Abstract
Practical methods of assessing resting energy expenditure (REE) could be useful in large populations of overweight and obese individuals during phases of weight loss and weight-loss maintenance to address weight regain. We compared predicted and measured REE using the MedGem handheld device and a traditional, indirect calorimeter in middle-aged men and women who were overweight and obese (body mass index ≥25.0 and <40.0). Each subject (n=88) completed traditional, indirect calorimetry and handheld calorimetry in random order. A subset of participants (n=10) completed each of these assessments at three different time points to examine their test-retest reliability. We found that MedGem estimates of REE were significantly greater than estimates with the traditional, indirect calorimeter and the predicted REE using the Harris-Benedict equation (P<0.01). Intra-class correlations were .70 (P=0.15) for repeated recordings with the MedGem and .84 (P=0.65) for traditional indirect calorimetry. The MedGem can overestimate REE in middle-aged overweight/obese individuals and has moderate test-retest reliability. Indirect calorimetry is the preferred measurement of REE in this population.
Keywords: Indirect calorimetry, MedGem, Overweight, Resting energy expenditure
OBESITY IS A MULTIFACTOR, CHRONIC DISEASE AND has become a major public health problem in the United States. Currently, 35.7% of US adults aged 20 years or older are obese, and 69.2% are overweight and obese.1 These data indicate a consistently increasing rate of obesity from a prevalence of 22.9% in 1988 to 1994.1,2 Obesity is associated with increased mortality and significant morbidity, including increased rates of hypertension, cardiovascular disease, type 2 diabetes, stroke, biliary disease, and osteoarthritis.3 In addition, this risk of mortality associated with obesity increases with higher body mass index (BMI).4 These data highlight the growing epidemic of obesity in the United States and the need for effective weight-loss interventions.
To lose weight, energy intake must be reduced to less than the energy utilized. It is desirable to know the energy expenditure of individuals to design and monitor effective weight-loss plans. Accurate assessment of energy intake would help facilitate one’s specific energy load (ie, number of calories) necessary to lose weight or maintain weight loss; enrich and enhance motivation to lose weight; increase willingness to adhere to long-term calorie intake modification; and to adopt a lifelong activity program.5
Determination of energy requirements can be estimated or measured using a variety of methods.6,7 Traditional, indirect calorimetry measures oxygen consumption (VO2) and carbon dioxide (VCO2) production. The resting energy expenditure (REE; kcal/day) and respiratory quotient (RQ) are estimated from the measured substrate oxidation rates.8,9 A portable, handheld calorimeter, MedGem (HealthETech),10 has been developed for easy, transportable assessment of one’s specific calorie requirements for the health fitness market. Using proprietary sensors that measure oxygen consumption and airflow, the MedGem device measures VO2 for the determination of REE, applying a constant RQ of 0.85 and a modified Weir equation. Prediction equations have been developed to estimate REE in adults, such as the Mifflin-St Jeor and Harris-Benedict equations, of which the Harris-Benedict equation is most commonly applied.11-13 The Harris-Benedict equation estimates REE based on an individual’s weight, height, sex, and age.
Knowledge of energy expenditure can be an important and effective tool to use in weight-management programs; however, the lack of practical, accurate methods to measure energy expenditure can impede the effectiveness of weight-loss programs.5 The primary aim of this study was to compare different methods of assessing energy expenditure in overweight and obese individuals.
METHODS
Design
The study had a random-order, crossover design in which both assessments were tested in all participants at the same visit. At study entry, the order in which subjects were tested (with traditional indirect calorimetry and the handheld device) was determined by computer-generated random-number list. To test reproducibility of instruments, a subset of subjects (n=10) had repeated measurements, again in random order, within 5 days of the initial visit.
Study Population
Eighty-nine individuals between the ages of 35 and 64 years with a BMI >25.0 but <40.0 and no or stable chronic disease were recruited through institutional e-mail advertisements. Participants were examined at the Massachusetts General Hospital Clinical Research Center in Boston, MA, or the Clinical Research Center at Massachusetts Institute of Technology in Cambridge, MA. Subjects were excluded if they had psychological barriers that could potentially interfere with completing the indirect calorimeter measurements (eg, claustrophobia); inability to hold the handheld mouthpiece; history of dental or facial trauma; acute or chronic disease known to alter energy expenditure (eg, cancer, respiratory distress, kidney disease, human immunodeficiency virus); use of steroids or weight-reduction–enhancing medications; unstable chronic disease (eg, diabetes, hypertension, hyperlipidemia); and, if female, pregnancy or nursing. Eligibility was determined by telephone interview. From the original sample of 89 subjects, one subject’s BMI increased to >40.0 after recruitment and the subject was excluded from data analysis. The Massachusetts General Hospital and Massachusetts Institute of Technology Institutional Review Boards approved the study and informed consent was obtained from each subject.
Measures
Anthropometric Assessment
Each subject underwent anthropometric measurements of height, weight, waist circumference, and hip circumference. Height (cm) was measured in triplicate using a Harpenden stadiometer (Croswell). Weight was assessed on a digital, electronic scale and was recorded to the nearest 0.1 kg and BMI was calculated. Circumferences were measured at the iliac waist and the broadest hip location (ie, around the buttocks). Waist-to-hip ratio was calculated as the iliac waist measure relative to the broadest hip circumference.14
Energy Assessment
Indirect calorimeter assessments were obtained in subjects after a 12-hour fast and at least 24-hour abstention from physical activity of moderate or greater intensity. A traditional, indirect calorimeter (Vmax 29N, SensorMedics) was calibrated before use according to manufacturer’s specifications. While the participant rested with head elevated at 45-degree angle, a canopy was placed over his head (allowing for uniform air flow). Oxygen consumption and carbon dioxide production were measured for a minimum of 20 minutes. Using the standard metabolic cart (Vmax29N), REE (kcal/day) and RQ were calculated from the substrate oxidation rates.8,9 Of note, basal metabolic rates can vary by populations, depending on population-specific factors, such as ethnicity, cardiovascular fitness, and medical comorbidities.15-17 The MedGem10 measures VO2 for the determination of REE using an RQ constant of 0.85. The handheld MedGem consists of two proprietary sensors that measure oxygen and air flow.
Total body composition was measured by dual-energy x-ray absorptiometry (DXA; QDR-4500A, Hologic Inc). A whole-body DXA scan was performed to determine body composition, which is a validated measure of fat mass and lean body mass.18
A brief, self-administered questionnaire developed for this study was completed to rate device preference and perceived benefit of knowledge regarding energy expenditure using an ordinal scale.
Statistical Analysis
Data for continuous outcomes were presented as mean±standard deviation and binary outcomes were presented as counts and proportions. Difference in means between the methods of REE measurements were examined using paired t tests. Repeated measures analysis of variance and intra-class correlation coefficient (ICC) and 95% CIs were used to evaluate the reproducibility of each method of REE over the three repeated measurements.19 The ICCs are based on the repeated measures analysis of variance (or ICC=[means square (between subjects)–means square (within subjects)]/[means square (between subjects)+MS (within subjects)]. The Cohen effect size to detect a difference in mean levels at a 5% type-1 error rate with 80% power between two methods was d=0.3 for overall comparison using both males and females. The effect sizes for sex-specific comparisons were d=0.35 for analysis of males and d=0.61 for analysis of females. All tests were two-tailed and P<0.05 was considered statistically significant for all analyses.
RESULTS AND DISCUSSION
Clinical and Anthropometric Characteristics
Body weight and anthropometric characteristics are presented in Table 1. Subjects ranged from overweight to obese, with mean BMI in the obese range and with no significant difference between sexes. There were no differences between females and males for hip circumference. Significant differences between sexes were observed for waist circumference, waist-to-hip ratio, total body fat by DXA, and total lean body mass (kg) by DXA (Table 1).
Table 1.
Demographic, anthropometric, and body composition of middle-age overweight/obese individuals participating in a study comparing energy assessment methods
| Males (n=23) | Females (n=65) | Total (n=88) | |
|---|---|---|---|
| mean ± standard deviation | |||
| Age (y) | 52.6±8.8 | 51.4±7.5 | 51.7±7.9 |
| Body mass index | 31.3±4.6 | 32.0±4.1 | 31.8±4.2 |
| Ideal body weight (%) | 136.6±19.4 | 139.1±16.3 | 138.4±17.1 |
| Iliac waist circumference (cm)*** | 109.0±12.7 | 103.4±10.5 | 104.8±11.3 |
| Hip circumference (cm) | 111.4±8.5 | 116.3±9.2 | 115.1±9.2 |
| Waist-hip ratio*** | 0.98±0.07 | 0.89±0.06 | 0.91±0.07 |
| Body fat (kg) by DXAa,*** | 29.6±8.9 | 36.3±7.7 | 34.5±8.5 |
| Body fat (%) by DXA*** | 29.9±5.3 | 41.0±4.1 | 38.0±6.6 |
| Lean body mass (kg) by DXA*** | 65.3±9.4 | 49.5±6.6 | 53.7±10.2 |
DXA=dual-energy x-ray absorptiometry.
P<0.001, for differences between males and females.
Energy Assessment
Of the 88 subjects, 45 were assigned to carry out the traditional, indirect calorimeter first, then the handheld device. There were significant differences in MedGem results compared with both measured resting energy expenditure (MREE) using a traditional indirect calorimeter (P<0.0001) and predicted resting energy expenditure (PREE) based on the Harris-Benedict equation13,20 (P<0.0001 for both) (Table 2). There was no significant difference between MREE and PREE (P=0.61). The MedGem’s estimates of REE were significantly higher (ie, 6%) than MREE and PREE for the total cohort and in the females, with a trend for similar differences in the males (Table 2). In overweight subjects (BMI=25 to 29.9; n=34), the MREE and PREE estimates were significantly higher than those with the MedGem (Δ=110.11; t=1.41; P=0.02 and Δ=109.26; t=1.56; P=0.03, respectively). This was also true in the obese subjects (BMI=30.0 to 39.9, n=54) (Δ=111.74; t=−1.96; P=0.002 and Δ=96.15; t=1.83; P=0.002, respectively). The measured RQ varied for males (0.90) and females (0.86) based on the differences in measured VCO2 and VO2 (see Table 2).
Table 2.
Predicted and estimated resting energy expenditure for middle-age overweight/obese individuals participating in a study comparing energy assessment methods
| Males | Females | Total | |
|---|---|---|---|
| mean ± standard deviation | |||
| MREEa | 1,873.5±309.3 | 1,524.6±226.2 | 1,615.8±292.6 |
| MRQb | 0.90±0.1 | 0.86±0.07 | 0.87±0.1 |
| MVCO2c | 237.0±58.6 | 185.6±29.3 | 199.0±44.9 |
| MVO2d | 262.3±41.1 | 215.9±33.4 | 228.0±40.9 |
| PREEe | 1,898.4±251.4 | 1,529.2±134.1 | 1,625.7±236.3 |
| MedGemf | 2,023.9±367.1 | 1,621.8±255.3 | 1,726.9±337.0 |
| Comparison of methods | mean difference | ||
| MedGem vs MREE | 150.4† | 97.2*** | 111.1*** |
| PREE vs MREE | 24.9 | 4.6 | 9.9 |
| MedGem vs PREE | 125.5 | 92.6*** | 101.2*** |
MREE=measured resting energy expenditure using traditional indirect calorimeter (kcal/24 h).
MRQ=measured respiratory quotient as determined with the traditional indirect calorimeter.
MVCO2=measured carbon dioxide eliminated as determined with the traditional indirect calorimeter.
MVO2=measured oxygen consumption as determined with the traditional indirect calorimeter.
PREE=predicted resting energy expenditure using the Harris-Benedict equation (kcal/24 h).
MedGem=use of MedGem (HealthETech) portable, handheld calorimeter to measure resting energy expenditure (kcal/24 h).
P=0.053.
P<0.001.
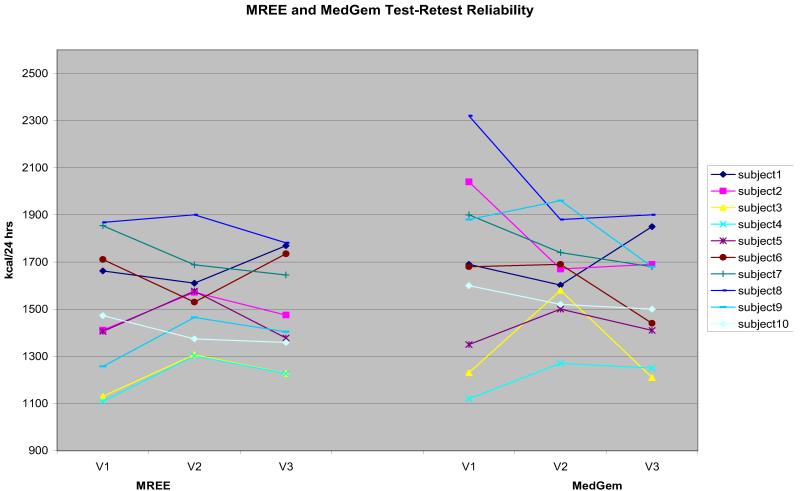
For the repeated measures analyses (Figure), we found the MedGem ICC to be .70 (95% CI 0.38 to 0.91) and the mean levels were not significantly different (P=0.15). The ICC for MREE using the traditional, indirect calorimetry was .84 (95% CI 0.61 to 0.95) and mean levels were not significantly different (P=0.65). The ICC assesses the consistency, or conformity, of measurements made by multiple observers (ie, independent visits/assessments) measuring the same quantity (ie, REE).21 An ICC of 1 represents perfect agreement, and a value of 0 means that there is no agreement at all. ICCs of 0 to 0.2 typically indicate poor agreement; 0.3 to 0.4 indicate fair agreement; 0.5 to 0.6 indicates moderate agreement; 0.7 to 0.8 indicates strong agreement; and >0.8 indicates almost perfect agreement.22
Figure.
Test-retest reliability of measured resting energy expenditure using traditional indirect calorimetry (kcal/24 h) and the MedGem (HealthETech) portable, handheld calorimeter. MREE=measured resting energy expenditure using traditional indirect calorimeter (kcal/24 h). MedGem=use of MedGem portable, handheld calorimeter to measure resting energy expenditure (kcal/24 h).
Acceptability of Energy Assessment
Both men and women preferred the traditional MREE procedure (61.4%) vs the handheld MedGem. Usefulness of knowing energy assessment data was rated “very helpful” (1.30±0.56 [1 standard deviation]), on a 5-point Likert scale with 1=very helpful) by 63 (72%) of the subjects. All the subjects rated knowledge of energy assessment between 1 (“very helpful”) and 3 (“helpful”) on the 5-point Likert scale. They were asked to rank three situations (meal planning, activity, label reading) during which such knowledge would be most helpful. Of these, meal planning was selected as the situation most likely to be helped.
Comparison of REE Assessment Methods
The present study found that a handheld device (MedGem) tended to overestimate REE when compared with a traditional indirect calorimeter (Vmax 29N) and with a predictive equation in a population of overweight/obese men and women aged 51.7±7.9 years (ie, mean=1,726.9 kcal/24 hours with MedGem vs mean=1,615.8 kcal/24 hours for MREE, and mean=1,625.7 kcal/24 hours for PREE). These comparisons were significant for the total sample as well as women (P<0.001), but were only marginally different (P=0.053) for males, perhaps because there were fewer males in the study or because of a small sample size (n=23) (Table 2).
These data are consistent with other studies that have found the MedGem to produce significantly higher measurements of energy expenditure than the indirect calorimeter.23,24 Melanson and colleagues found in overweight adults (n=41, 66% female; mean BMI=25.9 and 26.2 for males and females, respectively) that the repeated measured resting metabolic rate was significantly higher using the MedGem than the standard metabolic cart (mean difference=72 to 85±18 to 19 kcal/day).23 Another study assessing healthy subjects (n=17) and subjects with cancer (n=18) (BMI range=18 to 42) concluded that the MedGem had poor clinical agreement with a traditional indirect calorimeter (ie, VMax229).24 Specifically, less than half of the cancer patients (46.7%) and only one third (33.3%) of healthy subjects had measured REE with the MedGem within clinically acceptable limits of REE measured by indirect calorimetry. The MedGem can overestimate REE compared with the assessment from metabolic carts, as participants are required to sit up and hold the MedGem mouthpiece as opposed to lying down under a canopy. The latter condition allows participants to breathe more normally. This might also explain why participants preferred the metabolic cart measurement over the MedGem, particularly given that our sample was older than previous validation studies. Older individuals might also have dentures or other dental issues that make the MedGem mouthpiece more difficult to use, as well as potentially causing minor loss of air exchange. Although the metabolic carts are the preferred measurement method based on the results of our study, they are approximately the size of a desktop computer and require daily calibration, whereas the MedGem is smaller and lighter and more easily portable for use in field studies.
Our findings must also be considered in the context of other studies that have found the MedGem to be comparable with other methods of measuring energy expenditure. For example, a study comparing the MedGem to the Douglas Bag technique with adults who were, on average, overweight (n=63, 68% female; mean BMI=26.5±6.6) yielded high agreement (ICCs>.91; 1,657±324 kcal/day and 1,650±307 kcal/day, respectively) for measuring REE.25 In this healthy, middle-aged (mean age=41.3±11.2 years) population, the handheld device was as reliable as the Douglas Bag technique. In a small sample (n=15, 40% female, mean BMI=27.4±2.1), St-Onge and colleagues reported that REE determined using the Delta-Trac and MedGem showed no difference (6,455.1±417.6 kJ/day [1,542.8±99.8 kcal/day] vs 6,468.5±337.2 kJ/day [1,546.0±80.6 kcal/day], respectively).26 In this study, the REE was measured for 20 minutes using Delta-Trac, followed by a 10-minute measurement period using MedGem. Energy expenditure was again measured for 7 hours afterconsumption of a 2,510-kJ (600 kcal) breakfast and calculated as the average of the total measurement period for Delta-Trac and for eight readings using MedGem. These data suggest that the MedGem provides REE estimates similar to other indirect calorimeters; however, these samples, on average, had lower BMIs and current age than our study cohort. Given that our cohort had nearly 35% body fat, or on the upper end of normal, and the women had 41% body fat, this might explain the significant overestimation in this subsample (see Table 1). We also used different comparison methods than previous studies, which might have influenced the demonstrated differences. A larger study stratifying the sample by BMI (or body mass) and comparing more methods of REE assessment might be warranted.
We did not find any differences between the MREE and PREE using the Harris-Benedict equation,13,20 supporting the comparability of these two methods. This observation is important because the majority of energy utilization is based on REE and knowledge of energy expenditure has clinical implications in the weight management of individuals with overweight/obese BMIs. The Harris-Benedict equation is a commonly used formula to estimate energy intake goals for weight loss and it appears to be a valid assessment tool with clinical utility for overweight/obese individuals. In general, the Harris-Benedict equation tends to overestimate REE by approximately 5% to 15%,27 yet we found the Harris-Benedict equation to be remarkably consistent with REE measured by indirect calorimetry with an overestimation <1% (eg, 1,615.8 kcal/24 hours vs 1,625.7 kcal/24 hours; Table 2). Of note, we also calculated REE using the more recently developed Mifflin-St Jeor predictive equation.11 We found that the Mifflin-St Jeor estimate was less accurate than the Harris-Benedict equation or had a greater difference from our measured REE with indirect calorimetry (ie, the mean REE using the Mifflin-St Jeor equation was 1,561 kcal/24 hours). Our findings further validate the use of the Harris-Benedict equation in older, overweight/obese individuals.
We found the MedGem to be a less precise measure of REE, with an ICC of 0.71, compared with 0.84 for the conventional indirect calorimetry. Other studies have found it to be more reliable.28 There might be several reasons for the discrepancy in these results. First, our subjects tended to be older and more overweight than previous studies. We also conducted the repeated measures 5 days apart, compared with intervals that were closer together (eg, several times during the same day). Secondly, the MedGem assumes a consistent RQ of 0.85, but we found that the RQ actually varies between men and women. Third, there is a trend for the MedGem measurements to be lower at each subsequent visit and, therefore, more congruent with the MREE data. This could be a result of staff and participants becoming more skilled at using the MedGem, such as staff placing the nose clip more snugly to reduce air leakage and participants becoming less anxious using the device or learning to breathe more naturally into it. This would explain the lower ICC, as well as the differences with the traditional metabolic cart measurements. Whether the reproducibility of the MedGem measurements would have improved with shorter time intervals between testings in our population is unknown.
CONCLUSIONS
Our data suggest that using a traditional, indirect calorimeter and the predictive Harris-Benedict equation to assess REE in an older, overweight/obese population might be more reliable and accurate than using the MedGem. The MedGem yielded consistently higher estimates of REE compared with indirect calorimetry and the Harris-Benedict equation; however, it might still be useful for field studies, given that it is very portable. Additional studies are needed to identify how knowledge of energy expenditure can impact weight management and to assess whether subjects might benefit from knowing how energy requirements change based on weight, body composition, and activity.
ACKNOWLEDGEMENTS
The authors would like to thank the nursing and bionutrition staff from the Clinical Research Center at the Massachusetts General Hospital and the Clinical Research Center dual-energy x-ray absorptiometry facility at the Massachusetts Institute of Technology for providing expert care to research subjects and assistance with data collection.
FUNDING/SUPPORT
The project described was supported by the Earle Charlton Fund for Innovative Diabetes Research (Diabetes Research Center) and Grant Number 1 UL1 RR025758-01 (Massachusetts General Hospital Clinical Research Center) from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
STATEMENT OF POTENTIAL CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
Contributor Information
Ellen J. Anderson, Clinical Research Center and Department of Medicine and the Diabetes Research Center, Massachusetts General Hospital, Boston.
Louisa G. Sylvia, Bipolar Clinic and Research Program, Massachusetts General Hospital; Harvard Medical School, Boston, MA.
Martha Lynch, Inpatient Clinical, Department of Nutrition and Food Services, Massachusetts General Hospital, Boston.
Lillian Sonnenberg, Harvard Medical School, Boston, MA; Community Nutrition, Department of Nutrition and Food Services, Massachusetts General Hospital, Boston.
Hang Lee, Mallinckrodt General Clinical Research Center, Boston, MA.
David M. Nathan, Clinical Research Center, Department of Medicine and the Diabetes Research Center, Massachusetts General Hospital, Boston; Harvard Medical School, Boston, MA.
References
- 1.Ogden C, Carroll M, Curtin L, McDowell M, Tabak C, Flegal K. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics . Health, United States, 2011: With Special Feature on Socioeconomic Status and Health. Hyattsville, MD: [Accessed August 5, 2013]. 2012. p. 257. http://www.cdc.gov/nchs/data/hus/hus11.pdf. [PubMed] [Google Scholar]
- 3.Mokdad AH, Ford ES, Dowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors. JAMA. 2001;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Kit BK, Orpana H, Bi G. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakicic JM, Clark K, Coleman E, et al. Appropriate intervention strategies for weight loss and prevention of weight regain in adults. Med Sci Sports Exerc. 2001;33(12):2145–2156. doi: 10.1097/00005768-200112000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Sawaya AL, Tucker K, Tsay R, et al. Evaluation of four methods for determining energy intake in young and older women: Comparison with double labels water measurements of total energy expenditure. Am J Clin Nutr. 1996;63(4):491–499. doi: 10.1093/ajcn/63.4.491. [DOI] [PubMed] [Google Scholar]
- 7.Seale JL. Energy expenditure measurements in relation to energy requirements. Am J Clin Nutr. 1995;62(5 suppl):1042S–1046S. doi: 10.1093/ajcn/62.5.1042S. [DOI] [PubMed] [Google Scholar]
- 8.Ritz R, Cunningham J. Indirect calorimetry. In: Kacmarek RM, Hess D, Stoller JK, editors. Monitoring Respiratory Care. Mosby-Yearbook, Inc; St Louis, MO: 1993. pp. 411–413. [Google Scholar]
- 9.Cunningham JJ. Calculation of energy expenditure from indirect calorimetry: Assessment of the Weir equation. Nutrition. 1990;6(3):222–223. [PubMed] [Google Scholar]
- 10.HealthETech. BodyGem User Guide. HealthETEch, Inc; Golden, CO: 2001. [Google Scholar]
- 11.Mifflin M, St Jeor S, Hill L, Scott B, Daugherty S, Koh Y. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 12.Harris JA, Benedict FG. A Biometric Study of Basal Metabolism in Man. Carnegie Institute of Washington; Washington, DC: 1919. [Google Scholar]
- 13.Frankenfeld DC, Muth ER, Rowe WA. The Harris-Benedict studies of human and basal metabolism: History and limitations. J Am Diet Assoc. 1998;98(4):439–445. doi: 10.1016/S0002-8223(98)00100-X. [DOI] [PubMed] [Google Scholar]
- 14.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- 15.Miyake R, Ohkawara K, Ishikawa-Takata K, Morita A, Watanabe S, Tanaka S. Obese Japanese adults with type 2 diabetes have higher basal metabolic rates than non-diabetic adults. J Nutr Sci Vitaminol. 2011;57(5):348–354. doi: 10.3177/jnsv.57.348. [DOI] [PubMed] [Google Scholar]
- 16.Wong JE, Poh BK, Nik Shanita S, et al. Predicting basal metabolic rates in Malaysian adult elite athletes. Singapore Med J. 2012;53(11):744–749. [PubMed] [Google Scholar]
- 17.Manini T, Patel K, Bauer D, et al. European ancestry and resting metabolic rate in older African Americans. Eur J Clin Nutr. 2011;65(6):663–667. doi: 10.1038/ejcn.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viser M, Fuerst T, Lang T, Salamone L, Harris TB, Health A, Body Composition Study-Dual Energy X-Ray Absorptiometry and Body Composition Working Group Validity of the fan-beam dual energy x-ray absorptiometry for measuring fat-free mass and leg muscle mass. J Appl Physiol. 1999;87(4):1513–1520. doi: 10.1152/jappl.1999.87.4.1513. [DOI] [PubMed] [Google Scholar]
- 19.McGram KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. [Google Scholar]
- 20.Harris JA, Benedict FG. A Biometric Study of Basel Metabolism in Man. Carnegie Institution of Washington; Washington, DC: 1919. [Google Scholar]
- 21.Shrout P, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 22.Portney LG, Watkins MP. Foundations of Clinical Research Applications to Practice. Prentice Hall Inc; Englewood Cliffs, NJ: 2000. [Google Scholar]
- 23.Melanson EL, Coelho LB, Tran ZV, Haugen HA, Kearney JT, Hill JO. Validation of the BodyGem hand-held calorimeter. Int J Obes Relat Metab Disord. 2004;28(11):1479–1484. doi: 10.1038/sj.ijo.0802643. [DOI] [PubMed] [Google Scholar]
- 24.Reeves MM, Capra S, Bauer J, Davies PS, Battistutta D. Clinical accuracy of the MedGem indirect calorimeter for measuring resting energy expenditure in cancer patients. Eur J Clin Nutr. 2005;59(4):603–610. doi: 10.1038/sj.ejcn.1602114. [DOI] [PubMed] [Google Scholar]
- 25.Nieman DC, Trone GA, Austin MD. A new handheld device for measuring resting metabolic rate and oxygen consumption. J Am Diet Assoc. 2003;103(5):588–593. doi: 10.1053/jada.2003.50116. [DOI] [PubMed] [Google Scholar]
- 26.St-Onge M, Rubiano F, Jones A, Jr, Heymsfield SB. A new hand-held indirect calorimeter to measure postprandial energy expenditure. Obes Res. 2004;12(4):704–709. doi: 10.1038/oby.2004.82. [DOI] [PubMed] [Google Scholar]
- 27.Daly JM, Heymsfeld SB, Head CA, et al. Human energy requirements: Overestimation by widely used prediction equation. Am Clin Nutr. 1985;42(6):1170–1174. doi: 10.1093/ajcn/42.6.1170. [DOI] [PubMed] [Google Scholar]
- 28.McDoniel SO. Systematic review on the use of a handheld indirect calorimeter to assess energy needs in adults and children. Int J Sport Nutr Exerc Metab. 2007;17(5):491–500. doi: 10.1123/ijsnem.17.5.491. [DOI] [PubMed] [Google Scholar]