Abstract
Spino-cerebellar ataxia type 10 (SCA10) is an autosomal dominant disorder that is characterized by cerebellar ataxia, seizures and nystagmus with a fragmented pursuit. Schizophrenia has been reported with SCAs 1 and 2 yet in SCA 10, psychiatric manifestations are uncommon. We report a Hispanic family involving a father and his four children with SCA10 genetic mutation. Two of his children, a 20-year-old female and a 23-year-old male, presented with gradually progressive spino-cerebellar ataxia and paranoid schizophrenia. Neurological examination revealed ocular dysmetria, dysdiadokinesia, impaired finger-to-nose exam, gait ataxia and hyperreflexia in both the cases. Additionally, they had a history of psychosis with destructive behavior, depression and paranoid delusions with auditory hallucinations. Serology and CSF studies were unremarkable and MRI brain revealed cerebellar volume loss. Ultimately, a test for ATAXIN-10 mutation was positive thus confirming the diagnosis of SCA10 in father and his four children. We now endeavor to investigate the association between schizophrenia and SCA10.
Keywords: Ataxia, cerebellum, SCA10, schizophrenia, seizures
Introduction
Spinocerebellar Ataxia Type-10 (SCA10) is a rare autosomal dominant neurodegenerative disorder which is characterized by slowly progressive cerebellar ataxia and seizure disorder. It involves an expanded ATTCT pentanucleotide repeat in intron 9 of the ATXN10 gene, on chromosome 22q13.[1] It exhibits phenotypic variability and is commonly encountered in Latin America particularly among Mexican, Brazilian and Argentinian families.[1] In Mexico and Brazil, SCA10 represents the second most common type of autosomal dominant cerebellar ataxia.[1] It is characterized by pure cerebellar ataxia with seizure disorder among Mexicans, cerebellar ataxia without epilepsy among Brazilians and extrapyramidal signs in addition to ataxia and seizure disorder among Argentineans. Interestingly, all the reported families share a common American Indian ancestry.[1] A marked genetic anticipation with paternal inheritance is observed in this disorder, which may have its onset as early as 20 years of age. Clinical features include dysmetria of limbs, gait ataxia, dysarthria and gaze evoked nystagmus with fragmented pursuit. Complex partial seizures with or without secondary generalization is a hallmark of this disorder. Typically, the seizure frequency ranges between 20-80% in different families and contributes significantly to disease mortality.[1] Neuroimaging often reveals cerebellar atrophy. Here, we discuss the occurrence of psychiatric manifestations in SCA10.
Case Report
We report a Hispanic family of Guatemalan origin involving a father and his four children, two of whom were born from a different mother. We discuss two siblings from the same mother, a 20-years female and a 23-years male who presented with progressive difficulty in balance and coordination along with symptoms of paranoid schizophrenia. On examination, both siblings displayed ocular dysmetria, dysdiadokinesia, impaired finger-to-nose exam, gait ataxia and hyperreflexia. Additionally, they had a documented history of psychosis with destructive behavior, depression and paranoid delusions with auditory hallucinations. No suicidal ideation was observed. The psychiatric symptoms were managed with antipsychotic and antidepressant medication. Although results from serology and CSF analysis were unremarkable, MRI of the brain revealed cerebellar volume loss with peripheral vermian and cerebellar hemisphere atrophy [Figure 1]. A test for ATAXIN 10 mutation was positive not only in the siblings under discussion but also among the father and rest of his two children [Figure 2].
Figure 1.
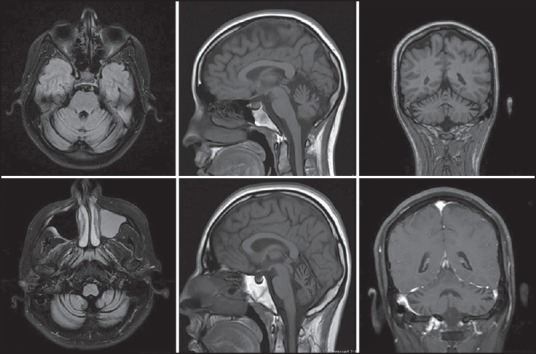
FLAIR Axial, Sagittal and Coronal section MRIs showing cerebellar atrophy in the 20 year female (Above) and 23 year male (Below)
Figure 2.
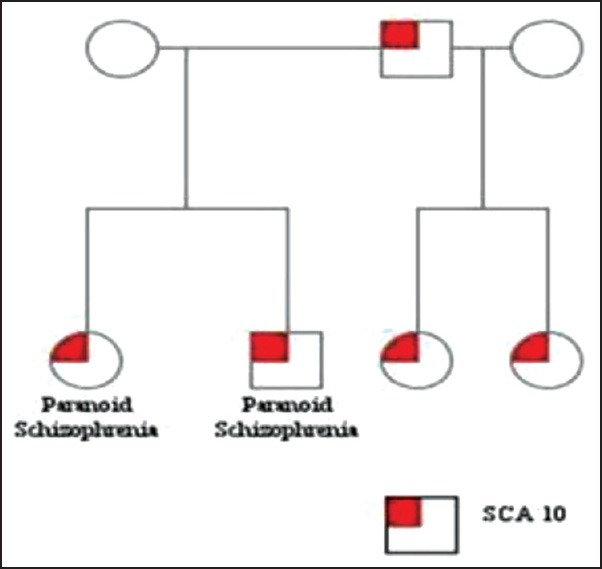
Pedigree of family with SCA-10 genetic mutation
However, the father and rest of his two children did not exhibit any psychiatric manifestations. Rather, they suffered from progressive gait disturbances and seizure disorder. Though there is a family history of seizures dating back to the great grandfather (paternal), the grandfather never developed any symptoms related to psychosis or seizures. Furthermore, the father has nine siblings of whom four sisters and one brother, presented with either gait disturbance or seizure activity at some point in their lives. Additionally, a third degree relative of the father, his uncle, presented with gait disturbance and seizure activity in the past. Genetic testing could not be performed on rest of the family and both the mothers denied any family history of neurological or mental disorders. It is noteworthy that the presenting cases did not suffer from seizure disorder, which is a commonly associated with SCA10. Instead, they suffered from paranoid schizophrenia which is rare and was absent in the rest of the family.
Discussion
SCAs are increasingly associated with some form of psychiatric and neurobehavioral dysfunction however; only SCAs 1 and 2 have been reported to co-present with major forms of psychosis.[2,3] Our review of literature revealed only one case report of new onset psychosis in a case of 37-year-old male with SCA10. This case study, like ours, supports the association of psychopathology with SCA10 rather than attributing it to coincidental occurrence.[4] On the basis of anticipation that is observed in multiple generations of families affected by this disorder, the likelihood of association between genetic mutations and schizophrenia was proposed.[5] More specifically, CAG/CTG triplet repeat expansions have been suggested to be involved in the genetics of schizophrenia.[6]
The neuropsychiatric implications of cerebellar dysfunction have long been overshadowed by the more prominent abnormalities in movement (dysmetria). Lately however, psychopathology is being increasingly reported in numerous cases of cerebellar degeneration including SCAs.[3,7] The evidence for cerebellar association with schizophrenia in particular, is revealed in numerous neuroimaging and clinical observational studies. Cerebellar abnormalities have been detected at structural, cellular, and functional levels and correlate with clinical measures. Structurally a reduction in the volume of cerebellar vermis and bilateral cerebellar hemispheres is observed in schizophrenics. Furthermore, a decrease in the size and density of Purkinje cells is also noted. Functional imaging in schizophrenia revealed abnormal cerebellar blood flow and clinically, cerebellar abnormalities correlated with clinical symptoms, cognitive deficits, and outcome measures in schizophrenia.[8]
Neurodegenerative disorders that primarily affect the striatum (chorea and Huntington's disease) may present with neurological or psychiatric symptoms. Interestingly, psychiatric symptoms are often associated with neurological symptoms in cerebellar disorders suggesting a striatal involvement. This association supports the possible involvement of striatum rather than cerebellum in the etiology of psychiatric symptoms. Alternatively, it may be hypothesized that the simultaneous occurrence of neurological and psychiatric disorders suggest an involvement of higher domains that are mediated by the frontal lobes and subserved by parallel circuits that link the frontal cortex, striatum, and thalamus. All of these frontostriatal circuits receive modulatory input from the cerebellum. Within the cerebellum however; the various functional domains (motor, vestibular, limbic, and cognitive) are topographically segregated and form parallel interfaces with the frontostriatal circuits.[3] The diffuse vermian and cerebellar hemisphere atrophy that was observed in our patients could possibly contribute to dysfunction across multiple domains.
It can be contemplated that due to different maternal lineages in this family, there is a possibilty of a primary mutation in the siblings with schizophrenia suggesting that the phenotypic differences occurred at a molecular level. In spite of an exhaustive literature review, we are unable to explain the non-occurrence of paranoid schizophrenia in individuals with seizures and vice versa. The absence of seizure disorder in the setting of paranoid schizophrenia makes this case unique. No topographical correlation or channel functioning could be identified to explain the absence of one of the two clinical features in presence of the other.
Conclusion
We present two previously unreported cases of SCA10 with paranoid schizophrenia. Although the association with seizures is frequently seen in patients with SCA10, it may be absent in some, as in our case. Unfortunately, the cause still remains unclear. It can however be debated that the occurrence of psychiatric and neurological manifestations in our cases was purely coincidental or result of a primary mutation. Nevertheless, it must be noted that in such a co-occurrence, motor symptoms can often be misjudged in context of a psychiatric illness or attributed to neuroleptic side effects, thus delaying neurological workup and diagnosis. These repercussions are particularly important from a neurological management and genetic counseling standpoint.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Teive HA, Munhoz RP, Arruda WO, Raskin S, Werneck LC, Ashizawa T. Spinocerebellar ataxia type 10: A review. Parkinsonism Relat Disord. 2011;17:655–61. doi: 10.1016/j.parkreldis.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Joo EJ, Lee JH, Cannon TD, Price RA. Possible association between schizophrenia and a CAG repeat polymorphism in the spinocerebellar ataxia type 1 (SCA1) gene on human chromosome 6p23. Psychiatr Genet. 1999;9:7–11. doi: 10.1097/00041444-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Rottnek M, Riggio S, Byne W, Sano M, Margolis RL, Walker RH. Schizophrenia in a patient with spinocerebellar ataxia 2: Coincidence of two disorders or a neurodegenerative disease presenting with psychosis? Am J Psychiatry. 2008;165:964–7. doi: 10.1176/appi.ajp.2008.08020285. [DOI] [PubMed] [Google Scholar]
- 4.Wexler E, Fogel BL. New-onset psychosis in a patient with spino-cerebellar ataxia type 10. Am J Psychiatry. 2011;168:1339–40. doi: 10.1176/appi.ajp.2011.11050737. [DOI] [PubMed] [Google Scholar]
- 5.Margolis RL, McInnis MG, Rosenblatt A, Ross CA. Trinucleotide repeat expansion and neuropsychiatric disease. Arch Gen Psychiatry. 1999;56:1019–31. doi: 10.1001/archpsyc.56.11.1019. [DOI] [PubMed] [Google Scholar]
- 6.Cardno AG, Murphy KC, Jones LA, Guy CA, Asherson P, De Azevedo MH, et al. Expanded CAG/CTG repeats in schizophrenia. A study of clinical correlates. Br J Psychiatry. 1996;169:766–71. doi: 10.1192/bjp.169.6.766. [DOI] [PubMed] [Google Scholar]
- 7.Leroi I, O’Hearn E, Marsh L, Lyketsos CG, Rosenblatt A, Ross CA, et al. Psychopathology in patients with degenerative cerebellar diseases: A comparison to Huntington's disease. Am J Psychiatry. 2002;159:1306–14. doi: 10.1176/appi.ajp.159.8.1306. [DOI] [PubMed] [Google Scholar]
- 8.Forsyth JK, Bolbecker AR, Mehta CS, Klaunig MJ, Steinmetz JE, O’Donnell BF, et al. Cerebellar-dependent eyeblink conditioning deficits in schizophrenia spectrum disorders. Schizophr Bull. 2012;38:751–9. doi: 10.1093/schbul/sbq148. [DOI] [PMC free article] [PubMed] [Google Scholar]


