Abstract
Since we last addressed the roles of NF-κB and JAK/STAT3 signaling in glioblastoma (GBM) five years ago, tremendous strides have been made in the understanding of these two pathways in glioma biology. Contributing to prosurvival mechanisms, cancer stem cell maintenance, and treatment resistance, both NF-κB and STAT3 have been characterized as major drivers of GBM. In this review, we address general improvements in the molecular understanding of GBM, the structure of NF-κB and STAT3 signaling, the ways in which these pathways contribute to GBM, and advances in preclinical and clinical targeting of these two signaling cascades.
Keywords: NF-κB, STAT3, glioblastoma, neurotherapeutics, signaling
Glioblastoma
Glioblastoma (GBM) is the commonest and deadliest malignant brain tumor [1]. Approximately 17,000 cases of malignant glioma are diagnosed annually in the U.S., and GBM accounts for over 80% of these [2]. Even with standard of care, median survival time is a dismal 12–15 months [3]. Historically, gliomas have been classified histologically, from World Health Organization Grades I–IV. Grades III and IV - encompassing anaplastic astrocytoma and GBM, respectively - are considered malignant. GBM is distinguished from lower grade gliomas by the presence of angiogenesis and/or necrosis, in addition to shared characteristics such as increased mitotic rates [4].
Standard first-line treatment for GBM is currently maximal surgical resection followed by concurrent radiation therapy and temozolomide (TMZ, an alkylating agent), followed by an adjuvant course of TMZ [5]. Options for therapy upon recurrence are currently manifold but dubiously beneficial. Surgical resection, TMZ rechallenge, and other traditional chemotherapies may be used under certain circumstances [5]. Another common treatment for recurrent GBM is bevacizumab, an anti-VEGF monoclonal antibody. Bevacizumab, however, has not been shown to improve survival for patients with GBM in either first-line or salvage settings [5–7], and its effects on the tumor vasculature can give false impressions of response, thus complicating radiological evaluation of GBM [8].
Despite the increase in life expectancy associated with maximal treatment, current therapy is considered palliative, and GBM is an essentially incurable disease [9]. Poor survival in cases of GBM has many roots in its biology and epidemiology. Complete surgical resection is impossible due to its diffuse nature [10]. Recovery from ablative treatment is stymied by the brain’s extremely limited capacity to regenerate [11]. Advances in chemotherapy are hindered because of the extra hurdle the blood-brain-barrier poses to delivery of a drug to its intended target [11], and GBM tumors invariably become resistant to any therapy that manages to be sufficiently and correctly delivered [12]. Finally, median age of presentation for GBM is high at 64 years of age [13], and older patients tend to tolerate radiation poorly [4], meaning that radiotherapy’s benefits are less striking than in younger patients [14]. In such cases, radiotherapy regimens may need to be shortened [15] or foregone completely [16]. For these reasons and several others, GBM remains an extremely challenging disease clinically despite intensive efforts to improve treatment.
Molecular Characterization and Classification
Because of the difficulties in treating GBM, numerous efforts have been made not only to develop new treatments for the disease, but also to refine the understanding of the basic biology of this devastating cancer. One area of particularly impressive progress is the molecular classification of GBM. Prominently, The Cancer Genome Atlas Project (TCGA) sought to improve the molecular landscape of cancer, first for GBM and then later for a panoply of other cancer types. Molecular - primarily transcriptional - classification has seen the emergence of four molecular subclasses of GBM (Table I). The defining feature of the Proneural subtype is amplification and/or high gene expression of PDGFRA [17]. Another major feature of Proneural GBM is IDH1 point mutations [17]. Almost 80% of IDH1-mutated GBM are associated with the glioma CpG island methylation phenotype (G-CIMP), which is itself associated with significantly improved prognosis and the Proneural subtype [18]. In fact, introduction of IDH1 mutations to primary human astrocytes is sufficient to induce an epigenomic profile mirroring G-CIMP, indicating that IDH1 mutations may actually be mechanistically responsible for G-CIMP [19]. Proneural GBM is distinct for its superior survival data in comparison to the other GBM subtypes. However, this improved survival may be largely or wholly due to the strong survival data for G-CIMP tumors; indeed, so clear is the difference between G-CIMP and non-G-CIMP Proneural GBM that recent analyses have proposed a Proneural “superfamily” dichotomized by methylation status [20].
Table I.
Subtypes of GBM and their Characteristics.
| Mesenchymal | Proneural | Classical | Neural | |
|---|---|---|---|---|
| Clinical Aspects | Worst prognosis *Treatment responsive |
Best prognosis *Treatment unresponsive Youngest patients |
*Treatment responsive | |
| Histology | Fetal astrocyte-like High necrosis/angiogenesis Evident epithelial-to-mesenchymal transition |
Neural stem cell- and oligodendrocyte-like Highest stem cell population Similar to secondary GBM and lower grade glioma |
Astrocyte-like | Neuron- and astrocyte-like Most similar to normal brain |
| Defining Molecular Features | Loss of NF1 and PTEN High angiogenic, astrocytic and mesenchymal markers High NF-κB activity High STAT3 activity |
High PDGFRA IDH1 and TP53 mutation/loss High oligodendrocytic and proneural genes High SOX and FGF families |
Frequent gain of chromsome 7 and loss of 10 High EGFR and FGFR3 activities CDKN2A loss High Notch and SHH |
High neuronal markers (FBXO3, GABRA1, GABRB2, SLC12A5, SYT1) |
| Differentially Overexpressed JAK/STAT & NF-κB Genes | ANGPTL4, IL1R1, IL4R, IL15RA, LIF, OSMR, RELB, SOCS3, STAT3/6, TRADD, TLR2/4, TNFAIP, TNFRSF1A/1B/10D/11A | IL17D | IRF3, SOCS2 |
Herein, treatment is defined as intensive treatment consisting of either concurrent radiation and chemotherapy, or more than three rounds of chemotherapy.
Another well-defined subtype is the Mesenchymal subtype. One of the defining features of this subtype is NF1 deletion and/or underexpression, particularly when in combination with PTEN loss. Overexpression of mesenchymal markers (YKL40, MET), astrocytic markers (CD44), and VEGF are prominent in the Mesenchymal subtype. Inflammatory signaling such as NF-κB and JAK/STAT3 signaling (discussed below) have also been demonstrated to be central to Mesenchymal GBM [17, 21, 22]. Overall, the genomic and transcriptomic profile of Mesenchymal GBM is suggestive of the epithelial-to-mesenchymal transition, hence the subtype’s name [17].
Classical GBM is defined primarily by enrichment for chromosome 7 amplification and chromosome 10 loss. EGFR amplification, homozygous deletion of CDKN2A, and overexpression of Notch and Sonic Hedgehog genes are also quite prominent in the Classical subtype [17]. The Neural subtype is mostly distinguished by high expression of neuronal markers like GABRA1 and SYT1 [17].
These molecular characterizations of GBM have been hugely exciting and promising for development of targeted therapy; however, recent studies have tempered the enthusiasm surrounding this paradigm. Sottoriva et al., recently demonstrated that genomic and transcriptomic analyses of multiple samples from the same tumor reveal distinct subclasses intratumorally [23]. Patel et al., have taken this approach a step further and performed single-cell RNA-seq to transcriptionally profile scores of individual cells from the same tumor. The results are striking: each tumor may be classifiable as a particular subtype, but individual cells can exhibit any of the multiple subtypes. Furthermore, the transcriptional phenotypes of the cells seem to exist spectrally rather than discretely [24]. The findings of both these studies call into question the clinical utility of the molecular subtyping scheme, as any targeted therapy tailored for a specific subtype may simply lead to the outgrowth of distinct subtypes which exist within a tumor but have been masked by technical limitations.
NF-κB Signaling
The NF-κB proteins are a family of transcription factors that mediate immune and inflammatory responses [25–27]. The family contains five structurally similar members that are classified into two groups. The first group, consisting of p65 (RelA), c-Rel and RelB, are synthesized in their mature forms and contain an N-terminal Rel homology domain (RHD) and a C-terminal transactivation domain (TAD). The second group, consisting of NF-κB1 (p105/p50) and NF-κB2 (p100/p52), are first synthesized as large precursors (p105 and p100) that are later processed into their mature forms (p50 and p52). Both p50 and p52 contain an RHD but neither possesses a TAD. Although these family members can dimerize in numerous combinations, only the NF-κB dimers that contain p65, RelB or c-Rel are competent transcription factors [28]. In the CNS, the p65/p50 heterodimer predominates, and it is this form that is referred to as NF-κB herein.
Typically, all NF-κB molecules are inactive and cytoplasmic. However, activation of these molecules is achieved in response to various stimuli using either the canonical or non-canonical pathway. Although noncanonical NF-κB appears to be involved in GBM, its role is currently poorly defined, and canonical signaling is currently considered the major contributor to this disease. In the canonical pathway (Figure 1), NF-κB molecules containing p65, c-Rel and/or p50 are sequestered through interactions with the Inhibitor of NF-κB (IκB) proteins. The IκB proteins contain multiple ankyrin repeat domains that enable them to bind to the RHD, and inhibit NF-κB’s ability to bind DNA. The pathway is triggered by various stimuli (discussed below) that activate the Inhibitor of NF-κB kinase (IKK) complex, which contains IKKα, IKKβ and IKKγ/NEMO. In NF-κB signaling, IKKβ phosphorylates the IκB proteins, which targets them for rapid degradation by the proteasome. This effectively liberates the NF-κB molecules, which then translocate into the nucleus and bind to their cognate DNA response elements [28].
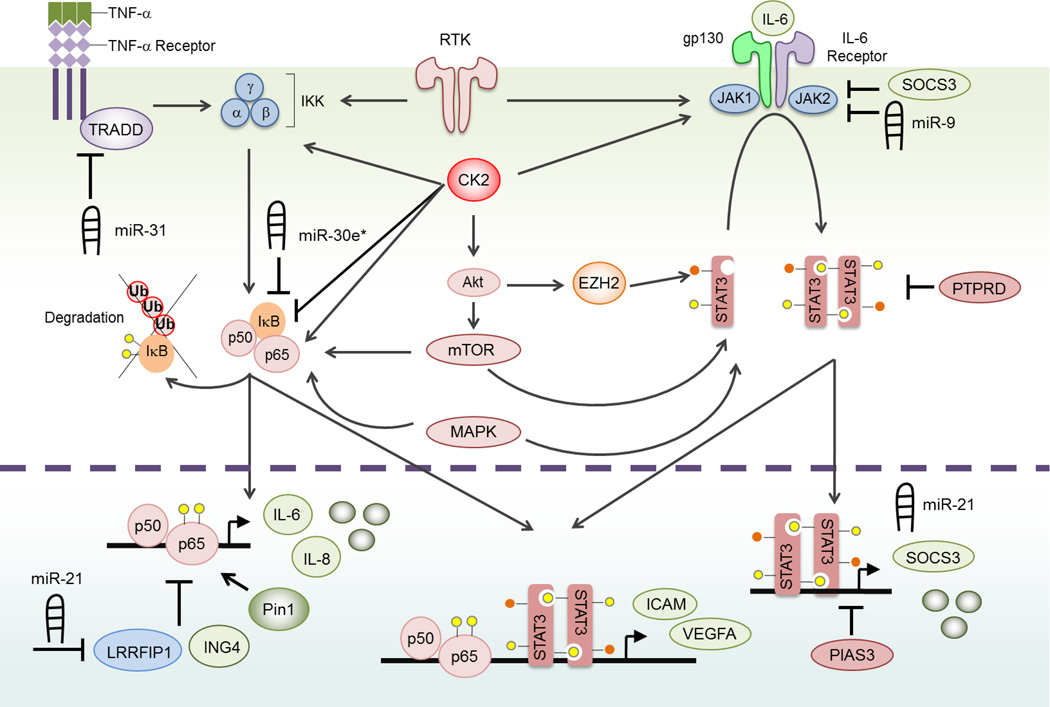
Figure 1. The NF-κB and STAT3 Signaling Pathways.
Canonical NF-κB, a homodimer of p65 and p50 proteins, is cytoplasmic and inactivated by IκB proteins. NF-κB is activated by numerous stimuli including pro-inflammatory cytokines. In this figure, we demonstrate TNF-α-mediated NF-κB activation. Specifically, TNF-α binds to the TNF-α receptor, which undergoes receptor trimerization and recruits TRADD and other molecules (not shown). Collectively, this activates the IKK complex, which is composed of three subunits: α, β and γ. IKKβ phosphorylates (yellow circle) IκB, which targets it for proteasomal mediated degradation. As a consequence, the newly liberated NF-κB molecules translocate to the nucleus, bind DNA and induce the expression of target genes such as IL-6 and IL-8. In the nucleus, Pin1 enhances NF-κB signaling while LRRFIP1 and ING4 inhibit NF-κB activity. Several miRs also influence NF-κB signaling. In particular, miR-31 inhibits NF-κB signaling by targeting TRADD, while miR-30e* and miR-21 enhance NF-κB signaling by targeting IκB and LRRFIP1, respectively. STAT3 signaling is activated by cytokines of the IL-6 family. These proteins bind to a receptor complex composed of a common gp130 subunit, and a cytokine specific receptor, i.e., IL-6 receptor. Members of the JAK family of kinases are associated with the gp130-receptor complex and are activated by ligand binding. JAKs trans- and auto-phosphorylate the receptor and STAT3 proteins. Next, STAT3 dimerizes, translocates to the nucleus, binds DNA and induces the expression of target genes including SOCS3 and miR-21. STAT3 signaling is inhibited by PIAS3, PTPRD, SOCS3 and miR-9. Some target genes contain response elements for both NF-κB and STAT3, such as ICAM and VEGFA. NF-κB and STAT3 signaling is also positively influenced by RTKs, which activate IKK and JAK kinases. We and others have shown that CK2 can directly phosphorylate NF-κB p65 and IκB to promote NF-κB signaling and that CK2 can interact with JAK1/2 to activate STAT3 signaling. CK2 also phosphorylates Akt. Akt signals through mTORC to positively impact STAT3 signaling. Recently, Akt was shown to interact with EZH2, a methyltransferase, to promote STAT3 methylation (orange circles) and activity. Finally, MAPK signaling also positively influences signals through both the NF-κB and STAT3 signaling pathway. In GBM, these pathways are aberrantly activated and ensure maximal NF-κB and STAT3 activation.
NF-κB in GBM
In GBM and many cancers, NF-κB is constitutively activated and correlates with increasing grade in astrocytic tumors. In particular, the Mesenchymal subclass of GBM is characterized by elevated levels of NF-κB signaling components (TRADD, RELB, TNFRSF1A), enhanced chemo- and radiation resistance and an overall poorer prognosis than patients with other types of GBM [17]. Interestingly, a recent paper demonstrated that Proneural patient-derived neurospheres can differentiate to a Mesenchymal phenotype via an NF-κB/TNF-α-dependent mechanism, which was also linked to radioresistance and poor prognosis [29]. These observations, while intriguing, leave open the question of how important NF-κB signaling is during glioma development and progression. Using gliomas as a test model of network analyses, Karlebach and Shamir found that dysregulation of the NF-κB pathway was one of four signaling pathways whose perturbation was minimally sufficient to support the malignant glioma phenotype [30]. However, in vivo the precise mechanism(s) of NF-κB activation in GBM is presently unknown, although there are numerous proteins and pathways dysregulated in GBM that may cause NF-κB activation.
TNF-α is one of the most potent activators of NF-κB [31]. TNF-α is a pro-inflammatory molecule and is secreted in the CNS by microglia, astrocytes and some neurons. TNF-α signals through two receptors, TNF receptor 1 (TNFR1) and TNFR2 [31]. TNFR2 is usually expressed on cells of the immune system (such as microglia) and oligodendrocytes, while TNFR1 is expressed on most cell types. The levels of TNFR1 expression in GBM and GBM-associated endothelial cells are elevated when compared with low grade gliomas or normal brain tissues [32–34].
NF-κB may also be activated by numerous growth factors or signaling pathways that are dysregulated in gliomas [35]. Specifically, NF-κB is activated by epidermal growth factor (EGF), and/or its receptor, (EGFR), the latter of which is frequently mutated and constitutively activated [36–38]. Moreover, it has been shown that oncogenic EGFR activates NF-κB by way of mTORC2, and this signaling cascade promotes chemoresistance [39]. Many gliomas also show allelic loss of PTEN, a tumor suppressor and negative regulator of the Akt pathway [40]. In the absence of PTEN, Akt is constitutively active and can activate NF-κB. Recently, elevated IGFBP2 levels and signaling was correlated with enhanced NF-κB signaling [41]. More specifically, in GBM, IGFBP2 binds to integrin β1 to activate an ILK/NF-κB cascade that promotes glioma growth.
NF-κB activity is carefully orchestrated by a number of positive and negative regulators. Data indicate that aberrant expression of these regulators can contribute to enhanced NF-κB activation. Specifically, we have shown that ING4, a negative regulator of NF-κB, is expressed at very low levels or is mutated in GBM, and that the lack of ING4 activity enables NF-κB to remain constitutively active [42] (Figure 1); conversely, Pin1, a positive regulator of NF-κB, is overexpressed in GBM, and also contributes to constitutive NF-κB activation [43] (Figure 1). PHF20 is another novel regulator of NF-κB in GBM; this protein binds methylated p65, which prevents recruitment of the phosphatase PP2A, thus prolonging the existence of an active NF-κB species in GBM [44]. Finally, the NFKBIA gene, which encodes IκBα, exhibits mono-allelic deletions in some types of GBM, is frequently lost in GBM in a subtype-specific manner, and this correlates with elevated NF-κB levels and poorer patient prognosis [45]. Interestingly, Pantane et al., demonstrated that NFKBIA deletions were increased when tumors were propagated as neurospheres when compared to parent tumor deletions, suggesting loss of NFKBIA is favorable for neurosphere formation and tumor-propagating properties [46]. These results support other reports noting the importance of NF-κB signaling in GSCs [47, 48].
Numerous microRNAs (miRs) are dysregulated and implicated in the clinical-pathologic features of gliomas, although few of these have been linked to NF-κB signaling [49]. Thus far, oncogenic miRs associated with NF-κB signaling in GBM include miR-21, miR-182 and miR-30e*. In particular, the levels of miR-21 are significantly elevated in GBM compared to non-tumor brain tissue, and inversely correlated with patient prognosis [50–54]. In GBM, overexpressed miR-21 enhances NF-κB signaling by targeting the DNA-binding protein LRRFIP1, an inhibitor of NF-κB signaling [55] (Figure 1). Regarding miR-182, 98% of gliomas exhibit elevated miR-182 levels, and miR-182 copy number is increased 2–3 fold in 35.6% of gliomas [56]. In GBM, TGF-β induces miR-182, which promotes NF-κB signaling by targeting several negative regulators of NF-κB, including CYLD, USP15, OPTN and TNIP1. Finally, miR-30e* is also elevated in GBM compared to normal brain, and sustains NF-κB signaling by targeting IκBα [57] (Figure 1). Presently, there are few tumor suppressive miRs associated with NF-κB signaling in GBM. However, we and others have found that miR-31 is largely downregulated or absent in GBM [58, 59] and this occurrence predominates in the Classical and Mesenchymal subtypes of GBM (unpublished observation). Moreover, reduced miR-31 levels are part of a ten miR expression signature that independently predicts patient survival [60]. In particular, we found that miR-31 inhibits NF-κB signaling by targeting TRADD, an upstream activator (unpublished observation; Figure 1). Moreover, as elevated levels of TRADD and loss of miR-31 are both observed in Mesenchymal GBM, our data may provide an explanation for this observed co-occurrence. The above miRs provide an interesting sampling of the miRs identified as regulating NF-κB, but the list of such miRs is long and growing [61–63].
NF-κB Signaling in Glioma: Preclinical Data
When last we wrote about NF-κB inhibitors in gliomas, we were hopeful new compounds would emerge to target NF-κB signaling. However, NF-κB remains a challenging therapeutic target and we still lack effective and specific compounds. Presently, the most promise comes from the use of proteasome inhibitors such as bortezomib (Velcade®), which blocks the degradation of IκBα and other proteins [64, 65]. Beginning in 2010, Phase I clinical trials were initiated to assess the side effects and maximum tolerated dose (MTD) of this drug. Although the nature of the study presently precludes a meaningful assessment of bortezomib efficacy in GBM, there were some data to indicate clinical efficacy [65]. Presently, however, bortezomib is not being further considered as a single agent therapy in the treatment of GBM. BAY-11, an IKK inhibitor, is a well-known inhibitor of NF-κB signaling and has more recently been shown to reverse chemoresistance, improve sensitivity to photodynamic (5-ALA) therapy, and promote senescence of GBM cells in vitro and in vivo [47, 66, 67]. Dehydroxymethylepoxyquinomicin (DHMEQ) is a unique small molecule inhibitor of NF-κB [68]. Preclinical testing of DHMEQ demonstrated inhibition of NF-κB activation and nuclear translocation, which led to decreased proliferation in GBM cells in vitro and decreased tumor growth in vivo. DHMEQ treatment also synergizes with TMZ and radiation, indicating strong therapeutic potential [69]. Recently, we published in vivo data demonstrating Withaferin A (WA), an IKKβ inhibitor, showed promise at inhibiting NF-κB and GBM growth [70]. Although WA is currently being used in a clinical trial of schizophrenia (NCT01793935), it is not under consideration for use in GBM.
JAK/STAT3 Signaling
JAK/STAT signaling is intimately involved in glioma biology. The pathway consists of four JAKs (JAKs1-3 and TYK2) and seven STATs (STATs1-4, 5a, 5b and 6) [71]. Of all the STATs, STAT3 is certainly the most eminent among cancers [72]. It can be activated by a variety of stimuli, including cytokines, growth factors and interferons [73]. Once an external factor has bound its receptor, JAKs are recruited to the cytoplasmic receptor tail and phosphorylate both themselves and the tail (Figure 1). STAT3 is then recruited to the tail, where it is phosphorylated on tyrosine 705 (and thus activated) by JAKs. The STATs will subsequently form homo- or heterodimers and localize to the nucleus, where they modulate gene expression [72]. STAT3 signaling is commonly activated by IL-6 family cytokines, including IL-6, Oncostatin M (OSM) and Leukemia Inhibitory Factor (LIF) [40]. STAT3 drives the transcription of a variety of genes which affect numerous aspects of cell survival and growth. Interestingly, a recent study of the transcriptomes of single GBM cells showed that STAT3 gene expression was increased in non-cycling cells preferentially, possibly indicating a subtler view of STAT3’s function in GBM than as simply driving growth in a general sense [24]. However, in general, STAT3 has a striking ability to promote tumor survival and invasion while suppressing anti-tumor immunity [72].
In addition to Tyr705 phosphorylation, the canonical marker of STAT3 activation, STAT3 may undergo post-translational modification (PTM) in a variety of other ways affecting its activity. Serine 727 phosphorylation is the best studied of these other modifications to date. Numerous pathways are capable of activating this pathway, including the MAPK and PI3K/mTOR cascades, and the site may be necessary for full transcriptional activation [74]. In glioma, Protein Kinase Cε has been shown to drive serine phosphorylation of STAT3 in a RAK/MEK/ERK-dependent fashion, and this modification of STAT3 enhances the invasive capacity and apoptosis resistance of glioma [75, 76]. Another recently identified STAT3 PTM is lysine 180 methylation. Intriguingly, this PTM appears to be restricted to glioma stem cells (GSCs). Akt phosphorylates EZH2, which in turn methylates STAT3. This methylation is necessary for STAT3 tyrosine phosphorylation, and mutation of this methylation site leads to declines in transcription of stem-associated genes [77]. The molecular means by which this modification is restricted to GSCs is as yet unknown, but further work will likely expand on the importance of this PTM in GBM biology.
JAK/STAT3 in GBM
STAT3 upregulation, hyperactivation, and nuclear accumulation is a well-known feature of GBM [78–80], and this aberrant STAT3 activation is associated with poor prognosis [81]. One important feature of STAT3’s involvement in GBM which has been highlighted in recent years is that STAT3 is closely associated with the Mesenchymal subtype of GBM. Beginning with the original TCGA study, it was shown that STAT3 mRNA levels increase in Proneural tumors which recur and thereafter acquire a more Mesenchymal-like phenotype [21], and it has been subsequently shown that STAT3 activation and target gene transcription increase in Proneural tumors undergoing a Mesenchymal shift after radiation treatment [82]. STAT3 and CEBPβ are the two master regulators of the Mesenchymal subtype, as identified in a bioinformatic study [22]. A recent miR-based “taxonomy” of GBM also showed upregulation of JAK/STAT pathway genes in subtypes corresponding to Mesenchymal GBM. Interestingly, in this study, it was shown that miR-9 upregulation in Proneural-like GBM suppresses JAK1, JAK2 and JAK3 transcription, which then leads to decreases in p-Tyr705-STAT3 and CEBPβ levels (Figure 1). MiR-9 thus acts as a negative regulator of mesenchymal differentiation in GBM [83]. Overall, STAT3’s connection to Mesenchymal GBM is intriguing and could potentially lead to preclinical and clinical studies with a more targeted focus on this subtype of GBM for STAT3 inhibition.
One important theme in the research concerning STAT3 in GBM over the past several years is the continual broadening of the mechanisms by which STAT3 is activated in GBM. IL-6 family cytokines are the prototypical activators of STAT3, and indeed, progress has been made in delineating their involvement in GBM. In vitro treatment of rat mesenchymal stem cells, but not astrocytes, with IL-6 is sufficient to induce malignant transformation and tumorigenic potential upon injection into nude mice [84]. In GSCs, IL-6 receptor and gp130 expression is elevated. Blocking IL-6 signaling or STAT3 activation directly in GSCs causes apoptosis, and treatment with anti-IL-6 antibodies in vivo slows glioma growth in both subcutaneous and intracranial murine models of glioma [85]. A similar role for the IL-6 family member erythropoietin and its receptor has also been described in GSCs [86].
STAT3 activation is by no means restricted to the IL-6 family, however. An area of increasing interest, particularly for its therapeutic potential, is STAT3 activation in response to receptor tyrosine kinases (RTKs). In an inducible model of glioma, overexpression of STAT3 was unable to induce tumors, while PDGFB overexpression induced some high-grade gliomas (HGGs). However, simultaneous overexpression of these genes produced a significantly higher number of HGGs, which displayed increased angiogenesis, necrosis and invasiveness [87]. PDGFRβ has also been shown to drive STAT3 activation in the glioma stem cell compartment, and STAT3 overactivation can compensate for PDGFRβ inhibition [88]. EGFR is increasingly seen as an important STAT3 activator in GBM. For example, EGFRvIII (a constitutively active mutant missing exons 2–7) cooperates with wild-type EGFR to cause hyperactivation of STAT3 and STAT5—but not Akt or ERK—in GBM [89], and STAT3-mediated coordination between EGFRvIII and c-MET has also been described [90]. (Of note, however, is the recent observation that EGFR varieties are rarely coexpressed at the single cell level in GBM [24].) A newly identified EGFR variant, termed EGFRvA [91], also drives STAT3 activation, as does a reported EGFR-SEPT14 chimera [92].
Another group of STAT3 regulators which is being increasingly recognized is miRs. Besides the previous example of miR-9, miR-124 negatively regulates STAT3 signaling at multiple points in glioma, and reconstitution of miR-124 in GSCs leads to differentiation and ablation of the immunosuppressive capacity of GSCs [93]. STAT3 is part of a miR-mediated post-transcriptional regulatory network found in GBM which involves numerous GBM-associated genes, including PTEN, PDGFRA, RUNX1, RB1 and VEGFA. In this network, overexpression of any single gene’s 3’ untranslated region caused slight increases in the expression of the other components [94]. While this network is evidently driven by miRs, the particular miRs driving the interactions have yet to be described. Thus, STAT3 interconnections with multiple pathways may exist at many levels, not just the protein level.
Loss of endogenous inhibitors of STAT3 is another means by which STAT3 may become hyperactivated. Low expression of the negative regulator PIAS3 has been previously described in GBM by our laboratory [78] (Figure 1). Work in the lab of Timothy Chan has identified PTPRD as a novel STAT3 tyrosine phosphatase whose activity is lost in approximately 50% of GBM by means of deletion, epigenetic silencing, and inactivating mutation [95] (Figure 1). Subsequent work in this lab demonstrated that in a murine model of glioma driven by PDGFB overexpression and p16 loss, PTPRD heterozygous—but not homozygous—loss could promote gliomagenesis and lead to worse survival. This loss of PTPRD led to increased STAT3 activation and protumorigenic macrophage infiltration. Why homozygous deletion did not demonstrate an equivalent pattern is unclear, although the authors speculated that it may be the result of compensatory negative regulation of STAT3 in the PTPRD−/− setting [96].
Being involved in such processes as hypoxia, angiogenesis, and immunosuppression, STAT3 is increasingly identified as a major component of the GBM tumor microenvironment [87, 97, 98]. A sampling of example roles of STAT3 in the GBM microenvironment has been provided throughout this section. However, it is important to emphasize that STAT3 is able to influence the microenvironment by activation in both tumor and stromal cells. An informative example is STAT3’s role in immunosuppression. While STAT3 activation is known to promote immunosuppressive (“M2-like”) phenotypes in GBM-associated macrophages/microglia [98], it is also true that STAT3 activation in tumor cells is able to promote a suppressive environment, as well [99, 100]. In these studies, it has been shown that STAT3 activation in GSCs leads to the release of cytokines suppressing T cell activation/proliferation while promoting an M2-like state in macrophages/microglia. Furthermore, GBM immunosuppression is enhanced by hypoxia, which is in turn driven by a STAT3/HIF1 signaling axis [101]. Overall, STAT3 is able to promote tumor development and progression via action in multiple compartments of GBM tissue, thus prominently displaying STAT3’s clinical promise for this disease.
Advances in Preclinical Studies of JAK/STAT3 in GBM
As demonstrated by the pervasive role of STAT3 in GBM described above, inhibition of JAK/STAT3 signaling has been of considerable interest in clinical and preclinical studies. The most common type of specific JAK/STAT inhibitors to date has been small molecule JAK inhibitors, and many of these have had positive results in studies of GBM in vitro and in vivo. One such compound is AZD1480, a JAK1/2 inhibitor [102]. This drug has been shown to potently inhibit activation of STAT3 and JAK2 in human and murine glioma cell lines. AZD1480 also blocks glioma cell proliferation and induces cell death in glioma cells while sparing murine primary astrocytes. AZD1480 treatment slowed the growth of tumors in a subcutaneous murine model using human xenografts, and in a similar intracranial model, AZD1480 significantly extended survival [102]. In another study, two JAK2 inhibitors, AG490 and WP1066, were used to demonstrate the importance of STAT3 in EGFRvIII-driven GBM. Inhibitors of EGFR, PI3K, mTOR, ERK1/2, NF-κB and JAK2 were all able to inhibit the invasive capacity of human glioma cells in vitro, but JAK2 inhibition was by far the most effective in this respect. Furthermore, intracranial injection of U87-MG human glioma cells transfected with vector or EGFRvIII resulted in aggressive tumors in each case; however, inhibition of JAK2 produced a survival benefit that was particularly profound in the EGFRvIII-expressing tumors, thus confirming in preclinical studies the connection between EGFR mutants and STAT3 [103]. Finally, it is worth noting that JAK inhibition is still an indirect means of inhibiting STAT3, which may have off-target or branching effects. For example, G5–7 is a recently described allosteric JAK2 inhibitor which, in GBM, blocks not only JAK2/STAT3 activation but also JAK2 interaction with EGFR, a necessary association for the activation of EGFR and downstream signaling [104]. Nevertheless, at least one promising drug directly targeting STAT3 is LLL12, which specifically inhibits the dimerization of STAT3 and thus its transcriptional capacity [105]. LLL12 effectively blocks glioma cell wound healing and colony formation while inducing apoptosis. However, in vivo studies have yet to be performed with this drug in GBM models, so it remains to be seen whether it will effectively cross the blood-brain barrier [105].
Another very exciting area in which STAT3 is emerging as a key player is GBM drug resistance. STAT3 has been identified as a driver of resistance to TMZ [106], radiation [82], and bevacizumab [107]. The case of bevacizumab and other antiangiogenic therapies provides the most intriguing example interrogated in recent years. In mice, antiangiogenic therapy, including sunitinib, VEGF knockout, and VEGFR small molecule inhibition, significantly increases the invasiveness of orthotopically implanted GBM while simultaneously failing to improve survival outcomes [108]. Subsequent work showed that VEGF antagonizes HGF signaling in GBM. VEGFR2 directly interacts with MET when both receptors are engaged with their respective growth factors, and PTP1B (a tyrosine phosphatase) is then recruited to the complex in order to dephosphorylate MET. Thus, when antiangiogenic therapy is applied to GBM, MET becomes hyperactivated, and indeed, inhibition of VEGF and MET effectively reduced invasive capacity of GBM tumors while improving survival [109]. As previously mentioned, MET is capable of driving STAT3 activation [90]. In bevacizumab-resistant GBM models, MET is upregulated, and p-Tyr705-STAT3 levels are increased twofold in bevacizumab resistant versus non-resistant GBM [110]. While inhibition of MET directly may be a promising avenue for synergy with antiangiogenic therapy, inhibition of STAT3 may prove another viable strategy, and STAT3 inhibition has been shown to be effective for this purpose in some studies [107].
Crosstalk Between NF-κB and JAK/STAT3 Signaling in GBM
NF-κB and STAT3 signaling have long been known to be intricately interwoven such that they cooperate in many pathological processes, cancer prominent among these [111, 112]. However, in recent years, much progress has been made in delineating the ways in which these two procancerous pathways interact specifically in GBM. One indirect example of their connection is in both pathways’ prominences in Mesenchymal GBM (Table I). NF-κB-pathway aberrance is one defining feature of Mesenchymal GBM as outlined in the papers originally identifying this subtype [17, 21], and STAT3 is a key driver of this particular phenotype [22]. Further, a shift to Mesenchymal GBM in response to therapy is also characterized by upregulations in NF-κB and STAT3 signaling [21, 29, 82].
This connection by subtype is indirect, but much work has been done to identify the direct means by which these two pathways interact in GBM. NF-κB and STAT3 are, for example, known to directly interact with one another in the promoter of ICAM1 post-irradiation [113]. Our lab has also recently demonstrated a feedback mechanism by which NF-κB can induce STAT3 signaling [70]. TNF-α treatment of GBM cell lines in vitro leads to robust NF-κB activation. As this NF-κB activity begins to wane after a period of several hours due to the accumulation of negative regulators, STAT3 activation waxes and in turn declines as NF-κB becomes reactivated. We showed that NF-κB-driven IL-6 production led to the observed STAT3 activation. In an orthotopic murine model of GBM, low-dose inhibition of NF-κB or STAT3 alone was insufficient to produce a survival benefit, but simultaneous low-dose inhibition of these pathways produced a significant increase in lifespan. Thus, NF-κB and JAK/STAT3 seem capable of feeding into one another, making them important co-conspirators in gliomagenesis. Although this study is informative, similar investigations in other cancer models have demonstrated ever more complex ligatures connecting these pathways by way of multiple miRNAs, PTEN/Akt signaling, and many others [114–118].
The astounding promiscuity of protein kinase CK2 concatenates the NF-κB and STAT3 cascades in GBM. CK2 is able to positively regulate both these pathways and thus act as a lateral enhancer of each. CK2 has long been known to enhance NF-κB signaling at many points in the pathway [119]. Our lab originally identified CK2 as being necessary for JAK/STAT activation in a variety of cancers [120], and we later confirmed such a role for CK2 in GBM [121] (Figure 1). Both NF-κB and STAT3 are activated in the context of GBM, but pharmacological and RNAi-mediated inhibition of CK2 activity attenuates these pathways simultaneously in in vitro and in vivo models of GBM [121]. Thus, CK2 inhibition may provide a novel route by which to inhibit both pathways using a single agent.
Expert Commentary
Although reports from the TCGA have validated many of the important signaling pathways in GBM, clinical trials of these targets have been largely ineffective, due to the highly heterogeneous nature of GBM and resistance to therapies. Therefore, more recent attempts have been made to include combinatorial approaches. For example, clinical trials testing the efficacy of combined bevacizumab with numerous tyrosine kinase inhibitors are currently underway (e.g., NCT00621686, NCT00720356 and NCT00892177). While these popular targets are continuing to be tested through alternative and combinatorial approaches in clinical trials, this allows for additional, previously discounted proteins and pathways a chance to be tested. Because there are no reported mutations in STAT3 or NF-κB proteins in GBM and they are instead dysregulated by positive and negative regulators, these pathways have not been the default targets for clinical trials, especially in GBM. However, the use of NF-κB and STAT3 inhibitors in clinical trials in GBM are continuing to gain momentum.
In searching the clinical trials database for “glioblastoma”, there are several trials testing inhibition of JAK/STAT3 and NF-κB signaling (Table II). Excitingly, the first JAK2/STAT3 inhibitor scheduled to be tested in patients with GBM is WP1066. This compound has been shown to be an effective inhibitor of JAK2/STAT3 activation and downstream gene expression while decreasing proliferation and reversing immune tolerance in human and murine GBM cells [122, 123]. In addition, numerous in vivo models have validated WP1066 as a remarkable inhibitor of tumor growth [87, 124]. Another, rather unconventional, therapeutic agent linked to STAT3 inhibition in GBM is curcumin [125, 126]. Treatment with curcumin inhibited JAK/STAT3 signaling and downstream gene expression, decreased proliferation, and induced cell cycle arrest in human and murine GBM cells. In vivo, tumor-bearing mice that were fed a curcumin diet exhibited decreased tumor growth and invasion and prolonged survival when compared to control diet. Given the overwhelming evidence demonstrating an important role of STAT3 in glioma progression, it will be exciting to follow these trials and hopefully more inhibitors of JAK/STAT3 will emerge in the future.
Table II.
Clinical Trials Evaluating JAK/STAT3 and NF-κB Inhibition in Glioblastoma
| Identifier | Intervention | Target/Pathway | Phase | Primary Outcome | Status |
|---|---|---|---|---|---|
| NCT01904123 | WP1066 | JAK2/STAT3 | I | Maximum tolerated dose | Not yet recruiting |
| NCT01712542 | Curcumin | STAT3 and others | 0 | Concentration of curcumin in tumor at time of surgery | Completed |
| NCT01654497 | Dexanabinol | NF-κB and others | I | Maximum tolerated dose | Recruiting |
| NCT00306618 | Panzem ® | NF-κB and others | II | 6 month progression free survival and overall survival | Completed |
| NCT00481455 | Panzem ® + TMZ | NF-κB and others; Chemotherapy | II | 6 month progression free survival and overall survival | Completed |
| NCT00006773 | Bortezomib | NF-κB and others; | I | Maximum tolerated dose | Terminated |
| NCT00544284 | Bortezomib + TMZ | NF-κB and others; Chemotherapy | I | Maximum tolerated dose | Completed |
| NCT01435395 | Bortezomib + Bevacizumab + TMZ | NF-κB and others; VEGF; Chemotherapy | I | Determination of progressive disease, complete or partial responses. | Recruiting |
| NCT00998010 | Bortezomib + TMZ + Radiation | NF-κB and others; Chemotherapy | II | Overall survival at 2 years | Recruiting |
| NCT00611325 | Bortezomib + Bevacizumab | NF-κB and others; VEGF | II | 6 month progression free survival | Completed |
| NCT00108069 | Bortezomib + Tamoxifen | NF-κB and others; SERM | II | Response – defined as stable disease or objective (partial or complete) | Completed |
| NCT00641706 | Bortezomib + Vorinostat | NF-κB and others; HDAC | II | 6 month progression free survival | Completed |
Inhibitors of the NF-κB pathway are also being tested in clinical trials for patients with GBM. Although no inhibitors of the upstream kinase IKK are being evaluated, there are several alternative inhibitors of NF-κB signaling being tested. Dexanabinol is a synthetic cannabinoid that inhibits phosphorylation of IκBα and prevents NF-κB nuclear translocation, transcriptional activity, and TNF-α and IL-6 expression [127]. 2-Methoxyestradiol (2ME2; Panzem®) is an estrogen metabolite that interferes with NF-κB activation and transcription in GBM cells and has demonstrated anti-tumor effects in vivo with optimal effectiveness in PTEN wild type tumors [128, 129]. In addition, the proteasomal inhibitor bortezomib also inhibits NF-κB signaling [28], and there are several clinical trials in GBM utilizing bortezomib in combination with other anti-cancer agents. As mentioned earlier, single agent bortezomib is ineffective in GBM, and therefore combination with other anti-cancer agents is being utilized. Other agents currently in clinical trials that are not listed in the table but do exhibit inhibitory effects related to NF-κB signaling include celecoxib, the COX-2 inhibitor; thalidomide and associated analogs, which decrease TNF-α levels; and dexamethasone, a corticosteroid with anti-inflammatory effects also linked to NF-κB inhibition. These exciting trials will lead us to a better understanding of the potential therapeutic benefits of targeting NF-κB and STAT3 in GBM.
Five-Year View
At times, research progress and hope for successful therapies for patients with GBM seem bleak. However, in reflecting over the past few years, substantial progress has indeed been made in the field. The revelation of the genetic molecular subtypes has opened many opportunities to exploit potential therapies for GBM. However, further reports revealed that there are multiple genetic subtypes within a single tumor. Thus, patients’ tumors could fall into multiple categories for genetic subtyping. This seems disconcerting at first, but essentially uncovers a more comprehensive view of all genetic aberrations within a tumor per patient. This heterogeneity also explains why therapies targeting one molecule or pathway are largely ineffective and that multiple targets should be exploited to account for the multiple subtypes per patient. For example, JAK/STAT3 inhibitors would potentially be therapeutic in Mesenchymal lineages of a tumor; whereas EGFR inhibitors may be helpful in Classical lineages within a tumor. Over the next five years, the clinical trials currently underway evaluating combination therapies for these subtype-specific targets will reveal optimal treatment regimens for patients.
Additionally, it is becoming increasingly more obvious that a promising therapeutic approach is immunotherapy [130]. Currently, there are numerous clinical trials for GBM testing peptide based vaccines, dendritic cell therapy, and stem cell transplants, and many of these are revealing promising preliminary results. Additional immune boosting mechanisms such as anti-PD-1 and anti-CTLA-4 are also being tested in GBM. These agents inhibit immunosuppressive activities of lymphocytes and instead promote cytotoxic lymphocytes capable of tumor killing. Over the next 5 years, immunotherapy trials will continue to be optimized, and combinations with other small molecule, tumor-specific inhibitors, will begin to be explored. For example, vaccine targeting immunotherapeutics combined with bevacizumab (NCT001498328, NCT01814813 and NCT02010606) or the mTOR inhibitor sirolimus (NCT001522820) are currently underway in clinical trials.
Although there are numerous combinations of therapies to test, some groups are re-evaluating the standard of care treatment protocols. For decades, the standard of care for patients has been surgical resection, followed by TMZ and radiation. Leder et al. [131] have developed a mathematical model for an alternative schedule of radiation that is optimized for targeting radioresistant cell populations and could potentially be beneficial for some patients. Altering the schedule of radiation treatment was sufficient to dramatically increase survival in pre-clinical studies and has provided alternative treatments that could be easily translated to the clinic. Over the next few years, personalized therapy will become increasingly present in the clinical setting. The right combination of therapeutics in conjunction with the right patient tumor profile will ultimately be the most advantageous treatment for patients with GBM.
Key Issues.
Glioblastoma (GBM) tumors are the most aggressive and malignant tumor of the CNS and remain essentially incurable.
The Cancer Genome Atlas (TCGA) and additional groups have determined that GBM tumors can be characterized genetically into 4 molecular subtypes.
Recently, studies have discovered that each patient’s tumor can harbor more than one molecular subtype, highlighting the complex heterogeneity in GBM.
Both NF-κB and STAT3 have been linked to GBM grade, the Mesenchymal molecular subtype, stem cell maintenance and resistance to therapies.
Multiple mechanisms of crosstalk between the NF-κB and STAT3 pathways have been discovered, revealing a vicious signaling cycle that occurs in GBM.
Clinical trials testing NF-κB and STAT3 inhibition are in progress, and although in their infancy, will soon provide answers to the questions regarding efficacy in patients with GBM.
Personalized therapies targeting the molecular subtype(s) in combination with additional approaches including immunotherapy are the future of GBM clinical research.
Acknowledgements
The authors would like to acknowledge Dr. Gordon P. Meares for critical review of the figures. Funding for this work was provided by grants from the National Institutes of Health (R01CA138517 to S.E.N. and R01NS057563 and R01NS050665 to E.N.B.); American Brain Tumor Association Basic Research Fellowship in Honor of Paul Fabbri (B.C.M.); William E. Cash Jr. Memorial Fund in Neuro-Oncology Research (B.C.M and S.E.N.) and the UAB Comprehensive Cancer Center (S.E.N.).
References
- 1.Porter KR, et al. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 2010;12(6):520–527. doi: 10.1093/neuonc/nop066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolecek TA, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 5.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 6. Chinot OL, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. *This is the much anticipated report of the Phase III trial (AVAglio) testing the addition of bevacizumab to standard of care (radiotherapy and chemotherapy) to patients with newly diagnosed GBM. The addition of bevacizumab did not improve overall survival and resulted in more adverse effects than placebo, but did improve progression-free survival and baseline quality of life.
- 7. Gilbert MR, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. *This is the complementary much anticipated report of the Phase III trial (RTOG 0825) testing the addition of bevacizumab to standard of care (radiotherapy and chemotherapy) to patients with newly diagnosed GBM. The addition of bevacizumab did not improve overall survival, and although progression-free survival was prolonged it did not reach statistical significance.
- 8.Wen PY, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 9.Ng K, et al. Genomic profiling of glioblastoma: convergence of fundamental biologic tenets and novel insights. J Neurooncol. 2012;107(1):1–12. doi: 10.1007/s11060-011-0714-2. [DOI] [PubMed] [Google Scholar]
- 10.Stummer W, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 11.Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci. 2011;12(9):495–508. doi: 10.1038/nrn3060. [DOI] [PubMed] [Google Scholar]
- 12.Van Meir EG, et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher JL, et al. Epidemiology of brain tumors. Neurol Clin. 2007;25(4):867–890. doi: 10.1016/j.ncl.2007.07.002. vii. [DOI] [PubMed] [Google Scholar]
- 14.Keime-Guibert F, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 15.Roa W, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 16.Glantz M, et al. Temozolomide as an alternative to irradiation for elderly patients with newly diagnosed malignant gliomas. Cancer. 2003;97(9):2262–2266. doi: 10.1002/cncr.11323. [DOI] [PubMed] [Google Scholar]
- 17. Verhaak RG, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. **This is the landmark report of the TCGA genetic analysis of GBM tumors. This report indicated that GBM subtypes can be molecularly classified into four subtypes: Classical, Mesenchymal, Proneural and Neural.
- 18.Noushmehr H, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turcan S, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan CW, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips HS, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 22. Carro MS, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463(7279):318–325. doi: 10.1038/nature08712. *This report demonstrated that STAT3 is a major transcriptional regulator of the Mesenchymal subtype of GBM.
- 23. Sottoriva A, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110(10):4009–4014. doi: 10.1073/pnas.1219747110. **This is a groundbreaking report that performed multi-sampling of tumors of patients with GBM and revealed that each patient can harbor more than one genetic subtype.
- 24. Patel AP, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. doi: 10.1126/science.1254257. **This groundbreaking report utilized single cell analysis to further demonstrate that multiple genetic subtypes are present in each patients tumor.
- 25.Basseres DS, Baldwin AS. NF-κB and IκB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25(51):6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann A, Baltimore D. Circuitry of NF-κB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 27.Karin M. NF-κB and cancer: mechanisms and targets. Mol Carcinog. 2006;45(6):355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- 28.Perkins ND. The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer. 2012;12(2):121–132. doi: 10.1038/nrc3204. [DOI] [PubMed] [Google Scholar]
- 29. Bhat KP, et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24(3):331–346. doi: 10.1016/j.ccr.2013.08.001. *This report demonstrated that NF-κB is a major driver of mesenchymal transformation in GBM and promotes radio-resistance.
- 30.Karlebach G, Shamir R. Minimally perturbing a gene regulatory network to avoid a disease phenotype: the glioma network as a test case. BMC Syst Biol. 2010;4:15. doi: 10.1186/1752-0509-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70(Suppl 1):i104–i108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi S, et al. Expression of nuclear factor-kappa B, tumor necrosis factor receptor type 1, and c-Myc in human astrocytomas. Neurol Med Chir (Tokyo) 2001;41(4):187–195. doi: 10.2176/nmc.41.187. [DOI] [PubMed] [Google Scholar]
- 33.Kargiotis O, Rao JS, Kyritsis AP. Mechanisms of angiogenesis in gliomas. J Neurooncol. 2006;78(3):281–293. doi: 10.1007/s11060-005-9097-6. [DOI] [PubMed] [Google Scholar]
- 34.Huang P, et al. Endothelial expression of TNF receptor-1 generates a proapoptotic signal inhibited by integrin alpha6beta1 in glioblastoma. Cancer Res. 2012;72(6):1428–1437. doi: 10.1158/0008-5472.CAN-11-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nogueira L, et al. The NFkappaB pathway: a therapeutic target in glioblastoma. Oncotarget. 2011;2(8):646–653. doi: 10.18632/oncotarget.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puliyappadamba VT, et al. Opposing effect of EGFRWT on EGFRvIII-mediated NF-kappaB activation with RIP1 as a cell death switch. Cell Rep. 2013;4(4):764–775. doi: 10.1016/j.celrep.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonavia R, et al. EGFRvIII promotes glioma angiogenesis and growth through the NF-kappaB, interleukin-8 pathway. Oncogene. 2012;31(36):4054–4066. doi: 10.1038/onc.2011.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang W, et al. EGFR-induced and PKCepsilon monoubiquitylation-dependent NF-kappaB activation upregulates PKM2 expression and promotes tumorigenesis. Mol Cell. 2012;48(5):771–784. doi: 10.1016/j.molcel.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka K, et al. Oncogenic EGFR signaling activates an mTORC2-NF-kappaB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1(6):524–538. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atkinson GP, Nozell SE, Benveniste ET. NF-kappaB and STAT3 signaling in glioma: targets for future therapies. Expert Rev Neurother. 2010;10(4):575–586. doi: 10.1586/ern.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes KM, et al. Insulin-like growth factor-binding protein 2-driven glioma progression is prevented by blocking a clinically significant integrin, integrin-linked kinase, and NF-kappaB network. Proc Natl Acad Sci U S A. 2012;109(9):3475–3480. doi: 10.1073/pnas.1120375109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nozell S, et al. The ING4 tumor suppressor attenuates NF-kappaB activity at the promoters of target genes. Mol Cell Biol. 2008;28(21):6632–6645. doi: 10.1128/MCB.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atkinson GP, et al. The prolyl isomerase Pin1 regulates the NF-kappaB signaling pathway and interleukin-8 expression in glioblastoma. Oncogene. 2009;28(42):3735–3745. doi: 10.1038/onc.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang T, et al. PHF20 regulates NF-kappaB signalling by disrupting recruitment of PP2A to p65. Nat Commun. 2013;4:2062. doi: 10.1038/ncomms3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bredel M, et al. NFKBIA deletion in glioblastomas. N Engl J Med. 2011;364(7):627–637. doi: 10.1056/NEJMoa1006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patane M, et al. Frequency of NFKBIA deletions is low in glioblastomas and skewed in glioblastoma neurospheres. Mol Cancer. 2013;12:160. doi: 10.1186/1476-4598-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nogueira L, et al. Blockade of the NFkappaB pathway drives differentiating glioblastoma-initiating cells into senescence both in vitro and in vivo. Oncogene. 2011;30(32):3537–3548. doi: 10.1038/onc.2011.74. [DOI] [PubMed] [Google Scholar]
- 48.Hjelmeland AB, et al. Targeting A20 decreases glioma stem cell survival and tumor growth. PLoS Biol. 2010;8(2):e1000319. doi: 10.1371/journal.pbio.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hummel R, Maurer J, Haier J. MicroRNAs in brain tumors : a new diagnostic and therapeutic perspective? Mol Neurobiol. 2011;44(3):223–234. doi: 10.1007/s12035-011-8197-x. [DOI] [PubMed] [Google Scholar]
- 50.Ciafre SA, et al. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334(4):1351–1358. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 51.Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68(19):8164–8172. doi: 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, et al. MicroRNA-21 down-regulates the expression of tumor suppressor PDCD4 in human glioblastoma cell T98G. Cancer Lett. 2008;272(2):197–205. doi: 10.1016/j.canlet.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 53.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65(14):6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 54.Lakomy R, et al. MiR-195, miR-196b, miR-181c, miR-21 expression levels and O-6-methylguanine-DNA methyltransferase methylation status are associated with clinical outcome in glioblastoma patients. Cancer Sci. 2011;102(12):2186–2190. doi: 10.1111/j.1349-7006.2011.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, et al. MicroRNA-21 targets LRRFIP1 and contributes to VM-26 resistance in glioblastoma multiforme. Brain Res. 2009;1286:13–18. doi: 10.1016/j.brainres.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 56.Song L, et al. TGF-beta induces miR-182 to sustain NF-kappaB activation in glioma subsets. J Clin Invest. 2012;122(10):3563–3578. doi: 10.1172/JCI62339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang L, et al. MicroRNA-30e* promotes human glioma cell invasiveness in an orthotopic xenotransplantation model by disrupting the NF-kappaB/IkappaBalpha negative feedback loop. J Clin Invest. 2012;122(1):33–47. doi: 10.1172/JCI58849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S, et al. Concomitant microRNA-31 downregulation and radixin upregulation predicts advanced tumor progression and unfavorable prognosis in patients with gliomas. J Neurol Sci. 2014;338(1–2):71–76. doi: 10.1016/j.jns.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 59.Hua D, et al. Human miR-31 targets radixin and inhibits migration and invasion of glioma cells. Oncol Rep. 2012;27(3):700–706. doi: 10.3892/or.2011.1555. [DOI] [PubMed] [Google Scholar]
- 60.Srinivasan S, Patric IR, Somasundaram K. A ten-microRNA expression signature predicts survival in glioblastoma. PLoS One. 2011;6(3):e17438. doi: 10.1371/journal.pone.0017438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang G, et al. MiR-196a exerts its oncogenic effect in glioblastoma multiforme by inhibition of IkappaBalpha both in vitro and in vivo. Neuro Oncol. 2014;16(5):652–661. doi: 10.1093/neuonc/not307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song L, et al. miR-486 sustains NF-kappaB activity by disrupting multiple NF-kappaB-negative feedback loops. Cell Res. 2013;23(2):274–289. doi: 10.1038/cr.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia H, et al. MiR-218 sensitizes glioma cells to apoptosis and inhibits tumorigenicity by regulating ECOP-mediated suppression of NF-kappaB activity. Neuro Oncol. 2013;15(4):413–422. doi: 10.1093/neuonc/nos296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin D, et al. Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme (GBM) Oncogene. 2005;24(3):344–354. doi: 10.1038/sj.onc.1208225. [DOI] [PubMed] [Google Scholar]
- 65.Phuphanich S, et al. Phase 1 clinical trial of bortezomib in adults with recurrent malignant glioma. J Neurooncol. 2010;100(1):95–103. doi: 10.1007/s11060-010-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coupienne I, et al. NF-kappaB inhibition improves the sensitivity of human glioblastoma cells to 5-aminolevulinic acid-based photodynamic therapy. Biochem Pharmacol. 2011;81(5):606–616. doi: 10.1016/j.bcp.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 67.Shukla S, et al. A DNA methylation prognostic signature of glioblastoma: identification of NPTX2-PTEN-NF-kappaB nexus. Cancer Res. 2013;73(22):6563–6573. doi: 10.1158/0008-5472.CAN-13-0298. [DOI] [PubMed] [Google Scholar]
- 68.Fukushima T, et al. Antitumor effect of dehydroxymethylepoxyquinomicin, a small molecule inhibitor of nuclear factor-kappaB, on glioblastoma. Neuro Oncol. 2012;14(1):19–28. doi: 10.1093/neuonc/nor168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brassesco MS, et al. Inhibition of NF- kappa B by Dehydroxymethylepoxyquinomicin suppresses invasion and synergistically potentiates temozolomide and gamma -radiation cytotoxicity in glioblastoma cells. Chemother Res Pract. 2013;2013:593020. doi: 10.1155/2013/593020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McFarland BC, et al. NF-kappaB-induced IL-6 ensures STAT3 activation and tumor aggressiveness in glioblastoma. PLoS One. 2013;8(11):e78728. doi: 10.1371/journal.pone.0078728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity. 2012;36(4):503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wagner KU, Schmidt JW. The two faces of Janus kinases and their respective STATs in mammary gland development and cancer. J Carcinog. 2011;10:32. doi: 10.4103/1477-3163.90677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19(21):2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 75.Aziz MH, et al. Protein kinase Cvarepsilon mediates Stat3Ser727 phosphorylation, Stat3-regulated gene expression, and cell invasion in various human cancer cell lines through integration with MAPK cascade (RAF-1, MEK1/2, and ERK1/2) Oncogene. 2010;29(21):3100–3109. doi: 10.1038/onc.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Y, et al. Knockdown of PKCepsilon expression inhibits growth, induces apoptosis and decreases invasiveness of human glioma cells partially through Stat3. J Mol Neurosci. 2014 doi: 10.1007/s12031-014-0341-4. [DOI] [PubMed] [Google Scholar]
- 77.Kim E, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell. 2013;23(6):839–852. doi: 10.1016/j.ccr.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brantley EC, et al. Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: implications for STAT-3 activation and gene expression. Clin Cancer Res. 2008;14(15):4694–4704. doi: 10.1158/1078-0432.CCR-08-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee K, et al. Proteome-wide discovery of mislocated proteins in cancer. Genome Res. 2013;23(8):1283–1294. doi: 10.1101/gr.155499.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6(5):675–684. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin GS, et al. STAT3 Tyr705 phosphorylation affects clinical outcome in patients with newly diagnosed supratentorial glioblastoma. Med Oncol. 2014;31(4):924. doi: 10.1007/s12032-014-0924-5. [DOI] [PubMed] [Google Scholar]
- 82.Halliday J, et al. In vivo radiation response of proneural glioma characterized by protective p53 transcriptional program and proneural-mesenchymal shift. Proc Natl Acad Sci U S A. 2014;111(14):5248–5253. doi: 10.1073/pnas.1321014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim TM, et al. A developmental taxonomy of glioblastoma defined and maintained by MicroRNAs. Cancer Res. 2011;71(9):3387–3399. doi: 10.1158/0008-5472.CAN-10-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cui X, et al. Interleukin-6 induces malignant transformation of rat mesenchymal stem cells in association with enhanced signaling of signal transducer and activator of transcription 3. Cancer Sci. 2014;105(1):64–71. doi: 10.1111/cas.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang H, et al. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells. 2009;27(10):2393–2404. doi: 10.1002/stem.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao Y, et al. Erythropoietin receptor signaling through STAT3 is required for glioma stem cell maintenance. Genes Cancer. 2010;1(1):50–61. doi: 10.1177/1947601909356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doucette TA, et al. Signal transducer and activator of transcription 3 promotes angiogenesis and drives malignant progression in glioma. Neuro Oncol. 2012;14(9):1136–1145. doi: 10.1093/neuonc/nos139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim Y, et al. Platelet-derived growth factor receptors differentially inform intertumoral and intratumoral heterogeneity. Genes Dev. 2012;26(11):1247–1262. doi: 10.1101/gad.193565.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan QW, et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell. 2013;24(4):438–449. doi: 10.1016/j.ccr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garnett J, et al. Regulation of HGF expression by DeltaEGFR-mediated c-Met activation in glioblastoma cells. Neoplasia. 2013;15(1):73–84. doi: 10.1593/neo.121536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou M, et al. A novel EGFR isoform confers increased invasiveness to cancer cells. Cancer Res. 2013;73(23):7056–7067. doi: 10.1158/0008-5472.CAN-13-0194. [DOI] [PubMed] [Google Scholar]
- 92.Frattini V, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 2013;45(10):1141–1149. doi: 10.1038/ng.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei J, et al. miR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma. Cancer Res. 2013;73(13):3913–3926. doi: 10.1158/0008-5472.CAN-12-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sumazin P, et al. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell. 2011;147(2):370–381. doi: 10.1016/j.cell.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Veeriah S, et al. The tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancers. Proc Natl Acad Sci U S A. 2009;106(23):9435–9440. doi: 10.1073/pnas.0900571106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ortiz B, et al. Loss of the tyrosine phosphatase PTPRD leads to aberrant STAT3 activation and promotes gliomagenesis. Proc Natl Acad Sci U S A. 2014;111(22):8149–8154. doi: 10.1073/pnas.1401952111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nilsson CL, et al. Quantitative phosphoproteomic analysis of the STAT3/IL-6/HIF1alpha signaling network: an initial study in GSC11 glioblastoma stem cells. J Proteome Res. 2010;9(1):430–443. doi: 10.1021/pr9007927. [DOI] [PubMed] [Google Scholar]
- 98.da Fonseca AC, Badie B. Microglia and macrophages in malignant gliomas: recent discoveries and implications for promising therapies. Clin Dev Immunol. 2013;2013:264124. doi: 10.1155/2013/264124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu A, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12(11):1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wei J, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9(1):67–78. doi: 10.1158/1535-7163.MCT-09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei J, et al. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2011;6(1):e16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McFarland BC, et al. Therapeutic potential of AZD1480 for the treatment of human glioblastoma. Mol Cancer Ther. 2011;10(12):2384–2393. doi: 10.1158/1535-7163.MCT-11-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zheng Q, et al. JAK2/STAT3 targeted therapy suppresses tumor invasion via disruption of the EGFRvIII/JAK2/STAT3 axis and associated focal adhesion in EGFRvIII-expressing glioblastoma. Neuro Oncol. 2014 doi: 10.1093/neuonc/nou046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He K, et al. Blockade of glioma proliferation through allosteric inhibition of JAK2. Sci Signal. 2013;6(283):ra55. doi: 10.1126/scisignal.2003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ball S, et al. The small molecule, LLL12, inhibits STAT3 phosphorylation and induces apoptosis in medulloblastoma and glioblastoma cells. PLoS One. 2011;6(4):e18820. doi: 10.1371/journal.pone.0018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kohsaka S, et al. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol Cancer Ther. 2012;11(6):1289–1299. doi: 10.1158/1535-7163.MCT-11-0801. [DOI] [PubMed] [Google Scholar]
- 107.de Groot J, et al. Modulating antiangiogenic resistance by inhibiting the signal transducer and activator of transcription 3 pathway in glioblastoma. Oncotarget. 2012;3(9):1036–1048. doi: 10.18632/oncotarget.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu KV, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22(1):21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu KV, Bergers G. Mechanisms of evasive resistance to anti-VEGF therapy in glioblastoma. CNS Oncol. 2013;2(1):49–65. doi: 10.2217/cns.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fan Y, Mao R, Yang J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4(3):176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kesanakurti D, et al. Essential role of cooperative NF-kappaB and Stat3 recruitment to ICAM-1 intronic consensus elements in the regulation of radiation-induced invasion and migration in glioma. Oncogene. 2013;32(43):5144–5155. doi: 10.1038/onc.2012.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iliopoulos D, et al. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39(4):493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee H, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15(4):283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xiang M, et al. STAT3 induction of miR-146b forms a feedback loop to inhibit the NF-kappaB to IL-6 signaling axis and STAT3-driven cancer phenotypes. Sci Signal. 2014;7(310):ra11. doi: 10.1126/scisignal.2004497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell. 2012;45(6):777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dominguez I, Sonenshein GE, Seldin DC. Protein kinase CK2 in health and disease: CK2 and its role in Wnt and NF-kappaB signaling: linking development and cancer. Cell Mol Life Sci. 2009;66(11–12):1850–1857. doi: 10.1007/s00018-009-9153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng Y, et al. A CK2-dependent mechanism for activation of the JAK-STAT signaling pathway. Blood. 2011;118(1):156–166. doi: 10.1182/blood-2010-01-266320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng Y, et al. Targeting protein kinase CK2 suppresses prosurvival signaling pathways and growth of glioblastoma. Clin Cancer Res. 2013;19(23):6484–6494. doi: 10.1158/1078-0432.CCR-13-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iwamaru A, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007;26(17):2435–2444. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- 123.Hussain SF, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67(20):9630–9636. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- 124.Kong LY, et al. Intratumoral mediated immunosuppression is prognostic in genetically engineered murine models of glioma and correlates to immunotherapeutic responses. Clin Cancer Res. 2010;16(23):5722–5733. doi: 10.1158/1078-0432.CCR-10-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weissenberger J, et al. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res. 2010;16(23):5781–5795. doi: 10.1158/1078-0432.CCR-10-0446. [DOI] [PubMed] [Google Scholar]
- 126.Senft C, et al. The nontoxic natural compound Curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer. 2010;10:491. doi: 10.1186/1471-2407-10-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Juttler E, et al. The cannabinoid dexanabinol is an inhibitor of the nuclear factor-kappa B (NF-kappa B) Neuropharmacology. 2004;47(4):580–592. doi: 10.1016/j.neuropharm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 128.Kumar AP. 2-Methoxyestradiol interferes with NF kappa B transcriptional activity in primitive neuroectodermal brain tumors: implications for management. Carcinogenesis. 2003;24(2):209–216. doi: 10.1093/carcin/24.2.209. [DOI] [PubMed] [Google Scholar]
- 129.Muh CR, et al. PTEN status mediates 2ME2 anti-tumor efficacy in preclinical glioblastoma models: role of HIF1alpha suppression. J Neurooncol. 2014;116(1):89–97. doi: 10.1007/s11060-013-1283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jackson CM, Lim M, Drake CG. Immunotherapy for brain cancer: Recent progress and future promise. Clin Cancer Res. 2014;20(14):3651–3659. doi: 10.1158/1078-0432.CCR-13-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leder K, et al. Mathematical modeling of PDGF-driven glioblastoma reveals optimized radiation dosing schedules. Cell. 2014;156(3):603–616. doi: 10.1016/j.cell.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]