Abstract
Object
Aneurysmal subarachnoid hemorrhage (aSAH) predisposes to delayed neurological deficits, including stroke and cognitive and neuropsychological abnormalities. Heparin is a pleiotropic drug that antagonizes many of the pathophysiological mechanisms implicated in secondary brain injury after aSAH.
Methods
The authors performed a retrospective analysis in 86 consecutive patients with Fisher Grade 3 aSAH due to rupture of a supratentorial aneurysm who presented within 36 hours and were treated by surgical clipping within 48 hours of their ictus. Forty-three patients were managed postoperatively with a low-dose intravenous heparin infusion (Maryland low-dose intravenous heparin infusion protocol: 8 U/kg/hr progressing over 36 hours to 10 U/kg/hr) beginning 12 hours after surgery and continuing until Day 14 after the ictus. Forty-three control patients received conventional subcutaneous heparin twice daily as deep vein thrombosis prophylaxis.
Results
Patients in the 2 groups were balanced in terms of baseline characteristics. In the heparin group, activated partial thromboplastin times were normal to mildly elevated; no clinically significant hemorrhages or instances of heparin-induced thrombocytopenia or deep vein thrombosis were encountered. In the control group, the incidence of clinical vasospasm requiring rescue therapy (induced hypertension, selective intraarterial verapamil, and angioplasty) was 20 (47%) of 43 patients, and 9 (21%) of 43 patients experienced a delayed infarct on CT scanning. In the heparin group, the incidence of clinical vasospasm requiring rescue therapy was 9% (4 of 43, p = 0.0002), and no patient suffered a delayed infarct (p = 0.003).
Conclusions
In patients with Fisher Grade 3 aSAH whose aneurysm is secured, postprocedure use of a low-dose intravenous heparin infusion may be safe and beneficial.
Keywords: subarachnoid hemorrhage, cerebral vasospasm, inflammation, heparin, vascular disorders
Aneurysmal subarachnoid hemorrhage (aSAH) is associated with numerous adverse sequelae.4,63 Patients who survive the initial hemorrhage are at high risk for secondary brain injury induced by vasospasm, microthrombi, oxidative damage, and inflammation, resulting in cerebral infarction, neuronal cell death, and white matter loss, and culminating in delayed focal neurological deficits and long-term cognitive and psychosocial deficits.11,24,42,48,53,54,58,62,70,77 Collectively, the neurological, cognitive, and neuropsychological abnormalities are referred to as aSAH-induced delayed neurological deficits (DNDs). To date, effective prophylactic therapy for aSAH-induced DNDs has been elusive.14,52
Heparin is a member of a family of polyanionic polysaccharides called glycosaminoglycans, composed of hexuronic acid and D-glucosamine residues joined by glycosidic linkages.6 Unfractionated heparin is a heterogeneous mixture of polysaccharide chains of varying lengths, ranging from 3 to 30 kD.37 Heparin (always used here to refer to unfractionated heparin) has the highest negative charge density of any known biological molecule and, as a result, has a high propensity to bind to positively charged molecules and surfaces. More than 100 heparin-binding proteins have been identified,76 with the growing list including numerous plasma proteins, proteins released from platelets, cytokines, chemokines, and other small biologically active molecules, as well as endothelial cells themselves. 10,23,27,51 Although clinically used almost exclusively for its anticoagulant properties, heparin binding can interrupt numerous other biological pathways.47
Heparin is a pleiotropic drug that antagonizes many of the molecular mechanisms that have been implicated in secondary brain injury after aSAH.60 Among its wide-ranging effects, heparin complexes with free hemoglobin itself, including oxyhemoglobin;1 it blocks the activity of free radicals, including reactive oxygen species;18 it antagonizes endothelin-mediated vasoconstriction;7,44,75 and it binds to several growth factors, thereby imparting antimitogenic25,26,36 and antifibrotic57 effects. Arguably, one of its most important effects is to act as a potent, nonconventional antiinflammatory agent.17,29,66,67,76 The antiinflammatory effect of heparin is unrelated to anticoagulant activity.45,55 Heparin binds to several cytokines and all chemokines; it inhibits the production and release of proinflammatory factors, including interleukin (IL)–1β, IL-6, and tumor necrosis factor–α;2,28,46 it inhibits the activation of nuclear factor κB;76 it inhibits RAGE (receptor for advanced glycation end products)–mediated inflammation;55 and it attenuates endothelial dysfunction by enhancing nitric oxide and prostacyclin release.50 Recent work has shown that low-dose intravenous heparin infusion significantly reduces neuroinflammation (neutrophils, activated phagocytic microglia, nuclear factor κB, tumor necrosis factor–α, and IL-1β) as well as transsynaptic apoptosis in a preclinical model of aSAH involving eloquent cortex.61 To our knowledge, no other compound has been identified that exhibits such a broad diversity of biological effects that are so intimately relevant to mechanisms implicated in aSAH-induced DNDs. The very multiplicity of mechanisms targeted by heparin suggests that it may be useful as a multitargeted prophylactic agent to ameliorate the wide-ranging abnormalities induced by aSAH.
For several years at our institution, some patients with high-grade aSAH who were treated surgically for a supratentorial saccular aneurysm have been managed postoperatively using a low-dose intravenous heparin infusion. Here, we report on a retrospective analysis of the outcome of these patients, comparing them with patients with similar baseline characteristics who were managed concurrently, but with conventional subcutaneous heparin twice daily as prophylaxis against deep vein thrombosis (DVT). Briefly, our experience suggests that a low, nonanticoagulating dose of heparin administered by constant intravenous infusion may be safe and beneficial in these patients.
Methods
The Institutional Review Board of the University of Maryland approved the protocol for a retrospective review. Patient records during the period between January 2006 and May 2011 were searched for the ICD-9-CM diagnosis code 473.3, which identified 411 patients who had undergone craniotomy for clipping of a supratentorial aneurysm. Computed tomography angiography (CTA) or catheter digital subtraction angiography (DSA) was used to confirm the presence of an aneurysm. In cases of aSAH, the severity of the hemorrhage was graded using the classification of Fisher et al.22 Of the 411 cases identified, 144 were excluded because they were Grade 0 (unruptured) supratentorial aneurysms, 177 were excluded because they were ruptured supratentorial aneurysms with Fisher Grade 1, 2, or 4 hemorrhages, or ruptured supratentorial aneurysms with Fisher Grade 3 hemorrhages that presented more than 36 hours after their ictus. These exclusions left 90 consecutive patients with ruptured supratentorial aneurysms presenting with Fisher Grade 3 hemorrhages within 36 hours of their ictus who underwent surgery. Of the 90 patients who met the initial screening criteria, 4 were excluded because they had either a giant or a fusiform aneurysm, or because they had a significant hemiparesis due to a preoperative intracerebral hematoma. The remaining 86 patients are the subject of this report.
The 86 patients all underwent craniotomy for aneurysm clipping within 48 hours of their ictus. All patients were treated by one of 2 faculty neurosurgeons, one of whose practice included the postoperative administration of a low-dose intravenous heparin infusion (heparin group), and the other whose practice did not (control group). All other aspects of patient management were identical for both surgeons. Patients in both groups were treated concurrently; there were no differences in variables such as treatment eras, ICU policy or ICU attending physicians, residents, or nurse practitioners. No patient was excluded because of a confirmed surgical injury or an untoward intraoperative event. There was no bias in assigning patients to one or the other surgeon; assignments were based simply on a predetermined call schedule. Both surgeons used temporary clipping for similar indications. Of the 86 patients who met the inclusion criteria, 43 patients were treated with a low-dose intravenous heparin infusion, and 43 were not.
The Maryland Low-Dose Intravenous Heparin Infusion Protocol
The protocol for the low-dose intravenous heparin infusion was as follows: 1) after surgery, CT scanning and CTA were performed to document the absence of any postoperative complication and to confirm aneurysm obliteration; 2) an intravenous infusion of unfractionated heparin, 8 U/kg/hr (no loading dose), was begun 12 hours after craniotomy; and 3) 12 hours later, the infusion was increased to 9 U/kg/hr intravenously, and after an additional 12 hours, the infusion was increased to 10 U/kg/hr intravenously. The infusion generally was maintained for 14 days (12–16 days) after the ictus.
Values of activated partial thromboplastin time (aPTT) were monitored twice daily for 5 days. The gradual increase in the rate of infusion typically yielded values of aPTT that were normal to mildly elevated (see Results). If values exceeded 45 seconds within the first 3 days after surgery (but not later), the infusion rate was decreased by one step for 12 hours before resuming the higher level.
Standard Management of Patients With aSAH
All patients were treated in accordance with the Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage, including initial maintenance of euvolemia, administration of nimodipine, and, when appropriate, drainage of CSF.4 For patients in the control group, prophylaxis against DVT included subcutaneous injections of unfractionated heparin (5000 U) twice daily. Patients treated with the low-dose intravenous heparin infusion did not receive subcutaneous heparin injections.
Radiological examinations, including CT, CTA, or DSA, as appropriate, were obtained routinely during the first 24 hours after surgery and periodically thereafter to check for hydrocephalus or clearance of intracranial blood, or at the discretion of the treating physician, or whenever there was neurological deterioration of any sort, focal or nonfocal. In the heparin group, CTA also was performed at the end of the infusion.
In the case of focal or nonfocal neurological deterioration suggestive of symptomatic vasospasm, even before radiographic confirmation of angiographic vasospasm, patients were given hypervolemic therapy consisting of a fluid bolus and an increase in the infusion rate of intravenous fluid.4 In addition, patients with symptomatic vasospasm were treated with induced hypertension using a phenylephrine infusion to increase systolic blood pressure by 20%, up to a maximum dose of 200 μg/min.49 When persistent clinical deterioration was accompanied by angiographic vasospasm that was not immediately responsive to induced hypertension, patients were treated with selective intraarterial verapamil or angioplasty.33,41 In some cases when imaging showed moderate to severe angiographic vasospasm, even in neurologically intact patients, hypervolemic therapy or induced hypertension was implemented.
Data Compilation
Patient medical records, including computerized hospital records and clinic notes, were reviewed by 2 neurosurgeons (N.B. and A.P.) for demographic, clinical, radiographic, and outcome data.
The severity of aSAH was scored based on the first available CT scan, obtained either before or after admission to the University of Maryland hospital. All CT scans were reviewed by 2 neurosurgeons (N.B. and A.P.) who together assigned a Fisher Grade.22 In accordance with the original description,22 CT scans with a pattern of aSAH fitting the criteria for Fisher Grade 3 aSAH but also showing moderate to large amounts of intraventricular or intracerebral hemorrhage were scored as Fisher Grade 3.
The occurrence of angiographic vasospasm, scored as a binary variable, was determined from a review of all CTA and DSA studies by 2 neurosurgeons (A.P. and N.B.) and independently by experienced neuroradiologists from the University of Maryland. When compared with baseline studies, any study showing evidence of angiographic vasospasm in any vascular territory, regardless of severity or of the clinical/neurological status of the patient, was scored as having angiographic vasospasm. The severity of angiographic vasospasm was not recorded.
The occurrence of clinical vasospasm, scored as a binary variable, was determined from a review of the neurological status of the patients and was scored when focal neurological deterioration was accompanied by angiographic vasospasm in the appropriate vascular territory, or when nonfocal neurological deterioration was accompanied by diffuse or multiterritory angiographic vasospasm.
The occurrence of new ischemia-related CT hypodensities was determined from a review of all CT scans by 2 neurosurgeons (N.B. and A.P.) and independently by experienced neuroradiologists from the University of Maryland. Findings on CT scans obtained during hospitalization and after discharge were correlated with CTA or DSA and neurological examination, to ascertain whether new hypodensities were attributable to vasospasm (within a discrete vascular or watershed territory), as opposed to being due to an intraparenchymal hemorrhage, intraventricular catheter placement, surgical retraction, or surgery-related loss of a perforating artery.30
Statistical Analysis
Calculations were performed using InStat (version 3.1, GraphPad Software, Inc.). Parametric data are reported as the mean ± SD. Parametric and nonparametric data were analyzed using Student’s unpaired t-test and the Mann-Whitney U-test, respectively. Rates were analyzed using a 2-way contingency table and Fisher’s exact test (2-tailed). p values < 0.05 were considered significant.
Results
Baseline Characteristics
Eighty-six patients with Fisher Grade 3 aSAH due to rupture of a supratentorial saccular aneurysm were included in this study, with 43 patients each in the control and the heparin groups. Their baseline characteristics are shown in Table 1. There were no statistically significant differences between groups in terms of age, sex, presenting neurological status (World Federation of Neurosurgical Societies [WFNS] grade and Glasgow Coma Scale [GCS] score), hypertension, use of statins,65 or use of tobacco or cocaine.9,72 Aneurysms in the control versus heparin groups were located at the posterior communicating artery (6 vs 14), anterior choroidal artery (1 vs 2), internal carotid artery bifurcation (2 vs 2), anterior communicating artery (26 vs 13), middle cerebral artery (MCA) bifurcation (6 vs 10), and pericallosal artery (2 vs 2).
TABLE 1.
Patient baseline characteristics
| Parameter | Control Group (n = 43)* | Heparin Group (n = 43)* | p Value |
|---|---|---|---|
| mean age (yrs) | 55.3 ± 12.9 | 54.5 ± 11.3 | 0.98† |
| female sex | 34 | 32 | 0.80 |
| WFNS grade | |||
| mean | 2.0 ± 1.3 | 2.5 ± 1.6 | 0.16† |
| median | 2.0 | 2.0 | 0.23† |
| ≥3 | 10 | 16 | 0.24 |
| GCS score | |||
| mean | 13.1 ± 2.9 | 12.2 ± 3.6 | 0.23† |
| median | 14 | 14 | 0.33† |
| ≤7 | 6 | 7 | 1.0 |
| hypertension | 21 | 20 | 1.0 |
| statin use | 8 | 6 | 0.77 |
| tobacco use | 22 | 25 | 0.67 |
| cocaine use | 3 | 1 | 0.62 |
Values represent the number of patients unless otherwise noted. Mean values are presented as the mean ± SD.
Student’s t-test or Mann-Whitney U-test; otherwise, from Fisher’s exact test (2-tailed).
Safety
The low-dose intravenous heparin infusion was started 12 hours after surgery. The average length of time between the start of heparin infusion and the ictus was 40.7 ± 7.9 hours. The protocol for the low-dose intravenous heparin infusion, which began with 8 U/kg/hr and progressed over 36 hours to 10 U/kg/hr intravenously, resulted in values of aPTT that were generally in the range of normal to mildly elevated (Fig. 1).
Fig. 1.
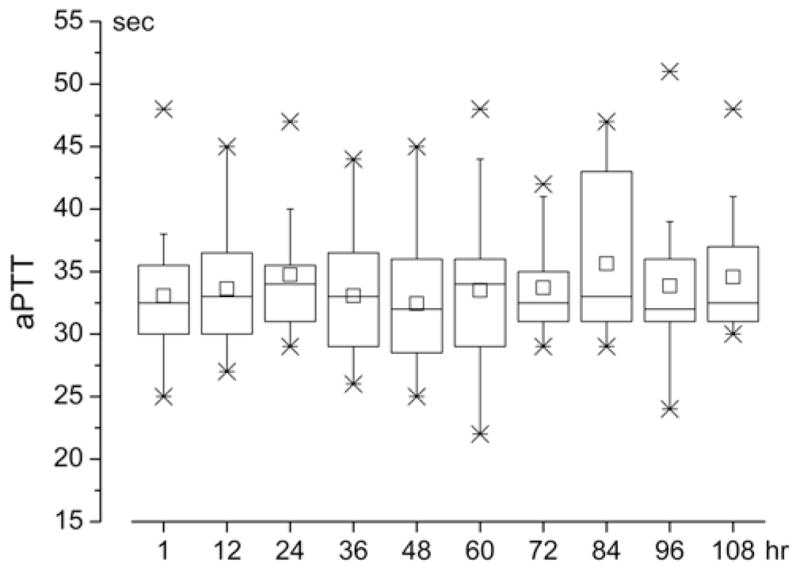
Values of aPTT associated with the Maryland low-dose intravenous heparin infusion protocol. Box plots as a function of time, of values of aPTT in patients administered the Maryland low-dose intravenous heparin infusion protocol (8 U/kg/hr by constant intravenous infusion for 12 hours, increasing by 1 U/kg/hr at 12-hour intervals to a final maintenance dose of 10 U/kg/hr intravenous). In the box plots, the large boxes indicate the 25th and 75th percentiles; whiskers, the 5th and 95th percentiles; x, the 1st and 99th percentiles; –, the maximum and minimum; horizontal line within the box, the median; and the small square, the mean. Normal aPTT at the University of Maryland, 25–38 seconds.
None of the 43 patients in the heparin group incurred a bleeding complication, DVT, or heparin-induced thrombocytopenia (HIT).12 One patient from the heparin group had a thin postoperative subdural hematoma that was diagnosed immediately after surgery on a postoperative CT scan. The heparin protocol was started at 12 hours despite this collection. Subsequent CT scans never showed expansion of this collection.
Vasospasm
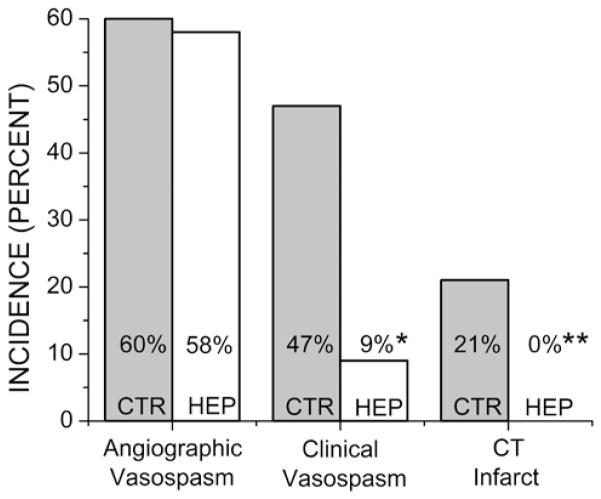
The incidence of angiographic vasospasm in the control and heparin groups was 26 (60%) of 43 patients and 25 (58%) of 43 patients, respectively (p = 1) (Fig. 2). In the control group, rescue therapy (induced hypertension or selective intraarterial verapamil with or without angioplasty; Table 2) was implemented in 21 (49%) of 43 patients, whereas in the heparin group, rescue therapy was implemented in 4 (9.3%) of 43 patients (p < 0.0001). In the control group, the reason for performing CTA that prompted initiation of rescue therapy was new focal neurological deficit (12 patients), new nonfocal neurological deterioration (8 patients), and discretionary investigation with no neurological deterioration (1 patient), for an overall incidence of symptomatic vasospasm of 47% (20 of 43 patients) (Fig. 2). In the heparin group, the reason for performing CTA that prompted initiation of rescue therapy was new focal neurological deficit (3 patients) and new nonfocal neurological deterioration (1 patient), for an overall incidence of symptomatic vasospasm of 9% (4 of 43 patients; p = 0.0002) (Fig. 2).
Fig. 2.
Vasospasm-related outcomes. The incidence of angiographic vasospasm, clinical vasospasm, and CT infarctions in the control (CTR) and heparin (HEP) groups, as indicated. *p = 0.0002; **p = 0.003.
TABLE 2.
Use of rescue therapy for vasospasm
| Rescue Therapy | Control Group (n = 43) | Heparin Group (n = 43) | p Value† |
|---|---|---|---|
| phenylephrine infusion* | 14 | 4 | 0.016 |
| microcatheter-directed therapy‡ | 12 | 3 | 0.021 |
Patients treated with a phenylephrine infusion had their blood pressure titrated to a systolic blood pressure that resulted in resolution of their symptoms or to a maximum of 200 mg/minute.
Fisher’s exact test (2-tailed).
Microcatheter-directed therapy involved selective angioplasty or intraarterial infusion of verapamil to a total dose no greater than 20 mg in a 24-hour period.
Computed Tomography Infarcts
Nine (21%) of 43 patients in the control group had vasospasm-related infarcts (Figs. 2 and 3A and B). The vascular territories involved were the left MCA (4 patients), right MCA (2 patients), anterior cerebral artery (2 patients), and bilateral anterior cerebral artery and right MCA territories (1 patient). In all cases that were scored as infarcts, accompanying neurological deficits confirmed the significance of the CT findings. There were no vasospasm-related infarcts in the heparin group (p = 0.003) (Figs. 2 and 3C and D).
Fig. 3.
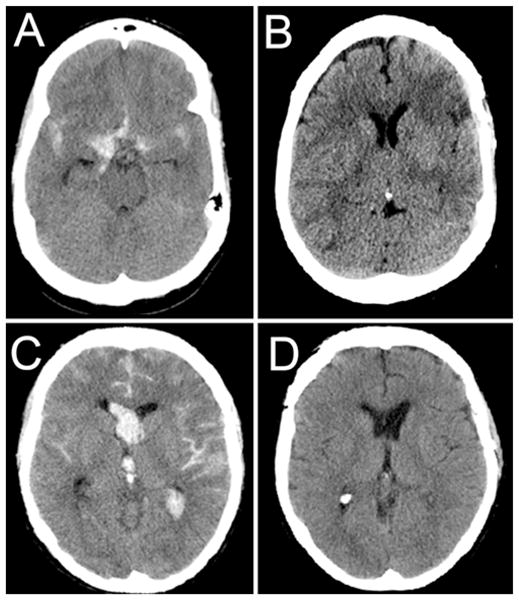
Computed tomography scans obtained in representative patients from the control and heparin groups. A and B: Admission (A) and follow-up (B) CT scans obtained in a 50-year-old patient who presented with a ruptured anterior communicating artery aneurysm. The patient was neurologically intact upon presentation. The patient underwent successful surgical clipping. The patient’s hospital course was complicated by clinical vasospasm with associated aphasia. Despite multiple intravascular interventions, the patient developed frontal and parietal infarcts. At follow-up, the patient’s aphasia was improving. C and D: Admission (C) and follow-up (D) CT scans obtained in a 47-year-old patient who presented with a ruptured anterior communicating artery aneurysm. The patient was comatose upon presentation with a GCS score of 6T. The patient underwent successful surgical clipping and was subsequently treated with a low-dose intravenous heparin infusion. The patient developed angiographic vasospasm but did not require rescue intervention. At follow-up, the patient was neurologically normal, including normal cognition and short-term memory.
Clinical Outcomes
Recommendations for discharge to home versus a rehabilitation center were made independently by physical therapists at the time of discharge. In the control group, 17 patients were discharged home and 25 were discharged to rehabilitation centers, whereas in the heparin group, 27 patients were discharged home and 16 were discharged to rehabilitation centers (p = 0.05).
In the control group 1 patient, who had not experienced clinical vasospasm, died of cardiopulmonary complications during an extended hospital stay. Of the 9 patients with vasospasm-related infarcts, 1 was discharged home with a mild residual aphasia, and 8 were transferred to rehabilitation centers with neurological deficits including hemiparesis, dense hemiplegia, dense aphasia, and bilateral lower-extremity weakness. At 6 weeks, 3 of these patients remained in rehabilitation; 2 of them had experienced vasospasm-related left MCA infarcts, and the third had developed chronic pulmonary failure worsened by a preexisting severe chronic obstructive pulmonary disease. The remaining 33 patients were seen at 6-week follow-up and were judged to have good to excellent neurological outcomes.
In the heparin group, no patient died in-hospital, and no patient had a persistent focal neurological deficit. Follow-up data were not available for 6 patients; 3 of these patients were discharged home directly from the hospital, and 3 were transferred to rehabilitation facilities, from which they had been discharged already by the time of their scheduled 6-week follow-up. No patient from the heparin group remained in rehabilitation at 6 weeks. Of the 37 patients who returned for 6-week follow-up, all self-reported good to excellent recovery with near-baseline or baseline memory and cognition.
Discussion
This is the first report to examine the use of a low-dose intravenous heparin infusion as prophylaxis against DNDs in the management of patients with aSAH. The use of an anticoagulating dose of heparin (2500–3500 U subcutaneously every 6 hours,39,40 or constant intravenous infusion71) has been advocated previously to reduce complications of aSAH and was reported to reduce ischemic events in patients with aSAH undergoing carotid ligation for aneurysm treatment.8,39,40,71 Anticoagulation with low-molecular-weight heparin (enoxaparin) was found to be promising in one study,74 but this finding was not replicated by a second group of investigators.35,59 Regardless, the use of heparin has not gained support among neurosurgeons, presumably because of the notion that an anticoagulating dose would be required, along with the concern that an anticoagulating dose might predispose to hemorrhagic complications.73 However, under physiological conditions, heparin does not have fibrinolytic activity,64 suggesting that the risk of bleeding may not be increased once a stable fibrin clot has formed. Our experience indicates that low-dose heparin administered by constant intravenous infusion may be safe and beneficial, independent of anticoagulation.
Safety
The specific protocol used for the low-dose intravenous heparin infusion, which began with 8 U/kg/hr and progressed over 36 hours to 10 U/kg/hr intravenously, combined with the delay of 12 hours after surgery before starting the infusion, yielded a favorable safety profile with no untoward side effects. Only modest elevations in aPTT were observed, and no hemorrhagic complications were identified.8,71 Furthermore, no case of HIT or DVT was identified. However, the absence of HIT, DVT, and hemorrhagic complications in the present series must be viewed with caution. The risk of HIT generally is low, with an incidence of approximately 3% associated with infusion of an anticoagulating dose,12 and of 1.4%–5.2% associated the dose used for DVT prophylaxis.34 The incidence of DVT in aSAH is 18%.56 Larger studies will be needed to establish the true safety of the low-dose intravenous heparin infusion protocol described here. Alternatively, nonanticoagulating N-desulfated heparin, which retains other biological properties and effects of heparin, including antiinflammatory effects,3,55 should be considered for use in future studies of aSAH, given its better safety profile regarding anticoagulation.
Efficacy
The incidence of angiographic vasospasm of any degree was not affected by the low-dose intravenous heparin infusion, consistent with the 2 groups being well balanced in terms of risk factors. Although we did not assess the severity of angiographic vasospasm in the 2 groups, significantly fewer patients in the heparin group developed symptomatic vasospasm and related infarcts on CT scanning.
Cerebral infarction and functional outcome are regarded as the preferred outcome measures to investigate the true impact of an intervention after aSAH.19 Cerebral infarction is strongly associated with poor outcome after aSAH,69 and symptomatic vasospasm is the most important factor predisposing to infarction.21 In the present study, the incidence of vasospasm-related infarcts in the control group was 21%, which is comparable to previous reports in patients with Fisher Grade 3 aSAH.30 By contrast, no patient in the heparin group developed a vasospasm-related infarct or lasting focal neurological deficit. Most of the patients with vasospasm-related infarcts in the control group (8 of 9) had poor functional outcomes, and most of these patients (7 of 8) were conscious and either neurologically normal or only confused upon presentation.
Patients who received the intravenous heparin infusion and who returned for evaluation at 6 weeks had overall favorable clinical outcomes, as judged by neurological examinations and by self-reported cognitive and memory functions. Further standardized testing will be required to confirm this tentative finding regarding cognition, but if true, such an effect of heparin could have a large impact on quality of life.
Starting a low-dose heparin infusion in patients several days after a substantial sentinel hemorrhage or after clinical vasospasm has developed may not be as beneficial (J. M. Simard, unpublished observation), reinforcing that this protocol needs to be started soon after the ictus. The protocol described here, including the maximum dose of 10 U/kg/hr, was developed before the US Food and Drug Administration changed its reference standard for heparin, 68 which resulted in a 10% lowering of its apparent potency. Future studies of heparin in aSAH will need to take this into account, as well as the possibility that higher doses of heparin may have greater efficacy.
Route and Manner of Administration
The dose and timing of heparin administration required for a beneficial effect in aSAH appear to differ from that required to alter coagulation. For a 70-kg patient, infusion of 10 U/kg/hr of heparin results in a total daily dose of 16,800 U, which yielded aPTT values that were normal to mildly elevated. By comparison, a total daily dose of 10,000 U (5000 U twice daily) was used in the control patients for DVT prophylaxis. It is possible that the higher daily dose received by the intravenous heparin group accounts for the better outcomes, compared with controls. However, the pharmacokinetics of heparin administered subcutaneously twice daily can be highly variable, due in part to a dose-dependent serum half-life that plateaus within 30 minutes.5,13,43 Evidence from other studies indicates that the route (intravenous vs subcutaneous) and the manner (continuous infusion vs twice daily injection) of administration of heparin can play significant roles in obtaining a favorable therapeutic effect unrelated to anticoagulation.16
The route and manner of administration also may account for the conflicting effects reported in trials with enoxaparin (1 subcutaneous injection daily) after aSAH.35,59,74 Notably, low-molecular-weight heparin exhibits an antiinflammatory profile similar to that of unfractionated heparin28,29 and may have a better safety profile regarding bleeding.15,31 However, continuous intravenous infusion of low-molecular-weight heparin20,38 has not been examined in patients with aSAH.
Study Limitations
This is a preliminary study of the potential safety and efficacy of low-dose intravenous heparin infusion in patients with high-grade aSAH undergoing craniotomy for clipping of a supratentorial aneurysm. This study has several limitations, including its open label, nonrandomized, retrospective design, all of which can introduce bias, as well as its modest sample size. We scored angiographic spasm as a binary variable (none vs any degree), precluding an assessment of whether heparin decreases the severity of angiographic vasospasm. However, the direct comparison of outcomes with those of patients having similar baseline characteristics who were treated concurrently at the same institution gives weight to our conclusion. Computed tomography scans may be unreliable when used by themselves to identify vasospasm-related infarcts,32 but all cases here that were scored as infarcts were corroborated by appropriate accompanying neurological deficits. Evaluating neurocognitive function based on self-reported information is not as robust as formal neuropsychological evaluation, but in practice, patients and their immediate family members are seldom reluctant to complain about life-altering neurocognitive abnormalities.
Conclusions
The Maryland low-dose intravenous heparin infusion protocol appeared to be safe and may be an efficacious prophylaxis against DNDs associated with severe aSAH. Patients administered a low-dose intravenous heparin infusion within 48 hours of their ictus experienced significantly fewer occurrences of symptomatic vasospasm and infarcts compared with controls. The favorable results reported in this study, bolstered by strong physiological60 and preclinical data,61 suggest that a multicenter dose and safety assessment of intravenous heparin infusion (conventional unfractionated heparin, low-molecular-weight heparin, or nonanticoagulating N-desulfated heparin),3,55 may be warranted.
Addendum
Because the US Food and Drug Administration changed its reference standard for heparin, we recently changed the Maryland low-dose intravenous heparin infusion protocol to 8 U/kg/hr progressing over 36 hours to 12 U/kg/hr.
Abbreviations used in this paper
- aPTT
activated partial thromboplastin time
- aSAH
aneurysmal subarachnoid hemorrhage
- CTA
CT angiography
- DND
delayed neurological deficit
- DSA
digital subtraction angiography
- DVT
deep vein thrombosis
- GCS
Glasgow Coma Scale
- HIT
heparin-induced thrombocytopenia
- MCA
middle cerebral artery
- WFNS
World Federation of Neurosurgical Societies
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Simard, Schreibman. Acquisition of data: all authors. Analysis and interpretation of data: Simard, Aldrich, Polifka, Beaty. Drafting the article: Simard, Schreibman, James, Polifka, Beaty. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Simard. Statistical analysis: Simard.
References
- 1.Amiconi G, Zolla L, Vecchini P, Brunori M, Antonini E. The effect of macromolecular polyanions on the functional properties of human hemoglobin. Eur J Biochem. 1977;76:339–343. doi: 10.1111/j.1432-1033.1977.tb11601.x. [DOI] [PubMed] [Google Scholar]
- 2.Attanasio M, Gori AM, Giusti B, Pepe G, Comeglio P, Brunelli T, et al. Cytokine gene expression in human LPS- and IFN-gamma-stimulated mononuclear cells is inhibited by heparin. Thromb Haemost. 1998;79:959–962. [PubMed] [Google Scholar]
- 3.Barry WH, Kennedy TP. Heparins with reduced anti-coagulant activity reduce myocardial reperfusion injury. Recent Patents Cardiovasc Drug Discov. 2011;6:148–157. doi: 10.2174/157489011795933855. [DOI] [PubMed] [Google Scholar]
- 4.Bederson JB, Connolly ES, Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 5.Boneu B, Dol F, Caranobe C, Sie P, Houin G. Pharmacokinetics of heparin and related polysaccharides. Ann N Y Acad Sci. 1989;556:282–291. doi: 10.1111/j.1749-6632.1989.tb22510.x. [DOI] [PubMed] [Google Scholar]
- 6.Casu B. Structure of heparin and heparin fragments. Ann N Y Acad Sci. 1989;556:1–17. doi: 10.1111/j.1749-6632.1989.tb22485.x. [DOI] [PubMed] [Google Scholar]
- 7.Chansel D, Ciroldi M, Vandermeersch S, Jackson LF, Gomez AM, Henrion D, et al. Heparin binding EGF is necessary for vasospastic response to endothelin. FASEB J. 2006;20:1936–1938. doi: 10.1096/fj.05-5328fje. [DOI] [PubMed] [Google Scholar]
- 8.Chimowitz MI, Pessin MS. Is there a role for heparin in the management of complications of subarachnoid hemorrhage? Stroke. 1987;18:1169–1172. doi: 10.1161/01.str.18.6.1169. [DOI] [PubMed] [Google Scholar]
- 9.Conway JE, Tamargo RJ. Cocaine use is an independent risk factor for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2001;32:2338–2343. doi: 10.1161/hs1001.097041. [DOI] [PubMed] [Google Scholar]
- 10.Coombe DR. Biological implications of glycosaminoglycan interactions with haemopoietic cytokines. Immunol Cell Biol. 2008;86:598–607. doi: 10.1038/icb.2008.49. [DOI] [PubMed] [Google Scholar]
- 11.Crowley RW, Medel R, Kassell NF, Dumont AS. New insights into the causes and therapy of cerebral vasospasm following subarachnoid hemorrhage. Drug Discov Today. 2008;13:254–260. doi: 10.1016/j.drudis.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Daneschvar HL, Daw H. Heparin-induced thrombocytopenia (an overview) Int J Clin Pract. 2007;61:130–137. doi: 10.1111/j.1742-1241.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 13.Dawes J, Prowse CV, Pepper DS. Absorption of heparin, LMW heparin and SP54 after subcutaneous injection, assessed by competitive binding assay. Thromb Res. 1986;44:683–693. doi: 10.1016/0049-3848(86)90169-6. [DOI] [PubMed] [Google Scholar]
- 14.Deshaies EM, Boulos AS, Popp AJ. Peri-operative medical management of cerebral vasospasm. Neurol Res. 2009;31:644–650. doi: 10.1179/174313209X382340. [DOI] [PubMed] [Google Scholar]
- 15.Duschek N, Vafaie M, Skrinjar E, Hirsch K, Waldhör T, Hübl W, et al. Comparison of enoxaparin and unfractionated heparin in endovascular interventions for the treatment of peripheral arterial occlusive disease: a randomized controlled trial. J Thromb Haemost. 2011;9:2159–2167. doi: 10.1111/j.1538-7836.2011.04501.x. [DOI] [PubMed] [Google Scholar]
- 16.Edelman ER, Karnovsky MJ. Contrasting effects of the intermittent and continuous administration of heparin in experimental restenosis. Circulation. 1994;89:770–776. doi: 10.1161/01.cir.89.2.770. [DOI] [PubMed] [Google Scholar]
- 17.Elsayed E, Becker RC. The impact of heparin compounds on cellular inflammatory responses: a construct for future investigation and pharmaceutical development. J Thromb Thrombolysis. 2003;15:11–18. doi: 10.1023/a:1026184100030. [DOI] [PubMed] [Google Scholar]
- 18.Engelberg H. Actions of heparin that may affect the malignant process. Cancer. 1999;85:257–272. doi: 10.1002/(sici)1097-0142(19990115)85:2<257::aid-cncr1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Etminan N, Vergouwen MD, Macdonald RL. Angiographic vasospasm versus cerebral infarction as outcome measures after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:33–40. doi: 10.1007/978-3-7091-1192-5_8. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Green B, Duffull SB, Kane-Gill SL, Bobek MB, Bies RR. Development of a dosage strategy in patients receiving enoxaparin by continuous intravenous infusion using modelling and simulation. Br J Clin Pharmacol. 2006;62:165–176. doi: 10.1111/j.1365-2125.2006.02650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fergusen S, Macdonald RL. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;60:658–667. doi: 10.1227/01.NEU.0000255396.23280.31. [DOI] [PubMed] [Google Scholar]
- 22.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 2008;72:455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 24.Hansen-Schwartz J. Cerebral vasospasm: a consideration of the various cellular mechanisms involved in the pathophysiology. Neurocrit Care. 2004;1:235–246. doi: 10.1385/NCC:1:2:235. [DOI] [PubMed] [Google Scholar]
- 25.Higashiyama S, Iwabuki H, Morimoto C, Hieda M, Inoue H, Matsushita N. Membrane-anchored growth factors, the epidermal growth factor family: beyond receptor ligands. Cancer Sci. 2008;99:214–220. doi: 10.1111/j.1349-7006.2007.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higashiyama S, Nanba D. ADAM-mediated ectodomain shedding of HB-EGF in receptor cross-talk. Biochim Biophys Acta. 2005;1751:110–117. doi: 10.1016/j.bbapap.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Hirsh J, Anand SS, Halperin JL, Fuster V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler Thromb Vasc Biol. 2001;21:1094–1096. doi: 10.1161/hq0701.093686. [DOI] [PubMed] [Google Scholar]
- 28.Hochart H, Jenkins PV, Preston RJ, Smith OP, White B, O’Donnell J. Concentration-dependent roles for heparin in modifying lipopolysaccharide-induced activation of mononuclear cells in whole blood. Thromb Haemost. 2008;99:570–575. doi: 10.1160/TH07-06-0424. [DOI] [PubMed] [Google Scholar]
- 29.Hochart H, Jenkins PV, Smith OP, White B. Low-molecular weight and unfractionated heparins induce a downregulation of inflammation: decreased levels of proinflammatory cytokines and nuclear factor-kappaB in LPS-stimulated human monocytes. Br J Haematol. 2006;133:62–67. doi: 10.1111/j.1365-2141.2006.05959.x. [DOI] [PubMed] [Google Scholar]
- 30.Hoh BL, Curry WT, Jr, Carter BS, Ogilvy CS. Computed tomographic demonstrated infarcts after surgical and endovascular treatment of aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 2004;146:1177–1183. doi: 10.1007/s00701-004-0349-6. [DOI] [PubMed] [Google Scholar]
- 31.Iba T, Takayama T. Enoxaparin attenuates endothelial damage with less bleeding compared with unfractionated heparin in endotoxemic rats. Shock. 2009;32:530–534. doi: 10.1097/SHK.0b013e3181a2e279. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahim GM, Weidauer S, Vatter H, Raabe A, Macdonald RL. Attributing hypodensities on CT to angiographic vasospasm is not sensitive and unreliable. Stroke. 2012;43:109–112. doi: 10.1161/STROKEAHA.111.632745. [DOI] [PubMed] [Google Scholar]
- 33.Joseph M, Ziadi S, Nates J, Dannenbaum M, Malkoff M. Increases in cardiac output can reverse flow deficits from vasospasm independent of blood pressure: a study using xenon computed tomographic measurement of cerebral blood flow. Neurosurgery. 2003;53:1044–1052. doi: 10.1227/01.neu.0000088567.59324.78. [DOI] [PubMed] [Google Scholar]
- 34.Junqueira DR, Perini E, Penholati RR, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2012;9:CD007557. doi: 10.1002/14651858.CD007557.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Juvela S, Siironen J, Varis J, Poussa K, Porras M. Risk factors for ischemic lesions following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2005;102:194–201. doi: 10.3171/jns.2005.102.2.0194. [DOI] [PubMed] [Google Scholar]
- 36.Kalmes A, Daum G, Clowes AW. EGFR transactivation in the regulation of SMC function. Ann N Y Acad Sci. 2001;947:42–55. doi: 10.1111/j.1749-6632.2001.tb03929.x. [DOI] [PubMed] [Google Scholar]
- 37.Kandrotas RJ. Heparin pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 1992;22:359–374. doi: 10.2165/00003088-199222050-00003. [DOI] [PubMed] [Google Scholar]
- 38.Kane-Gill SL, Feng Y, Bobek MB, Bies RR, Pruchnicki MC, Dasta JF. Administration of enoxaparin by continuous infusion in a naturalistic setting: analysis of renal function and safety. J Clin Pharm Ther. 2005;30:207–213. doi: 10.1111/j.1365-2710.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 39.Kapp JP, Neill WR, Neill CL, Hodges LR, Smith RR. The three phases of vasospasm. Surg Neurol. 1982;18:40–45. doi: 10.1016/0090-3019(82)90011-8. [DOI] [PubMed] [Google Scholar]
- 40.Kapp JP, Neill WR, Salter JE, Barnes TY. Systemic heparin in the early management of ruptured intracranial aneurysms: review of 104 consecutive cases and comparison with concurrent controls. Neurosurgery. 1987;20:564–570. doi: 10.1227/00006123-198704000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Keuskamp J, Murali R, Chao KH. High-dose intraarterial verapamil in the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;108:458–463. doi: 10.3171/JNS/2008/108/3/0458. [DOI] [PubMed] [Google Scholar]
- 42.Kranc KR, Pyne GJ, Tao L, Claridge TD, Harris DA, Cadoux-Hudson TA, et al. Oxidative degradation of bilirubin produces vasoactive compounds. Eur J Biochem. 2000;267:7094–7101. doi: 10.1046/j.1432-1327.2000.01812.x. [DOI] [PubMed] [Google Scholar]
- 43.Kroon C, ten Hove WR, de Boer A, Kroon JM, van der Pol JM, Harthoorn-Lasthuizen EJ, et al. Highly variable anticoagulant response after subcutaneous administration of high-dose (12,500 IU) heparin in patients with myocardial infarction and healthy volunteers. Circulation. 1992;86:1370–1375. doi: 10.1161/01.cir.86.5.1370. [DOI] [PubMed] [Google Scholar]
- 44.Kuwahara-Watanabe K, Hidai C, Ikeda H, Aoka Y, Ichikawa K, Iguchi N, et al. Heparin regulates transcription of endothelin-1 gene in endothelial cells. J Vasc Res. 2005;42:183–189. doi: 10.1159/000084656. [DOI] [PubMed] [Google Scholar]
- 45.Lever R, Smailbegovic A, Page CP. Locally available heparin modulates inflammatory cell recruitment in a manner independent of anticoagulant activity. Eur J Pharmacol. 2010;630:137–144. doi: 10.1016/j.ejphar.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Zheng Z, Li X, Ma X. Unfractionated heparin inhibits lipopolysaccharide-induced inflammatory response through blocking p38 MAPK and NF-κB activation on endothelial cell. Cytokine. 2012;60:114–121. doi: 10.1016/j.cyto.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Lindahl U, Lidholt K, Spillmann D, Kjellén L. More to “heparin” than anticoagulation. Thromb Res. 1994;75:1–32. doi: 10.1016/0049-3848(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 48.Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- 49.Meyer R, Deem S, Yanez ND, Souter M, Lam A, Treggiari MM. Current practices of triple-H prophylaxis and therapy in patients with subarachnoid hemorrhage. Neurocrit Care. 2011;14:24–36. doi: 10.1007/s12028-010-9437-z. [DOI] [PubMed] [Google Scholar]
- 50.Morrison AM, Wang P, Chaudry IH. A novel nonanticoagulant heparin prevents vascular endothelial cell dysfunction during hyperdynamic sepsis. Shock. 1996;6:46–51. doi: 10.1097/00024382-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Mulloy B. The specificity of interactions between proteins and sulfated polysaccharides. An Acad Bras Cienc. 2005;77:651–664. doi: 10.1590/s0001-37652005000400007. [DOI] [PubMed] [Google Scholar]
- 52.Otten ML, Mocco J, Connolly ES, Jr, Solomon RA. A review of medical treatments of cerebral vasospasm. Neurol Res. 2008;30:444–449. doi: 10.1179/174313208X284089. [DOI] [PubMed] [Google Scholar]
- 53.Provencio JJ, Vora N. Subarachnoid hemorrhage and inflammation: bench to bedside and back. Semin Neurol. 2005;25:435–444. doi: 10.1055/s-2005-923537. [DOI] [PubMed] [Google Scholar]
- 54.Pyne-Geithman GJ, Caudell DN, Prakash P, Clark JF. Glutathione peroxidase and subarachnoid hemorrhage: implications for the role of oxidative stress in cerebral vasospasm. Neurol Res. 2009;31:195–199. doi: 10.1179/174313209X393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao NV, Argyle B, Xu X, Reynolds PR, Walenga JM, Prechel M, et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am J Physiol Cell Physiol. 2010;299:C97–C110. doi: 10.1152/ajpcell.00009.2010. [DOI] [PubMed] [Google Scholar]
- 56.Ray WZ, Strom RG, Blackburn SL, Ashley WW, Sicard GA, Rich KM. Incidence of deep venous thrombosis after subarachnoid hemorrhage. Clinical article. J Neurosurg. 2009;110:1010–1014. doi: 10.3171/2008.9.JNS08107. [DOI] [PubMed] [Google Scholar]
- 57.Rider CC. Heparin/heparan sulphate binding in the TGF-beta cytokine superfamily. Biochem Soc Trans. 2006;34:458–460. doi: 10.1042/BST0340458. [DOI] [PubMed] [Google Scholar]
- 58.Rothoerl RD, Ringel F. Molecular mechanisms of cerebral vasospasm following aneurysmal SAH. Neurol Res. 2007;29:636–642. doi: 10.1179/016164107X240224. [DOI] [PubMed] [Google Scholar]
- 59.Siironen J, Juvela S, Varis J, Porras M, Poussa K, Ilveskero S, et al. No effect of enoxaparin on outcome of aneurysmal subarachnoid hemorrhage: a randomized, double-blind, placebo-controlled clinical trial. J Neurosurg. 2003;99:953–959. doi: 10.3171/jns.2003.99.6.0953. [DOI] [PubMed] [Google Scholar]
- 60.Simard JM, Schreibman D, Aldrich EF, Stallmeyer B, Le B, James RF, et al. Unfractionated heparin: multitargeted therapy for delayed neurological deficits induced by subarachnoid hemorrhage. Neurocrit Care. 2010;13:439–449. doi: 10.1007/s12028-010-9435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simard JM, Tosun C, Ivanova S, Kurland DB, Hong C, Radecki L, et al. Heparin reduces neuroinflammation and transsynaptic neuronal apoptosis in a model of subarachnoid hemorrhage. Transl Stroke Res. 2012;3 (Suppl 1):155–165. doi: 10.1007/s12975-012-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stein SC, Levine JM, Nagpal S, LeRoux PD. Vasospasm as the sole cause of cerebral ischemia: how strong is the evidence? Neurosurg Focus. 2006;21(3):E2. doi: 10.3171/foc.2006.21.3.2. [DOI] [PubMed] [Google Scholar]
- 63.Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–396. doi: 10.1056/NEJMra052732. [DOI] [PubMed] [Google Scholar]
- 64.Triantaphyllopoulos DC, Triantaphyllopoulos E. Solubility of fibrin clots in solutions of heparin. Nature. 1964;204:1096–1098. doi: 10.1038/2041096b0. [DOI] [PubMed] [Google Scholar]
- 65.Tseng MY. Summary of evidence on immediate statins therapy following aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011;15:298–301. doi: 10.1007/s12028-011-9596-6. [DOI] [PubMed] [Google Scholar]
- 66.Tyrell DJ, Kilfeather S, Page CP. Therapeutic uses of heparin beyond its traditional role as an anticoagulant. Trends Pharmacol Sci. 1995;16:198–204. doi: 10.1016/s0165-6147(00)89022-7. [DOI] [PubMed] [Google Scholar]
- 67.Tyrrell DJ, Horne AP, Holme KR, Preuss JM, Page CP. Heparin in inflammation: potential therapeutic applications beyond anticoagulation. Adv Pharmacol. 1999;46:151–208. doi: 10.1016/s1054-3589(08)60471-8. [DOI] [PubMed] [Google Scholar]
- 68.US Food and Drug Administration. Heparin: change in reference standard. [Accessed August 13, 2013];FDA.gov. ( http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm184687.htm)
- 69.Vergouwen MD, Etminan N, Ilodigwe D, Macdonald RL. Lower incidence of cerebral infarction correlates with improved functional outcome after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2011;31:1545–1553. doi: 10.1038/jcbfm.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1761–1770. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]
- 71.Wang DZ, Futrell N, Taylon C, Millikan C. Anticoagulation for prevention of cerebral infarcts following subarachnoid hemorrhage. Surg Neurol. 1995;44:270–274. doi: 10.1016/0090-3019(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 72.Weir BK, Kongable GL, Kassell NF, Schultz JR, Truskowski LL, Sigrest A. Cigarette smoking as a cause of aneurysmal subarachnoid hemorrhage and risk for vasospasm: a report of the Cooperative Aneurysm Study. J Neurosurg. 1998;89:405–411. doi: 10.3171/jns.1998.89.3.0405. [DOI] [PubMed] [Google Scholar]
- 73.Weyer GW, Nolan CP, Macdonald RL. Evidence-based cerebral vasospasm management. Neurosurg Focus. 2006;21(3):E8. doi: 10.3171/foc.2006.21.3.8. [DOI] [PubMed] [Google Scholar]
- 74.Wurm G, Tomancok B, Nussbaumer K, Adelwöhrer C, Holl K. Reduction of ischemic sequelae following spontaneous subarachnoid hemorrhage: a double-blind, randomized comparison of enoxaparin versus placebo. Clin Neurol Neurosurg. 2004;106:97–103. doi: 10.1016/j.clineuro.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 75.Yokokawa K, Mandal AK, Kohno M, Horio T, Murakawa K, Yasunari K, et al. Heparin suppresses endothelin-1 action and production in spontaneously hypertensive rats. Am J Physiol. 1992;263:R1035–R1041. doi: 10.1152/ajpregu.1992.263.5.R1035. [DOI] [PubMed] [Google Scholar]
- 76.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122:743–752. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 77.Zhang ZD, Macdonald RL. Contribution of the remodeling response to cerebral vasospasm. Neurol Res. 2006;28:713–720. doi: 10.1179/016164106X151990. [DOI] [PubMed] [Google Scholar]