Abstract
Objective:
To evaluate neuropsychiatric co-morbidities (depression, psychosis and anxiety) in non-demented patients with Parkinson's disease (PD).
Background:
Non-motor symptoms like neuropsychiatric co-morbidities are common in Parkinson's disease and may predate motor symptoms. Currently there is scarcity of data regarding neuropsychiatry manifestations in Indian patients with PD.
Methods:
In this cross-sectional study consecutive 126 non-demented patients with PD (MMSE ≥25) were enrolled. They were assessed using Unified Parkinson's disease rating scale (UPDRS), Hoehn & Yahr (H&Y) stage, Schwab and England (S&E) scale of activity of daily life. Mini-international neuropsychiatric interview (MINI) was used for diagnosis of depression, psychosis and anxiety. Beck's depression inventory (BDI), Brief psychiatric rating scale (BSRS) and Hamilton rating scale for anxiety (HAM-A) scales were used for assessment of severity of depression, psychosis and anxiety respectively.
Results:
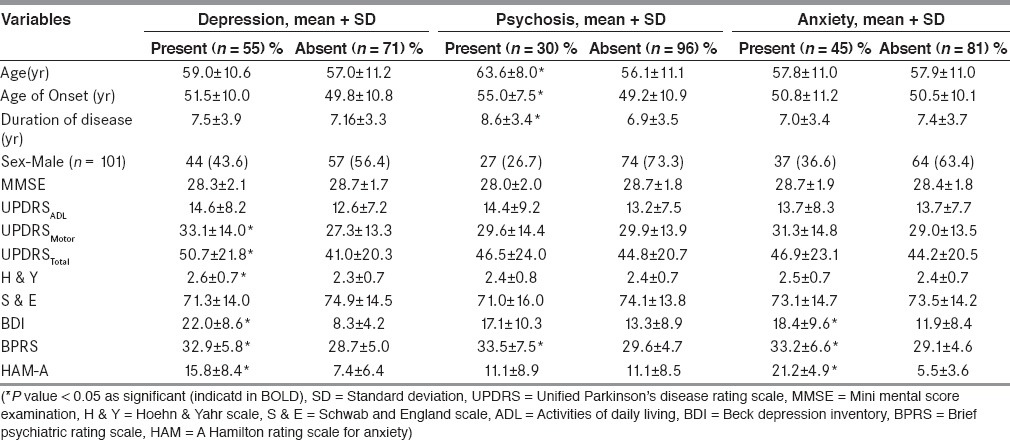
Mean age and duration of disease was 57.9 ± 10.9 years and 7.3 ± 3.6 years respectively. At least one of the neuropsychiatric co-morbidity was present in 64% patients. Depression, suicidal risk, psychosis and anxiety were present in 43.7%, 31%, 23.8% and 35.7% respectively. Visual hallucinations (20.6%) were most frequent, followed by tactile (13.5%), auditory (7.2%) and olfactory hallucinations (1.6%). Patients with depression had higher motor disability (UPDRS-motor score 33.1 ± 14.0 vs 27.3 ± 13.3; and UPDRS-total 50.7 ± 21.8 vs 41.0 ± 20.3, all p values <0.05). Patients with psychosis were older (63.6 ± 8.0 years vs 56.1 ± 11.1 years, p < 0.05) and had longer duration of illness (8.6 ± 3.4 years vs 6.9 ± 3.5, p < 0.05).
Conclusions:
About two third patients with Parkinson's disease have associated neuropsychiatric co-morbidities. Depression was more frequent in patients with higher disability and psychosis with longer duration of disease and older age. These co-morbidities need to be addressed during management of patients with PD.
Keywords: Anxiety, comorbidity, depression, neuropsychiatry, Parkinson's disease, psychosis
Introduction
Non-motor symptoms such as neuropsychiatric co-morbidity (NPC), autonomic dysfunctions, sleep disorders, and sensory symptoms can appear many years before development of motor symptoms of Parkinson's disease (PD). They are of major concern once motor symptoms are controlled with drugs. Neuropsychiatric co-morbidities such as depression, anxiety, psychosis, and apathy are frequent.[1,2] They contribute to poor quality of life,[3,4] distress for caregiver,[5] and increased risk for admission to nursing homes.[6,7] Prevalence of neuropsychiatric co-morbidities varies widely among reported series due to age of patients, duration of illness, cognitive impairment, assessment tools, and social structure of society in different cohort studied.[1,8,9,10,11] Cultural, social, and family structure of Indian subcontinent is different from western world and may affect neuropsychiatric symptoms of patients. Literature is sparse regarding neuropsychiatric co-morbidities in Indian patients with PD. The objective of the present study was to evaluate prevalence of depression, psychosis, and anxiety and their severity in non-demented Indian patients with PD.
Subjects and Methods
Clinical and Epidemiological Data Collection
Consecutive non-demented (MMSE ≥ 25) patients with PD, attending movement disorder clinic of a tertiary care university hospital in India, satisfying Queen Square PD brain bank clinical diagnostic criteria were recruited.[12] Exclusion criteria were history of alcohol or substance abuse, significant head trauma with loss of consciousness, psychiatric disorder preceding onset of motor symptoms of PD (other than depression), presence of dementia,[13] and structural brain abnormality on imaging. Patients with PD with age of onset of symptoms between 20 and 40 years were classified as young onset Parkinson's disease (YOPD) and those with age more than 40 years as late onset PD (LOPD).
A semi-structured interview was performed to obtain detailed information on demographic variables, disease history, drug therapy, and response to levodopa. Total levodopa equivalent daily dose (LEDD) was calculated in patients who were receiving levodopa with or without entacapone and dopamine agonists as reported by Lim et al.[14] Clinical assessment included Unified Parkinson's disease rating scale (UPDRS),[15] Hoehn & Yahr (H&Y) staging for PD,[16] and Schwab and England (S & E)[17] scale for activities of daily living. All assessments were done in “on state” by a trained neurologist. This study was approved by institutional human research ethics committee. Complete evaluation of each subject required 90-120 minutes and was completed in one or two interviews, depending on patient's cooperation.
Neuropsychiatric assessment
Neuropsychiatric co-morbidities mainly depression (including suicidal ideation), psychosis, and anxiety were diagnosed according to mini-international neuropsychiatric interview (MINI),[18,19,20] whereas severity of depression, psychosis, and anxiety were measured according to Beck's depression inventory (BDI),[21] brief psychiatric rating scale (BSRS),[22] and Hamilton rating scale for anxiety (HAM-A),[9,23,24] respectively. Patients were classified as non-depressed if BDI score was ≤10, mild depression if it was between 11 and 20, moderate depression if it was between 21 and 30, and severe depression if it was >30. Suicidal risk was assessed according to MINI and “thought of better to be dead/wish of death” was considered as mild risk whereas thinking, planning or attempting suicide as moderate to severe death risk. Anxiety was also graded according to total HAM-A score into mild (score <17), moderate (score 18-24), and severe (score ≥25).
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (statistical package for social sciences, SPSS Inc). Student's t test was used for comparisons of normally distributed continuous data and χ2 test for categorical variables. Non-parametric tests were used for comparison of various scores due to skewedness and non-linearity of these data. In addition, multivariate analyses were carried out to adjust other confounding factors including age and gender. All P values were two tailed and <0.05 was considered significant.
Results
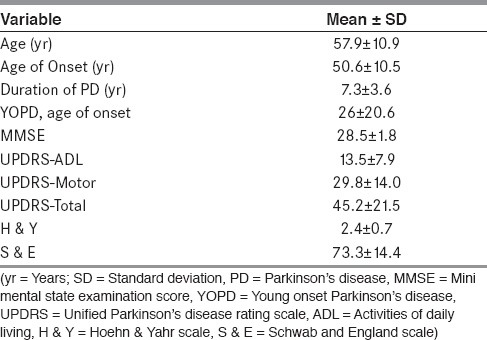
Among the 126 PD patients recruited for the study, mean age was 57.9 (±10.9) years, mean age of onset of PD was 50.6 (±10.5) years, and median duration of illness was 7.3 (± 3.6) years. Male to female ratio was 4:1. Demographic and clinical characteristics are shown in Table 1.
Table 1.
Demographics and clinical characteristics of Patients with PD (n = 126)
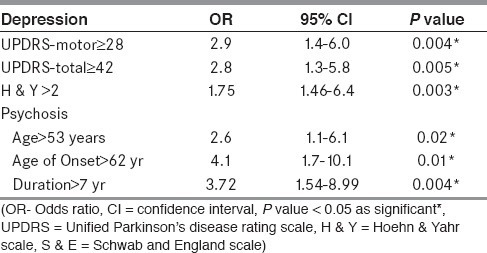
Details of neuropsychiatric co-morbidities are given in Table 2. Depression was significantly associated with higher motor disability as measured on UPDRS-motor, UPDRS-total scores, and more H & Y stage (P <0.05). No association was observed between depression and other demographic or clinical feature. All patients with PD developed depression after onset of PD. The median duration of depression was 12 months (minimum and maximum duration of 1 month and 60 months; range, 59 months). Receiver operating characteristic (ROC) curve was drawn between UPDRS-motor vs. depression (area under the curve 0.63; P = 0.012, 95% CI = 0.53-0.73) and UPDRS-total score vs. depression (area under the curve 0.64; P = 0.008, 95% CI = 0.54-0.74). Association of UPDRS-motor score ≥28 (sensitivity 0.60 and 1-specificity 0.35) and UPDRS-total score ≥42 (sensitivity 0.62 and 1-specificity 0.37) with depression is shown in Table 3.
Table 2.
Neuropsychiatric co-morbidities in patients with PD (n = 126)
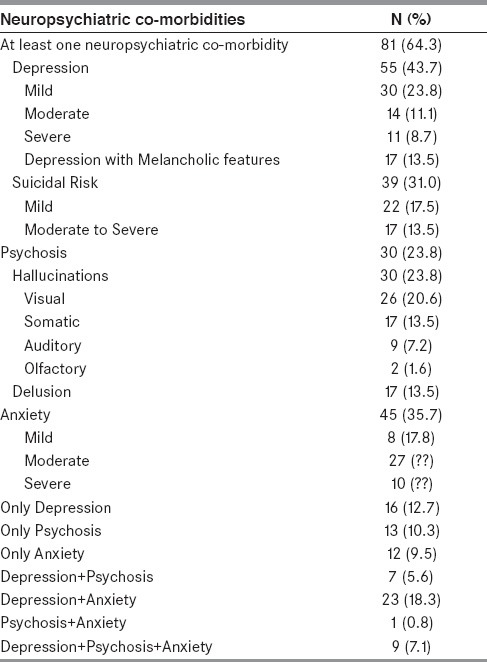
Table 3.
Odds ratio of depression and psychosis with various clinical features
Suicidal risk or death risk was noted in 39 patients (31.0%). However, none of them had attempted suicide. Suicidal risk and death risk significantly correlated with higher H&Y stage (2.7 ± 0.8 vs. 2.3 ± 0.7, p = 0.015), higher UPDRS-motor score (35.0 ± 14.5 vs. 27.5 ± 13.2, P = 0.005), and higher UPDRS — total scores (53.9 ± 23.1 vs. 41.3 ± 19.6, P = 0.004), respectively. Out of 39 patients with suicidal risk/death ideation, 36 patients (92.3%) had depression (OR 42.9, 95% CI = 11.9-154.9; P < 0.001) and 23 patients (59.0%) had anxiety (OR 4.3, 95% CI = 1.9-9.5, P < 0.001). However, multivariate analysis after adjusting for other clinical variables such as age and gender showed significant association between depression and suicidal risk (P < 0.001) but not with anxiety.
In patients with psychosis, hallucination was most frequent (n = 30, 23.8%) as shown in Table 2. Most frequent content of visual hallucinations were human beings, either dead or living relatives, who would be talking with patient or making a running commentary. Other reported visual hallucinations were animals, monsters, and shadows. Most frequent somatic hallucinations were feeling of insects creeping over skin followed by unusual positive sensation over trunk and limbs. Eight patients reported auditory hallucinations, and all of them had associated visual hallucination. They had either conversation between them self and another person (n = 3) or conversation between two or more persons (n = 6). Delusions with religious content (n = 8) were most frequent among delusions, while delusion of guilt (n = 3), delusion of jealousy (n = 3), delusion of mind reading (n = 1), delusion of grandiosity (n = 1), and delusion of persecution (n = 1) were other types of delusions. Most patients with delusion of religious content (n = 5) had firm belief that a God/Goddess or a supernatural power is punishing them because of their previous non-religious work and they cannot get rid of this disease by any means. Additional two patients had beliefs that some special worship might be helpful to cure them. They further believed that their disability prevented them to do that despite their motor improvement during “on state”. They would fight with their spouse for not doing rituals properly. One patient had belief that God is calling him to his place (heaven) as he loves him and this disease is a mode of a call to heaven.
Patients with psychosis were significantly older in age (63.6 ± 8.0 years vs. 56.1 ± 11.1 years; P < 0.001), were older at onset of PD (55.0 ± 7.5 years vs. 49.2 ± 10.9 years; P = 0.002) and had the disease for a longer time (8.6 ± 3.4 years vs. 6.9 ± 3.5 years; P = 0.024) as compared to those without psychosis [Table 4]. To detect a best cut-off value of these parameters, ROC curves were drawn between psychosis and age of patient at presentation (area under the curve 0.69, P = 0.002, 95% CI = 0.59-0.79), age of onset (area under the curve 0.66, P = 0.008, 95% CI = 0.56-0.76), and duration of illness (area under the curve 0.66, P = 0.01, 95% CI = 0.54-0.77). Association of these parameters viz. age of onset of PD >53 years (sensitivity 0.63, 1-specificity 0.37), age of presentation of patients >62 years (sensitivity 0.67, 1-specificity 0.32) and duration of illness >7 years (sensitivity 0.7, 1-specificity 0.38) with psychosis in PD patients is shown in Table 3.
Table 4.
Clinical variables in patients with and without neuropsychiatric co-morbidities (n = 126)
Frequent falls were only clinical variable significantly associated with psychosis (OR 3.7, 95% CI = 1.1-12.7, P = 0.02), but this significance was lost on multivariate analysis. Presence of psychosis was not associated with class, dose, or combinations of anti-Parkinsonian medicines though there was a trend toward higher daily dose of levodopa (494.3 ± 218.2 mg vs. 415.3 ± 179.5 mg; P = 0.08) and higher “LEDD” (732.5 ± 508.5 mg vs. 650.6 ± 423 mg; P = 0.38).
Anxiety was present in 45 (35.7%) patients with PD. Most of them had moderate anxiety. None of demographic or clinical variables were found to be significantly associated with anxiety. Dual co-morbidity (anxiety + depression) was present in 23 (18.3%) patients.
Spearman's rank correlation between UPDRS-motor score significantly correlated with BDI score (Spearman's r = 0.29, P = 0.001) and with BPRS score (Spearman's r = 0.19, P = 0.03) though strength of correlation was weak but not with HAM-A score (Spearman's r = 0.05, P = 0.58), suggesting increasing severity of depression and psychosis with disease severity of PD. However, there was no such correlation between increasing disease severity and presence of anxiety.
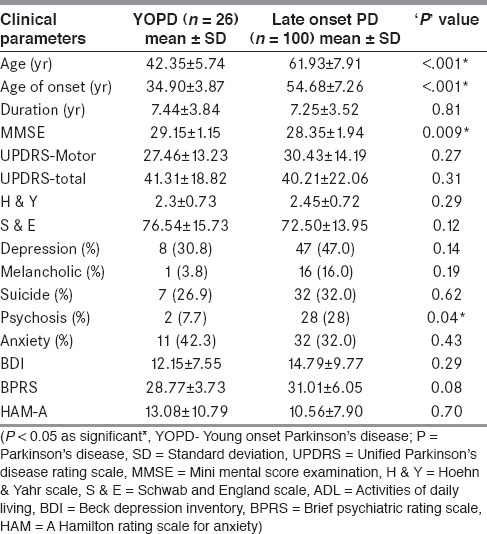
Twenty-six patients (20.6%) in cohort were classified as YOPD [Table 5]. Duration of illness and severity was comparable in both groups. MMSE was higher in YOPD (29.1 vs. 28.3; P = 0.009) as compared to late onset PD. YOPD patients had lower prevalence of psychosis as compared to late onset PD (30% vs. 47%, P = 0.14 and 7.7% vs. 28%, P = 0.03, respectively). There was a trend towards higher frequency of anxiety but lesser frequency of depression in YOPD patients [Table 5].
Table 5.
Comparison of neuropsychiatric co-morbidities in YOPD vs. late onset PD
Discussion
The present study comprehensively evaluated prevalence and severity of neuropsychiatric co-morbidities in Indian patients with PD. At least one neuropsychiatric symptom was seen in 64.3% patients with PD. Depression was most frequent neuropsychiatric co-morbidity (43.7%) followed by anxiety (36%), suicidal risk (31%), and psychosis (24%). Moderate to severe depression with suicidal or death ideation was noted in approximately one-third of patients with PD. Psychosis was more frequent among late onset patients with PD who were older at the time evaluation. Interestingly, delusion of religious content was most frequent delusion in Indian patients with PD.
Recent reviews show that depression is a common neuropsychiatric co-morbidity and causes significant problem in management of PD, affecting 4% to 70% patients with PD.[25,26,27,28] Though etiology of depression in PD is unclear, biochemical changes, psychosocial factors, situational stressors along with reaction to motor disability have all been implicated in its causation.[29,30] In the present study, depression was significantly associated with severity of motor disability as observed by Gotham et al.[31] As disability and depression go hand in hand with severity of PD or can precede motor symptoms, central neurotransmitter mechanisms seem to play a central role in their pathogenesis.[32,33,34,35,36] Overall prevalence of depression in patients with PD in present study was similar to western patients with PD, despite different social and family structure. Old-age homes are rare in Indian society, and elderly are still regarded with respect and care in familiar environment though joint family practice is now changing, especially in big cities. While still living in nuclear families, family ties are rather strong with network of people from neighboring villages or relatives living nearby in these cities, who are easily available to provide physical and emotional support in case of need. Despite the different social structure from western world, there was no difference in prevalence of depression in Indian patients, suggesting biological factors involved in the genesis of depression with limited role of social and familial/emotional factors. It is important to highlight here that only one-third of our patients with depression were taking antidepressant therapy, suggesting under recognition and under treatment of depression in Indian PD patients. Death risk or suicidal risk was quite high in the present study and was significantly associated with presence of depression and motor disability as reported by others.[37] Hence, direct query about suicide ideation and intent is required in patients with depression.
Psychosis is variously described in 20-40% of patients with PD and is associated with perceptual disorders such as illusions and hallucinations.[38,39] Pathophysiology of psychosis in PD is still not well established.[40,41,42] Risk factors include older age, longer duration of illness, and presence of depression, cognitive decline, daytime somnolence, and poor visual acuity.[10,11,40] In the present study, 23.8% of PD had psychosis, which was associated with older age, older age at onset of disease, and longer duration of illness as reported in other studies.[10,11] The present study did not find any association between dose and combination of various dopaminergic agents with psychosis, though patients with psychosis were taking higher dose of levodopa and levodopa equivalent doses. Although dopaminergic agents are considered to cause hallucination,[9,40] they are not the sole causative factor as hallucinations were well known in PD prior to dopaminergic era.[39] Our findings confirm contributory role of factors other than dopaminergic agents in genesis of psychosis. Auditory and somatic hallucinations present in this study was also reported by others.[10,43] “Delusion with religious content” was commonest delusion whereas “delusion of infidelity” was rather rare in present study (though it has been reported more commonly in other studies). All patients with delusion with religious content were well educated and these beliefs could not be explained by their sociocultural background. This interesting observation reflects influence of cultural ethos on delusion as most of elderly from Indian subcontinent become more religious during their later part of life (as is doctrine of “Vaanprastha period of Ancient Indian Philosophy”, which is a period in one's life when he gradually withdraws himself from the material world). Higher reporting of these unusual types of hallucinations and delusion in present study may be a reflection of a more vigilant observation of caregiver. As the present study included non-demented patients with PD, content of psychosis in the present study is likely to be more reliable than cohort of patients with PD with cognitive impairment.
Prevalence of anxiety in patients with PD is estimated to range from 20% to 52%.[44,45,46] Diagnosing anxiety in PD can be difficult because of co-existence of overlapping symptoms (such as tremor, numbness, tingling sensations, etc.) and coexisting depression further complicates the issue.[47] Anxiety in PD may be secondary reaction to disability of PD or because of neurochemical changes due to disease itself. Reported co-existence of anxiety with depression in 15-92% of PD patients,[9,44] while in general population, its coexistence is only in 3%.[48] In the present study, anxiety was present in 45% of patients with PD whereas depression and anxiety co-existed in 18%. Relatively less frequent coexistence of anxiety and depression, despite more frequent anxiety suggests that along with central neurotransmitter changes in PD, some other factors have contributory role in etiopathogenesis of anxiety.
In the present study, patients with YOPD had higher frequency of anxiety as compared to later onset PD, which is consistent with other report.[49] Difficulty in execution of responsibilities by patients with YOPD might play role in etiopathogenesis of anxiety, as younger (age 20-40 years) family members are expected to be active and money earning member of their family with more social and family responsibilities. However, depression, on the other hand, was less YOPD as compared to later onset PD, pointing to a complex etiopathogenesis of anxiety and depression in YOPD.
To conclude, the present comprehensive study has evaluated neuropsychiatric co-morbidities in a cohort of non-demented patients with PD. Prevalence of these co-morbidities in Indian patients with PD was similar to those reported in literature with some interesting difference suggesting complex etiopathogenesis of these comorbidities. The present study suffers from inherent limitations of cross-sectional study; hence, a well-designed cohort study is required to confirm these findings.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Aarsland D, Larsen JP, Lim NG, Janvin C, Karlsen K, Tandberg E, et al. Range of neuropsychiatric disturbances in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:492–6. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown RG, MacCarthy B. Psychiatric morbidity in patients with Parkinson's disease. Psychol Med. 1990;20:77–87. doi: 10.1017/s0033291700013246. [DOI] [PubMed] [Google Scholar]
- 3.Karlsen KH, Tandberg E, Arsland D, Larsen JP. Health related quality of life in Parkinson's disease: A prospective longitudinal study. J Neurol Neurosurg Psychiatry. 2000;69:584–9. doi: 10.1136/jnnp.69.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behari M, Srivastava AK, Pandey RM. Quality of life in patients with Parkinson's disease. Parkinsonism Relat Disord. 2005;11:221–6. doi: 10.1016/j.parkreldis.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Larsen JP, Larsen JP. Mental symptoms in Parkinson's disease are important contributors to caregiver distress. Int J Geriatr Psychiatry. 1999;14:866–74. [PubMed] [Google Scholar]
- 6.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: A population-based, prospective study. J Am Geriatr Soc. 2000;48:938–42. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 7.Goetz CG, Stebbins GT. Mortality and hallucinations in nursing home patients with advanced Parkinson's disease. Neurology. 1995;45:669–71. doi: 10.1212/wnl.45.4.669. [DOI] [PubMed] [Google Scholar]
- 8.Kulisevsky J, Pagonabarraga J, Pascual-Sedano B, García-Sánchez C, Gironell A Trapecio Group Study. Prevalence and correlates of neuropsychiatric symptoms in Parkinson's disease without dementia. Mov Disord. 2008;23:1889–96. doi: 10.1002/mds.22246. [DOI] [PubMed] [Google Scholar]
- 9.Nuti A, Ceravolo R, Piccinni A, Dell’Agnello G, Bellini G, Gambaccini G, et al. Psychiatric comorbidity in a population of Parkinson's disease patients. Eur J Neurol. 2004;11:315–20. doi: 10.1111/j.1468-1331.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- 10.Holroyd S, Currie L, Wooten GF. Prospective study of hallucinations and delusions in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2001;70:734–8. doi: 10.1136/jnnp.70.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetz CG, Leurgans S, Pappert EJ, Raman R, Stemer AB. Prospective longitudinal assessment of hallucinations in Parkinson's disease. Neurology. 2001;57:2078–82. doi: 10.1212/wnl.57.11.2078. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology. 2001;57:1497–9. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 13.4th ed. Washington: 1994. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 14.Lim SY, Evans AH, Miyasaki JM. Impulse control and related disorders in Parkinson's disease: Review. Ann N Y Acad Sci. 2008;1142:85–107. doi: 10.1196/annals.1444.006. [DOI] [PubMed] [Google Scholar]
- 15.Fahn S, Elton RL. Members of the UPDRS Development Committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Development in Parkinson's Disease. Florham Park: Macmillan Health Care Information; 1987. pp. 153–63. [Google Scholar]
- 16.Hoehn MH, Yahr MD. Parkinsonism: Onset, progression, and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 17.Schwab JF, England AC. Projection technique for evaluating surgery in Parkinson's disease. In: Gillingham FJ, Donaldson MC, editors. Third Symposium on Parkinson's Disease. Edinburgh, UK: E & S Livingston, 1969; 1968. pp. 152–7. [Google Scholar]
- 18.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 19.Dissanayaka NN, Sellbach A, Matheson S, O’Sullivan JD, Silburn PA, Byrne GJ, et al. Anxiety disorders in Parkinson's disease: Prevalence and risk factors. Mov Disord. 2010;25:838–45. doi: 10.1002/mds.22833. [DOI] [PubMed] [Google Scholar]
- 20.Schneider CB, Pilhatsch M, Rifati M, Jost WH, Wodarz F, Ebersbach G, et al. Utility of the WHO-Five wellbeing index as a screening tool for depression in Parkinson's disease. Mov Disord. 2010;25:777–83. doi: 10.1002/mds.22985. [DOI] [PubMed] [Google Scholar]
- 21.Leentjens AF, Verhey FR, Luijckx GJ, Troost J. The validity of the Beck Depression Inventory as a screening and diagnostic instrument for depression in patients with Parkinson's disease. Mov Disord. 2000;15:1221–4. doi: 10.1002/1531-8257(200011)15:6<1221::aid-mds1024>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:790–812. [Google Scholar]
- 23.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 24.Berrios GE, Campbell C, Politynska BE. Autonomic failure, depression and anxiety in Parkinson's disease. Br J Psychiatry. 1995;166:789–92. doi: 10.1192/bjp.166.6.789. [DOI] [PubMed] [Google Scholar]
- 25.Cummings JL. Depression and Parkinson's disease: A review. Am J Psychiatry. 1992;149:443–54. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- 26.Sano M, Stern Y, Williams J, Cote L, Rosenstein R, Mayeux R. Coexisting dementia and depression in Parkinson's disease. Arch Neurol. 1989;46:1284–6. doi: 10.1001/archneur.1989.00520480026014. [DOI] [PubMed] [Google Scholar]
- 27.Ravina B, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D, et al. The impact of depressive symptoms in early Parkinson disease. Neurology. 2007;69:342–7. doi: 10.1212/01.wnl.0000268695.63392.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dooneief G, Mirabello E, Bell K, Marder K, Stern Y, Mayeux R. An estimate of incidence of depression in idiopathic Parkinson's disease. Arch Neurol. 1992;49:305–7. doi: 10.1001/archneur.1992.00530270125028. [DOI] [PubMed] [Google Scholar]
- 29.Zesiewicz TA, Gold M, Chari G, Hauser RA. Current issues in depression in Parkinson's disease. Am J Geriatr Psychiatry. 1999;7:110–8. [PubMed] [Google Scholar]
- 30.Cummings JL, Masterman DL. Depression in patients with Parkinson's disease. Int J Geriatr Psychiatry. 1999;14:711–8. [PubMed] [Google Scholar]
- 31.Gotham AM, Brown RG, Marsden CD. Depression in Parkinson's disease: A quantitative and qualitative analysis. J Neurol Neurosurg Psychiatry. 1986;49:381–9. doi: 10.1136/jnnp.49.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starkstein SE, Preziosi TJ, Bolduc PL, Robinson RG. Depression in Parkinson's disease. J Nerv Ment Dis. 1990;178:27–31. doi: 10.1097/00005053-199001000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson's disease: Loss of dopamine and noradrenaline innervations in limbic system. Brain. 2005;128:1314–22. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- 34.Miguelez C, Grandoso L, Ugedo L. Locus coeruleus and dorsal raphe neuron activity and response to acute antidepressant administration in a rat model of Parkinson's disease. Int J Neuropsychopharmacol. 2011;14:187–200. doi: 10.1017/S146114571000043X. [DOI] [PubMed] [Google Scholar]
- 35.Fukunishi I, Hosokawa K, Ozaki S. Depression antedating onset of Parkinson's disease. Jpn J Psychiatry Neurol. 1991;45:7–11. doi: 10.1111/j.1440-1819.1991.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 36.Shiba M, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Anxiety disorders and depressive disorders preceding Parkinson's disease: A case-control study. Mov Disord. 2000;15:669–77. doi: 10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Nazem S, Siderowf AD, Duda JE, Brown GK, Ten Have T, Stern MB, et al. Suicidal and death ideation in Parkinson's disease. Mov Disord. 2008;23:1573–9. doi: 10.1002/mds.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papapetropoulos S, Mash DC. Psychotic symptoms in Parkinson's disease. From description to etiology. J Neurol. 2005;252:753–64. doi: 10.1007/s00415-005-0918-5. [DOI] [PubMed] [Google Scholar]
- 39.Fe´nelon G, Goetz CG, Karenberg A. Hallucinations in Parkinson disease in the prelevodopa era. Neurology. 2006;66:93–8. doi: 10.1212/01.wnl.0000191325.31068.c4. [DOI] [PubMed] [Google Scholar]
- 40.Diederich NJ, Goetz CG, Stebbins GT. Repeated visual hallucinations in Parkinson's disease as disturbed external/internal perceptions: Focused review and a new integrative model. Mov Disord. 2005;20:130–40. doi: 10.1002/mds.20308. [DOI] [PubMed] [Google Scholar]
- 41.Graham JM, Grunewald RA, Sagar HJ. Hallucinations in idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;63:434–40. doi: 10.1136/jnnp.63.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goetz CG, Pappert EJ, Blasucci LM. Intravenous levodopa in chronically treated hallucinating Parkinson's patients: High dose pharmacological challenge does not precipitate visual hallucinations. Neurology. 1998;50:515–7. doi: 10.1212/wnl.50.2.515. [DOI] [PubMed] [Google Scholar]
- 43.Fénelon G, Soulas T, Zenasni F, Cleret de Langavan L. The changing face of Parkinson's disease-associated psychosis: A cross-sectional study based on the new NINDS-NIMH criteria. Mov Disord. 2010;25:763–6. doi: 10.1002/mds.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen JJ. Anxiety, depression, and psychosis in Parkinson's disease: Unmet needs and treatment challenges. Neurol Clin. 2004;22:S63–90. doi: 10.1016/j.ncl.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Menza MA, Robertson-Hoffman DE, Bonapace AS. Parkinson's disease and anxiety: Comorbidity with depression. Biol Psychiatry. 1993;34:465–70. doi: 10.1016/0006-3223(93)90237-8. [DOI] [PubMed] [Google Scholar]
- 46.Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, et al. Nonmotor fluctuations in Parkinson's disease: Frequent and disabling. Neurology. 2002;59:408–13. doi: 10.1212/wnl.59.3.408. [DOI] [PubMed] [Google Scholar]
- 47.Schiffer RB, Kurlan R, Rubin A, Boer S. Evidence for atypical depression in Parkinson's disease. Am J Psychiatry. 1988;145:1020–2. doi: 10.1176/ajp.145.8.1020. [DOI] [PubMed] [Google Scholar]
- 48.Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML. High occurrence of mood and anxiety disorders among older adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry. 2010;67:489–96. doi: 10.1001/archgenpsychiatry.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein MB, Heuser IJ, Juncos JL, Uhde TW. Anxiety disorder in patients with Parkinson's disease. Am J Psychiatry. 1990;147:217–20. doi: 10.1176/ajp.147.2.217. [DOI] [PubMed] [Google Scholar]


