Abstract
Background
Current approaches to the management of type 2 diabetes focus on the early initiation of novel pharmacologic therapies and bariatric surgery.
Objective
The purpose of this study was to revisit the use of intensive, outpatient, behavioral weight management programs for the management of type 2 diabetes.
Design
Prospective observational study of 66 patients with type 2 diabetes and BMI ≥ 32 kg/m2 who enrolled in a program designed to produce 15% weight reduction over 12 weeks using total meal replacement and low- to moderate-intensity physical activity.
Results
Patients were 53 ± 7 years of age (mean ± SD) and 53% were men. After 12 weeks, BMI fell from 40.1 ± 6.6 to 35.1 ± 6.5 kg/m2. HbA1c fell from 7.4 ± 1.3% to 6.5 ± 1.2% (57.4 ± 12.3 to 47.7 ± 12.9 mmol/mol) in patients with established diabetes: 76% of patients with established diabetes and 100% of patients with newly diagnosed diabetes achieved HbA1c <7.0% (53.0 mmol/mol). Improvement in HbA1c over 12 weeks was associated with higher baseline HbA1c and greater reduction in BMI.
Conclusions
An intensive, outpatient, behavioral weight management program significantly improved HbA1c in patients with type 2 diabetes over 12 weeks. The use of such programs should be encouraged among obese patients with type 2 diabetes.
Introduction
In response to the evidence that near-normal glycemia can reduce the long-term complications of type 2 diabetes and the approval of new classes of antihyperglycemic medications, consensus guidelines have been published for the management of type 2 diabetes. Although essentially all of them recognize the role of overnutrition, physical inactivity, and obesity in the pathogenesis of type 2 diabetes, few, if any, recommend lifestyle intervention as first line therapy for its management. Indeed, in 2008, the American Diabetes Association and the European Association for the Study of Diabetes concluded that because lifestyle interventions often fail to achieve or maintain glycemic goals either because of failure to lose weight, weight regain, progressive disease, or a combination of factors, metformin therapy should be initiated at the diagnosis of type 2 diabetes (1). More recently, Roux-en-Y gastric bypass (RYGB), now termed “metabolic surgery”, has been proposed as first line therapy for obese patients (body mass index (BMI) >30 kg/m2) with type 2 diabetes (2,3). Although a recent trial demonstrated that 12 months of medical therapy plus bariatric surgery achieved better glycemic outcomes than medical therapy alone in obese patients with uncontrolled type 2 diabetes (4), another small, short-term, clinical trial suggested that a very-low-energy diet, similar to those consumed by patients following RYGB, can produce similar improvements in glycemia, beta-cell function, and insulin sensitivity as RYGB (5).
The purpose of this study was to determine if 12 weeks of a very-low-energy diet combined with physical activity of low- to moderate-intensity was effective for the management of type 2 diabetes. Our hypothesis was that although traditional low intensity lifestyle interventions may fail to achieve glycemic goals, more intensive, outpatient, multidisciplinary, behavioral obesity management programs may be an appropriate first-line treatment for type 2 diabetes.
Methods
The University of Michigan Investigational Weight Management Program is a 2-year, outpatient, multidisciplinary, behavioral, obesity management program. The program is offered to obese members of a managed care health plan who are required to participate in one of three weight management programs in order to receive enhanced benefits (6) and to patients referred by University of Michigan-affiliated health care providers. To be eligible, patients must have BMI >32 kg/m2 with a diagnosis of type 2 diabetes or BMI >35 kg/m2. The study was reviewed and approved by the University of Michigan Institutional Review Board and all patients provided written informed consent.
Patients are seen by an endocrinologist for an initial assessment, once in the first month, and quarterly thereafter. At the first visit, antihyperglycemic medication regimens are reviewed and modified. Sulfonylureas and thiazolidinediones are tapered and discontinued. When necessary, weight neutral or weight negative medications are substituted for weight potentiating medications. If patients are taking fewer than 15 units of insulin per day, insulin is discontinued. For patients taking ≥ 15 units of insulin per day, short-acting insulin analogues are discontinued and the dose of long-acting insulin analogues are reduced by 50%. GLP-1 agonists were substituted for insulin in 3 patients who discontinued insulin. Metformin is discontinued only if the HbA1c is ≤ 6.5% (47.5 mmol/mol) and the patient requests that metformin be discontinued.
Patients are seen by a dietitian weekly for the first month, every other week for the next 2 months, and monthly thereafter. The initial focus of the program is on 15% weight reduction over 12 weeks using intensive energy restriction. Most patients receive intensive caloric restriction (800 kcal/day) in the form of total meal replacement (HMR®, Boston, MA) employing chocolate or vanilla shakes or chicken soup (160–170 kcal/per packet). Additional calories are prescribed for those who weigh more than 160 kg (an additional 160–170 kcal/day for every 23 kg over 160 kg). Patients are asked to gradually increase their physical activity (low to moderate intensity) to 40 minutes per day (either in divided bouts or all at once) over the first 12 weeks. Patients are asked to keep diaries listing the number of shakes they consume, deviations from the prescribed diet, hunger/satiety, and physical activity. Diaries are reviewed weekly with the dietitian. After approximately 12 weeks, patients continue to receive intensive behavioral counseling, are transitioned to regular foodstuffs, and are asked to perform 40 to 90 minutes of moderate to vigorous physical activity per day for weight maintenance.
Glucose and HbA1c were measured by the Chemistry Laboratory of the Michigan Diabetes Research Center. Glucose assays were performed using a Cobas Mira Chemistry Analyzer (Roche Diagnostics Corporation, Indianapolis, IN). Intra-assay coefficients of variation are 2% at 84 and 283 mg/dl (4.7 and 15.7 mmol/l). Inter-assay coefficients of variation are 3.6% at 92 mg/dl (5.1 mmol/l) and 2.8% at 310 mg/dl (17.2 mmol/l). HbA1c was measured using a Tosoh G7 HPLC Analyzer (Tosoh Biosciences Inc, South San Francisco, CA). The method uses a non-porous ion exchange column and a high performance liquid chromatography system. At an HbA1c of 5.8% (39.9 mmol/mol), the day-to-day coefficient of variation of the assay is 1.7% and at an HbA1c of 9.7% (82.5 mmol/mol), it is 1.6%.
The demographic and clinical characteristics of the study population were described using means ± standard deviation (SD) or number (%). The demographic and clinical characteristics of patients included in the study vs. those excluded from the study and those with established diabetes vs. those with newly diagnosed diabetes were compared using t-tests or chi-square tests. BMI, weight, and measures of glycemia at baseline and follow-up were described using means ± standard deviation or number (%) and then compared using paired t-tests. To examine the demographic and clinical factors associated with a change in HbA1c between baseline and follow-up, we used analysis of variance. We used stepwise regression with a p-value to enter the model set at 0.15 and a p-value to stay in the model set at 0.05 to further examine factors associated with change in HbA1c. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Results
Between 2010 and 2013, 367 patients enrolled in the Investigational Weight Management Program and completed at least 12 weeks of follow-up. Of these, 93 (25%) were diagnosed with type 2 diabetes. Sixty-six (71%) had baseline HbA1c or blood glucose levels and HbA1c levels measured 3 to 6 months after baseline and are the focus of this study. Twenty-seven (29%) were missing baseline or follow-up measures of glycemia and were excluded from the study. Those excluded did not differ from those included with respect to age, sex, race/ethnicity, baseline or follow-up BMI. They did, however, have slightly shorter durations of diabetes (3.4 ± 5.0 years) compared to those included in the study (6.2 ± 6.1 years) (p=0.03).
Of the 66 patients included in this study, 58 had established diabetes and had baseline and follow-up measures of HbA1c. Eight were newly diagnosed with diabetes based on standard 75 gram oral glucose tolerance tests. They did not have baseline measurements of HbA1c but had follow-up measures of HbA1c. Table 1 shows the characteristics of the study population including the total population (n=66), those with established diabetes (n=58), and those with newly diagnosed diabetes (n=8). In general, the population was middle-aged, white, and well educated. There were approximately equal numbers of men and women. Very few patients reported smoking. Mean duration of diabetes was relatively short.
Table 1.
Characteristics of the study population
| People with baseline HbA1c or OGTT and follow-up HbA1c (n=66) | Established diabetes (n=58) | Newly diagnosed diabetes (n=8) | p-value (established vs. newly diagnosed diabetes) | |
|---|---|---|---|---|
| Age (years) | 53 ± 7 | 53 ± 7 | 53 ± 8 | 0.8268 |
| Sex | 0.4591 | |||
| Male | 35 (53%) | 32 (55%) | 3 (38%) | |
| Female | 31 (47%) | 26 (45%) | 5 (63%) | |
| Race/ethnicity | 1.00 | |||
| Non-Hispanic White | 60 (91%) | 52 (90%) | 8 (100%) | |
| Other (Hispanic, Black, or Asian) | 6 (9%) | 6 (10%) | 0 | |
| Education (missing 4) | 0.7952 | |||
| <College degree | 20 (31%) | 17 (29%) | 3 (38%) | |
| College degree | 21 (32%) | 18 (32%) | 3 (38%) | |
| Professional or graduate degree | 24 (37%) | 22 (39%) | 2 (25%) | |
| Smoking | 1.00 | |||
| Current smoker | 2 (3%) | 2 (3%) | 0 | |
| Never smoker/ex-smoker | 90 (97%) | 56 (97%) | 8 (100%) | |
| Duration of diabetes (years) (missing 4) | 6.2 ± 6.1 | 7.1 ± 6.0 | 0.2 ± 0.2 | 0.0020 |
Table 2 shows baseline and follow-up BMI, weight, and HbA1c levels. Baseline BMI was 40.1 ± 6.6 kg/m2. Patients newly diagnosed with diabetes tended to have higher BMIs than those with established diabetes. For those with established diabetes, baseline HbA1c was 7.4 ± 1.3% (57.4 ± 12.3 mmol/mol). Sixty percent of patients with established diabetes had baseline HbA1c >7.0% (53.0 mmol/mol). For patients with newly diagnosed diabetes, mean fasting glucose was 117 ± 15 mg/dl (6.5 ± 0.8 mmol/l) and 2-hour post glucose load glucose was 220 ± 31 mg/dl (12.2 ± 1.7 mmol/l). At baseline, 93% of patients with established diabetes and none of the patients with newly diagnosed diabetes were taking antihyperglycemic medications.
Table 2.
Baseline and follow-up BMI, weight, and measures of glycemia
| Diabetic patients with baseline HbA1c/OGTT and follow-up HbA1c (n=66) | Established diabetes (n=58) | Newly diagnosed diabetes (n=8) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | p-value | Baseline | Follow-up | p-value | Baseline | Follow-up | p-value | |
| BMI (kg/m2) | 40.1 ± 6.6 | 35.1 ± 6.5 | <0.0001 | 39.8 ± 6.6 | 34.9 ± 6.4 | <0.0001 | 42.1 ± 6.7 | 36.6 ± 7.1 | <0.0001 |
| Weight (kg) | 117 ± 23 | 102 ± 21 | <0.0001 | 117 ± 23 | 103 ± 22 | <0.0001 | 118 ± 23 | 102 ± 20 | 0.0003 |
| HbA1c (%)(mmol/mol) | — | 6.4 ± 1.1 46.6 ± 12.5 |
— | 7.4 ± 1.3 57.4 ± 12.3 |
6.5 ± 1.2 47.7 ± 12.9 |
<0.0001 | — | 5.7 ± 0.4 38.3 ± 4.3 |
— |
| HbA1c categories | |||||||||
| ≥7.0% (≥53.0 mmol/mol) | — | 14 (21%) | — | 35 (60%) | 14 (24%) | — | 0 (0%) | — | |
| <7.0% (<53.0 mmol/mol) | — | 52 (79%) | — | 23 (40%) | 44 (76%) | — | — | 8 (100%) | — |
| <6.5% (<47.5 mmol/mol) | — | 44 (67%) | — | 15 (26%) | 36 (62%) | — | — | 8 (100%) | — |
| <6.0% (<42.1 mmol/mol) | — | 28 (42%) | — | 5 (9%) | 21 (36%) | — | — | 7 (88%) | — |
| Fasting glucose (mg/dl) (mmol/l) | — | — | — | — | — | — | 117 ± 15 6.5 ± 0.8 |
— | — |
| 2hr glucose (mg/dl) (mmol/l) | — | — | — | — | — | — | 220 ± 31 12.2 ± 1.7 |
— | — |
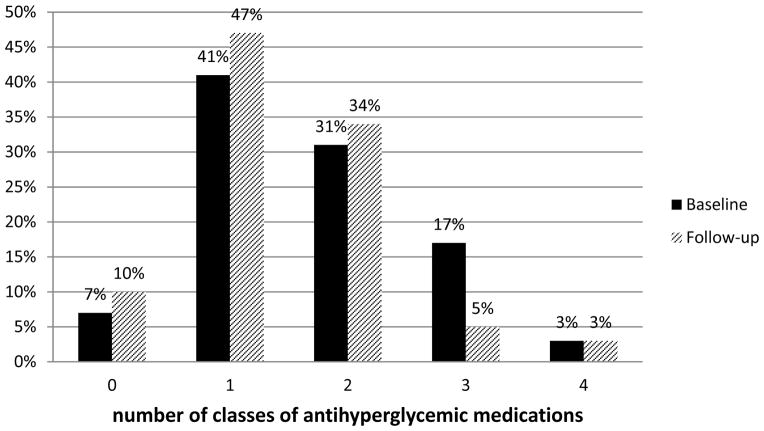
At follow-up, mean BMI was 35.1 ± 6.5 kg/m2, a decrease of 5.0 kg/m2, and mean weight was 102 ± 21 kg, a decrease of 15 kg. Patients with newly diagnosed diabetes tended to have greater reductions in BMI than patients with established diabetes. At follow-up, mean HbA1c for the entire cohort was 6.4 ± 1.1% (46.6 ± 12.5 mmol/mol) with significantly lower HbA1c in patients with newly diagnosed diabetes (5.7 ± 0.4% or 38.3 ± 4.3 mmol/mol) compared to those with established diabetes (6.5 ± 1.2% or 47.7 ± 12.9 mmol/mol) (p=0.0002). At follow-up, only 21% of patients had HbA1c ±7.0% (53.0 mmol/mol). One hundred percent of patients with newly diagnosed diabetes and 76% of patients with established diabetes had HbA1c <7.0% (53.0 mmol/mol), and 100% of patients with newly diagnosed diabetes and 62% of those with established diabetes had HbA1c <6.5% (47.5 mmol/mol). Figure 1 shows the distribution of HbA1c levels at baseline and follow-up. At follow-up, six of 8 patients with newly diagnosed diabetes were treated with metformin. Fewer patients with established diabetes were treated with 3 or 4 classes of antihyperglycemic medications and more were treated with 0, 1, or 2 classes of antihyperglycemic medications (Figure 2).
Figure 1.
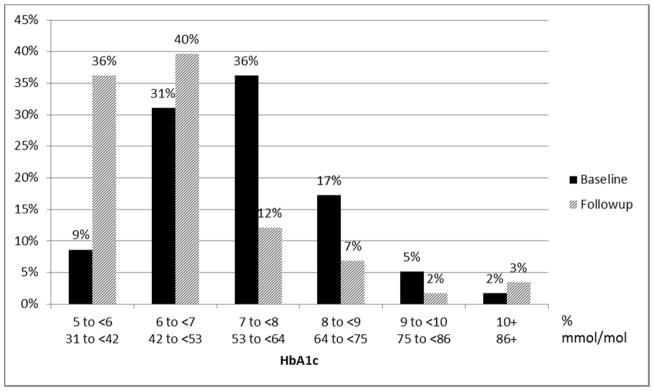
Percent distribution of HbA1c levels at baseline and follow-up for patients with established diabetes (n=58)
Figure 2.
Percent distribution of numbers of classes of antihyperglycemic medications at baseline and follow-up for patients with established diabetes (n=58)
Table 3 shows the demographic and clinical factors associated with change in HbA1c among patients with established diabetes. Age, sex, education, and duration of diabetes were not associated with change in HbA1c from baseline to follow-up. The improvement in HbA1c tended to be greater for Hispanics, Blacks or Asians compared to whites, and for those with higher baseline BMIs. Change in HbA1c was strongly associated with baseline HbA1c. Patients with the lowest HbA1c at baseline had the least improvement and patients with the highest HbA1c at baseline had the greatest improvement in HbA1c at follow-up (p=0.0147).
Table 3.
Demographic and clinical factors associated with change in HbA1c among patients with established diabetes (n=58)
| N | Change in HbA1c | p-value | |
|---|---|---|---|
| Age (years) | 0.2910 | ||
| <52 | 19 | −0.8 | |
| 52–57 | 22 | −1.1 | |
| ≥58 | 17 | −0.5 | |
| Sex | 0.9817 | ||
| Male | 32 | −0.8 | |
| Female | 26 | −0.8 | |
| Race/ethnicity | 0.2036 | ||
| NonHispanic White | 52 | −0.7 | |
| Other (Hispanic, Black, or Asian) | 6 | −1.3 | |
| Education (missing=1) | 0.3774 | ||
| <College | 17 | −1.1 | |
| College degree | 18 | −0.6 | |
| Professional or graduate degree | 22 | −0.7 | |
| Duration of diabetes (years) | 0.7021 | ||
| <3 | 20 | −1.0 | |
| 3–7 | 18 | −0.8 | |
| ≥8 | 20 | −0.7 | |
| Baseline BMI (kg/m2) | 0.2607 | ||
| <36 | 19 | −0.5 | |
| 36–40 | 19 | −0.8 | |
| ≥41 | 20 | −1.1 | |
| Baseline HbA1c | 0.0147 | ||
| <6.8% <50.8 mmol/mol |
18 | −0.3 −26.5 |
|
| 6.8–7.6% | 20 | −0.8 | |
| 50.8–59.6 mmol/mol | −32.3 | ||
| ≥7.7% ≥60.7 mmol/mol |
20 | −1.3 −37.4 |
Table 4 shows the classes of antihyperglycemic medication taken by patients with established diabetes at baseline and follow-up. Most patients (43/58, 74%) were taking metformin at baseline and 40 of them (93%) continued to take metformin follow-up. Six patients began taking metformin and 3 patients discontinued taking metformin between baseline and follow-up. At baseline, one-half of patients (29/58) were taking oral antihyperglycemic medications other than metformin (28 of 29 were taking sulfonylureas or thiazoladinediones). Most such patients (17/29, 59%) discontinued taking them between baseline and follow-up. Twelve of 58 patients (21%) were taking glucagon-like peptide-1 (GLP-1) agonists at baseline and 10 of them (83%) continued to take them at follow-up. Two patients discontinued GLP-1 agonists and three patients began GLP-1 agonists between baseline and follow-up. Fourteen of 58 patients (24%) took insulin at baseline. Two patients discontinued taking insulin between baseline and follow-up and none began taking insulin.
Table 4.
Changes in classes of antihyperglycemic medications used by patients with established diabetes at baseline and follow-up (n=58)
| Metformin | Baseline | Follow-up | N |
|---|---|---|---|
| Yes | No | 3 | |
| No | No | 9 | |
| No | Yes | 6 | |
| Yes | Yes | 40 | |
| Other oral antihyperglycemic medications* | |||
| Yes | No | 17 | |
| No | No | 28 | |
| No | Yes | 1 | |
| Yes | Yes | 12 | |
| Insulin | |||
| Yes | No | 2 | |
| No | No | 44 | |
| No | Yes | 0 | |
| Yes | Yes | 12 | |
| GLP-1 agonists | |||
| Yes | No | 2 | |
| No | No | 43 | |
| No | Yes | 3 | |
| Yes | Yes | 10 |
includes sulfonylureas and thiazoladinediones
To further examine factors associated with reduction in HbA1c between baseline and follow-up in patients with established diabetes, we performed stepwise regression to explore age, sex, race/ethnicity, education, duration of diabetes, baseline BMI, change in BMI, baseline HbA1c, and classes of medications used at baseline and follow-up as explanatory variables. Factors significantly associated with change in HbA1c between baseline and follow-up were change in body mass index, baseline HbA1c, GLP-1 agonist initiation, and insulin treatment at baseline and follow-up. Each one unit reduction in BMI was associated with a 0.24% (2.66 mmol/mol) absolute reduction in HbA1c at follow-up (p<0.0001). Each one percent (1 mmol/mol) higher baseline HbA1c was associated with a 0.41% (0.41 mmol/mol) absolute reduction in HbA1c at follow-up (p<0.0001). Initiating a GLP-1 agonist between baseline and follow-up, compared to not taking a GLP-1 agonist at baseline or follow-up, was associated with a 1.29% (14.13 mmol/mol) reduction in HbA1c at follow-up (p=0.0084). Taking insulin at both baseline and follow-up, compared to not taking insulin at baseline or follow-up, was associated with a 0.90% (9.83 mmol/mol) higher HbA1c at follow-up (p=0.0010). The model explained 54% of the variance in HbA1c.
Discussion
With the focus on metformin as first line therapy for type 2 diabetes, the promotion of new classes of oral antihyperglycemic medications, and the near-universal enthusiasm for bariatric or “metabolic” surgery for the treatment for type 2 diabetes, the role of intensive, outpatient, behavioral weight management programs for the management of type 2 diabetes has been largely overlooked. Others have previously demonstrated that 12 weeks of very-low-energy diet improves insulin secretion, insulin sensitivity, and HbA1c in subjects with type 2 diabetes (7) and that 20 weeks of intermittent very-low-energy diet in subjects with type 2 diabetes improves weight loss and glycemic control more than moderate caloric restriction alone (8). In this study, we demonstrate that patients with type 2 diabetes who met eligibility criteria for bariatric surgery but instead elected to enroll in an intensive, outpatient, behavioral weight management program that employed a very-low-energy diet in the form of meal replacement and low-to moderate-intensity physical activity decreased BMI by 5 kg/m2 over 12 weeks, reduced the use of antihyperglycemic medications, and improved glycemic control. Among patients with established diabetes, HbA1c fell from 7.4% ± 1.3% (57.4 ± 12.3 mmol/mol) at baseline to 6.5% ± 1.2% (47.7 ± 12.9 mmol/mol) at follow-up: 62% of patients with established diabetes and all patients with newly diagnosed diabetes had HbA1c <6.5% (47.5 mmol/mol) at 12 weeks follow-up.
Patients with newly diagnosed diabetes and racial and ethnic minority groups tended to have greater reductions in HbA1c. In contrast to what has been seen with bariatric surgery (9,10), we found that among patients with established diabetes, duration of diabetes was not associated with improvement in HbA1c, although it should be noted that mean duration of diabetes was relatively short (7.1 ± 6.0 years). In multivariate analysis, factors significantly associated with improvement in HbA1c were change in body mass index, higher baseline HbA1c, and GLP-1 agonist initiation. Although initiation of GLP-1 agonists was associated with a dramatic reduction in HbA1c, it should be noted that only 3 patients initiated such therapy. Their baseline HbA1c levels were high (8.6 ± 1.1% or 70.5 ± 11.8 mmol/mol) and all 3 had discontinued insulin therapy. Patients treated with insulin at both baseline and follow-up had smaller improvements in HbA1c than those not treated with insulin at baseline or follow-up, perhaps related to their longer durations of diabetes and reduced beta-cell function or the appetite stimulation or anabolic effects associated with insulin therapy.
The major limitations of our study were that it was observational and of short duration. All patients elected to participate in the program. Patients were not randomized to the intensive weight management program or usual care. Outcomes would likely have been less favorable among less motivated patients (6). Because physicians followed usual clinical practice guidelines and initiated metformin for all patients with type 2 diabetes, antihyperglycemic medication use was likely more frequent than it would have been if there were formal protocols to test medication discontinuation. Finally, we present only short-term results. Additional follow-up is planned and is needed to determine the longer-term benefits of this treatment.
In summary, we have demonstrated that 12 weeks of very-low-energy diet and counseling in low- to moderate-intensity physical activity delivered as part of an intensive, outpatient, behavioral weight management program was associated with substantial short-term weight loss, reduced need for pharmacologic therapy, and dramatic improvement in HbA1c. The role of such therapy should be revisited in the management of patients with both newly diagnosed and established type 2 diabetes.
Acknowledgments
Additional support was provided by the A. Alfred Taubman Medical Institute and the Robert C. and Veronica Atkins Foundation.
Support: The work was supported by the Michigan Nutrition and Obesity Research Center (Grant Number DK089503), the Michigan Center for Diabetes Translational Research (Grant Number P30DK092926), and the Chemistry Laboratory of the Michigan Diabetes Research Center (Grant Number P30DK020572) from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Health Management Resources (HMR), Inc. (Boston, MA) donated Healthy Solutions meal replacement products used in this study.
Abbreviations
- BMI
Body Mass Index
- SD
Standard deviation
- HbA1c
Hemoglobin A1c
- RYGB
Roux-en-Y gastric bypass
- GLP-1
Glucagon-like peptide 1
Footnotes
Reprints: Reprints will not be available from the author.
Clinical Trial Registry: NCT02043457
AER has received consulting fees from NovoNordisk and WHH has received consulting fees from sanofi. LNM, ATK, and CEF have no potential conflicts of interest to report.
Authors’ contributions to manuscript
AER designed research; AER, ATK, and CEF conducted research; LNM and WHH analyzed data or performed statistical analysis; AER, LNM, and WHH wrote the paper; and AER had primary responsibility for final content. All authors read and approved the final manuscript.
References
- 1.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy. Diabetologia. 2009;52:17–30. doi: 10.1007/s00125-008-1157-y. [DOI] [PubMed] [Google Scholar]
- 2.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. discussion 350–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35:1420–1428. doi: 10.2337/dc11-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, McMahon DJ, Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell function in type 2 diabetic patients. Diabetes. 2013;62:3027–3032. doi: 10.2337/db12-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothberg AE, McEwen LN, Fraser T, Burant CF, Herman WH. The impact of a managed care obesity intervention on clinical outcomes and costs: A prospective observational study. Obesity. 2013;21:2157–2162. doi: 10.1002/oby.20597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahleova H, Mari A, Nofrate V, Matoulek M, Kazdova L, Hill M, Pelikanova T. Improvement in β-cell function after diet-induced weight loss is associated with decrease in pancreatic polypeptide in subjects with type 2 diabetes. J Diabetes Complications. 2012;26:442–449. doi: 10.1016/j.jdiacomp.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Williams KV, Mullen ML, Kelley DE, Wing RR. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care. 1998;21:2–8. doi: 10.2337/diacare.21.1.2. [DOI] [PubMed] [Google Scholar]
- 9.Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, Kennedy L, Kashyap SR, Kirwan JP, Rogula T, Kroh M, Chand B, Schauer PR. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258:628–637. doi: 10.1097/SLA.0b013e3182a5034b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogrady B. Bariatric surgery benefits in type 2 diabetes linked to diabetes duration. Family Practice News Digital Network; [Accessed January 8, 2014]. http://www.familypracticenews.com. [Google Scholar]