Abstract
The vast majority of people living with HIV infection reside in resource-limited settings. As compared to resource-rich settings, there are important differences in the epidemiology and outcomes of HIV infection in resource-limited settings. Nonetheless, little HIV neurology research occurs in these regions. We will first review clinical, epidemiological and translational HIV neurology research originating from resource-limited settings. We will then discuss the barriers to conducting neurological research such as limited human resources, diagnostics and access to medications. Finally, we will review existing initiatives to build capacity for research in resource-limited settings. Despite the barriers, there is growing interest in and opportunities for collaborative international neurological research. Including viral and human populations from across the globe in HIV neurology research may lead to important implementation science, clinical and basic science discoveries.
Keywords: HIV, global health, neurology, HIV-associated neurocognitive disorders
GLOBAL EPIDEMIOLOGY AND THE BURDEN OF HIV/AIDS
Thirty-four million people are living with HIV/AIDS worldwide, of whom 23 million live in sub-Saharan Africa and 3.5 million live in South-East Asia.[1] Anti-retroviral therapy (ART) coverage has expanded dramatically from 400,000 people in 2003 to 8 million at the end of 2011.[2] The greatest increase in ART coverage has occurred in sub-Saharan Africa where 6.2 million people gained access to treatment between 2003 and 2011. Furthermore, there are five countries in this region now recognized for providing universal access to ART, namely Botswana, Namibia, Rwanda, Swaziland, and Zambia.[2]
There are differences between resource-rich and resource-limited settings in the epidemiology and outcomes of HIV infection and its associated disorders. Therefore, research conducted in the United States and Europe may not always be generalizable to resource-limited settings. Variations in HIV prevalence in resource-limited as compared to resource-rich settings may be due to behavioral as well as biological factors including mucosal levels of immune activation or co-infections with tuberculosis, malaria or schistosomiasis.[3] Similar variations in the prevalence of HIV-associated disorders have been observed. For example, pruritic papular eruption (PPE) is much more common in resource-limited settings.[4] Similarly, malignancies such as Kaposi’s sarcoma, cervical cancer, and non-Hodgkins lymphoma are more likely to be associated with HIV in resource-limited as compared to resource-rich settings; many non-AIDS defining cancers are also more likely to be associated with HIV including Hodgkins lymphoma, vaginal, testicular, renal, thymic, and uterine cancers.[5]
It is not clear whether the outcomes of HIV infection in resource-limited settings will be comparable to resource-rich settings. For example, unlike resource-rich settings, increasing coverage with ART has not been associated with a decrease in the incidence of Kaposi’s sarcoma in resource-limited settings.[5] In contrast, immunologic outcomes of HIV infection after initiation of ART in resource-limited settings are thought to be comparable to those observed in resource-rich settings.[6] However, these outcome estimates from resource-limited settings may be overly optimistic as substantial early mortality rates and loss-to-follow-up may have introduced survivorship bias.[6] Indeed, high early mortality rates have been reported in many resource-limited settings.[7]
Nonetheless, most HIV research has taken place in resource-rich settings and the results extrapolated to resource-limited settings. However, there have been instances in which observations from research and clinical care in resource-limited settings have informed the care of HIV-infected individuals in resource-rich settings. For example, one cancer that was initially reported in association with HIV in sub-Saharan Africa, squamous cell carcinoma of the conjunctiva, led to the discovery that this cancer was also more likely to be found in individuals with HIV in the United States.[5] In addition, a number of landmark HIV prevention trials have recently been performed in resource-limited settings including trials for male circumcision[8–10] and pre-exposure prophylaxis.[11–14]
In summary, there is a massive burden of HIV infection in resource-limited settings, despite the successes of ART treatment programs in the past decade. Furthermore, there are important differences in the epidemiology and outcomes of HIV infection between resource-rich and resource-poor settings. While most HIV research is conducted in resource-rich settings, research conducted in resource-limited settings has the potential to make unique contributions to our knowledge. Although there is a growing body of HIV research originating from resource-limited settings, little research on more specialized topics, such as the neurological complications of HIV or the pathophysiology of HIV in the central nervous system, originates from these regions.
HIV NEUROLOGY RESEARCH IN RESOURCE-LIMITED SETTINGS
Over the past decade the quantity of HIV neurology research conducted in resource-limited settings has grown substantially. The bulk of this research is comprised of descriptive clinical and epidemiological studies of the effects of HIV on the peripheral and central nervous systems. However, in the past few years, the number of translational research studies and clinical trials has increased as well.
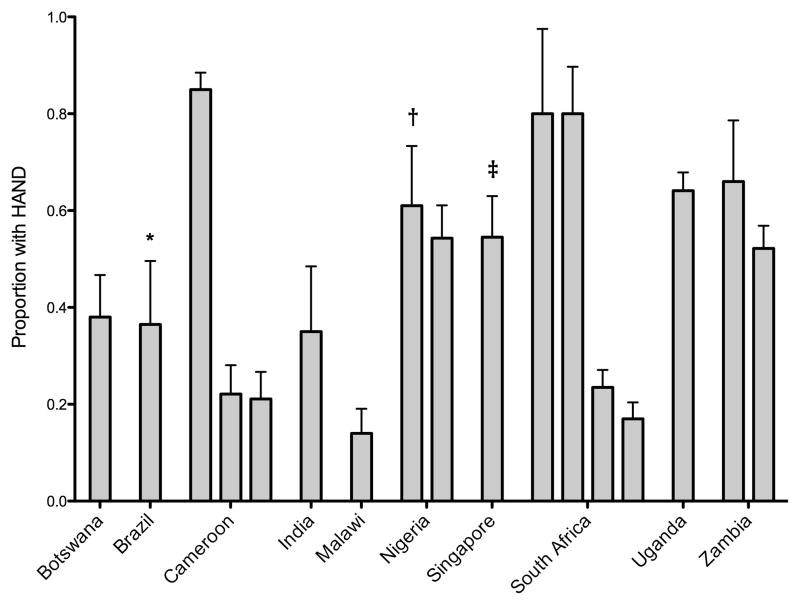
The first detailed reports of neurological disorders associated with HIV in resource-limited settings date back to the late 1980’s and early 1990’s.[15,16] The first major multi-country clinical and epidemiological study on HIV-associated neurocognitive disorders (HAND) in resource-limited settings was conducted in 1994.[17] In 2005, the International HIV Dementia Scale (IHDS)[18] was developed and this screening tool has been used widely for clinical and epidemiological studies of HIV-associated cognitive disorders (Figure 1).[19–35] The IHDS has been the subject of a number validation studies in resource-limited settings, [26,31,36–40] and in a recent systematic review, demonstrates moderate sensitivity and specificity.[38] However, the IHDS appears to be strongly affected by level of education, which may be a potential challenge in using the IHDS in resource-limited settings.[23,41]
Figure 1.
Prevalence of suspected HIV-associated neurocognitive disorders in resource-limited settings as ascertained using screening tools.
* Also used Mini-Mental State Examination.
† Used the Community Screening Instrument for Dementia.
‡ Also used the Montreal Cognitive Assessment.
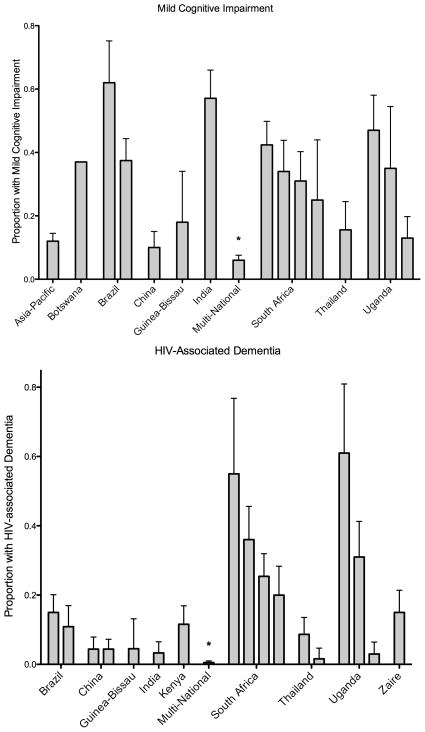
In addition, there are an increasing number of studies in resource-limited settings that have employed comprehensive neuropsychological batteries to diagnose HAND (Figure 2).[17,31,37,40,42–56] However, wide variation in prevalence estimates is observed whether using screening tools or comprehensive batteries. This may be due to differing criteria for defining impairment or lack of normative data. Although the Frascati criteria sought to standardize research criteria for HAND,[57] substantial variation in HAND definitions exists even in recent studies. Normative data is not available for most resource-limited settings. For example, in a large multi-national study of HIV-associated cognitive disorders among ART-eligible individuals, a comprehensive neuropsychological test battery was administered, but due to a lack of normative data, this battery could not be used to assign HAND diagnoses; instead, a standardized clinical assessment was used.[50] Studies which seek to use comprehensive batteries must enroll an appropriate HIV-negative control group to generate normative data, requiring larger sample sizes and adding to the expense of the research.[17,18,37,40,43–48,51,53–56,58]
Figure 2.
Prevalence of HIV-associated neurocognitive disorders in resource-limited settings as ascertained using neuropsychological test batteries or clinical assessment.
* Used clinical assessment for diagnosis.
Instead, many studies explore change over time or group level differences rather than assigning diagnoses. Several studies have demonstrated significant improvements on neuropsychological test performance after initiation of ART.[55,59–62] Of note, while these improvements on neuropsychological test performance varied significantly by country, the magnitude of improvement did not vary significantly by ART regimen.[59] Most studies additionally demonstrate significant group level differences in cognition between HIV-infected and –uninfected individuals.[36,50,63–65]
In addition, there is a growing body of literature exploring the development of HIV infected children. Infants and children with HIV in resource-limited settings generally demonstrate developmental deficits as compared to their HIV-uninfected peers,[66–68] though one small Thai study did not demonstrate significant impairment.[69] Peripheral neuropathy has also been an important topic of study as the prevalence of distal symmetric peripheral neuropathy ranges from 20%–60% in resource limited settings.[43,50,70–72] The high prevalence of peripheral neuropathy is likely due in part to the fact that until recently stavudine was recommended as first-line ART in most resource-limited settings.[56,73] A limitation of many of these studies is that only few regions have access to advanced diagnostics such as such as nerve conduction studies[74] and epidermal nerve fiber densities,[75] and thus most assessments are based on neuropathy screening tools.[76–78]
Translational research in resource-limited settings is also on the rise in regions such as South Africa and Thailand. While studies of the systemic immunologic response to ART have not demonstrated clade differences when the treatment setting is comparable,[6] similar studies of the potential effects of clade differences on HAND prevalence have not been conclusive.[24,27,44,47,48,51,79–81] Translational research has explored HIV viral reservoirs,[82,83] volumetric analyses,[84] or using magnetic resonance spectroscopy to monitor changes in brain inflammation and neuronal injury.[85,86]
BARRIERS TO NEUROLOGICAL RESEARCH IN RESOURCE LIMITED SETTINGS
There are several important barriers to conducting high quality neurological research in resource-limited settings including limited human resources, diagnostics, and access to medications.
First, there are few neurologists to partner with in most resource limited settings. The World Health Organization estimates there are 0.03 neurologists per 100,000 population in Africa and 0.07 in South-East Asia, as compared to nearly 5 in Europe.[87] As compared to Europe, Africa has over 150 times fewer neurologists and South-East Asia has over 70 times fewer neurologists. Furthermore, most neurologists from resource-limited settings are concentrated in urban centers[88] and few are significantly involved in research given the competing demands of clinical, academic and administrative duties.
Increasing the number of neurologists is daunting as there are limited training opportunities in many of these regions. For example, there are no neurology training programs in the entire East African community (Kenya, Uganda, Tanzania, Rwanda and Burundi), and until the past few years, only a handful on the African continent (Nigeria, Senegal, South Africa, Morocco, Egypt). Recently new post-graduate neurology training programs have been started in Ethiopia and Cameroon and neurology research training is being offered in Uganda through the Medical Education Partnership Initiative (MEPI). Nonetheless, most individuals from sub-Saharan Africa who wish to seek training in Neurology must go abroad, often at great personal cost, in order to obtain advanced training.
Accessibility of diagnostics in resource-limited settings is another challenge to conducting research. At least one computed tomography (CT) and magnetic resonance imaging (MRI), and electroencephalography (EEG) machine can be found in most resource-limited settings.[89] However, these technologies are rarely found outside of major urban centers[88] and the cost is prohibitive to the vast majority of patients in resource-limited settings. Electromyography and nerve conduction studies are not routinely available either. Basic laboratory testing for cerebrospinal fluid (CSF) is not widely available. For example, in Kenya, many public hospitals are only able to perform gram stain and Ziehls-Neelson staining on CSF and measure CSF glucose and protein with a urinanalysis dipstick. Cell counts, cryptococcal antigen testing and culture are not routinely available. Patients in resource-limited settings are often afraid to get lumbar punctures.[90] For patients, lumbar punctures are associated with death, as they are only done in the most critically ill patients and are very painful as use of local anesthesia is not routine. Finally, lumbar punctures are likely more frequently unsuccessful in resource-limited settings as they are typically performed with an intravenous catheter.
Few medications for neurological disorders are available. For example, multiple sclerosis is treated with pulse or chronic corticosteroids; disease-modulating therapy is prohibitively expensive. Dopaminergic agents for treatments of Parkinson’s disease are available in major urban centers though newer medications are unaffordable to most. Anti-epilepsy drugs such as phenobarbital, carbemazepine, phenytoin and valproate are available, though most are very expensive.[91]
BUILDING CAPACITY
While there is a great need for HIV and HIV neurology research in resource-limited settings, it is essential to conduct research in a way that sustainably builds research capacity in-country and addresses local priorities. Heavy criticism has been levied against “helicopter research” where researchers take samples and leave, or conduct research without partnering with and building capacity in local organizations and communities.[92,93] To build capacity to conduct research, it will be essential to continue to increase training opportunities and partnerships and to improve the quality and accessibility of diagnostics and treatments in resource limited settings.
There are several key initiatives that seek to develop international research partnerships and create opportunities for trainees. My discussion will focus on those funded by the United States, though there are numerous important Canadian and European initiatives as well. The Fogarty International Center of the U.S. National Institutes of Health recently initiated a new program for early stage investigators from the U.S. and low- and low-middle income countries (LMIC) entitled Global Health Program for Fellows and Scholars and which is offered through five support centers.[94] This program replaced the Fogarty International Clinical Research Scholars and Fellows which provided opportunities for mentored clinical research in developing countries. This program served 220 U.S. and 214 international medical students and junior trainees between 2003–2011, and 70 U.S. and 54 international post-doctoral fellows between 2008–2012.[95,96] Furthermore, the Fogarty International Center offers a career development award specifically designed for international research.[97] Over the past decade, the U.S. National Institutes of Health offered more than 120 planning grants and over 20 full research grants specifically for international neurology research through the initiative “Brain Disorders in the Developing World: Research Across the Lifespan.”[98]
Another important U.S. initiative is the MEPI, also supported through the Fogarty International Center, which funds institutions in sub-Saharan African countries to develop and expand medical education, train new health care workers, and build clinical and research capacity as part of a retention strategy for medical school faculty. MEPI supports over 30 regional partners in around a dozen African countries as well as more than 20 U.S. and foreign collaborators.[99] Finally, there are a few other important training opportunities to mention including: the Doris Duke International Clinical Research Training Fellowship which offers opportunities for U.S. medical students;[100] the Global Health Research Capacity Strengthening Program which offers opportunities for Canadian and LMIC citizens;[101] and the Wellcome Trust which provides a number of opportunities primarily for citizens and institutions of the U.K. and LMIC.[102]
CONCLUSION
In conclusion, the burden of HIV/AIDS is greatest in resource-limited settings and there is a great need for collaborative international research that addresses local priorities, sustainably builds research capacity in-country, and advances scientific inquiry. Despite many barriers, there is growing interest in and opportunities for collaborative international neurological research. As the response to the HIV epidemic in resource-limited settings moves from an emergency response focused on ART initiation to management of a chronic disease focused on retention in care, health maintenance, and quality of life, the potential exists for important implementation science, clinical and basic science discoveries by including both viral and human populations from across the globe.
Acknowledgments
Dr. Meyer’s time was supported by the Fogarty International Center of the National Institutes of Health (K01TW008764). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- AIDS
Acquired Immune Deficiency Syndrome
- ART
anti-retroviral therapy
- CSF
cerebrospinal fluid
- CT
computed tomography
- EEG
electroencephalography
- HAND
HIV-associated neurocognitive disorders
- HIV
human immunodeficiency virus
- IHDS
International HIV Dementia Scale
- LMIC
Lower-middle income country
- MMSE
Mini-Mental State Examination
- MRI
magnetic resonance imaging
- MEPI
Medical Education Partnership Initiative
- PPE
pruritic papular eruption
References
- 1.Anonymous. Number of people (all ages) living with HIV. World Health Organization; [Accessed 25 Nov 2013]. http://www.who.int/gho/hiv/epidemic_status/cases_all/en/index.html. [Google Scholar]
- 2.Anonymous. Anti-retroviral coverage among all age groups. World Health Organization; [Accessed 25 Nov 2013]. http://www.who.int/gho/hiv/epidemic_response/ART_text/en/ [Google Scholar]
- 3.Kaul R, Cohen C, Chege D, et al. Biological Factors that May Contribute to Regional and Racial Disparities in HIV Prevalence. Am J Reprod Immunol. 2011;65:317–324. doi: 10.1111/j.1600-0897.2010.00962.x. [DOI] [PubMed] [Google Scholar]
- 4.Eisman S. Pruritic Papular Eruption in HIV. Dermatol Clin. 2006;24:449–457. doi: 10.1016/j.det.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Casper C. The Increasing Burden of HIV-Associated Malignancies in Resource-Limited Regions. Annu Rev Med. 2011;62:157–170. doi: 10.1146/annurev-med-050409-103711. [DOI] [PubMed] [Google Scholar]
- 6.Achhra A, Phanuphak P, Amin J. Long-term immunological outcomes in treated HIV-infected individuals in high-income and low-middle income countries. Current Opinion in HIV and AIDS. 2011;6:258–265. doi: 10.1097/COH.0b013e3283476c72. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, Nadkarni G, Yang W, et al. Early Mortality in Adults Initiating Antiretroviral Therapy (ART) in Low- and Middle-Income Countries (LMIC): A Systematic Review and Meta-Analysis. PLos One. 2011;6:e28691. doi: 10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auvert B, Taljaard D, Lagarde E, et al. Randomized, Controlled Intervention Trial of Male Circumcision for Reduction of HIV Infection Risk: The ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey R, Moses S, Parker C, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomized controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 10.Gray R, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 11.Baeten J, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. NEJM. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2013 doi: 10.1016/S0140-6736(13)61127-7. Online First http://dx.doi.org/10.1016/S0140-6736(13)61127-7. [DOI] [PubMed]
- 13.Grant R, Lama J, Anderson P, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. NEJM. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thigpen M, Kebaabetswe P, Paxton L, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. NEJM. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 15.Howlett W, Nkya W, Mmuni K, et al. Neurological disorders in AIDS and HIV disease in the northern zone of Tanzania. AIDS. 1989;3:289–296. doi: 10.1097/00002030-198905000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Perriëns JH, Mussa M, Luabeya MK, et al. Neurological complications of HIV-1-seropositive internal medicine inpatients in Kinshasa, Zaire. J Acquir Immune Defic Syndr. 1992;5:333–340. [PubMed] [Google Scholar]
- 17.Maj M, Satz P, Janssen R, et al. WHO Neuropsychiatric AIDS study, cross-sectional phase II. Neuropsychological and neurological findings. Arch Gen Psychiatry. 1994;51:51–61. doi: 10.1001/archpsyc.1994.03950010051007. [DOI] [PubMed] [Google Scholar]
- 18.Sacktor N, Wong M, Nakasujja N, et al. The International HIV Dementia Scale: a new rapid screening test for HIV Dementia. AIDS. 2005;19 [PubMed] [Google Scholar]
- 19.Lawler K, Mosepele M, Ratcliffe S, et al. Neurocognitive impairment among HIV-positive individuals in Botswana: a pilot study. J Int AIDS Soc. 2010;13:15. doi: 10.1186/1758-2652-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filho S, de Melo H. Frequency and risk factors for HIV-associated neurocognitive disorder and depression in older individuals with HIV in northeastern Brazil. International Psychogeriatrics. 2012;24:1648–1655. doi: 10.1017/S1041610212000944. [DOI] [PubMed] [Google Scholar]
- 21.Njamnshi A, Djientcheu V, Fonsah J, et al. The International HIV Dementia Scale Is a Useful Screening Tool for HIV-Associated Dementia/Cognitive Impairment in HIV-Infected Adults in Yaounde-Cameroon. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2008;49:393. doi: 10.1097/qai.0b013e318183a9df. [DOI] [PubMed] [Google Scholar]
- 22.Njamnshi A, Bissek A, Ongolo-Zogo P, et al. Risk factors for HIV-associated neurocognitive disorders (HAND) in sub-Saharan Africa: The case of Yaoundé-Cameroon. Journal of the Neurological Sciences. 2009;285:149–153. doi: 10.1016/j.jns.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atashili J, Gaynes B, Pence B, et al. Prevalence, characteristics and correlates of a positive-dementia screen in patients on antiretroviral therapy in Bamenda, Cameroon: a cross-sectional study. BMC Neurology. 2013;13:86. doi: 10.1186/1471-2377-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riedel D, Ghate M, Nene M, et al. Screening for human immunodeficiency virus (HIV) dementia in an HIV clade C-infected population in India. Journal of Neurovirology. 2006;12:34–38. doi: 10.1080/13550280500516500. [DOI] [PubMed] [Google Scholar]
- 25.Patel VN, Mungwira RG, Tarumbiswa TF, et al. High prevalence of suspected HIV-associated dementia in adult Malawian HIV patients. International Journal of STD & AIDS. 2010;21:356–358. doi: 10.1258/ijsa.2010.009554. [DOI] [PubMed] [Google Scholar]
- 26.Oshinaike O, Akinbami A, Ojo O, et al. Comparison of the Minimental State Examination Scale and the International HIV Dementia Scale in Assessing Cognitive Function in Nigerian HIV Patients on Antiretroviral Therapy. AIDS Research and Treatment. 2012:581531. doi: 10.1155/2012/581531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salawu FK, Bwala SA, Wakil MA, et al. Cognitive function in HIV-seropositive Nigerians without AIDS. J Neurol Sci. 2008;267:142–146. doi: 10.1016/j.jns.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Chan L, Kandiah N, Chua A. HIV-associated neurocognitive disorders (HAND) in a South Asian population-contextual application of the 2007 criteria. BMJ Open. 2012;2:e000662. doi: 10.1136/bmjopen-2011-000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins RN, Remien RH, Mellins CA, et al. Screening for HIV-Associated Dementia in South Africa: Potentials and Pitfalls of Task-Shifting. AIDS Patient Care and STDs. 2011;25:587–593. doi: 10.1089/apc.2011.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joska J, Fincham D, Stein DJ, et al. Clinical correlates of HIV-associated neurocognitive disorders in South Africa. AIDS Behav. 2010;14:371–378. doi: 10.1007/s10461-009-9538-x. [DOI] [PubMed] [Google Scholar]
- 31.Singh D, Sunpath H, John S, et al. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts-a preliminary report. Afr J Psychiatry. 2008;11:282–286. [PubMed] [Google Scholar]
- 32.Ganasen K, Fincham D, Smit J, et al. Utility of the HIV Dementia Scale (HDS) in identifying HIV dementia in a South African sample. Journal of the Neurological Sciences. 2008;269:62–64. doi: 10.1016/j.jns.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 33.Nakku J, Kinyanda E, Hoskins S. Prevalence and factors associated with probable HIV dementia in an African population: A cross-sectional study of an HIV/AIDS clinic population. BMC Psychiatry. 2013;13:126. doi: 10.1186/1471-244X-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holguin A, Banda M, Willen E, et al. HIV-1 effects on neuropsychological performance in a resource-limited country, Zambia. AIDS Behav. 2011;15:1895–1901. doi: 10.1007/s10461-011-9988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birbeck GL, Kvalsund MP, Byers PA, et al. Neuropsychiatric and Socioeconomic Status Impact Antiretroviral Adherence and Mortality in Rural Zambia. American Journal of Tropical Medicine and Hygiene. 2011;85:782–789. doi: 10.4269/ajtmh.2011.11-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Royal W, Cherner M, Carr J, et al. Clinical features and preliminary studies of virological correlates of neurocognitive impairment among HIV-infected individuals in Nigeria. J Neurovirol. 2012;18:191–199. doi: 10.1007/s13365-012-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues R, Oliveira R, Grinsztejn B, et al. Validity of the International HIV Dementia Scale. Arq Neuropsiquiatr. 2013;71:376–379. doi: 10.1590/0004-282X20130042. [DOI] [PubMed] [Google Scholar]
- 38.Haddow L, Floyd S, Copas A, et al. A Systematic Review of the Screening Accuracy of the HIV Dementia Scale and International HIV Dementia Scale. PLOS One. 2013;8:e61826. doi: 10.1371/journal.pone.0061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalermchai T, Valcour V, Sithinamsuwan P, et al. Trail Making Test A improves performance characteristics of the International HIV Dementia Scale to Identify Symptomatic HAND. J Neurovirol. 2013;19 doi: 10.1007/s13365-013-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joska JA, Westgarth-Taylor J, Hoare J, et al. Validity of the International HIV Dementia Scale in South Africa. AIDS Patient Care and STDs. 2011;25:95–101. doi: 10.1089/apc.2010.0292. [DOI] [PubMed] [Google Scholar]
- 41.Waldrop-Valverde D, Nehra R, Sharma S, et al. Education effects on the International HIV Dementia Scale. Journal of Neurovirology. 2010;16:264–267. doi: 10.3109/13550284.2010.497808. [DOI] [PubMed] [Google Scholar]
- 42.Lawler K, Jeremiah K, Mosepele M, et al. Neurobehavioral effects in HIV-positive individuals receiving highly active antiretroviral therapy (HAART) in Gaborone, Botswana. PLoS ONE. 2011;6:e17233. doi: 10.1371/journal.pone.0017233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright E, Brew B, Arayawichanont A, et al. Neurologic Disorders are prevalent in HIV-positive outpatients in the Asia-Pacific region. Neurology. 2008;71:50–56. doi: 10.1212/01.wnl.0000316390.17248.65. [DOI] [PubMed] [Google Scholar]
- 44.de Almeida S, Ribeiro C, de Pereira A, et al. Neurocognitive impairment in HIV-1 clade C-versus B-infected individuals in Southern Brazil. J Neurovirol. 2013;19 doi: 10.1007/s13365-013-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heaton R, Cysique L, Jin H, et al. Neurobehavioral effects of human immunodeficiency virus infection among former plasma donors in rural China. Journal of Neurovirology. 2008;14:536–549. doi: 10.1080/13550280802378880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Qiao L, Ding W, et al. An initial screening for HIV-associate neurocognitive disorders of HIV-1 infected patients in China. J Neurovirol. 2012;18:120–126. doi: 10.1007/s13365-012-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi Y, Townend J, Vincent T, et al. Neurologic manifestations of human immunodeficiency virus-2: dementia, myelopathy, and neuropathy in West Africa. J Neurovirol. 2011;17:166–175. doi: 10.1007/s13365-011-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta J, Satishchandra P, Gopukumar K, et al. Neuropsychological deficits in human immunodeficiency virus type 1 clade C-seropositive adults from South India. J Neurovirol. 2007;13:195–202. doi: 10.1080/13550280701258407. [DOI] [PubMed] [Google Scholar]
- 49.Sebit MB. Neuropsychiatric HIV-1 infection study: in Kenya and Zaire cross-sectional phase I and II. Cent Afr J Med. 1995;41:315–322. [PubMed] [Google Scholar]
- 50.Robertson K, Kumwenda J, Supparatpinyo K, et al. A multinational study of neurological performance in antiretroviral therapy-naïve HIV-1-infected persons in diverse resource-constrained settings. J Neurovirol. 2011;17:438–447. doi: 10.1007/s13365-011-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joska JA, Westgarth-Taylor J, Myer L, et al. Characterization of HIV-Associated Neurocognitive Disorders Among Individuals Starting Antiretroviral Therapy in South Africa. AIDS Behav. 2011;15:1197–1203. doi: 10.1007/s10461-010-9744-6. [DOI] [PubMed] [Google Scholar]
- 52.Cross H, Combrinck M, Joska J. HIV-associated neurocognitive disorders: Antiretroviral regimen, central nervous system penetration effectiveness, and cognitive outcomes. S Afr Med J. 2013;103:758–762. doi: 10.7196/samj.6677. [DOI] [PubMed] [Google Scholar]
- 53.Pumpradit W, Ananworanich J, Lolak S, et al. Neurocognitive impairment and psychiatric comorbidity in well-controlled human immunodeficiency virus-infected Thais from the 2NN Cohort Study. Journal of Neurovirology. 2010;16:76–82. doi: 10.3109/13550280903493914. [DOI] [PubMed] [Google Scholar]
- 54.Wong MH, Robertson K, Nakasujja N, et al. Frequency of and risk factors for HIV dementia in an HIV clinic in sub-Saharan Africa. Neurology. 2007;68:350–355. doi: 10.1212/01.wnl.0000252811.48891.6d. [DOI] [PubMed] [Google Scholar]
- 55.Sacktor N, Nakasujja N, Skolasky R, et al. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology. 2006;67:311–314. doi: 10.1212/01.wnl.0000225183.74521.72. [DOI] [PubMed] [Google Scholar]
- 56.Sacktor N, Nakasujja N, Skolasky R, et al. Benefits and risks of stavudine therapy for HIV-associated neurologic complications in Uganda. Neurology. 2009;72:165–170. doi: 10.1212/01.wnl.0000339042.96109.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antinori A, Arendt G, Becker J, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh D, Joska J, Goodkin K, et al. Normative scores for a brief neuropsychological battery for the detection of HIV-associated neurocognitive disorder (HAND) among South Africans. BMC Research Notes. 2010;3:28. doi: 10.1186/1756-0500-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robertson K, Jiang H, Kumwenda J, et al. Improved Neuropsychological and Neurological Functioning Across Three Antiretroviral Regimens in Diverse Resource-Limited Settings: AIDS Clinical Trials Group Study A5199, the International Neurological Study. Clinical Infectious Diseases. 2012;55:868–876. doi: 10.1093/cid/cis507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joska J, Gouse H, Paul R, et al. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. Journal of Neurovirology. 2010;16:101–114. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- 61.Carvalhal A, Rourke S, Belmonte-Abreu P, et al. Evaluation of Neuropsychological Performance of HIV-Infected Patients with Minor Motor Cognitive Dysfunction Treated with Highly Active Antiretroviral Therapy. Infection. 2006;34:357–360. doi: 10.1007/s15010-006-6610-6. [DOI] [PubMed] [Google Scholar]
- 62.Sacktor N, Nakasujja N, Okonkwo O, et al. Longitudinal neuropsychological test performance among HIV seropositive individuals in Uganda. J Neurovirol. 2013;19:48–56. doi: 10.1007/s13365-012-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clifford DB, Mitike M, Mekonnen Y, et al. Neurological evaluation of untreated human immunodeficiency virus infected adults in Ethiopia. Journal of Neurovirology. 2007;13:67–72. doi: 10.1080/13550280601169837. [DOI] [PubMed] [Google Scholar]
- 64.Cysique L, Jin H, Franklin D, et al. Neurobehavioral effects of HIV-1 infection in China and the United States: A pilot study. J Int Neuropsychol Soc. 2007;13:781–790. doi: 10.1017/S1355617707071007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanmogne G, Kuate C, Cysique L, et al. HIV-associated neurocognitive disorders in sub-Saharan Africa: a pilot study in Cameroon. BMC Neurology. 2010;10:60. doi: 10.1186/1471-2377-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drotar D, Olness K, Wiznitzer M, et al. Neurodevelopmental Outcomes of Ugandan Infants with Human Immunodeficiency Virus Type 1 Infection. Pediatrics. 1997;100:e5. doi: 10.1542/peds.100.1.e5. [DOI] [PubMed] [Google Scholar]
- 67.Ruel T, Boivin M, Boal H, et al. Neurocognitive and Motor Deficits in HIV-infected Ugandan Children with High CD4 Cell Counts. CLIN INFECT DIS. 2012;54:1001–1009. doi: 10.1093/cid/cir1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tahan T, Bruck I, Burger M, et al. Neurological Profile and Neurodevelopment of 88 Children Infected with HIV and 84 Seroreverter Children Followed from 1995–2002. The Brazilian Journal of Infectious Disease. 2006;10:322–326. doi: 10.1590/s1413-86702006000500004. [DOI] [PubMed] [Google Scholar]
- 69.Vanprapar N, Kongstan N, Tritilanant P, et al. Developmental Screening by the Cognitive Adaptive Test/Clinical Linguistic and Auditory Milestone Scale (CAT/CLAMS) in HIV-infected Children. J Med Assoc Thai. 2005;88:S211–214. [PubMed] [Google Scholar]
- 70.Maritz J, Benatar M, Dave J, et al. HIV Neuropathy in South Africans: Frequency, Characteristics, and Risk Factors. Muscle and Nerve. 2010;41:599–606. doi: 10.1002/mus.21535. [DOI] [PubMed] [Google Scholar]
- 71.Wadley A, Cherry CL, Price P, et al. HIV Neuropathy Risk Factors and Symptom Characterization in Stavudine-Exposed South Africans. Journal of Pain and Symptom Management. 2011;41:700–706. doi: 10.1016/j.jpainsymman.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 72.Evans D, Takuva S, Rassool M, et al. Prevalence of peripheral neuropathy in antiretroviral therapy naive HIV-positive patients and the impact on treatment outcomes--a retrospective study from a large urban cohort in Johannesburg, South Africa. J Neurovirol. 2012;18:162–171. doi: 10.1007/s13365-012-0093-2. [DOI] [PubMed] [Google Scholar]
- 73.Pahuja M, Grobler A, Glesby M, et al. Effects of a reduced dose of stavudine on the incidence and severity of peripheral neuropathy in HIV-infected adults in South Africa. Antivir Ther. 2012;17:737–743. doi: 10.3851/IMP2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Konchalard K, Wangphonpattanasiri K. Clinical and electrophysiologic evaluation of peripheral neuropathy in a group of HIV-infected patients in Thailand. J Med Assoc Thai. 2007;90:774–781. [PubMed] [Google Scholar]
- 75.Shikuma C, McArthur JC, Ebenezer G, et al. Ethnic Differences in Epidermal Nerve Fiber Density. Muscle and Nerve. 2013:462–464. doi: 10.1002/mus.23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kandiah P, Atadzhanov M, Kvalsund MP, et al. Evaluating the diagnostic capacity of a single-question neuropathy screen (SQNS) in HIV positive Zambian adults. J Neurol Neurosurg Psychiatry. 2010;81:1380–1381. doi: 10.1136/jnnp.2009.183210. [DOI] [PubMed] [Google Scholar]
- 77.Cettomai D, Kwasa J, Birbeck GL, et al. Screening for HIV-associated Peripheral Neuropathy in Resource-Limited Settings. Muscle and Nerve. 2013;48:516–524. doi: 10.1002/mus.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mehta SA, Ahmed A, Kariuki BW, et al. Implementation of a validated peripheral neuropathy screening tool in patients receiving antiretroviral therapy in Mombasa, Kenya. Am J Trop Med Hyg. 2010;83:565–570. doi: 10.4269/ajtmh.2010.09-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joseph J, Achim C, Boivin M, et al. Global NeuroAIDS Roundtable. J Neurovirol. 2013;19:1–9. doi: 10.1007/s13365-013-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sacktor N, Nakasujja N, Skolasky R, et al. HIV Subtype D Is Associated with Dementia, Compared with Subtype A, in Immunosuppressed Individuals at Risk of Cognitive Impairment in Kampala Uganda. CLIN INFECT DIS. 2009;49:780–786. doi: 10.1086/605284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sacktor N, Nakasujja N, Robertson K, et al. HIV-associated cognitive impairment in sub-Saharan Africa--the potential effect of clade diversity. Nature Clinical Practice Neurology. 2007;3:436–443. doi: 10.1038/ncpneuro0559. [DOI] [PubMed] [Google Scholar]
- 82.Valcour V, Ananworanich J, Agsalda M, et al. HIV DNA Reservoir Increases Risk for Cognitive Disorders in cART-Naive Patients. PLoS ONE. 2013;8:e70164. doi: 10.1371/journal.pone.0070164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valcour V, Shiramizu B, Sithinamsuwan P, et al. HIV DNA and cognition in a Thai longitudinal HAART initiation cohort: the SEARCH 001 Cohort Study. Neurology. 2009;72:992–998. doi: 10.1212/01.wnl.0000344404.12759.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heaps J, Joska JA, Hoare J, et al. Neuroimaging markers of human immunodeficiency virus infection in South Africa. J Neurovirol. 2012;18:151–156. doi: 10.1007/s13365-012-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valcour V, Chalermchai T, Sailasuta N, et al. Central Nervous System Viral Invasion and Inflammation during Acute HIV infection. The Journal of Infectious Diseases. 2012;206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sailasuta N, Ross W, Ananworanich J, et al. Changes in Brain Magnetic Resonance Spectroscopy after Treatment during Acute HIV infection. PLOS One. 2012;7:e49272. doi: 10.1371/journal.pone.0049272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.World Health Organization, World Federation of Neurology. Atlas: Country Resources for Neurologic Disorders. Geneva: 2004. pp. 1–62. [Google Scholar]
- 88.Jowi J. Provision of Care to People with Epilepsy in Kenya. East African Medical Journal. 2007;84:97–99. [PubMed] [Google Scholar]
- 89.The Global Campaign against Epilepsy, World Health Organization, International Bureau for Epilepsy et al. Atlas: Epilepsy Care in the World. Geneva: 2005. pp. 1–96. [Google Scholar]
- 90.Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. CLIN INFECT DIS. 2008;46:1694–1701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meyer A, Dua T, Boscardin W, et al. Critical determinants of the epilepsy treatment gap: A cross-national analysis in resource-limited settings. Epilepsia. 2012;53:2178–2185. doi: 10.1111/epi.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferreira M, Gendron F. Community-based participatory research with traditional and indigenous communities of the Americas: Historical context and future directions. International Journal of Critical Pedagogy. 2011;3:153–168. [Google Scholar]
- 93.Flicker S, Travers R, Guta A, et al. Ethical Dilemmas in Community-Based Participatory Research: Recommendations for Institutional Review Boards. Journal of Urban Health: Bulletin of the New York Academy of Medicine. 2007;84:478–493. doi: 10.1007/s11524-007-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anonymous. Global Health Program for Fellows and Scholars. Fogarty International Center, U.S. National Institutes of Health; [Accessed 30 Dec 2013]. ( http://www.fic.nih.gov/Programs/Pages/scholars-fellows-global-health.aspx) [Google Scholar]
- 95.Heimburger D, Carothers C, Gardner P, et al. Nurturing the Global Workforce in Clinical Research: The National Institutes of Health Fogarty International Clinical Scholars and Fellows Program. Am J Trop Med Hyg. 2011;85:971–978. doi: 10.4269/ajtmh.2011.11-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anonymous. The Fogarty International Research Scholars Program. Vanderbilt University School of Medicine; [Accessed 30 Dec 2013]. ( http://fogartyscholars.org/program-history/the-scholars-program/) [Google Scholar]
- 97.Anonymous. International Research Scientist Development Award. Fogarty International Center, U.S. National Institutes of Health; [Accessed 30 Dec 2013]. ( http://www.fic.nih.gov/programs/Pages/research-scientists.aspx) [Google Scholar]
- 98.Anonymous. Brain Disorders in the Developing World: Research Across the Lifespan. Fogarty International Center, U.S. National Institutes of Health; [Accessed 30 Dec 2013]. ( http://www.fic.nih.gov/Programs/Pages/brain-disorders.aspx) [Google Scholar]
- 99.Anonymous. Medical Education Partnership Initiative (MEPI) Fogarty International Center, U.S. National Institutes of Health; [Accessed 14 Jan 2014]. http://www.fic.nih.gov/programs/Pages/medical-education-africa.aspx. [Google Scholar]
- 100.Anonymous. International Clinical Research Fellowship. Doris Duke Charitable Foundation; [Accessed 30 Dec 2013]. ( http://www.ddcf.org/Programs/Medical-Research/Goals-and-Strategies/Build-the-Clinical-Research-Career-Ladder/International-Clinical-Research-Fellowship/) [Google Scholar]
- 101.Anonymous. [Accessed 30 Dec 2013];Global Health Research Capacity Strengthening Program, funded by Canadian Institutes of Health Research and Quebec Population Health Research Network. ( http://www.pifrsm-ghrcaps.org/)
- 102.Anonymous. International Funding. Wellcome Trust; [Accessed 30 Dec 2013]. ( http://www.wellcome.ac.uk/Funding/International/index.htm) [Google Scholar]