Abstract
The gene that codes for the CD44 family members consists of 20 exons, nine of which encode the standard form of the molecule. The other exons can be inserted in various combinations into the membrane proximal region of the extracellular domain of the protein, giving rise to variant isoforms (CD44v). CD44 variants, especially the CD44v6, have been reported to regulate tumor invasion, progression, and metastasis of carcinomas. Producing a high affinity monoclonal antibody against human CD44v6 provides a powerful tool to monitor and trace CD44v6 function in different biological fluids. In this study, a synthetic peptide from CD44v6 was conjugated to keyhole limpet hemocyanin (KLH) and injected into BALB/c mice. Splenocytes from the immunized mice were fused with murine SP2/0 myeloma cells followed by selection of antibody producing hybridoma cells. After screening of hybridoma colonies by ELISA, high affinity antibodies were selected and purified by affinity chromatography. Western blot, immunocytochemistry, and flow cytometry experiments were used to characterize the antibodies. Six stable hybridoma cell lines, designated as 1H1, 1H2, 2A12, 2G11, 3H3, and 3H7, were obtained. Flow cytometry and immunocytochemistry results showed that the new monoclonal antibodies recognized CD44v6 on the cell surface. This novel panel of anti-CD44v6 antibodies has the potential for investigating the role of CD44v6 in cancer pathogenesis.
Introduction
The CD44 family includes a large group of transmembrane glycoproteins formed by alternative splicing and post-translational modifications. It has been reported that the extensive heterogeneity in CD44 molecular structure may be involved in various important cellular functions such as: (1) interaction between cell and extracellular matrix, (2) initiating several signaling pathways through combination with intracellular molecules, and (3) acting as an anchor protein by binding to the cytoskeleton.(1) As an adhesion molecule, CD44 may participate in various biological processes, including angiogenesis, lymphogenesis, wound healing, inflammation, and cancer metastasis.(2) The CD44 gene is located on the short arm of chromosome 11 in humans. CD44 can exist in many isoforms derived from a single gene by alternative mRNA splicing. The gene includes 20 exons, nine of which encode the standard form of the molecule (CD44s). The other exons can be inserted in different combinations into the membrane proximal region of the extracellular domain of the protein, giving rise to variant isoforms (CD44v). These variant exons are designated as v1–v10.(3)
CD44 variants, predominantly CD44v6, have been reported to regulate invasion, progression, and metastasis of carcinomas in rat experimental models.(4) In addition, CD44 expression has been shown to be associated with tumor progression of various human malignancies, including liver carcinoma,(5) colon carcinoma,(6) breast carcinoma,(7) lymphoma,(8) and lung carcinoma.(9) It has been suggested that CD44v6 is probably capable of helping cancer cells adhere to the vascular endothelium and basement membranes, as well as enhancing the motility of cancer cells.(10–13)
In this study, murine monoclonal antibodies against a synthetic peptide of CD44v6 were produced and characterized. These antibodies may serve as tools for monitoring CD44v6 in different biological systems.
Materials and Methods
Cell lines and antibodies
Human acute megakaryocytic leukemia cell line Mo7e, human leukemic monocytic cell line U937, human promyelocytic leukemia cell line HL60, human T-cell leukemia cell line Jurkat, and human prostate carcinoma LnCap were purchased from National Cell Bank of Iran (Pasteur Institute of Iran, Tehran, Iran). Cell lines were cultured according to the manufacturer's instructions. Secondary FITC-conjugated sheep anti-mouse Ig and irrelevant (ENV11) antibodies were obtained from Avicenna Research Institute.
Synthesis of peptide, preparation of immunogen, and confirmation of peptide conjugation
A 43 amino acid long peptide (CQA TPS STT EET ATQ KEQ WFG NRW HEG YRQ TPK EDS HST TGT A) from CD44v6, including an additional cysteine residue at the C-terminal end for conjugation, was synthesized (Chinapeptide Corp., Shanghai, China). The peptide was chemically conjugated with the carrier protein keyhole limpet hemocyanin (KLH, Sigma-Aldrich, St. Louis, MO).
Conjugation of the peptide to carrier proteins was performed as described previously.(14) Briefly, a total of 2 mg KLH (Sigma-Aldrich) and 2 mg of synthetic peptide CD44v6 were dissolved in 140 μL sterile water and 800 μL phosphate-buffered saline (PBS), respectively. Combination of KLH (140 μL), CD44v6 (800 μL), and 1% glutaraldehyde (Sigma-Aldrich) (60 μL) was incubated at room temperature for 2 h with gentle shaking. The same procedure was performed for conjugation of the peptide to bovine serum albumin (BSA, Sigma-Aldrich).(15) The technique for evaluation of the efficiency of conjugation has been described previously.(14)
Preparation of hybridoma cells and production of monoclonal antibodies
Monoclonal antibody production was performed according to the hybridoma technology.(14,16,17) Briefly, two female BALB/c mice (6–8 weeks old) were injected three times with peptide-KLH conjugate. The primary immunization was administered intraperitoneally (i.p.) with antigen and Freund's complete adjuvant (Sigma-Aldrich). Three weeks later a booster injection was delivered i.p. in Freund's incomplete adjuvant, followed by a similar booster injection 2 weeks later. Venous blood was obtained from the tail of the immunized mice 7 days after the second booster vaccination, and serum antibody titers were evaluated by indirect enzyme-linked immunosorbent assay (ELISA). Splenocytes were collected from the mouse with the highest antibody titer and fused with SP2/0 mouse myeloma cells. The cells were washed twice with RPMI 1640 medium. Pre-warmed 50% polyethylene glycol (PEG) 1500 (Sigma-Aldrich) was added to the cell pellet slowly with continuous shaking. Fused cells were washed with RPMI medium and distributed in 96-well plates followed by selection with HAT medium (Sigma-Aldrich). Hybridoma supernatants were initially screened by indirect ELISA against the CD44v6 peptide. Positive clones were subcloned four times by limiting dilution method.
Antibody purification and isotype determination
Antibodies against CD44v6 were purified from culture supernatants by affinity chromatography using a Hi-Trap protein G column (GE Healthcare, Uppsala, Sweden) according to the manufacturer's instructions. Briefly, culture supernatants were filtered through 0.45 μm filters and pH was adjusted to 7.5. The elution was performed using glycine-HCl (0.1 M, pH 2.7). The eluted antibodies were dialyzed against PBS at pH 7.5, and reactivity of the purified antibodies was determined by ELISA, as described previously.(18,19) The isotypes of the antibodies were determined by ELISA using anti mouse isotype antibodies (Sigma-Aldrich) as reported previously.(20)
Analysis of antibody titers by ELISA
Peptide-specific antibodies in the mouse sera and in hybridoma culture supernatants were determined by indirect ELISA. Briefly, 96-well polystyrene microtiter plates (Nunc, Roskilde, Denmark) were coated with the CD44v6 peptide, BSA-peptide, and KLH peptide conjugated (all at 10 μg/mL) overnight at 4°C. The plates were washed three times in PBS–Tween-20 (0.05%, PBS-T), and the unbound sites were blocked with 100 μL of 2.5% skim milk at 37°C for 1.5 h. The plates were washed as described above. For mouse serum titrations, different dilutions of mouse sera were added to the wells starting from 1:500 (37°C for 1.5 h). After washing, HRP-conjugated rabbit anti-mouse immunoglobulin (Ig, 1:1000 v/v; Avicenna Research Institute, Tehran, Iran) was added and incubated for 1.5 h at 37°C. The plates were washed, and the substrate solution containing tetramethylbenzidine (TMB) was added. The reaction was stopped by 20% H2SO4 after 15 min and the absorbance was measured at 450 nm by an ELISA reader (BioTek). The mouse with the highest titer of specific antibody was chosen for fusion. To screen for antibody production by hybridoma cells, the same procedure was performed on the hybridoma cell culture supernatants.
RNA isolation and polymerase chain reaction
Total RNA was extracted from cell lines according to guanidinium thiocyanate-phenol-chloroform extraction method(18) using RNA-Bee reagent (Tel-Test, Friendswood, TX) according to the manufacturer's instructions. The extracted RNA was treated with deoxyribonuclease (DNAase I, Sigma-Aldrich) to eliminate any potential DNA contamination.(19) First-strand cDNA was synthesized using 1 μg total RNA in 20 μL of reaction mixture consisting of 4 μL 5X RT buffer, 2 μL 20 mM dNTPs (Roche, Mannheim, Germany), 1 μL 10 pmol/μL random hexamers (N6; Roche), 2 μL deionized sterile H2O, and 1 μL Moloney murine leukemia virus reverse transcriptase (200 U/μL; Fermentas, Vilnius, Lithuania). The mixture was incubated at 42°C for 45 min, followed by 90°C for 5 min.
Polymerase chain reaction (PCR) amplification was performed using CD44 and CD44v6 primers: 5′-GAC ACA TAT TGC TTC AAT GCT TCA-3′ and 5′-GGC AAC TCC TAG TAG TAC AAC-3′ as sense and 5′-GAT GCC AAG ATG ATC AGC CAT TCT G-3' and 5′-CAG CTG TCC CTG TTG TCG AAT-3′ as antisense, respectively. Briefly, 25 μL of PCR mixture was prepared using 2.5 μL of 10× PCR buffer, 1 μL of 25 mM MgCl2, 1.5 μL dNTPs (10 mM), 0.5 μL of each primer (10 pmol/μL), 0.1 μL of Taq-DNA polymerase (5 U/μL; CinnaGen, Tehran, Iran), and 3 μL of cDNA. PCR was performed with 37 cycles and each cycle of amplification consisted of 92°C for 30 s, 62°C for 30 s, 72°C for 1 min, and a final cycle of 72°C for 10 min. PCR products were visualized by running on agarose gel (1.5%) electrophoresis containing ethidium bromide. After electrophoresis, images were taken using a gel documentation system (LMS-20E, UVP, Upland, CA).
Western blot analysis
Western blotting was performed to detect the minimum amount of CD44v6 that could be recognized by the anti-CD44v6 antibodies. To do this, 250 and 500 ng of BSA and peptide-BSA were loaded onto a 10% SDS-PAGE gel. Proteins were then transferred onto Immobilon-PVDF (Millipore, Billerica, MA) membranes. Membranes were blocked by incubating overnight at +4°C with 5% non-fat milk in PBS. Proteins were incubated for 1.5 h with monoclonal antibodies against CD44v6. After extensive washing, membranes were incubated with peroxidase-conjugated sheep anti-mouse Ig (1:2500, Avicenna Research Institute) for 1 h at room temperature, followed by washing and developing with ECL chemiluminescence detection system (GE Healthcare, Piscataway, NJ).
Flow cytometric analysis
Cell surface CD44v6 negative cell lines (HL60, Jurkat, and LnCap)(21–23) and positive cell lines (U937 and Mo7e)(23,24) were used to determine the reactivity of monoclonal antibodies by flow cytometry. Approximately, 0.5×106 HL60, Jurkat, LnCap, U937, and Mo7e cells were suspended in cold PBS and incubated with 5% sheep serum for 30 min. Cells were then fixed with 1% formaldehyde for 10 min followed by incubation with monoclonal antibodies (10 μg/mL) and an irrelevant antibody (ENV11, 10 μg/mL) as a negative control for 60 min at 4°C in PBS-0.1% BSA. After washing twice with the PBS, FITC conjugated sheep anti-mouse antibody (Avicenna Research Institute; dilution 1:50) was added, and the cells were incubated for an additional 45 min in the dark. After washing twice, the cells were analyzed on a flow cytometer (Partec, Munster, Germany). Data were analyzed by using FlowJo software (Treestar, San Carlos, CA).
Immunocytochemistry
Briefly, 30,000 U937cells were suspended in PBS and air dried on glass slides at room temperature for 1 h. After using immunocytochemistry (ICC), fixation buffer (BD Biosciences, Franklin Lakes, NJ), the cells were incubated with 5% sheep serum for 15 min at room temperature. The cells were subsequently incubated with CD44v6 and irrelevant antibodies (10 μg/mL) and negative control (PBS alone), followed by inculcation with FITC conjugated sheep anti-mouse Ig secondary antibody (1:50; Avicenna Research Institute). The cells were extensively washed with PBS between incubations. Microscopic images were acquired by Olympus BX51 microscope (Tokyo, Japan), motorized fluorescence illuminator BX-RFA equipped with Olympus DP70 digital camera, sutter excitation, and emission filter wheels. Images were acquired using the Olympus U-MWIB2 filter set.
Results
Confirmation of CD44v6-BSA and CD44v6-KLH conjugations
BSA and KLH were selected as carrier proteins to be conjugated with the CD44v6 synthetic peptide. A smear pattern of CD44v6-conjugated BSA in contrast to a sharp unconjugated BSA band (67 kDa) revealed that synthetic peptide CD44v6 was conjugated to BSA molecules (data not shown). Since KLH has a very high molecular weight (4,500–13,000 kDa) and may not be easily monitored in SDS-PAGE, simultaneous conjugation of CD44v6 peptide to KLH and BSA using identical procedures would allow us to conclude that the CD44v6-KLH conjugation was also done in the same way as the CD44v6-BSA conjugation.(15,25) CD44v6-KLH was used to immunize mice, and CD44v6-BSA was only used to confirm the conjugation.
Development of monoclonal antibodies
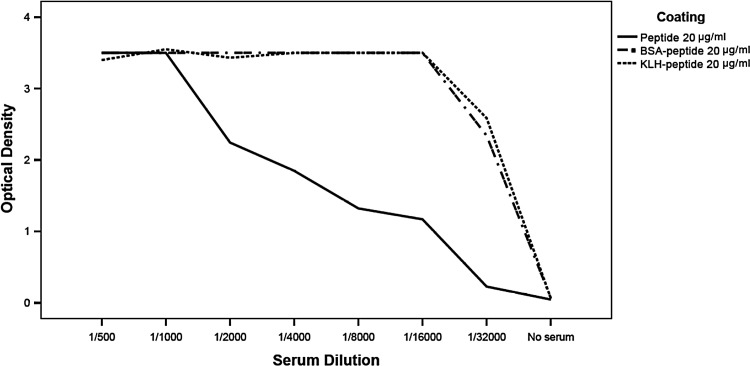
Fusion experiment was performed for hybridoma production from the mouse with higher serum antibody titer for anti-KLH-conjugated CD44v6 synthetic peptide. The hybridoma supernatants were screened by indirect ELISA after fusion. Immunoreactivity against the peptide and KLH were tested. In this experiment, mouse sera were diluted from 1:500 to 1:32,000. Obtained data demonstrated that the mice were suitable for cell fusion (Fig. 1). Culture supernatants from growing hybridomas were screened by ELISA. Clones specific for purified CD44v6 were subcloned. Six stable hybridoma cell lines, designated as 1H1, 1H2, 2A12, 2G11, 3H3, and 3H7, were obtained and further characterized. The isotyping of obtained monoclonal antibodies indicated that they all belonged to IgG1 subclass.
FIG. 1.
Titration of immunized mouse serum by ELISA. Wells were coated with CD44v6 peptide, KLH-peptide, or BSA-peptide, and the immunized mouse serum was titrated in ELISA, using peroxidase conjugated sheep anti-mouse immunoglobulin as the secondary antibody. The mouse with higher serum antibody titer for anti-KLH-conjugated CD44v6 synthetic peptide was used for cell fusion.
Analysis of CD44 and CD44v6 gene expression in cell lines
Figure 2 displays the CD44 and CD44v6 gene expressions in Mo7e, U937, HL60, LnCap, and Jurkat cell lines. As shown, 84 and 481 bp bands of amplified CD44v6 and CD44 mRNA, respectively, were found in Mo7e, U937, and HL60 cell lines. On the contrary, neither 84 nor 481 bp bands were detected in LnCap and Jurkat cell lines. Therefore, we concluded that LnCap and Jurkat cell lines did not express CD44 and CD44v6 genes (Fig. 2).
FIG. 2.
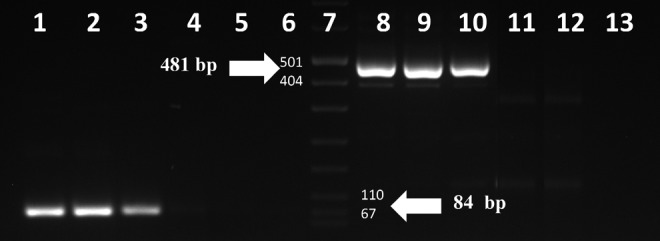
RT-PCR analysis CD44v6 transcripts in studied cell lines. Lanes 1–5, analysis of CD44v6 (84 bp) in Mo7e, U937, HL60, LnCap, and Jurkat cell lines, respectively; lane 6, negative control (no DNA) for CD44v6; lane 7, DNA size marker, Gene Ruler, marker VIII; lanes 8–12, analysis of CD44 (481 bp) in Mo7e, U937, HL60, LnCap, and Jurkat cell lines, respectively; lane 13, negative control (no DNA) for CD44.
Western blot analysis with anti-CD44v6 monoclonal antibodies
Western blot studies were done to test whether obtained monoclonal antibodies would recognize BSA-CD44v6. Six stable clones, 1H1, 1H2, 2A12, 2G11, 3H3, and 3H7, were tested against two different concentrations of BSA-CD44v6. Western blot experiment indicated that monoclonal antibodies against CD44v6 recognized BSA-CD44v6 under non-reducing conditions (Fig. 3).
FIG. 3.
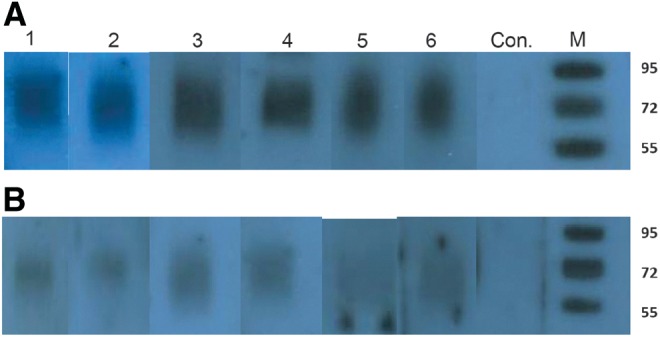
Western blot analysis of different CD44v6 antibodies using 500 ng (A) and 250 ng (B) BSA-CD44v6 conjugate. Lanes 1–6, monoclonal antibody clones 1H1, 1H2, 2A12, 2G11, 3H3, and 3H7, respectively; Con., BSA alone blotted onto the membrane and tested for reactivity with 3H7 monoclonal antibody clone (negative control); M, marker.
Flow cytometric analysis
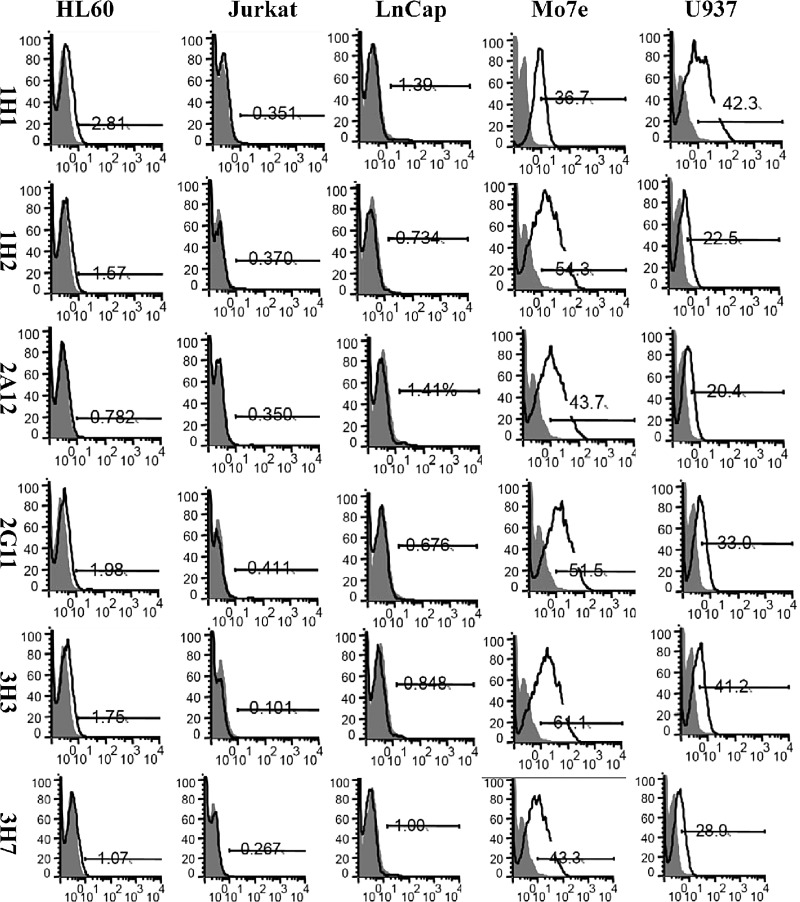
CD44v6 negative (HL60, Jurkat, and LNCap) and positive (U937 and Mo7e) cell lines were used to analyze cell surface expression of CD44v6. All six anti-CD44v6 monoclonal antibodies were examined to determine whether they recognized CD44v6 on the cell surfaces. Figure 4 shows the flow cytometry results for all cell lines. Positive staining patterns were obtained for Mo7e and U937 cells with 1H1, 1H2, 2A12, 2G11, 3H3, and 3H7 clones. None of the monoclonal antibodies detected the membrane CD44v6 in HL60, Jurkat, and LnCap cell lines.
FIG. 4.
Flow cytometric analyses of reactivities of anti-CD44v6 monoclonal antibody clones 1H1, 1H2, 2A12, 2G11, 3H3, and 3H7 with HL60, Jurkat, LnCap, Mo7e, and U937 cell lines. 1H1, 1H2, 2A12, 2G11, 3H3, and 3H7 monoclonal antibodies were reactive with Mo7e and U937 cells. None of the monoclonal antibodies reacted with HL60, Jurkat, and LnCap cell lines.
Immunocytochemistry
The reactivities of 1H1, 1H2, 2A12, 2G11, 3H3, and 3H7 antibodies were investigated on U937 cell line by immunocytochemistry. Acquired images revealed that the monoclonal antibodies recognized CD44v6 molecules in U937 cell line, although reactivity of clone 2A12 was very weak (Fig. 5). In this experiment, the irrelevant antibody and PBS alone (negative controls) showed no reactivity (Fig. 5A, B).
FIG. 5.
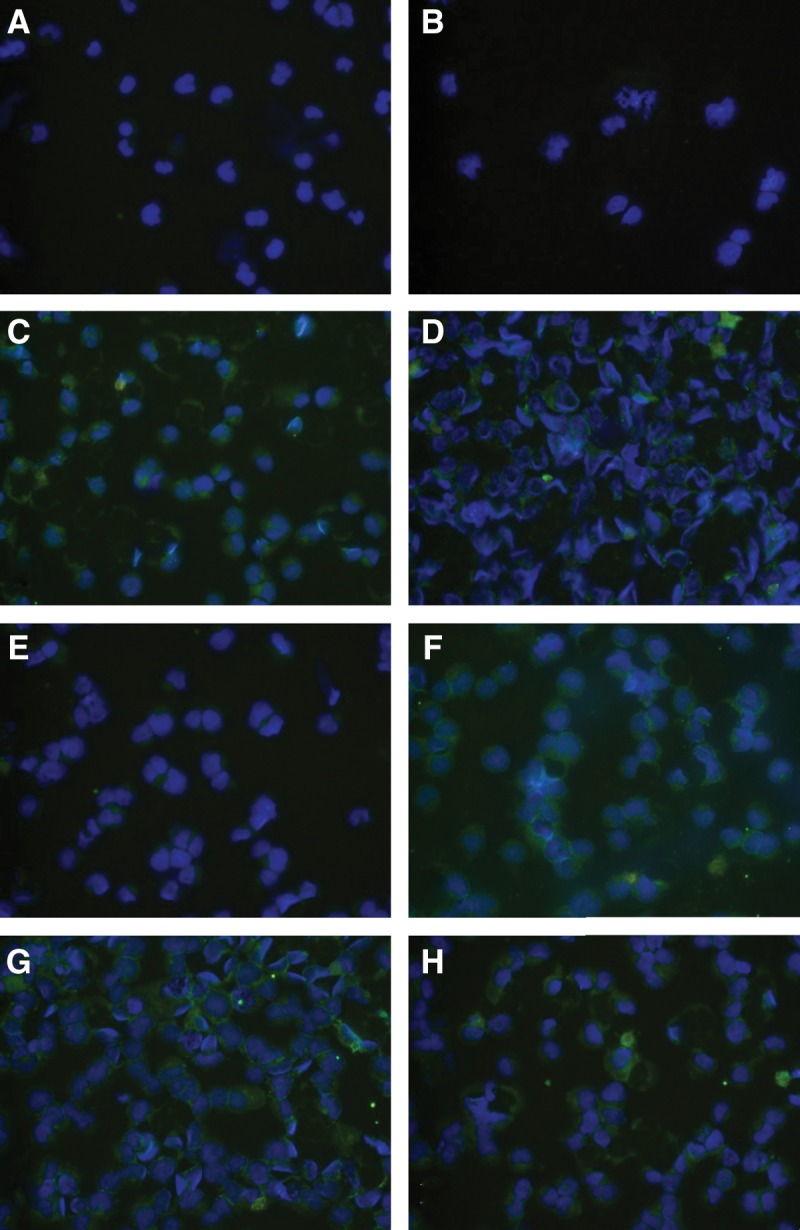
Immunocytochemical staining using anti-CD44v6 peptide antibodies on CD44v6 positive U937 cell line. (A) No antibody (negative control). (B) Irrelevant antibody (negative control). (C–H) Anti-CD44v6 antibody clones 1H1, 1H2, 2A12, 2G11, 3H3 and 3H7, respectively. DAPI was used for the nuclei staining and FITC-conjugated (green) secondary antibody for immunoflourescence staining (magnification, 200×).
Discussion
Previous studies have reported the involvement of CD44v6 in regulating invasion, progression, and metastasis of carcinomas and the useful of the monoclonal antibodies against this molecule in monitoring of certain cancers.(4,26–29) Anti-CD44v6 antibodies have also been found to have diagnostic applications in some malignancies such as head and neck squamous cell carcinoma (HNSCC)(30–32) and in noninvasive imaging of breast cancer.(33,34) Because of its high expression in HNSCC, CD44v6 has been targeted with a monoclonal antibody (U36) for diagnosis of this cancer in humans.(31,32) The humanized anti-CD44v6 monoclonal antibody has also been conjugated with the near-infrared fluorescent dye and was used for molecular imaging of ductal carcinoma in mice.(35)
Considering the importance of the CD44v6 monoclonal antibodies for diagnosis and management of cancer, we aimed to produce a panel of monoclonal antibodies against this molecule and to characterize them to possibly find new antibodies with useful detection and monitoring potentials. In this regard, we used a synthetic CD44v6 peptide and conjugated it with KLH as carrier protein for immunization. The use of synthetic peptide immunogens as tools for generating specific immunological reagents for a variety of purposes has increased markedly in recent years.(36) Using this approach, after fusion of myeloma cells with splenocytes from immunized mice, several hybridoma clones were obtained, of which six were reactive with CD44v6 peptide coupled to KLH. In order to characterize the antibodies their reaction with CD44v6 positive Mo7e and U937 cell lines and CD44v6 negative cell lines HL60, LnCap and Jurkat were investigated by flow cytometry method. All monoclonal antibodies reacted with CD44v6 positive cell lines Mo7e and U937 in flow cytometry. This would be an important characteristic of the antibodies since targeting of tumor cells with monoclonal antibodies for immunotherapy of cancer requires the reactivity of the antibodies with the native target protein on the tumor cell surface. In this context, our monoclonal antibodies seem to meet the preliminary requirement for use in immunotherapy of CD44 expressing tumors. In addition, our antibodies reacted with the CD44v6 positive U937 cell line in immunochemistry studies, which is also an important characteristic for these antibodies to make them suitable candidates for diagnosis of CD44v6 positive tumors. As in other studies, CD44v6 antibodies for immunotherapy and diagnosis of CD44 expressing tumors have been used.(37–39) None of the monoclonal antibodies were reactive with CD44v6 negative cell lines LnCap and Jurkat cells. We showed the lack of CD44v6 gene expression in these cell lines by RT-PCR, which support the production of highly specific monoclonal antibodies against CD44v6.
With respect to HL60 cells, these cells have been known as non-surface CD44v6 expressing cells.(23,40) As our results show, the monoclonal antibodies produced in the present study did not react with this cell line. However, in line with a previous study,(23) when the CD44v6 transcript was surveyed by RT-PCR using CD44v6-specific primers, a clear band of the right size was amplified. The discrepancy between protein and transcript expressions in this cell line might represent transcription of the gene but not continuation to translation to the protein. Additionally, one may argue that DNA contamination might result in amplification of the gene in PCR. This was ruled out in our experiments as we administered DNAase I treatment on all extracted RNA samples to avoid contaminating DNA amplification in RT PCR.
In conclusion, in this study six anti-CD44v6 monoclonal antibodies were produced. The antibodies demonstrated reactivity with CD44v6 positive cell lines, suggesting their potential as a diagnostic tool for CD44v6 positive tumors. Further studies are needed to determine the functional role of these antibodies and their usefulness in the immunotherapy of cancer.
Acknowledgment
This work was extracted from a thesis written by one of the authors (S.Z.) and supported by grants from Shiraz University of Medical Sciences (no. 5634) and Avicenna Research Institute (no. 90-009).
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Hertweck MK, Erdfelder F, and Kreuzer KA: CD44 in hematological neoplasias. Ann Hematol 2011;90:493–508 [DOI] [PubMed] [Google Scholar]
- 2.Davern SM, Lankford PK, Foote LJ, and Kennel SJ: Monoclonal antibodies to CD44 epitopes on mouse endothelium. Hybrid Hybridomics 2002;21:339–349 [DOI] [PubMed] [Google Scholar]
- 3.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, and Bell JI: Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA 1992;89:12160–12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, and Herrlich P: A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 1991;65:13–24 [DOI] [PubMed] [Google Scholar]
- 5.Coradini D, Zorzet S, Rossin R, Scarlata I, Pellizzaro C, Turrin C, Bello M, Cantoni S, Speranza A, Sava G, Mazzi U, and Perbellini A: Inhibition of hepatocellular carcinomas in vitro and hepatic metastases in vivo in mice by the histone deacetylase inhibitor HA-But. Clin Cancer Res 2004;10:4822–4830 [DOI] [PubMed] [Google Scholar]
- 6.Mulder JW, Kruyt PM, Sewnath M, Oosting J, Seldenrijk CA, Weidema WF, Offerhaus GJ, and Pals ST: Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet 1994;344:1470–1472 [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann M, Heider KH, Sinn HP, von Minckwitz G, Ponta H, and Herrlich P: CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet 1995;345:615–619 [DOI] [PubMed] [Google Scholar]
- 8.Stauder R, Eisterer W, Thaler J, and Gunthert U: CD44 variant isoforms in non-Hodgkin's lymphoma: a new independent prognostic factor. Blood 1995;85:2885–2899 [PubMed] [Google Scholar]
- 9.Hirata T, Fukuse T, Naiki H, Hitomi S, and Wada H: Expression of CD44 variant exon 6 in stage I non-small cell lung carcinoma as a prognostic factor. Cancer Res 1998;58:1108–1110 [PubMed] [Google Scholar]
- 10.Harn HJ, Ho LI, Shyu RY, Yuan JS, Lin FG, Young TH, Liu CA, Tang HS, and Lee WH: Soluble CD44 isoforms in serum as potential markers of metastatic gastric carcinoma. J Clin Gastroenterol 1996;22:107–110 [DOI] [PubMed] [Google Scholar]
- 11.Dong WG, Sun XM, Yu BP, Luo HS, and Yu JP: Role of VEGF and CD44v6 in differentiating benign from malignant ascites. World J Gastroenterol 2003;9:2596–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marhaba R, and Zoller M: CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol 2004;35:211–231 [DOI] [PubMed] [Google Scholar]
- 13.Kuhn S, Koch M, Nubel T, Ladwein M, Antolovic D, Klingbeil P, Hildebrand D, Moldenhauer G, Langbein L, Franke WW, Weitz J, and Zoller M: A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Mol Cancer Res 2007;5:553–567 [DOI] [PubMed] [Google Scholar]
- 14.Mahmoudian J, Jeddi-Tehrani M, Bayat AA, Mahmoudi AR, Vojgani Y, Tavangar B, Hadavi R, and Zarei S: A monoclonal antibody against leptin. Hybridoma 2012;31:372–377 [DOI] [PubMed] [Google Scholar]
- 15.Tabatabaei-Panah AS, Zarnani AH, Montaser-Kouhsari S, Chamankhah M, Ghods R, Bayat AA, Kazemi-Sefat GE, Mahmoudi SAR, Karampour MO, and Shojaeian S: Production and characterization of anti-Her2 monoclonal antibodies. Yakhteh 2008;10:109–120 [Google Scholar]
- 16.Hajighasemi F, Khoshnoodi J, and Shokri F: Development of two murine monoclonal antibodies recognizing human nG1m(a)-like isoallotypic markers. Hybridoma 2008;27:473–479 [DOI] [PubMed] [Google Scholar]
- 17.Kohler G, and Milstein C: Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol 1976;6:511–519 [DOI] [PubMed] [Google Scholar]
- 18.Shabani M, Asgarian-Omran H, Jeddi-Tehrani M, Vossough P, Faranoush M, Sharifian RA, Toughe GR, Kordmahin M, Khoshnoodi J, Roohi A, Tavoosi N, Mellstedt H, Rabbani H, and Shokri F: Overexpression of orphan receptor tyrosine kinase Ror1 as a putative tumor-associated antigen in Iranian patients with acute lymphoblastic leukemia. Tumour Biol 2007;28:318–326 [DOI] [PubMed] [Google Scholar]
- 19.Zhou G, Chiu D, Qin D, Niu L, Cai J, He L, Tan D, and Xu K: Expression of CD44v6 and integrin-beta1 for the prognosis evaluation of pancreatic cancer patients after cryosurgery. Diagn Pathol 2012;8:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazemi T, Tahmasebi F, Bayat AA, Mohajer N, Khoshnoodi J, Jeddi-Tehrani M, Rabbani H, and Shokri F: Characterization of novel murine monoclonal antibodies directed against the extracellular domain of human HER2 tyrosine kinase receptor. Hybridoma 2011;30:347–353 [DOI] [PubMed] [Google Scholar]
- 21.Verkaik NS, Trapman J, Romijn JC, Van der Kwast TH, and Van Steenbrugge GJ: Down-regulation of CD44 expression in human prostatic carcinoma cell lines is correlated with DNA hypermethylation. Int J Cancer 1999;80:439–443 [DOI] [PubMed] [Google Scholar]
- 22.Dougherty GJ, Landorp PM, Cooper DL, and Humphries RK: Molecular cloning of CD44R1 and CD44R2, two novel isoforms of the human CD44 lymphocyte "homing" receptor expressed by hemopoietic cells. J Exp Med 1991;174:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legras S, Gunthert U, Stauder R, Curt F, Oliferenko S, Kluin-Nelemans HC, Marie JP, Proctor S, Jasmin C, and Smadja-Joffe F: A strong expression of CD44–6v correlates with shorter survival of patients with acute myeloid leukemia. Blood 1998;91:3401–3413 [PubMed] [Google Scholar]
- 24.Zada AA, Singh SM, Reddy VA, Elsasser A, Meisel A, Haferlach T, Tenen DG, Hiddemann W, and Behre G: Downregulation of c-Jun expression and cell cycle regulatory molecules in acute myeloid leukemia cells upon CD44 ligation. Oncogene 2003;22:2296–2308 [DOI] [PubMed] [Google Scholar]
- 25.Walker J: Methods in molecular medicine. In: Molecular Diagnosis of Infectious Diseases, 2nd ed, Decker J. (Ed.). Humana Press, Totowa, NJ, 2004, pp. 260–262 [Google Scholar]
- 26.Sandstrom K, Nestor M, Ekberg T, Engstrom M, Anniko M, and Lundqvist H: Targeting CD44v6 expressed in head and neck squamous cell carcinoma: preclinical characterization of an 111In-labeled monoclonal antibody. Tumour Biol 2008;29:137–144 [DOI] [PubMed] [Google Scholar]
- 27.Stroomer JW, Roos JC, Sproll M, Quak JJ, Heider KH, Wilhelm BJ, Castelijns JA, Meyer R, Kwakkelstein MO, Snow GB, Adolf GR, and van Dongen GA: Safety and biodistribution of 99mTechnetium-labeled anti-CD44v6 monoclonal antibody BIWA 1 in head and neck cancer patients. Clin Cancer Res 2000;6:3046–3055 [PubMed] [Google Scholar]
- 28.Van Hal NL, Van Dongen GA, Ten Brink CB, Herron JN, Snow GB, and Brakenhoff RH: Sequence variation in the monoclonal-antibody-U36-defined CD44v6 epitope. Cancer Immunol Immunother 1997;45:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heider KH, Sproll M, Susani S, Patzelt E, Beaumier P, Ostermann E, Ahorn H, and Adolf GR: Characterization of a high-affinity monoclonal antibody specific for CD44v6 as candidate for immunotherapy of squamous cell carcinomas. Cancer Immunol Immunother 1996;43:245–253 [DOI] [PubMed] [Google Scholar]
- 30.Nestor M, Ekberg T, Dring J, van Dongen GA, Wester K, Tolmachev V, and Anniko M: Quantification of CD44v6 and EGFR expression in head and neck squamous cell carcinomas using a single-dose radioimmunoassay. Tumour Biol 2007;28:253–263 [DOI] [PubMed] [Google Scholar]
- 31.de Bree R, Roos JC, Quak JJ, den Hollander W, Snow GB, and van Dongen GA: Radioimmunoscintigraphy and biodistribution of technetium-99m-labeled monoclonal antibody U36 in patients with head and neck cancer. Clin Cancer Res 1995;1:591–598 [PubMed] [Google Scholar]
- 32.de Bree R, Roos JC, Plaizier MA, Quak JJ, van Kamp GJ, den Hollander W, Snow GB, and van Dongen GA: Selection of monoclonal antibody E48 IgG or U36 IgG for adjuvant radioimmunotherapy in head and neck cancer patients. Br J Cancer 1997;75:1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermeulen JF, van Brussel AS, van der Groep P, Morsink FH, Bult P, van der Wall E, and van Diest PJ: Immunophenotyping invasive breast cancer: paving the road for molecular imaging. BMC Cancer 2012;12:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koppe M, Schaijk F, Roos J, Leeuwen P, Heider KH, Kuthan H, and Bleichrodt R: Safety, pharmacokinetics, immunogenicity, and biodistribution of (186)Re-labeled humanized monoclonal antibody BIWA 4 (Bivatuzumab) in patients with early-stage breast cancer. Cancer Biother Radiopharm 2004;19:720–729 [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen JF, van Brussel AS, Adams A, Mali WP, van der Wall E, van Diest PJ, and Derksen PW: Near-infrared fluorescence molecular imaging of ductal carcinoma in situ with CD44v6-specific antibodies in mice: a preclinical study. Mol Imaging Biol 2013;15:290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsurumi Y, Hayakawa M, Shibata Y, and Abiko Y: Production of antibody against a synthetic peptide of Porphyromonas gingivalis 40-kDa outer membrane protein. J Oral Sci 2003;45:111–116 [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Huang K, Li X, Lin X, Zhu Z, and and Wu Y: Generation of a stable anti-human CD44v6 scFv and analysis of its cancer-targeting ability in vitro. Cancer Immunol Immunother 2010;59:933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng J, Persson M, Tolmachev V, Siavaev I, Orlova A, Kairemo K, and Anniko M: Targeting of a head and neck squamous cell carcinoma xenograft model using the chimeric monoclonal antibody U36 radioiodinated with a closo-dodecaborate-containing linker. Acta Otolaryngol 2004;124:1078–1085 [DOI] [PubMed] [Google Scholar]
- 39.Nestor M, Sundstrom M, Anniko M, and Tolmachev V: Effect of cetuximab in combination with alpha-radioimmunotherapy in cultured squamous cell carcinomas. Nucl Med Biol 2011;38:103–112 [DOI] [PubMed] [Google Scholar]
- 40.Moll J, Khaldoyanidi S, Sleeman JP, Achtnich M, Preuss I, Ponta H, and Herrlich P: Two different functions for CD44 proteins in human myelopoiesis. J Clin Invest 1998;102:1024–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]