Abstract
Background:
Therapeutic plasma exchange (PE) or plasmapheresis is the treatment of choice in many neurological disorders. Even though it is safe in experienced hands, there is a major concern about its safety among physicians.
Objectives:
To analyze our experience with 230 patients who underwent PE for various neurological disorders.
Materials and Methods:
Retrospective review of PE procedures done during a period of 48 months, from July 2007 to June 2011 in a tertiary care teaching hospital in South India. Indications, clinical results and technical factors are discussed.
Results:
The main indication for PE was GBS (203 patients; 88.3%). Age of patients ranged from 14-65 (mean = 42.3 years). The most common complications were paraesthesias and/or cramps (36.1%) and hypotension (32.2%). Four pregnant patients who underwent PE had good recovery with one intrauterine death. There was no mortality.
Conclusion:
The analysis of 240 cases of PE done in our department shows that the procedure is safe, with only minimal procedure related complications and no mortality.
Keywords: Continuous flow method, Guillain-Barré syndrome, plasmapheresis, therapeutic plasma exchange
Introduction
The term ‘Apheresis’ is used to describe the process of removal of abnormal blood constituents by extracorporeal blood purification methods. Even though both terms are often used synonymously in the literature, plasma exchange (PE) involves the separation and removal of plasma from corpuscular blood and the replacement of it with various fluids, while plasmapheresis only refers to the removal of plasma. Most neurological disorders that are treated with PE are associated with presumed aberrant humoral immune responses, including myasthenia gravis, Guillain-Barré syndrome (GBS), and chronic inflammatory demyelinating polyneuropathy (CIDP).[1] It was first employed in 1952 in patients with multiple myeloma to control the hyperviscosity. By the 1970s PE had evolved as a treatment modality in a number of neurological disorders.[2]
The separation of plasma from the blood's cellular elements can be achieved with centrifugation devices or with permeable blood filters. Usually 1.5-2 L or 30-40 ml/Kg of plasma is removed at each cycle in most neurological disorders. This requires replacement with plasma expanders like albumin or plasma. There is no consensus on the ideal replacement solution for plasma discarded during PE. But, to reduce the complications due to plasma, the use of albumin and cross-matching plasma have been recommended.[3] Except for distinct diseases like hemolytic uremic syndrome or thrombotic thrombocytopenic purpura in which substitution is clearly by fresh-frozen plasma, colloid replacement can be achieved with the use of fresh-frozen plasma, albumin, albumin and saline, or albumin and plasma expander solutions.[4]
In addition to PE, the immunoadsorption technique represents a newer approach that allows a more selective removal of circulating antibodies. By binding to a ligand, removal of immunoglobulin fractions can be achieved.[1] After adsorption, the cellular blood components and plasma are combined and reinfused.
A variety of possible mechanisms for the actions of therapeutic PE have been proposed, including removal of antibody, alloantibody, immune complexes, monoclonal protein, toxin or cytokine(s) and the replenishment of a specific plasma factor[5,6,7,8]
In experienced hands, PE is extremely safe. Although hypotension and fluid-electrolyte imbalance may occur either during or following the procedure, most of these problems are rapidly recognized and reversed, and are rarely serious.[9,10] Probably the greatest risk to the patients is related to the vascular access like pneumothorax, thrombosis and infection.[11] Immunosuppression due to removal of plasma from the body can occur and, such patients are prone for systemic infections. Bleeding due to anticoagulant use can occur rarely. Deaths from PE have been reported, but have generally been related to preexisting illness.[12]
Here we report the experience on PE in 230 patients with various neurological diseases treated in the Neurology department of a tertiary care teaching hospital in South India.
Materials and Methods
We retrospectively reviewed all the PE procedures performed during a period of 48 months, from July 2007 to June 2011 in neurology intensive care unit (NICU).
Diagnosis and indication for PE were established with proper clinical and laboratory evaluation. Informed consent was obtained from every patient prior to the procedure. ECG, Chest X ray, Serology for HIV, Hepatitis B and C and blood grouping were done and cardiac and respiratory statuses were assessed. The subclavian vein was catheterized in 213 patients and femoral vein was accessed in 27 patients with a double lumen catheter, under local anesthesia, under aseptic precautions. A chest X-ray was routinely taken after subclavian catheterization, to assure proper placement of the catheter. The goal was to perform five exchanges on an alternate day schedule to reach a therapeutic target of 150-200 ml of plasma removed per Kg of body weight.
Patients were preloaded with 1.5-2 L of normal saline to get an adequate hydration status before the procedure. The blood pump speed was set at of 90 ml/min and gradually increased up to a maximum of 130 ml/min taking care to prevent clotting due to low speed and filter breakage due to high speed. Blood pressure (BP) and pulse were monitored every 15-30 minute intervals during the sessions and patients were closely observed for changes in appearance, development of symptoms (e.g., lightheadedness, nausea, parasthesias, etc.), and overall status. Hypotension was treated by lowering the pump speed or temporarily stopping the procedure and infusion of normal saline or fresh frozen plasma (FFP). During the procedure intravenous heparin was given 1000 U every 30 minutes to prevent clot formation in the extracorporeal circuit. Once target plasma filtrate was obtained, procedure was stopped. As replacement fluid, 3 units of FFP were infused to all patients immediately after the completion of the each exchange. All PE procedures were administered by staff nurses trained in dialysis units under the supervision of a senior resident in Neurology assigned to the case.
The Dialysis machine marketed by Fresenius Medical Care (4008 B) was used for doing the PE and, the procedures were done by continuous flow method using either the Plasmaflux PsU 2S (Fresenius Medical Care) or Plasmaflow (Gambro) plasma filters.
All patients were kept in NICU till the course of the PE was over.
Results
Altogether 979 PE procedures were performed on 230 patients. There were 131 male patients and 99 female patients. Age ranged from 14 to 65 years (mean 42.3). Number of patients in different age-groups is given in the Table 1.
Table 1.
Age distribution

Neurological diseases for which PE were done are given in Table 2. The three subtypes of GBS, namely, acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN) and acute motor sensory axonal neuropathy (AMSAN) constituted the indication for the procedure in 203 patients (88.3%).
Table 2.
Neurological diseases
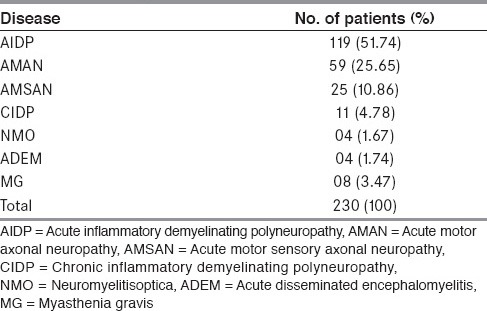
Other comorbid medical conditions were present in 153 patients (hypertension in 71 patients, diabetes mellitus in 31 patients, chronic obstructive airway disease in 27 patients and history of mild angina pectoris in 24 patients). All patients were relatively stable in their medical condition prior to PE.
Four patients were pregnant, three of them in second trimester and one in first trimester.
PE was done on an average of 7 days after the onset of symptoms in GBS. In patients with acute disseminated encephalomyelitis (ADEM) and neuromyelitisoptica (NMO), it was done after inadequate response with steroids. The patients with myasthenia gravis (MG) who underwent PE were in myasthenic crisis.
Even though the goal of our PE protocol was five exchanges in all the patients, only 123 (51.3%) patients could complete all the five sessions [Table 3]. Various reasons for incomplete PE sessions were filter related problems like poor filtration, filter block or clot formation (n = 47, 19.6%), significant improvement after less number of exchanges (n = 44, 19.3%), significant hemodynamic instability during procedure (n = 12, 5%), catheter infection (n = 11, 4.8%), catheter displacement (n = 5, 2.2%) and allergic reactions (n = 4, 1.7%).
Table 3.
Number of exchanges

Average volume of plasma removed was 1.23 Liters per session, ranging from 900 ml to 2 L.
Complications of PE were either local (related to the venous catheter) or systemic (related to the PE procedure) [Table 4].
Table 4.
Complications of plasma exchange
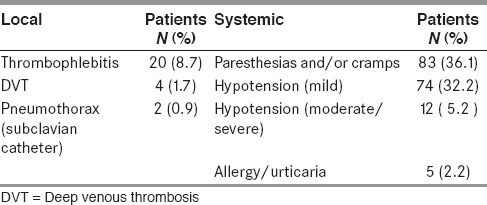
Fever due to possible catheter related phlebitis occurred in 20 (8.7%) patients. Of these, 14 (70%) patients had femoral and 6 (30%) had subclavian catheters. Four patients with femoral and five patients with subclavian catheters responded promptly to systemic antibiotics and PE was continued. The catheter was removed and further sessions cancelled in the other 11 patients (10 with femoral and one with subclavian) in whom the fever was prolonged and response to antibiotics was poor. Subsequently, they also responded very well to intravenous (IV) antibiotics. Deep vein thrombosis of lower limb occurred in 4 (1.7%) patients with femoral catheter which was confirmed with doppler ultrasound. The most feared complication of subclavian catheterization, the pneumothorax, occurred in 2 (0.9%) cases. Both of them had good recovery with intercostal drainage.
Among the systemic complications, perioral and/or limb paresthesias and muscle cramps were the most common, occurring in 88 (36.1%) patients. These were mild and transient and never resulted in termination of the procedure.
Mild transient hypotension (systolic BP < 100 mmHg with only minimal or no symptoms) occurred in 79 (32.2%) patients during at least one of their PE cycles. This was readily corrected by reducing the pump speed or administering intravenous 0.9% saline. PE was continued in all of them without any significant symptoms or complications during or after the procedure.
Twelve (5.2%) patients developed severe prolonged hypotension (systolic BP <80 mmHg) during procedure and required temporary stoppage of the exchange and administration of FFP or 0.9% saline. Eleven of these patients were having GBS and eight among them were hemodynamically unstable even before the procedure, most probably due to autonomic dysfunction.
Five patients (2.2%) developed urticarial rash which was managed with IV antihistamines and corticosteroids.
Three of the four pregnant women who underwent PE completed their pregnancy and delivered without any maternal or fetal complications while one patient who was in her first trimester of gestation had an intra-uterine fetal death.
In 31 patients aged more than 60 yrs, we did more rigorous monitoring, because of the higher likelihood of hemodynamic complications. Of these, 20 patients completed the cycles without any complications. In the remaining, early termination of the procedure was done due to hypotension.
Significant cardiac, renal or hepatic abnormality did not occur during or after the PE and there was no worsening of associated medical disorders like COPD, diabetes or hypertension in any of the patients.
There was no procedure related mortality in any of the 230 patients.
Six patients expired due to various reasons. All of them had GBS with respiratory muscle involvement and were on ventilator support. Three patients had severe hemodynamic instability and died one week after their last sessions, possibly due to autonomic dysfunction. Two patients died after 3 weeks, one due to lung collapse with consolidation and another with aspiration pneumonia with septicemia (both were on ventilator). One patient died on the second day after the first cycle, due to refractory hypotension.
Discussion
We report a series of 230 cases that underwent 979 cycles of PE over 48 months for various neurological diseases. The major indication for the procedure was GBS (203 patients, 88.3%). In GBS, the recommended treatment options are PE or Intravenous immunoglobulin (IVIG) and both have been found to be equally effective and significantly better than the conservative treatment for recovery from the disability.[13,14,15] Because of the ease of administration and less chance of complications, IVIG is preferred by most physicians. According to the report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology on plasmapheresis in 2011, it was found that PE is extremely safe in experienced hands.[16]
Although complication can occur, most of these are rapidly recognized and reversed, and are rarely serious. The deaths from PE have been reported, but have generally been related to underlying or preexisting illness than the procedure itself.
Majority of patients were adults and middle aged persons (Mean age: 42.3; range: 14-65). We did not employ PE in patients in the pediatric age group and the youngest patient in our cohort was 14-year-old. In children, therapeutic PE procedures are associated with multiple and unique challenges. The procedures are often performed using evidence or experience extrapolated from adult clinical practice, which may not be evidence based. In addition to the clinical challenges, relevant psychological issues, modification of protocols and technical hardware are often necessary for safe and effective treatment in children.[17] Old age is also a high risk for the procedure due to higher chance of hemodynamic alterations during the procedure. Data regarding the use of PE in elderly patients is sparse. In a study in patients aged 65 years or older, complications occurred during 11.5% of treatments, compared to 3.9% in the younger group.[18]
In our cohort, 32 patients were above 60 years. Of these, 20 patients tolerated the procedure well and in the remaining the procedure had to be discontinued due to hypotension.
Four pregnant patients, all suffering from GBS also tolerated the procedure well without any maternal complications, but one of them who were in her first trimester of gestation had intra uterine fetal death. Data regarding the safety of PE in pregnant women with neurological diseases is lacking. As the number of pregnant patients is small, conclusion regarding the safety in this group is difficult. But it can be reasonably concluded that in significantly disabled patients, where maternal prognosis is the prime concern it would be better to do the procedure and might not produce any significant complications with meticulous monitoring.
Hypotension, allergic reactions, nausea-vomiting, paresthesia and arrhythmias are the most common complications of PE which may be seen in 3-20% of the procedures. These events are usually mild and resolves without treatment. Because of problems related to vascular access, 4-5% of PE may have to be terminated.[18] In our series there was a 7% incidence such complications (catheter infection: 4.8% and catheter displacement: 2.2%). Complications of PE did not differ significantly between GBS and other disorders in our cohort except severe prolonged hypotension (systolic BP < 80 mm of Hg) which was much higher (11 out of 12) in GBS, most likely contributed by autonomic dysfunction.
The most common complication in our series was paresthesias and cramps (36.1%). This is attributed the large fluid shifts between the intra and extra vascular compartments with associated electrolyte imbalances and the citrate content of the anticoagulant in the FFP which chelates the calcium. The reported incidence of these symptoms ranges from 1.5%-9%.[19] Similarly, there was a much higher incidence of hypotension in our series (32.2%) compared to the 2.6-8.1% incidence in previous reports.[20] However, the previous lower rates of the reported adverse effects could be explained. First, the vast majority of (88.3%) of our patients were having GBS, a disorder prone to have autonomic dysfunction. Most of the previous studies on PE were based on data gathered from apheresis registries of various countries, for various neurological as well as non-neurological diseases, of varying severity. Unfortunately our country does not have a national apheresis registry. Second, most of the PE trials took into account the adverse events reported per session of PE where as ours is a patient wise data. Finally, we used FFP as the replacement fluid which has been associated with higher incidence of hypotension and other adverse events. The French Cooperative Group on plasma exchange in GBS has recommended albumin in place of FFP, as replacement fluid,[3] but we preferred FFP over albumin owing to the higher cost of the latter. Nevertheless, the high incidence of adverse events in our study is in agreement with some previous studies, including one large study from India.[21] The overall mortality rate in PE, neurological and non-neurological indications combined together, is estimated to be 1-3 per 10,000 procedures.[22] Globally, neurological disorders constitute the leading indication for PE, followed by hematological, renal and rheumatologic disorders. Hyperviscosity syndrome, cryoglobulinemia, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome and idiopathic thrombocytopenia are some of the leading haematological indications. The complication and mortality rates do not vary significantly among different clinical indications.
No mortality due to the PE occurred in this series and the cause of death of six patients was due to the underlying illnesses and their complications.
Conclusion
This is, to the best of our knowledge, the largest series of PE in neurological disorders from our country. Our results show that PE is not only a safe and effective treatment but also a cost effective alternative to IVIG, for various immune mediated neurological disorders.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Weinstein R. Therapeutic apheresis in neurological disorders. J Clin Apher. 2000;15:74–128. doi: 10.1002/(sici)1098-1101(2000)15:1/2<74::aid-jca6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Srauss RG, Ciavarella D, Gilcher RO, Kasprisin DO, Kiprov DD, Klein HG, et al. An overview of current management. J Clin Apher. 1993;8:189–94. doi: 10.1002/jca.2920080402. [DOI] [PubMed] [Google Scholar]
- 3.Korach JM, Berger P, Giraud C, Le Perff-Desman C, Chillet P. Role of replacement fluids in the immediate complications of plasma exchange. French Registry Cooperative Group. Intensive Care Med. 1998;24:452–8. doi: 10.1007/s001340050595. [DOI] [PubMed] [Google Scholar]
- 4.Clark WF, Rock GA, Buskard N, Shumak KH, LeBlond P, Anderson D, et al. Therapeutic plasma exchange: An update from the Canadian Apheresis Group. Ann Intern Med. 1999;131:453–62. doi: 10.7326/0003-4819-131-6-199909210-00011. [DOI] [PubMed] [Google Scholar]
- 5.The use of therapeutic plasmapheresis for neurological disorders. National Institutes of Consensus Development Conference. Transfus Med Rev. 1998;2:48–53. doi: 10.1016/s0887-7963(88)70031-0. [DOI] [PubMed] [Google Scholar]
- 6.Hartung HP, Archelos JJ, Zielasek J, Gold R, Koltzenburg M, Reiners KH, et al. Circulating adhesion molecules and inflammatory mediators in demyelination: A review. Neurology. 1995;45(6 Suppl 6):S22–32. doi: 10.1212/wnl.45.6_suppl_6.s22. [DOI] [PubMed] [Google Scholar]
- 7.Reeves JH, Butt WW, Shann F, Layton JE, Stewart A, Waring PM, et al. Continuous plasma filtration in sepsis syndrome. Plasmafiltration in Sepsis Study Group. Crit Care Med. 1997;27:2096–104. doi: 10.1097/00003246-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Goto H, Matsuo H, Nakane S, Izumoto H, Fukudome T, Kambara C, et al. Plasmapheresis affects T helper type 1/T helper type 2 balance of circulating peripheral lymphocytes. Ther Apher. 2001;5:494–6. doi: 10.1046/j.1526-0968.2001.00386.x. [DOI] [PubMed] [Google Scholar]
- 9.Shariatmadar S, Nassiri M, Vincek V. Effect of plasma exchange on cytokines measured by multianalyte bead array in thrombotic thrombocytopenic purpura. Am J Haematol. 2005;79:83–8. doi: 10.1002/ajh.20342. [DOI] [PubMed] [Google Scholar]
- 10.Couriel D, Weistein R. Complications of therapeutic plasma exchange: Recent assessment. J Clin Apher. 1994;9:1–5. doi: 10.1002/jca.2920090102. [DOI] [PubMed] [Google Scholar]
- 11.Mokryzicki MH, Kaplan AA. Therapeutic plasma exchange: Complications and management. Am J Kidney Dis. 1994;23:817–27. doi: 10.1016/s0272-6386(12)80135-1. [DOI] [PubMed] [Google Scholar]
- 12.Bouget J, Chevret S, Chastang C, Raphael JC. Plasma exchange morbidity in GBS: Results from the French prospective, randomised, multicentre study. French Cooperative Group. Crit Care Med. 1993;21:651–8. doi: 10.1097/00003246-199305000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Hartung HP, Willison HJ, Keiseier BC. Acute immunoinflammatory neuropathy: Update on Guillain-Barre syndrome. Curr Opin Neurol. 2002;15:571–7. doi: 10.1097/00019052-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 14.van der Meche FG, Schmitz PI. A randomised trial comparing intravenous immunoglobulin and plasma exchange in Guillain-Barre Syndrome. Dutch Guillain Barre Study Group. N Engl J Med. 1992;326:1123–9. doi: 10.1056/NEJM199204233261705. [DOI] [PubMed] [Google Scholar]
- 15.Randomised Trial of Plasma exchange, Intravenous Immunoglobulin and Combined treatments in GuillainBarre Syndrome. Plasma exchange/Sandoglobulin Guillain-Barre Syndrome Trial Group. Lancet. 1997;349:225–30. [PubMed] [Google Scholar]
- 16.Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A. Evidence-based guideline update: Plasmapheresis in neurologic disorders: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;76:294–300. doi: 10.1212/WNL.0b013e318207b1f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong EC, Balogun RA. Therapeutic apheresis in pediatrics: Technique adjustments, indications and nonindications, a plasma exchange focus. J Clin Apher. 2012;27:132–7. doi: 10.1002/jca.21224. [DOI] [PubMed] [Google Scholar]
- 18.Basic-Jukic N, Brunetta B, Kes P. Plasma exchange in elderly patients. Ther Apher Dial. 2010;14:161–5. doi: 10.1111/j.1744-9987.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 19.Stegmayr B, Ptak J, Wikstrom B, Berlin G, Axelsson CG, Griskevicius A, et al. World apheresis registry 2003-2007 data. Transfus Apher Sci. 2008;39:247–54. doi: 10.1016/j.transci.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Shemin D, Briggs D, Greenan M. Complications of therapeutic plasma exchange: A prospective study of 1,727 procedures. J Clin Apher. 2007;22:270–6. doi: 10.1002/jca.20143. [DOI] [PubMed] [Google Scholar]
- 21.Sharma RR, Saluja K, Jain A, Dhawan HK, Thakral B, Marwaha N. Scope and application of therapeutic apheresis: Experience from a tertiary care hospital in North India. Transfus Apher Sci. 2011;45:239–45. doi: 10.1016/j.transci.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Ward DM. Conventional apheresis therapies: A review. J Clin Apher. 2011;26:230–8. doi: 10.1002/jca.20302. [DOI] [PubMed] [Google Scholar]


