Abstract
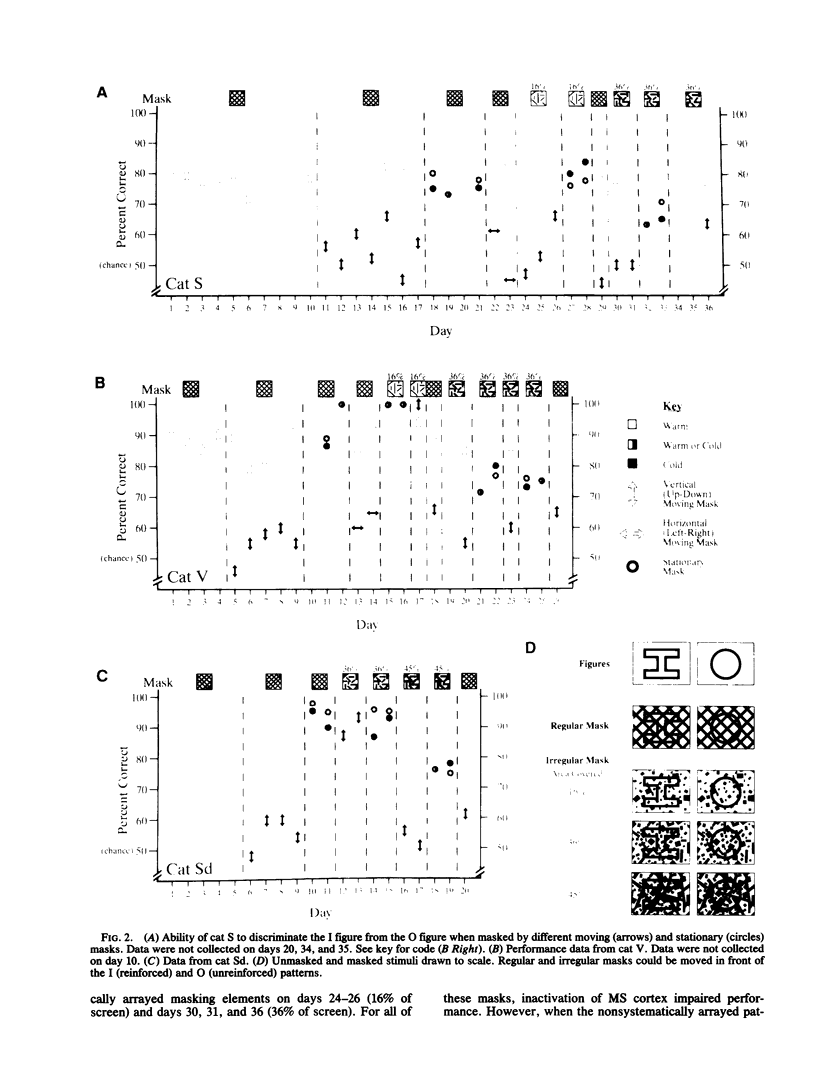
Extrastriate visual areas on the banks of the middle suprasylvian sulcus were inactivated by cooling to assess the behavioral contribution of this cortical region to the extraction of a stationary figure from a moving mask. Cooling blocked figure-ground separation when the mask was moving but had no influence when the mask was static. This difference provides strong evidence that the areas bounding the middle suprasylvian sulcus contribute to the neural separation of stationary from moving visual stimuli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright T. D. Centrifugal directional bias in the middle temporal visual area (MT) of the macaque. Vis Neurosci. 1989;2(2):177–188. doi: 10.1017/s0952523800012037. [DOI] [PubMed] [Google Scholar]
- Albright T. D. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol. 1984 Dec;52(6):1106–1130. doi: 10.1152/jn.1984.52.6.1106. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Zumbroich T. J. Stimulus selectivity and functional organization in the lateral suprasylvian visual cortex of the cat. J Physiol. 1987 Aug;389:569–603. doi: 10.1113/jphysiol.1987.sp016673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénita M., Condé H. Effects of local cooling upon conduction and synaptic transmission. Brain Res. 1972 Jan 14;36(1):133–151. doi: 10.1016/0006-8993(72)90771-8. [DOI] [PubMed] [Google Scholar]
- Campbell A., Jr Deficits in visual learning produced by posterior temporal lesions in cats. J Comp Physiol Psychol. 1978 Feb;92(1):45–57. doi: 10.1037/h0077442. [DOI] [PubMed] [Google Scholar]
- Cornwell P., Herbein S., Corso C., Eskew R., Warren J. M., Payne B. Selective sparing after lesions of visual cortex in newborn kittens. Behav Neurosci. 1989 Dec;103(6):1176–1190. doi: 10.1037//0735-7044.103.6.1176. [DOI] [PubMed] [Google Scholar]
- Diamond I. T., Hall W. C. Evolution of neocortex. Science. 1969 Apr 18;164(3877):251–262. doi: 10.1126/science.164.3877.251. [DOI] [PubMed] [Google Scholar]
- Gizzi M. S., Katz E., Schumer R. A., Movshon J. A. Selectivity for orientation and direction of motion of single neurons in cat striate and extrastriate visual cortex. J Neurophysiol. 1990 Jun;63(6):1529–1543. doi: 10.1152/jn.1990.63.6.1529. [DOI] [PubMed] [Google Scholar]
- Hughes H. C., Sprague J. M. Cortical mechanisms for local and global analysis of visual space in the cat. Exp Brain Res. 1986;61(2):332–354. doi: 10.1007/BF00239523. [DOI] [PubMed] [Google Scholar]
- Jasper H. H., Shacter D. G., Montplaisir J. The effect of local cooling upon spontaneous and evoked electrical activity of cerebral cortex. Can J Physiol Pharmacol. 1970 Sep;48(9):640–652. doi: 10.1139/y70-094. [DOI] [PubMed] [Google Scholar]
- Kiefer W., Krüger K., Strauss G., Berlucchi G. Considerable deficits in the detection performance of the cat after lesion of the suprasylvian visual cortex. Exp Brain Res. 1989;75(1):208–212. doi: 10.1007/BF00248543. [DOI] [PubMed] [Google Scholar]
- Mendola J. D., Payne B. R. Direction selectivity and physiological compensation in the superior colliculus following removal of areas 17 and 18. Vis Neurosci. 1993 Nov-Dec;10(6):1019–1026. doi: 10.1017/s0952523800010129. [DOI] [PubMed] [Google Scholar]
- Newsome W. T., Paré E. B. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J Neurosci. 1988 Jun;8(6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome W. T., Wurtz R. H., Dürsteler M. R., Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985 Mar;5(3):825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak T., Horn K. M., Maunsell J. H. Deficits in speed discrimination following lesions of the lateral suprasylvian cortex in the cat. Vis Neurosci. 1989 Oct;3(4):365–375. doi: 10.1017/s0952523800005538. [DOI] [PubMed] [Google Scholar]
- Payne B. R., Conners C., Cornwell P. Survival and death of neurons in cortical area PMLS after removal of areas 17, 18, and 19 from cats and kittens. Cereb Cortex. 1991 Nov-Dec;1(6):469–491. doi: 10.1093/cercor/1.6.469. [DOI] [PubMed] [Google Scholar]
- Payne B. R. Evidence for visual cortical area homologs in cat and macaque monkey. Cereb Cortex. 1993 Jan-Feb;3(1):1–25. doi: 10.1093/cercor/3.1.1. [DOI] [PubMed] [Google Scholar]
- Payne B. R., Pearson H. E., Berman N. Role of corpus callosum in functional organization of cat striate cortex. J Neurophysiol. 1984 Sep;52(3):570–594. doi: 10.1152/jn.1984.52.3.570. [DOI] [PubMed] [Google Scholar]
- Payne B. R. Representation of the ipsilateral visual field in the transition zone between areas 17 and 18 of the cat's cerebral cortex. Vis Neurosci. 1990 May;4(5):445–474. doi: 10.1017/s0952523800005204. [DOI] [PubMed] [Google Scholar]
- Payne B. R., Siwek D. F., Lomber S. G. Complex transcallosal interactions in visual cortex. Vis Neurosci. 1991 Mar;6(3):283–289. doi: 10.1017/s0952523800006283. [DOI] [PubMed] [Google Scholar]
- Payne B. R., Siwek D. F. The visual map in the corpus callosum of the cat. Cereb Cortex. 1991 Mar-Apr;1(2):173–188. doi: 10.1093/cercor/1.2.173. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., von Grünau M. W., Poulin C. Centrifugal organization of direction preferences in the cat's lateral suprasylvian visual cortex and its relation to flow field processing. J Neurosci. 1987 Apr;7(4):943–958. doi: 10.1523/JNEUROSCI.07-04-00943.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanides F., Hoffmann J. Cyto- and myeloarchitecture of the visual cortex of the cat and of the surrounding integration cortices. J Hirnforsch. 1969;11(1):79–104. [PubMed] [Google Scholar]
- Shipp S., Grant S. Organization of reciprocal connections between area 17 and the lateral suprasylvian area of cat visual cortex. Vis Neurosci. 1991 Apr;6(4):339–355. doi: 10.1017/s095252380000657x. [DOI] [PubMed] [Google Scholar]
- Spear P. D., Miller S., Ohman L. Effects of lateral suprasylvian visual cortex lesions on visual localization, discrimination, and attention in cats. Behav Brain Res. 1983 Dec;10(2-3):339–359. doi: 10.1016/0166-4328(83)90039-6. [DOI] [PubMed] [Google Scholar]
- Sugiyama M. The projection of the visual cortex on the Clare-Bishop area in the cat. A degeneration study with the electron microscope. Exp Brain Res. 1979 Aug 1;36(3):433–443. doi: 10.1007/BF00238514. [DOI] [PubMed] [Google Scholar]
- Symonds L. L., Rosenquist A. C., Edwards S. B., Palmer L. A. Projections of the pulvinar-lateral posterior complex to visual cortical areas in the cat. Neuroscience. 1981;6(10):1995–2020. doi: 10.1016/0306-4522(81)90039-7. [DOI] [PubMed] [Google Scholar]
- Yin T. C., Greenwood M. Visual response properties of neurons in the middle and lateral suprasylvian cortices of the behaving cat. Exp Brain Res. 1992;88(1):1–14. doi: 10.1007/BF02259124. [DOI] [PubMed] [Google Scholar]
- von Grünau M., Frost B. J. Double-opponent-process mechanism underlying RF-structure of directionally specific cells of cat lateral suprasylvian visual area. Exp Brain Res. 1983;49(1):84–92. doi: 10.1007/BF00235544. [DOI] [PubMed] [Google Scholar]