Abstract
Background:
Liver is one of the most important organs affected by exercise. According to the literature a few study to date has investigated the effects of estrogen supplementation on exercise-induced oxidative stress in liver tissue of rats.
Objectives:
We aimed to investigate the effects of estrogen supplementation on oxidative stress markers in liver tissue of exercised rats.
Materials and Methods:
Male rats (n = 35) were divided as estrogen supplemented (n = 18) and non-supplemented groups (n = 17); these groups were further divided as rest and eccentric exercised groups. Eccentric exercise groups were further divided as rats killed after 1 hour and 48 hours of eccentric exercise. Estrogen (10 mg/kg) was administered subcutaneously for 30 days. Eccentric exercise was applied as treadmill run (15° downhill, 20 m/min) consisting of periods of "5 min" run and 2 min rest repeated 18 times. The rat liver was examined biochemically and histologically. Activities of GST, GSH-Px, CAT, SOD and MDA concentration were also measured spectrophotometrically.
Results:
Some disruptions were detected in experimental groups compared with the control group. Additionally, exercise training caused an increase in SOD and decrease in GSH-Px activities in some experimental groups. SOD activities increased significantly in group 3 (Estrogen (-), eccentric exercise (+) killed (after 1 h), compared with group 5 (Estrogen (-), eccentric exercise (+) killed (after 48 h). On the other hand, GSH-Px activities were also significantly decreased in groups 3, 4 and 5 compared with the control group. Leukocyte infiltration in liver increased after 48 hours compared with after 1 hour and estrogen supplementation was not able to prevent this infiltration.
Conclusions:
Estrogen seemed to be not very effective to prevent eccentric exercise-induced liver damage.
Keywords: Antioxidants, Exercise, Estrogens, Liver
1. Background
There has been recently a great deal of interest in the role of inflammatory responses and oxidative stress related to tissue damages and fatigue from exercise. Exhaustive physical exercise is known to induce free radicals in vivo and guide to oxidative damage in multiple tissues in rats (1-4). It is also well known that regular exercise plays a protective role against lifestyle-related diseases and has many health beneficial effects including improvement of antioxidant status in liver tissue (4-6).
However, exhaustive exercise can produce a large quantity of reactive oxygen species (ROS) due to increased oxygen consumption (7). ROS are scavenged by a sophisticated antioxidant defense system, which includes enzymes, superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px), and non-enzyme glutathione (GSH) (8). On the other hand, NF-κB is a well-known nuclear transcription factor involved in regulating expression of various genes and enzymes including antioxidants, which play critical roles in the first step of inflammation (9, 10). Since oxidative stress and inflammation contribute to fatigue, tissue damage and impaired recovery from exhaustive exercise, much research has focused on supplementation of nutraceutical agents for reducing these effects. Additionally, people tried various strategies to improve the body’s strength and enhance recovery from fatigue, including the use of stimulants, which cause long-term harm to the body (11).
Estrogen exerts a variety of important physiological effects, suggested to be mediated via the two known estrogen receptors (ERs), alpha and beta. 17b-estradiol (E2) binds estrogen receptors alpha (ER-alpha) with a higher affinity than estrogen receptors beta (ER-beta) and promotes higher rates of ER-alpha-mediated transcriptional activity at the estrogen response elements (ERE). Estrogen is believed to have a high antioxidant capacity, membrane stabilizing properties and a gene regulatory effect. Through one or all, of these interrelating properties it has been suggested that estrogen could play a role in reducing tissue damage (12). However, there are some contradictions about estrogen supplementation effects.
2. Objectives
The liver is one of the most important organs affected by exercise, and to the best of our knowledge and literature data, a few study to date investigated the effects of estrogen supplementation on exercise-induced oxidative stress in liver tissue of rats. Therefore, the present study aimed to investigate positive or negative effects of estrogen supplementation on oxidative stress markers in liver tissue of exercised rats.
3. Materials and Methods
3.1. Animals
Thirty-five male Sprague-Dawley rats (ATADEM, 22.04.2011, B.30.2.ATA.0.23.71-514) were housed according to gender in cages of 6-7 animals, in an environmentally controlled room with reversed light/dark cycles. All animals were allowed free access to food and water and fed with AIN-93 purified rodent diet. After a week of acclimatization, animals were randomly assigned to experimental groups as follows; eighteen exercised, vehicle-injected males (MV); eighteen exercised, estrogen-injected males (ME). Animals were approximately 12 weeks of age and sexually mature at the start of experiment. And then, the rats were divided into six groups (Table 1).
Table 1. Experimental Groups of Study.
| Group | n | Treatment |
|---|---|---|
| 1 | 6 | Control |
| 2 | 6 | Estrogen (+), eccentric exercise (-) |
| 3 | 5 | Estrogen (-), eccentric exercise (+) killed (after 1 h) |
| 4 | 6 | Estrogen (+), eccentric exercise (+) killed (after 1 h) |
| 5 | 6 | Estrogen (-), eccentric exercise (+) killed (after 48 h) |
| 6 | 6 | Estrogen (+), eccentric exercise (+) killed (after 48 h) |
3.2. Drug Administration
Animals received daily physiological doses of either 10 µg/kg 1 body mass β-estradiol 3-benzoate (13) in sesame oil and absolute ethanol or virgin sesame oil and absolute ethanol vehicle alone. Injections were made subcutaneously into the neck fold for 30 consecutive days. The animals were acutely exercised 24 hours following the final injection. Estrogen treatment protocols similar to those used in this study were reported to induce adaptations in liver as well as other tissues of gonadally intact male (14-16).
3.3. Exercise Administration
Acute exercise was performed on a motorized rodent treadmill with an electric shock grid. Animals ran at 20 m/min on a 15% acute exercise on a motorized rodent treadmill with an electric shock grid. Animals ran at 20 m/min on a 15% grade for 90 minutes. All animals completed 90 minutes of exercise (17, 18). At the end of exercise, all rats were killed under isoflurane anesthesia by taking blood from the heart.
3.4. Histological Procedures
Liver samples for light microscopic examination were fixed in 10% formaldehyde solution for 48 hours, dehydrated in a graded alcohol series and cleared in xylene. After dehydration, specimens were embedded in fresh paraffin (Agar, Cambridge, UK). Sections were cut using a Leica RM2125RT microtome (Leica, Germany). In this stage, each paraffin block was cut to 7 μm thickness. The sections were stained with Hematoxylin-Eosin (H&E) for light microscopic examination.
3.5. Immunostaining Procedures
NF-κB is a protein complex, which controls transcription of DNA. NF-κB is involved in cellular responses to stimuli such as stress, cytokines and free radicals. Therefore, our aim for using NF-κB staining method was to detect possible pathologies about oxidative stress in liver tissue. Immunohistochemical staining was performed on 7 μm thick paraffin-embedded biopsies. Immunohistochemical staining for NF-κB protein was performed by an automated method on the VENTANA BenchMark GX System (Ventana Medical Systems, Inc.) with an ultraView Universal DAB Detection Kit. The antigenic determinant sites for NF-κB were unmasked in citrate buffer with steam for 60 minutes, after deparaffinization step. The primary antibody used for NF-κB staining, a rabbit anti-human NF-κB/p65 primary antibody (Santa Cruz, CA) was used at a dilution of 1:50 for 32 minutes at 37°C. Then, the slides were incubated with the diluted antibody, followed by application of ultraView Universal DAB detection kit (Ventana Medical Systems, Inc.). DAB was used as a chromogenic and hematoxylin as a counter stain.
3.6. Tissue Sample Preparation
Livers were collected and tissues were homogenized with Malondialdehyde (MDA) 1.15% KCI, and for GST, GSH-Px ve SOD NaCl 0.9% was used. Tissue samples were centrifuged with Yellow Line DI 18 basic. Homogenates were centrifuged at 15000 rpm for 15 minutes at +4 °C. The supernatant was collected and frozen at -80 °C until assayed (19).
3.7. Measurements
The CAT enzyme activity was determined according to the method proposed by Aebi (20). The kinetic analysis of CAT was started after H2O2 addition and the color reaction was measured at 240 nm. Data was corrected by the protein content and expressed as percentage of control. GSH-Px activity was measured spectrophotometrically at 340 nm by NADPH consumption for three minutes at 37 °C (21). The reaction was initiated by adding H2O2 to a final concentration of 0.4 mM. The GSH-Px activity was determined using the molar extinction coefficient 6220 /M cm and expressed as percentage of control. The SOD enzyme activity was determined according to the method proposed by Misra and Fridovich (22). The principle of method is based on the inhibition of Nitro Blue Tetrazolium (NBT) reduction by the xanthine-xanthine oxidase system as a superoxide generator. One unit of SOD was defined as the amount of enzyme causing 50% inhibition in the NBT reduction rate. SOD activity was also expressed as unit per g-1 protein. The kinetic analysis of SOD was started after adrenaline addition, and the color reaction was measured at 480 nm. Data was corrected by the protein content and expressed as percentage of control. MDA concentrations were estimated by spectrophotometric measurement of the color generated by the reaction of thiobarbituric acid and MDA at 532 nm (23). Results were expressed as nanomoles per gram protein (nmol.g-1 protein). GST activity of the cell supernatant was measured using 1-chloro-2,4-dinitrobenzene and it was assayed at 340 nm according to the method of Habig (24). Results were expressed as (U/mg-protein).
3.8. Statistical Analysis
Results given are means ± SEM. Mann-Whitney U test was used to compare the group means. P value less than 0.05 was considered as significant.
4. Results
4.1. Histopathological Results
Sections obtained from every group were evaluated in two different steps:
1st step: H&E dyed sections for detection of cell cytoplasm and nuclei,
2nd step: NF-κB immunostained for detection of oxidative stress.
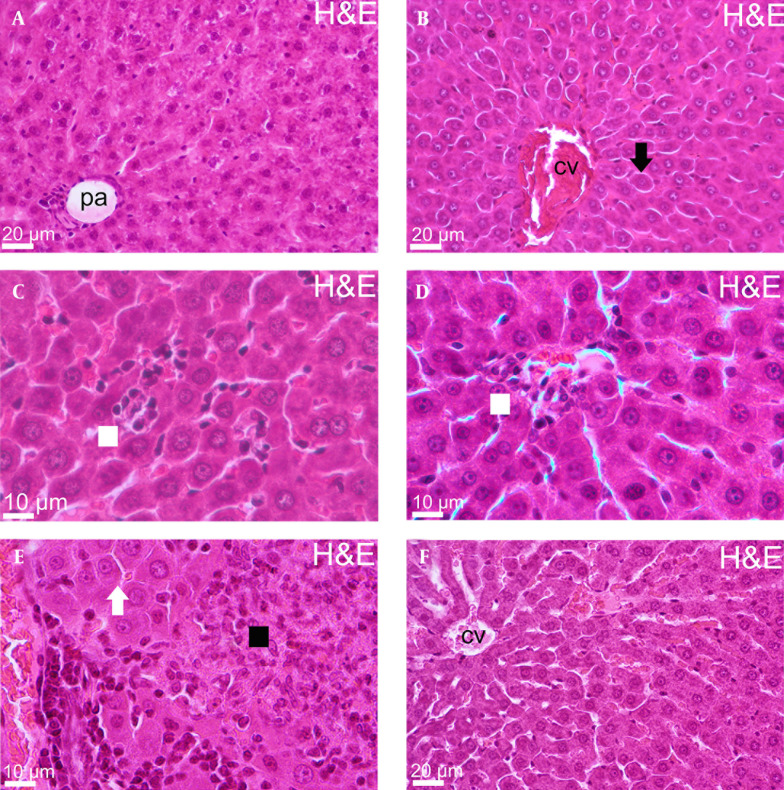
According to histopathological data, central vein and hepatocytes, lined from central vein radially and sinusoids, between hepatocyte cords, were seen as healthy appearance in liver tissue of control groups. However, there were some disruptions in other experimental groups. Three important findings were detected for group 2. Firstly, hepatocytes lost their hexagonal shape and connection with each other. Additionally, hepatocytes had more eosinophilic cytoplasm and finally, congestion was remarkable in central veins. The sinusoids were full of inflammatory cells in group 3 histopathological sections. Spotty necrosis and some hepatocytes with pushed aside picnotic nuclei and eosinophilic cytoplasm were observed in some area. In addition, congestion was mildly remarkable in small central veins the same as group 2. Hyalinization was observed in portal area in liver tissue of group 4. Additionally, increased cytoplasmic eosinophilia was detected in hepatocytes located on central area as well as dilatation in central area sinusoids. On the other hand, endothelial damage in brunches of hepatic artery and portal vein, necrotic focus and inflammatory cell invasion and congestion were detected at portal area for the fifth group. Finally, eosinophilic hepatocytes, dilatation and congestion were observed in Group 6 sections (Figure 1).
Figure 1. Liver Sections Stained With H&E of all Experimental Groups.
The liver sections of whole groups named as (A) Control group, (B) Group 2, (C) Group 3, (D) Group 4, (E) Group 5 and (F) Group 6. (Portal area = pa, central vein = cv, black arrow = degenerative hepatocyte, white square = inflammatory cell infiltration, black square = necrotic focus).
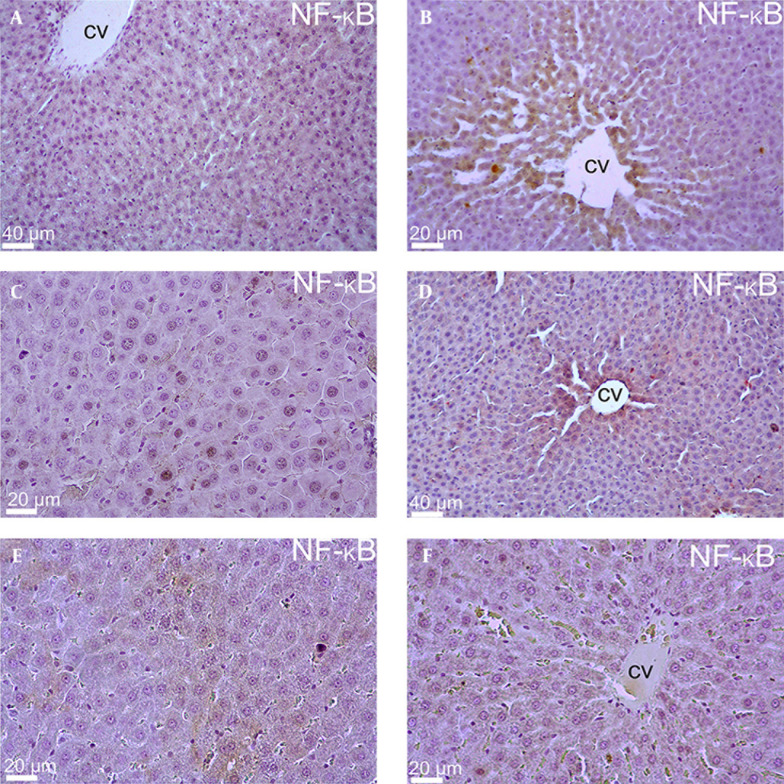
When the NF-κB immuno-staining data was evaluated, there was no significant immunopositivity in Group 1 (Control group). However, mild immunopositivity was detected in hepatocytes located around the central vein in the experimental groups that were subjected to estrogen supplementation (Groups 2, 4 and 6). On the other hand, cytoplasmic and nuclear positivity were rarely detected in mid-zonal area in Groups 3 and 5. (Figure 2).
Figure 2. Liver Sections Stained With NF-κB of all Experimental Groups.
The liver sections of whole groups named as (A) Control group, (B) Group 2, (C) Group 3, (D) Group 4, (E) Group 5 and (F) Group 6. (Central vein = cv).
4.2. Biochemical Results
Some differences were observed in the level of some antioxidant parameters when biochemical data was evaluated. For this study, there were serious increases and decreases in SOD and GSH-Px activities in some experimental groups. At first, no significant differences were found between all experimental groups regarding the level of MDA, CAT and GST activities (P > 0.05). Additionally, there were no significant differences between control group and groups 2, 3, 4 and 6 regarding the level of SOD activity. However, a significant difference in SOD activity was detected between groups 3 and 5. SOD activities were increased in group 3 compared to group 5 (P < 0.05). On the other hand, there were no significant differences between control group and groups 2 and 6 regarding the level of GSH-Px activity. However, GSH-Px activity was significantly (P < 0.05) decreased in groups 3, 4 and 5 compared with the control group (Table 2).
Table 2. Malondialdehyde (MDA) Level and Superoxide Dismutase (SOD), Catalase (CAT), Glutathione S-transferase (GST) and Glutathione Peroxidase (GPx) Activities in Liver Tissues a.
| Group | n | MDA (nmol.g-1 protein ) | SOD (U/mg protein) | CAT (U/mg protein) | GST (U/mg protein) | GPx (U/mg protein) |
|---|---|---|---|---|---|---|
| 1: Control | 6 | 235.90 ± 52.82 | 770.41 ± 79.31 | 346.02 ± 42.63 | 1.41 ± 0.17 | 43.90 ± 18.34 |
| 2: Estrogen (+), eccentric exercise (-) | 6 | 222.01 ± 52.06 | 840.35 ± 94.83 | 302.46 ± 95.37 | 1.22 ± 0.16 | 24.60 ± 8.27 |
| 3: Estrogen (-), eccentric exercise (+) killed (after 1 h) | 5 | 225.84 ± 27.45 | 703.19 ± 64.81 | 291.42 ± 52.66 | 1.37 ± 0.17 | 11.23 ± 4.20 |
| 4: Estrogen (+), eccentric exercise (+) killed (after 1 h) | 6 | 210.49 ± 24.39 | 717.61 ± 59.45 | 339.33 ± 51.12 | 1.49 ± 0.15 | 15.26 ± 5.79 |
| 5: Estrogen (-), eccentric exercise (+) killed (after 48 h) | 6 | 187.21 ± 17.86 | 941.67 ± 64.05 | 459.56 ± 71.89 | 1.31 ± 0.25 | 10.91 ± 2.18 |
| 6: Estrogen (+), eccentric exercise (+) killed (after 48 h) | 6 | 184.56 ± 9.21 | 753.88 ± 39.05 | 407.14 ± 82.69 | 1.21 ± 0.07 | 43.43 ± 28.27 |
aThe results are means ± SEM. The groups were compared by Mann-Whitney U test. P < 0.05 was considered significant.
There was really a serious damage in rat liver occurred by estrogen supplementation and exhausted exercise, but some disruption should not be overlooked according to the obtained data. There were no necrotic areas in any experimental groups except group 5. As a consequent, this issue may be explained as some disruptions detected in experimental groups may form the first few steps of necrotic process.
5. Discussion
Exercise is a protective method of some situations for life such as improving person's physical and mental health, ensuring self-confidence and achieving maximum performance in physical activities. However, we can talk about the beneficial sides of sports, when performed properly and consciously. Some applications such as drug usage, overload and balance problems may lead to injuries, permanent damages and some side effects of drug (doping) usage (25).
Recently, there is an increased interest on the topic related with estrogen and its inflammatory response on some tissues. Estrogen has serious effects on some organs, especially brain, heart and skeletal muscles, nerve tissue and liver (26). There are some studies showing the effects of estrogen supplementation on exercise-induced liver damage and leukocyte infiltration (27). Although some studies claim estrogen beneficial effects on some tissue damage, there are still contradictions about this issue (28, 29).
Our study attempted to examine estrogen supplementation in relation to exhaustive exercise-induced liver damage in a rat model. In the histopathological results of this study, there were some changes in liver tissue in only estrogen supplementation group compared with the control group. However, the damage level was higher in exercise groups because of exercise adverse effects. Inflammatory cell infiltration and NF-κB-p65 immunopositivity were observed both in groups 3 and 4. Sinusoidal narrowing was seen in groups 5 and 6 as well as inflammatory cell infiltration and NF-κB-p65 immunopositivity. According to the histopathological results, estrogen supplementation (groups 4 and 6) could not prevent the liver damage.
There are some studies regarding estrogen supplementation in exercise-induced tissues damage, but there is no data about estrogen supplementation on exhaustive exercise-induced liver damage according to the literature (30, 31). By this way, the present study would be the important data about estrogen and exercise-induced liver damage, but the present findings indicated that estrogen may not be very effective to prevent eccentric exercise-induced liver damage.
It is well known that SOD, CAT and GSH-Px are regarded as the first line of defense by the anti-oxidant enzyme system against ROS generated during exhaustive exercise. The current study showed that some of these enzymes in liver increased as a compensatory mechanism in response to an increase in oxidative stress due to exhaustive exercise.
SOD is one of the first lines of defense of anti-oxidant enzyme system against reactive oxygen species generated during exhaustive physical exercise (32). Previous studies reported that SOD and GSH-Px activities in serum are increased with oxidative stress due to exercise-induced fatigue (33, 34). In our study, exercise increased SOD and decreased GSH-Px activities in some experimental groups. SOD activity was statistically increased only in group 3, compared with the control group. On the other hand, GSH-Px activities were also significantly decreased in groups 3, 4 and 5 compared with the control group. These effects were not induced by estrogen intake, because estrogen supplementations were not performed for groups 3 and 5. There were no statistically differences regarding MDA, SOD, CAT, GST and GSH-Px in experimental groups with estrogen supplementation. Although some numerical changes in MDA, SOD, CAT, GST and GSH-Px parameters were observed, estrogen had no significant effect on liver damage.
In conclusion, there was really a serious damage in rat liver occurred by estrogen supplementation and exhausted exercise, but some disruption should not be overlooked according to the obtained data. This issue may be explained as some disruptions detected in experimental groups may form the first few steps of necrotic process. Additionally, this report demonstrated that estrogen supplementation had no effect on liver damage occurred by exhausted exercise. Therefore, further studies must be performed about estrogen supplementation and exercise-induced tissue damage to explain the possible mechanism.
Footnotes
Authors’ Contributions:Study concept and design: Can Serpil, Gul Mustafa. Acquisition of data: Can Ismail, Ozabacigil Fatma, Selli Jale and Yigit Serdar. Analysis and interpretation of data: Gedikli Semin. Drafting of the manuscript: Aksak Karamese Selina. Critical revision of the manuscript for important intellectual content: Bacak Gulsum. Statistical analysis: Sahin Gonul Zisan. Administrative, technical and material support: Cigsar Gulsen.
References
- 1.Frankiewicz-Jozko A, Faff J, Sieradzan-Gabelska B. Changes in concentrations of tissue free radical marker and serum creatine kinase during the post-exercise period in rats. Eur J Appl Physiol Occup Physiol. 1996;74(5):470–4. doi: 10.1007/BF02337728. [DOI] [PubMed] [Google Scholar]
- 2.Radak Z, Nakamura A, Nakamoto H, Asano K, Ohno H, Goto S. A period of anaerobic exercise increases the accumulation of reactive carbonyl derivatives in the lungs of rats. Pflugers Arch. 1998;435(3):439–41. doi: 10.1007/s004240050537. [DOI] [PubMed] [Google Scholar]
- 3.Huang CC, Tsai SC, Lin WT. Potential ergogenic effects of L-arginine against oxidative and inflammatory stress induced by acute exercise in aging rats. Exp Gerontol. 2008;43(6):571–7. doi: 10.1016/j.exger.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Gunduz F, Senturk UK, Kuru O, Aktekin B, Aktekin MR. The effect of one year's swimming exercise on oxidant stress and antioxidant capacity in aged rats. Physiol Res. 2004;53(2):171–6. [PubMed] [Google Scholar]
- 5.Mallikarjuna K, Nishanth K, Hou CW, Kuo CH, Sathyavelu Reddy K. Effect of exercise training on ethanol-induced oxidative damage in aged rats. Alcohol. 2009;43(1):59–64. doi: 10.1016/j.alcohol.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Radak Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, et al. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18(6):749–50. doi: 10.1096/fj.03-0509fje. [DOI] [PubMed] [Google Scholar]
- 7.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107(4):1198–205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 8.Michiels C, Raes M, Toussaint O, Remacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med. 1994;17(3):235–48. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 9.Djordjevic J, Djordjevic A, Adzic M, Niciforovic A, Radojcic MB. Chronic stress differentially affects antioxidant enzymes and modifies the acute stress response in liver of Wistar rats. Physiol Res. 2010;59(5):729–36. doi: 10.33549/physiolres.931862. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad ST, Arjumand W, Nafees S, Seth A, Ali N, Rashid S, et al. Hesperidin alleviates acetaminophen induced toxicity in Wistar rats by abrogation of oxidative stress, apoptosis and inflammation. Toxicol Lett. 2012;208(2):149–61. doi: 10.1016/j.toxlet.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Gore RK, Webb TS, Hermes ED. Fatigue and stimulant use in military fighter aircrew during combat operations. Aviat Space Environ Med. 2010;81(8):719–27. doi: 10.3357/asem.2755.2010. [DOI] [PubMed] [Google Scholar]
- 12.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150(5):2109–17. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 13.Kendrick ZV, Steffen CA, Rumsey WL, Goldberg DI. Effect of estradiol on tissue glycogen metabolism in exercised oophorectomized rats. J Appl Physiol (1985). 1987;63(2):492–6. doi: 10.1152/jappl.1987.63.2.492. [DOI] [PubMed] [Google Scholar]
- 14.Kenagy R, Weinstein I, Heimberg M. The effects of 17 beta-estradiol and progesterone on the metabolism of free fatty acid by perfused livers from normal female and ovariectomized rats. Endocrinology. 1981;108(5):1613–21. doi: 10.1210/endo-108-5-1613. [DOI] [PubMed] [Google Scholar]
- 15.Koot RW, Amelink GJ, Blankenstein MA, Bar PR. Tamoxifen and oestrogen both protect the rat muscle against physiological damage. J Steroid Biochem Mol Biol. 1991;40(4-6):689–95. doi: 10.1016/0960-0760(91)90292-d. [DOI] [PubMed] [Google Scholar]
- 16.Rooney TP, Kendrick ZV, Carlson J, Ellis GS, Matakevich B, Lorusso SM, et al. Effect of estradiol on the temporal pattern of exercise-induced tissue glycogen depletion in male rats. J Appl Physiol (1985). 1993;75(4):1502–6. doi: 10.1152/jappl.1993.75.4.1502. [DOI] [PubMed] [Google Scholar]
- 17.Warren GL, Lowe DA, Inman CL, Orr OM, Hogan HA, Bloomfield SA, et al. Estradiol effect on anterior crural muscles-tibial bone relationship and susceptibility to injury. J Appl Physiol (1985). 1996;80(5):1660–5. doi: 10.1152/jappl.1996.80.5.1660. [DOI] [PubMed] [Google Scholar]
- 18.Tsivitse SK, McLoughlin TJ, Peterson JM, Mylona E, McGregor SJ, Pizza FX. Downhill running in rats: influence on neutrophils, macrophages, and MyoD+ cells in skeletal muscle. Eur J Appl Physiol. 2003;90(5-6):633–8. doi: 10.1007/s00421-003-0909-0. [DOI] [PubMed] [Google Scholar]
- 19.Hara M, Abe M, Suzuki T, Reiter RJ. Tissue changes in glutathione metabolism and lipid peroxidation induced by swimming are partially prevented by melatonin. Pharmacol Toxicol. 1996;78(5):308–12. doi: 10.1111/j.1600-0773.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 20.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 21.Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–21. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 22.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–5. [PubMed] [Google Scholar]
- 23.Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993;39(12):2522–6. [PubMed] [Google Scholar]
- 24.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–9. [PubMed] [Google Scholar]
- 25.Schneider BS, Tiidus PM. Neutrophil infiltration in exercise-injured skeletal muscle: how do we resolve the controversy? Sports Med. 2007;37(10):837–56. doi: 10.2165/00007256-200737100-00002. [DOI] [PubMed] [Google Scholar]
- 26.Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M. Minireview: neuroprotective effects of estrogen-new insights into mechanisms of action. Endocrinology. 2001;142(3):969–73. doi: 10.1210/endo.142.3.8033. [DOI] [PubMed] [Google Scholar]
- 27.Tiidus PM, Holden D, Bombardier E, Zajchowski S, Enns D, Belcastro A. Estrogen effect on post-exercise skeletal muscle neutrophil infiltration and calpain activity. Can J Physiol Pharmacol. 2001;79(5):400–6. [PubMed] [Google Scholar]
- 28.Tiidus PM. Estrogen and gender effects on muscle damage, inflammation, and oxidative stress. Can J Appl Physiol. 2000;25(4):274–87. doi: 10.1139/h00-022. [DOI] [PubMed] [Google Scholar]
- 29.Dabbagh A, Rajaei S. The role of anesthetic drugs in liver apoptosis. Hepat Mon. 2013;13(8) doi: 10.5812/hepatmon.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao L, Wang Y, Duan Y, Bu S. Effects of treadmill exercise training on liver fat accumulation and estrogen receptor alpha expression in intact and ovariectomized rats with or without estrogen replacement treatment. Eur J Appl Physiol. 2010;109(5):879–86. doi: 10.1007/s00421-010-1426-6. [DOI] [PubMed] [Google Scholar]
- 31.Kendall B, Eston R. Exercise-induced muscle damage and the potential protective role of estrogen. Sports Med. 2002;32(2):103–23. doi: 10.2165/00007256-200232020-00003. [DOI] [PubMed] [Google Scholar]
- 32.Mestre-Alfaro A, Ferrer MD, Sureda A, Tauler P, Martinez E, Bibiloni MM, et al. Phytoestrogens enhance antioxidant enzymes after swimming exercise and modulate sex hormone plasma levels in female swimmers. Eur J Appl Physiol. 2011;111(9):2281–94. doi: 10.1007/s00421-011-1862-y. [DOI] [PubMed] [Google Scholar]
- 33.Feng Z, Bai L, Yan J, Li Y, Shen W, Wang Y, et al. Mitochondrial dynamic remodeling in strenuous exercise-induced muscle and mitochondrial dysfunction: regulatory effects of hydroxytyrosol. Free Radic Biol Med. 2011;50(10):1437–46. doi: 10.1016/j.freeradbiomed.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Taysi S, Oztasan N, Efe H, Polat MF, Gumustekin K, Siktar E, et al. Endurance training attenuates the oxidative stress due to acute exhaustive exercise in rat liver. Acta Physiol Hung. 2008;95(4):337–47. doi: 10.1556/APhysiol.95.2008.4.2. [DOI] [PubMed] [Google Scholar]