal microscope). Color images available online at www.liebertpub.com/jmf
Bioassay-guided fractionation
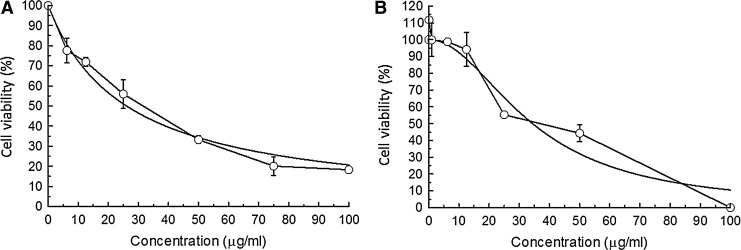
Since the methanolic extract was active on MDA-MB-231 tumor cells, column chromatography was performed. The results for fractions F1–F10 show that fraction F4 had the greatest cytotoxic activity on MDA-MB-231 breast cancer cells. The IC50 for F4 was 32.4 μg/mL, similar to the value found with TdM on this same cancer cell line (Fig. 3). Cytotoxic activity was also found with F2, F3, and F5 (resulting in an IC50 of 64.3, 59.4, and 94.0 μg/mL, respectively), whereas F1 and F6–F10 had no cytotoxic activity (Table 2). In all cases of cytotoxic activity, the effect was dose dependent.
FIG. 3.
A comparison of the effect of TdM extract (A) and the F4 fraction (B) on MDA-MB-231 cells. The IC50 values are similar. F4 is inactive at the lowest concentrations. Beginning with 10 μg/mL, the activity gradually increases.
Table 2.
Cytotoxic Effect of TdM Fractions on MDA-MB-231 Cells
| Fraction (eluent) | IC50 (μg/mL) |
|---|---|
| F1 (A, 60:40) | NA |
| F2 (A, 40:60) | 64.3±5.0 |
| F3 (A, 20:80) | 59.4±1.6 |
| F4 (B, 90:10) | 32.4±2.0 |
| F5 (B, 80:20) | 94.0±3.0 |
| F6 (B, 70:30) | NA |
| F7 (A, 60:40) | NA |
| F8 (A, 50:50) | NA |
| F9 (A, 30:70) | NA |
| F10 (B, 20:80) | NA |
The effect of the fractions of methanolic extract on tumor cells was examined by an MTT assay. The cells were treated with various concentrations for 72 h. Data from three independent experiments are expressed as the mean±SD.
TdM, methanolic extract of Turnera diffusa; NA, no activity; A, mixture hexane/CH2Cl2; B, mixture CH2Cl2/MeOH.
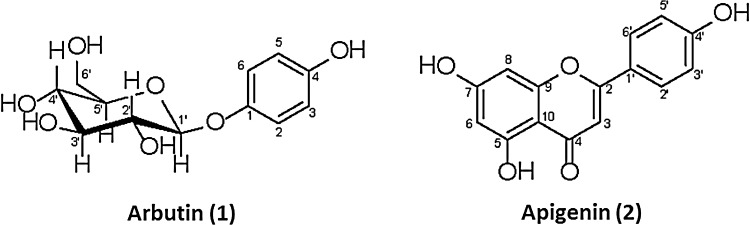
With the two fractions exhibiting the best cytotoxic activity, F3 and F4, a phytochemical study was carried out to identify the compounds responsible for this effect. Two major constituents were isolated and identified from these two fractions (Fig. 4). From F3, arbutin (1) was obtained as a white solid. 1H-NMR (500 MHz, D2O) δ 3.45–3.55 (m, 4H, H2′-H5′), 3.76 (dd, J=12.4, 5.6 Hz, 1H, H-6′b), 3.93 (dd, J=12.4, 2.0 Hz, 1H, H-6′a), 4.99 (d, J=7.6 Hz, 1H, H-1′), 6.88 (d, J=9.0 Hz, 2H, H6-H2), and 7.06 (d, J=9.0 Hz, 2H, H5-H3); 13C-NMR (125 MHz, D2O) δ 63.5 (C-6′), 72.4 (H-4′), 75.9 (C-2′), 78.5 (C-3′), 79.0 (C-5′), 104.2 (C-1′), 119.1 (C-3,C-5), 121.4 (C-2, C-6), 153.3 (C-4), and 154.2 (C-1). From F4, arbutin (1) and apigenin (2) were obtained, the latter as a yellow powder. Apigenin showed a characteristic flavonoid reaction with AlCl3. 1H-NMR (500 MHz, DMSO-d6) δ 6.18 (1H, d, J=2.1 Hz, H6), 6.47 (1H, d, J=2,1 Hz, H8), 6.74 (1H, s, H-3), 6.92 (2H, dd, J=8.8, 2.1 Hz, H-3′, H-5′), and 7.91(2H, dd, J=8.8, 2.1 Hz, H2′, H6′); 13C-NMR (125 MHz, DMSO-d6) δ 94.7 (C-8), 99.6 (C-6), 103.5 (C-3), 104.3 (C-10), 116.7 (C-5′), 116.7 (C-3′), 121.2 (C-1′), 129.2 (C-2′,C-6′), 158.0 (C-9), 161.8 (C-5), 162.1 (C-4′), 164.5 (C-7), 164.9 (C-2), and182.4 (C-4). The NMR data of compounds 1 and 2 are in agreement with reported data.34,35
FIG. 4.
Structures of compounds isolated from the two fractions of the methanolic extract of T. diffusa having the greatest cytotoxic activity.
Annexin assay
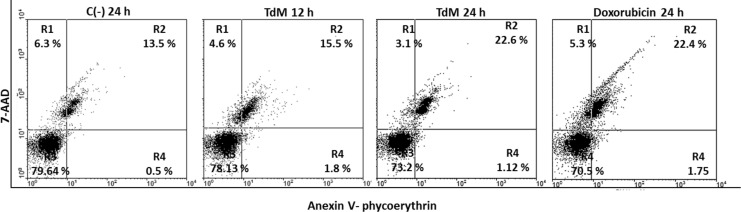
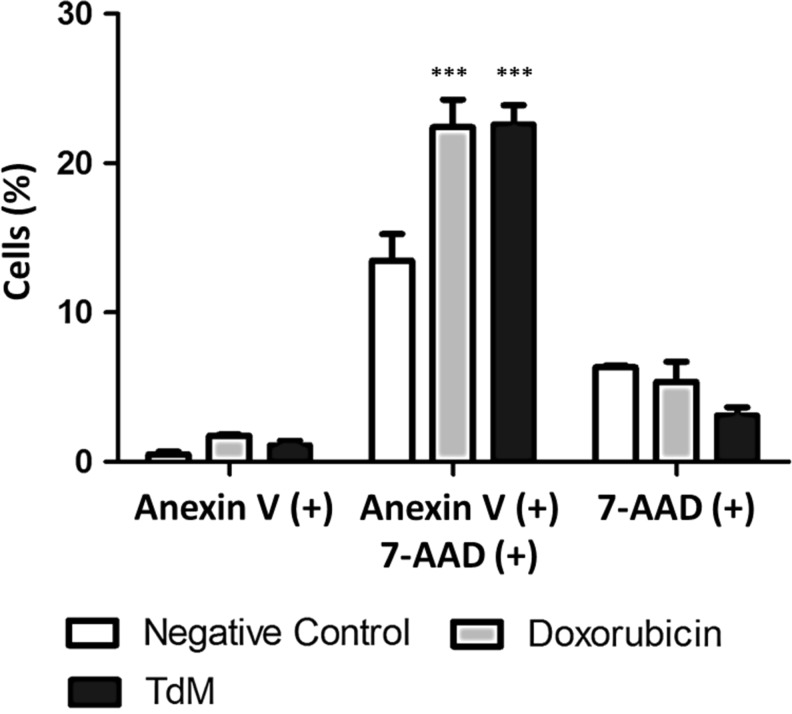
MDA-MB-231 breast cancer cells exposed to TdM for 24 h were positive to Annexin V (PE) and 7AAD, indicating that they were in the latter stages of apoptosis. The percentage of these cancer cells stained with Annexin V (+) was 22.6% for those treated with TdM, 22.4% for those treated with doxorubicin (the positive control), and 13.5% for those treated with DMSO (the negative control) (Figs. 5 and 6).
FIG. 5.
Cytometric analysis of MDA-MB-231 cells treated with different concentrations of TdM and doxorubicin for 24 h. Cells were double stained with Annexin V and 7-aminoactinomycin D (7AAD). Necrotic cells are positive to 7AAD, the early stages of apoptotic cells to Annexin V, and the later stages of apoptosis to both these fluorochromes. R1: 7AAD (+); R2: Annexin V (+), 7AAD (+); R3: Annexin V (−), 7AAD (−); and R4: Annexin V (+). Later apoptosis and necrosis is observed in cells exposed to TdM at 24 h.
FIG. 6.
Representation of the effect of three distinct treatments (TdM, doxorubicin, or the negative control) on MDA-MB-231 cells. A significant difference between cells treated with TdM and control negative is observed. ***Significant differences between control untreated and treated cell, p<.03.
Discussion
Many of the drugs used in anticancer therapy are either compounds isolated from plants or semisynthetic derivatives of the same. Therefore, it is crucial to keep searching for plants that have compounds or precursors of compounds with antitumor activity.
In the present study, the methanolic extract (TdM) of T. diffusa showed important cytotoxic activity on MDA-MB-231 breast cancer cells, although there was a lesser effect (evidenced by higher IC50 values) on the other cancer cell lines tested. This difference in activity can be attributed to the distinct type of cells and cellular receptors. For instance, MDA-MB-231 cells express only epidermal growth factor and transforming growth factor alpha. Contrarily, T-47D cells express calcitonin, prolactin, glucocorticoids, androgen receptors, progesterone receptors, and estrogen receptors. In addition, although both MDA-MB-231 and T-47D cells were derived from a metastasis in pleural effusion, the former were taken from breast adenocarcinoma and the latter from ductal carcinoma (ATCC).
The National Cancer Institute (NCI) has established the criterion of IC50≤30 μg/mL for an extract to be considered active.36 TdM showed this level of cytotoxic activity for MDA-MB-231 cells (IC50=30.67±3.5 μg/mL), while for the other tested cancer cell lines this extract showed a lesser effect. This activity was selective, given that the IC50 of TdM was 63.24 μg/mL with normal fibroblasts.
An activity-guided fractionation process led to the identification of the two fractions, F3 and F4, with the greatest activity. Arbutin (1) was isolated from F3, while arbutin (1) and apigenin (2) were isolated from F4. The latter fraction showed the best cytotoxic activity. Both of these compounds have been previously reported from T. diffusa.
Extensive studies with apigenin (2), a trihydroxyflavone, have demonstrated its anticancer properties.37 This compound has been found to induce apoptosis in a variety of tumor cell lines.38,39 Lee et al.40 revealed the effect of apigenin on TNF-alpha, which, in part, explains the activity of this compound on MDA-MB-231 cells. On the other hand, arbutin (1) is a hydroquinone-D-glucopyranoside that leads to the expression of genes inducing apoptosis in human melanoma cells41 and therefore is also probably involved in the observed effect. The Annexin assay suggests that the TdM extract induces apoptosis and necrosis in MDA-MB-231 cells.
When comparing the IC50 value of F4 to that of the methanolic extract (TdM; Fig. 3), it is apparent that although 1 and 2 strongly contributed to the observed activity, there was probably a synergistic effect exercised by other compounds present in the extract. Indeed, it is generally assumed that a plant extract acts as a phytocomplex, wherein a variety of constituents interact with multiple targets. In the present study, it is likely that such a synergy existed given that it is a complete extract with numerous metabolites that are likely to induce different activities.
In conclusion, the current study demonstrates for the first time that the methanolic extract of T. diffusa has a cytotoxic effect on MDA-MB-231 breast cancer cells, and that this activity, at least in part, owes itself to the activity of apigenin. The present results provide scientific evidence of yet another medicinal activity of T. diffusa, in this case as a possible alternative therapy for cancer.
Acknowledgments
The authors gratefully acknowledge CONACYT, Mexico (grant 134627) and SIP-IPN (grants 20100347, 20121209) for financial support. M.C.A.-F. is grateful to PIFI-IPN and CONACYT for the scholarships awarded. M.C.-C. and F.J.-M. are fellows of the EDI/IPN and COFAA/IPN programs. The authors thank the personnel of the Organic Chemistry Department, ENCB, IPN for NMR technical assistance.
Author Disclosure Statement
The authors declare that there is no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Formad D: Global cancer statistics. CA. Cancer J Clin 2011;61:69–90 [DOI] [PubMed] [Google Scholar]
- 2.Desantis C, Howlader N, Cronin KA, Jemal A: Breast cancer incidence rates in US women are no longer declining. Cancer Epidemiol Biomarkers Prev 2011;20:733–739 [DOI] [PubMed] [Google Scholar]
- 3.Hartwell JL: Plants Used Against Cancer. Quarterman, Lawrence, MA, 1982 [Google Scholar]
- 4.Gueritte F, Fahy J: The vinca alkaloids. In: Anticancer Agents from Natural Products (Cragg GM, Kingston DGI, Newman DJ, eds.). Taylor and Francis, Boca Raton, FL, 2005, pp. 123–135 [Google Scholar]
- 5.Roussi F, Gueritte F, Fahy J: The vinca alkaloids, In: Anticancer Agents from Natural Products, 2nd ed. (Cragg GM, Kingston DGI, Newman DJ, eds.). Taylor and Francis, Boca Raton, FL, 2012, pp. 177–198 [Google Scholar]
- 6.Lee KH, Xiao Z: Podophyllotoxin and analogs. In: Anticancer Agents from Natural Products (Cragg GM, Kingston DGI, Newman DJ, eds.). Taylor and Francis, Boca Raton, FL, 2005, pp. 71–87 [Google Scholar]
- 7.Lee KH, Xiao Z: Podophyllotoxin and analogs. In: Anticancer Agents from Natural Products, 2nd ed. (Cragg GM, Kingston DGI, Newman DJ, eds.). Taylor and Francis, Boca Raton, FL, 2012, pp. 95–122 [Google Scholar]
- 8.Kingston DGI: Taxol and its analogs. In: Anticancer Agents from Natural Products, 2nd ed. (Cragg GM, Kingston DGI, Newman DJ, eds.). Taylor and Francis, Boca Raton, FL, 2012, pp. 123–175 [Google Scholar]
- 9.Kingston DGI: Taxol and its analogs. In: Anticancer Agents from Natural Products, 2nd ed. (Cragg GM, Kingston DGI, Newman DJ, eds.). Taylor and Francis, Boca Raton, FL, 2005, pp. 89–122 [Google Scholar]
- 10.Kingston DGI, Newman DJ: Taxoids: cancer-fighting compounds from nature. Curr Opin Drug Discov Dev 2007;10:130–144 [PubMed] [Google Scholar]
- 11.Rahier NJ, Thomas CJ, Hecht SM: Camptothecin and its analogs. In: Anticancer Agents from Natural Products (Cragg GM, Kingston DGI, Newman DJ, eds.). Taylor and Francis, Boca Raton, FL, 2005, pp. 5–21 [Google Scholar]
- 12.Tyler VE: Damiana-history of an herbal hoax. Pharm Hist 1983;25:55–60 [PubMed] [Google Scholar]
- 13.Andrade-Cetto A, Heinrich M: Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J Ethnopharmacol 2005;99:325–348 [DOI] [PubMed] [Google Scholar]
- 14.Alarcon-Aguilar FJ, Roman-Ramos R, Flores-Saenz JL, Aguirre-García F: Investigation on the hypoglycaemic effects of extracts of four medicinal plants in normal and alloxan diabetic mice. Phytother Res 2002;16:382–386 [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Dasmahapatra AK, Khan SI, Khan IA: Anti-aromatase activity of the constituents from damiana (Turnera diffusa). J Ethnopharmacol 2008;120:387–393 [DOI] [PubMed] [Google Scholar]
- 16.Arletti R, Benelli A, Cavazzuti E, Scarpetta G, Bertolini A: Stimulating property of Turnera diffusa and Pfaffia paniculata extracts on the sexual behavior of male rats. Psychopharmacology 1999;143:15–19 [DOI] [PubMed] [Google Scholar]
- 17.Estrada-Reyes R, Ortiz-López P, Gutiérrez-Ortíz J, Martínez-Mota L: Turnera diffusa Wild (Turneraceae) recovers sexual behavior in sexually exhausted males. J Ethnopharmacol 2009;123:423–429 [DOI] [PubMed] [Google Scholar]
- 18.Zava DT, Dollbaum CM, Blen M: Estrogen and progestin bioactivity of foods, herbs and spices. Proc Soc Exp Biol Med 1998;217:369–378 [DOI] [PubMed] [Google Scholar]
- 19.Hernández T, Canales M, Avila JG, Duran A, Caballero J, Romo de Vivar A, Lira R: Ethnobotany and antibacterial activity of some plants used in traditional medicine of Zapotitlan de las Salinas, Puebla (México). J Ethnopharmacol 2003;88:181–188 [DOI] [PubMed] [Google Scholar]
- 20.Aguilera-Carbo AF, Augur C, Prado-Barragan LA, Aguilar CN, Favela-Torres E: Extraction and analysis of ellagic acid from novel complex sources. Chem Papers 2008;62:440–444 [Google Scholar]
- 21.Salazar R, Pozos ME, Cordero P, Pérez J, Salinas MC, Waksman N: Determination of the antioxidant activity of plants from northeast Mexico. Pharm Biol 2008;46:166–170 [Google Scholar]
- 22.Spencer KC, Seigler DS: Tetraphyllin B from Turnera diffusa. Planta Med 1981;43:175–178 [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Pawar RS, Ali Z, Khan IA: Phytochemical investigation of Turnera diffusa. J Nat Prod 2007;70:289–292 [DOI] [PubMed] [Google Scholar]
- 24.Popoca J, Aguilar A, Alonso D, Villarreal ML: Cytotoxic activity of selected plants used as antitumorals in Mexican traditional medicine. J Ethnopharmacol 1998;59:173–177 [DOI] [PubMed] [Google Scholar]
- 25.Weng C-J, Yen G-C: Chemopreventive effect of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenols, polyphenols, and their derivatives. Cancer Treat Rev 2011;38:76–87 [DOI] [PubMed] [Google Scholar]
- 26.Betancur LAG, Saez J, Granados H, Salazar A, Ossa JE: Antitumor and antiviral activity of Colombian medicinal plant extracts. Mem Inst Oswaldo Cruz 1999;94:531–534 [DOI] [PubMed] [Google Scholar]
- 27.Popoca J, Aguilar A, Alonso D, Villarreal ML: Cytotoxic activity of selected plants used as antitumorals in Mexican traditional medicine. J Ethnopharmacol 1998;59:173–177 [DOI] [PubMed] [Google Scholar]
- 28.Moo-Puc R, Robledo D, Freile-Pelegrín Y: In vitro cytotoxic and antiproliferative activities of marine macroalgae from Yucatán, México. Ciencias Marinas 2009;35:345–358 [Google Scholar]
- 29.Quintero A, Pelcastre A, Solano JD: Antitumoral activity of new pyrimidine derivatives of sesquiterpene lactones. J. Pharm Pharmaceut Sci 1999;2:108–112 [PubMed] [Google Scholar]
- 30.Regasini LO, Lopes AA, Silva DHS, Furlan M, Young MCM, Maria DA, Barreiro EJ, Bolzani VS: Antiproliferative effect of Pterogyne nitens on melanoma cells Rev Ciềnc Farm Básica Apl 2007;28:335–340 [Google Scholar]
- 31.Gao S, Xu Y, Valeriote A, Gunatilaka AAL: Pierreiones A-D, solid tumor selective pyranoisoflavones and others cytotoxic constituents from Antheroporum pierrei. J Nat Prod 2011;74:852–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng Y, Sk UH, Zhang Y, Ren X, Zhang L, et al. : Rational incorporation of selenium into temozolomide elicits superior antitumor activity associated with both apoptotic and autophagic cell death. PLoS One 2012;7:e35104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voisin T, El Firar A, Fasseu M, Rouyer-Fessard C, Descatoire V, et al. : Therapeutics, targets, and chemical biology: aberrant expression of OX1 receptors for orexins in colon cancers and liver metastases an openable gate to apoptosis. Cancer Res 2011;71:3341–3351 [DOI] [PubMed] [Google Scholar]
- 34.Nycz JE, Maleck G, Morag M, Nowak G, Ponikiewski L, Kusz J, Switlicka A: Arbutin: isolation, X-ray structure and computional studies. J Mol Struc 2010;980:13–17 [Google Scholar]
- 35.She G, Guo Z, Lv H, She D: New flavonoid glycosides from Elsholtzia rugulosa Hemsl. Molecules 2009;14:4190–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso-Castro AJ, Villareal ML, Salazar Olivo LA, Gómez-Sánchez M, Domínguez F, García-Carranca A: Mexican medicinal plants used for cancer treatment: pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol 2011;133:945–972 [DOI] [PubMed] [Google Scholar]
- 37.Clere N, Faure S, Martinez MC, Andriantsitohaina R: Anticancer properties of flavonoids: roles in various stages of carcinogenesis. Cardiovasc Hematol Agents Med Chem 2011;9:62–77 [DOI] [PubMed] [Google Scholar]
- 38.Kim BR, Jeon YK, Nam MJ: A mechanism of apigenin-induced apoptosis is potentially related to anti-angiogenesis and anti-migration in human hepatocellular carcinoma cells. Food Chem Toxicol 2011;49:1626–1632 [DOI] [PubMed] [Google Scholar]
- 39.Lu HF, Chie YJ, Yang MS, Lu KW, Fu JJ, Yang JS, Chen HY, Hsia TC, Ma CY, Ip SW, Chung JG: Apigenin induces apoptosis in human lung cancer H460 cells through caspase-and mitochondria-dependent pathways. Hum Exp Toxicol 2010;30:1053–1061 [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Zhou HY, Cho SY, Kim YS, Lee YS, Jeong CS: Antiinflammatory mechanism of apigenin: inhibition of cyclooxigenase-2 expression, adhesion of monocytes to human umbilical vein endotelial cells and expression of cellular adhesion molecules. Arch Pharm Res 2007;30:1318–1327 [DOI] [PubMed] [Google Scholar]
- 41.Nawarak J, Huang-Liu R, Kao SH, Liao HH, Sinchaikul S, Chen ST, Cheng SL: Proteomic analysis of A375 human malignant melanoma cells in response to arbutin treatment. Biochim Biophys Acta 2009;1794:159–167 [DOI] [PubMed] [Google Scholar]