Abstract
Background:
Orthognathic surgery has traditionally been performed using stone model surgery. This involves translating desired clinical movements of the maxilla and mandible into stone models that are then cut and repositioned into class I occlusion from which a splint is generated. Model surgery is an accurate and reproducible method of surgical correction of the dentofacial skeleton in cleft and noncleft patients, albeit considerably time-consuming. With the advent of computed tomography scanning, 3D imaging and virtual surgical planning (VSP) have gained a foothold in orthognathic surgery with VSP rapidly replacing traditional model surgery in many parts of the country and the world. What has yet to be determined is whether the application and feasibility of virtual model surgery is at a point where it will eliminate the need for traditional model surgery in both the private and academic setting.
Methods:
Traditional model surgery was compared with VSP splint fabrication to determine the feasibility of use and accuracy of application in orthognathic surgery within our institution.
Results:
VSP was found to generate acrylic splints of equal quality to model surgery splints in a fraction of the time. Drawbacks of VSP splint fabrication are the increased cost of production and certain limitations as it relates to complex craniofacial patients.
Conclusions:
It is our opinion that virtual model surgery will displace and replace traditional model surgery as it will become cost and time effective in both the private and academic setting for practitioners providing orthognathic surgical care in cleft and noncleft patients.
Orthognathic surgery requires precise evaluation of complex dentofacial deformities of the craniofacial skeleton. The success of the surgical plan is not only dependent on the accuracy of the skeletal and dental diagnosis of the deformity but also is unequivocally dependent on presurgical prediction of the proposed jaw movements. It is the task of the surgeon to first define the original position of the dentofacial skeleton and then to estimate the desired final position and finally to develop a 3-dimensional representation of the movements necessary to accomplish the intended goal.1 Traditionally, this has involved detailed preoperative clinical examination, standard facial photography, cephalometric radiographs with tracings, dental impressions, and articulator-mounted models. The end goal of all of these steps is to develop a representative blueprint of the current relationship of the maxilla/mandible and the associated dentofacial skeletal dysplasia. That relationship then is used to facilitate model surgery to determine the feasibility of the proposed jaw movements and to subsequently directly fabricate surgical guide splints which are critical for the accurate intraoperative positioning of the maxilla and/or mandible. This traditional analytical model surgery integrates the quantitative data and allows transfer of the anticipated 3D movements directly to the patient to facilitate the intraoperative position of the maxilla and/or the mandible.2 This technique has stood the test of time and has allowed for accurate and reproducible surgical correction of the dentofacial skeleton. This technique, however, requires an extensive process of analytical and radiographic analysis, dental model fabrication and splint preparation which require an extensive time commitment, and a firm grasp of dental materials and has the potential to have inaccuracies amplified during the algorithmic process. The advent of virtual surgical planning (VSP) has recently called into question the efficacy and accuracy of traditional analytical model surgery.
Maxillofacial surgery as a discipline was not an organized specialty until the latter half of the 20th century requiring particularly trained surgeons with masterful knowledge of both anatomy and surgical techniques to accomplish successful bony reconstruction.3 Orthognathic surgery in patients with dentofacial abnormalities is an original field within maxillofacial surgery. Modern practices within this particular field have undergone evolutionary development and refinement since its derivation by the first teachers in the early 1900s. The historic development traces its roots back to 1906 when the first surgery to correct at prognathic mandible was performed on a Washington University medical student by plastic surgery pioneer Vilray Blair.4 This ushered in decades of jaw surgery eclipsed by Obwegeser’s introduction of the sagittal split osteotomy in the 1950s and Bell’s research on the vascularization of the upper jaw leading to the safe downfracture of the maxilla in a LeFort I osteotomy.5
In present-day orthognathic surgery, the spectrum of surgical intervention ranges from simple single-jaw and double-jaw surgery to complex cleft craniomaxillofacial orthognathic surgery. Albeit more than 20 years ago and likely an underestimation, a survey performed in 1990 estimated that the current number of people in the United States benefiting from orthognathic surgery was more than 1.5 million.6 With the likelihood of craniomaxillofacial surgeons facing a growing number of patients requiring orthognathic surgery, it is imperative for the clinician to have a sound understanding of dental facial proportions, development of the craniofacial skeleton, orthodontic preparation for surgical intervention requiring a collaborative team approach with the patient’s orthodontist, and the ability to plan and execute single- and double-jaw surgery.
Throughout the last 100 years, the field has undergone significant refinement and development as it relates to technique modification, innovation as it relates to rigid fixation, and recent technological advancements in presurgical planning and splint fabrication. Any discussion surrounding orthognathic surgery in present-day medicine now includes the argument of traditional model surgery versus VSP. In review of recent literature, one can identify a number of articles defining and celebrating the use of computer-aided design/computer-aided manufacturing (CAD/CAM) in development of surgical planning for the treatment of complex craniomaxillofacial deformities.3,7–12 In addition to the gaining popularity of VSP within orthognathic surgery, a series of recent investigations performed at multiple institutions have confirmed the accuracy of this technique.10 As VSP is proving both highly accurate and efficient, the future of traditional model surgery comes into question. It is our objective in this article to (1) define both traditional model surgery and VSP and (2) determine the accuracy and relevance of the 2 methods.
TRADITIONAL MODEL SURGERY
One of the most crucial aspects of orthognathic surgical proficiency is for the clinician to have a mastery of presurgical planning. With that being said, however, it is well recognized that treatment planning for 2-jaw surgery is one of the most challenging topics in maxillofacial surgery.13,14 The traditional method of presurgical planning allows for quantitative analysis of various occlusal relationships and discrepancies as they relate to overbite, overjet, and dental relationships of the first molar and canine. There are a multitude of analyses available to the clinician to delineate the various malocclusions into a quantitative analysis that helps define the sella turcica to traditional A and B points of the maxilla and mandible, respectively. The following presurgical imaging, dental analysis, and model surgery then allow for a surgeon to transfer records completed with a presurgical examination to a set of casts that can then be studied to plan 3-dimensional maxillary and mandibular movements to correct a dentofacial deformity. Performing model surgery facilitates a surgeon to determine the blueprint for surgery and to directly fabricate an accurate dental template to facilitate precise and accurate surgical movements.
The process begins with presurgical clinical measurements including occlusal discrepancy, cuspid position, molar position, dental and skeletal midlines, overjet, overbite, and occlusal plane cants. Intraoral and extraoral photographs can be used to help determine aesthetic goals. Vertical and anteroposterior skeletal position can be determined both clinically and radiographically using various 3-view plain films and lateral cephalograms.2,13–15 The identification of the underlying deformity followed by the feasibility of surgical correction with various tracings and overlays in an effort to predict surgical movements is then possible. This radiographic evaluation enables quantitative analysis to assist in indentifying the current position of the maxilla and mandible to the cranial base.
To initiate analytical model surgery, maxillary and mandibular impressions are taken and stone casts poured (Fig. 1). These are subsequently mounted with a face-bow transfer onto an anatomic articulator to relate the maxilla to the cranial base. The face-bow transfer must accurately capture the orientation of the maxilla to assess the occlusal plane angle, occlusal cant, and horizontal arch rotation as well as the vertical movements desired with surgery.16 Simple hinge articulators can be used if the treatment plan calls for a maxillary advancement only. For more complex maxillary and bimaxillary surgeries, a semiadjustable articulator should be used.17 Predictive movements in a 3-dimensional model align the relationship of the maxilla and mandible as it relates to the Frankfort horizontal and then subsequently relate the maxillomandibular unit in the traditional yaw, pitch, and roll alignment in the X, Y, and Z coordinates. This has been a time-tested method with relatively accurate preoperative and postoperative predictions in tracings as stone models are a 1:1 representation of the patient’s facial structure.1
Fig. 1.
Maxillary and mandibular stone models.
Dental landmarks and horizontal and vertical reference marks are made directly on the casts to help quantify the amount of impaction of down fracture, anteroposterior, transverse, and rotational movements that will be needed. Impaction of down fracture movements can be quantified with horizontal lines. Anteroposterior repositioning is quantified by measuring the labial surface of the central incisor to the incisal guide pin (Fig. 2). Transverse expansion or constriction can be quantified by comparison with the study model casts. Finally, rotational movements can be measured using vertical reference marks. A Boley gauge can then be used to determine the exact measurements of the surgical movements needed following a mock model surgical set up into the ideal occlusion.18,19 Comparison of these movements can be made using clinical and cephalometric records to confirm the surgical treatment plan.1
Fig. 2.

Maxillary cast markings.
The exhaustive process of model surgery now starts by separating the maxillary cast and segmenting the cast to mimic the cuts that will be made during surgery (Fig. 3). The maxillary cast is then remounted according to the prescribed movements determined in the treatment plan. The mandibular cast is then separated and remounted into the ideal occlusion. Once the remounting of both the maxillary and mandibular cast is verified with the treatment plan, an acrylic splint can be fabricated with the maxillary and mandibular casts in their ideal position (Fig. 4). For single-jaw surgeries, a single splint is sufficient to transfer the specific 3-dimensional movements from the model to the patient. For bimaxillary surgeries, an intermediate splint is required to stabilize the maxillary segment to the native mandible.20 Once the maxilla has been stabilized, a final splint can be used to determine the position of the segmented mandible (Fig. 5). Splint fabrication can use self-cure or light-cure acrylic.
Fig. 3.
Maxillary cast segmentation.
Fig. 4.
Mounted maxillary and mandibular casts with final acrylic splint.
Fig. 5.
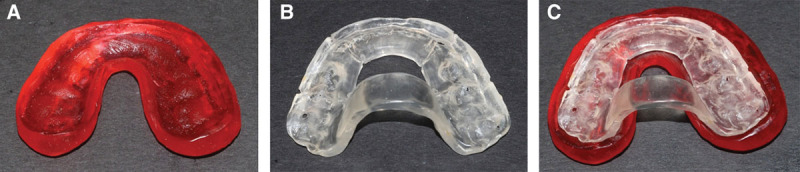
Acrylic splints from traditional model surgery: (A) intermediate splint, (B) final splint, and (C) final splint mounted within intermediate splint.
As may be apparent to an informed reader, with the multiple analyses that are required, including the clinical analysis, the cephalometric and radiographic analysis, and the analytical model surgery, there is the potential for inconsistencies. Those inaccuracies can be intrinsic to the initial quantitative measures. They can also be secondary to less than optimal cephalometric analysis and tracing and utilization of various programs for predictive movements, or it could be due to the inherent nature of analytical model surgery, which encompasses various materials and methods of dentistry. Those materials and methods include face-bow transfers, bite registrations, impressions of the maxilla and the mandible, stone and plaster analysis, model surgery requiring actual cutting of the stone and casts, and then subsequent repositioning of the casts in the predicted surgical movement. Inherently, all of the materials, methods, and potential for errors can be transmitted and even amplified during the multistep transfer of information to obtain the final and intermediate splint for surgical intervention. This process is time-consuming and tedious and requires a firm understanding of dental and medical principles. As mentioned, it has withstood the test of time and has been a traditional method of training orthognathic surgery to the novice surgeon. The ability to quantify the analytical information from the clinical and radiographic examination and transpose that into a 3D analysis via model surgery and subsequently manipulate the models into their new position essentially allows the clinician to perform the LeFort I osteotomy and bilateral sagittal split osteotomy in the 3-dimensional plane so that they will have a firm understanding of the preexisting relationship and the predicted movements.
VIRTUAL SURGICAL PLANNING
With the advent of computed tomography (CT) scanning and, in particular, cone beam dental CTs over the past 10 years, 3D imaging and VSP have gained a foothold in orthognathic surgery. VSP, or CAD/CAM, is rapidly replacing traditional model surgery in many parts of the country and the world. VSP entails obtaining a maxillofacial CT with 3D reconstruction, which provides superior quality to plain radiographs, and innately aligns the maxilla and the mandible in the appropriate relationship, eliminating the need for a face-bow transfer and aligning the Frankfort horizontal plane to the maxilla and the mandible. The quality of the images and the applications are impressive; however, it is yet to be determined whether or not the significant advantages and time-saving technologic innovations of medical modeling and splint fabrication are at a point where they will eliminate traditional cephalometric analysis and analytical model surgery.
VSP through medical modeling uses the stone models obtained from the patient as is done in traditional model surgery and a standard medical maxillofacial CT with 1-mm cuts. The models are laser scanned and the digital data are combined with that obtained from the maxillofacial CT scan of the patient to create an accurate 3-dimensional model of the patient’s maxillofacial and mandibular anatomy, including the dental arches. Over the last 5 years, different groups have published their experiences and recommendations regarding accurately representing the patient’s natural head position. These techniques include data acquisition through cone beam CT, use of a face-bow jig with an attached gyroscope, and patient-specific bite registration mounted to a fiducial face bow to generate numerical values for the pitch, roll, and yaw of the patient’s head.3,10,12,21,22 As technology has progressed in CT imaging, it is now possible to use medical CT images with 3D reconstruction as the patient’s Frankfurt horizontal is normalized through this process.
For CT-guided splint fabrication, 2 original stone models are provided to the splint manufacturer. If multisegment LeFort surgery is planned, the maxillary stone model is cut and repositioned to create the desirable occlusion by articulating it to the mandibular teeth. The splint manufacturer requires that the modified maxillary model and the mandibular model are stabilized in the desired final occlusion and delivered to them along with the original maxillary model. In cases with no segmentation of the maxillary arch, 2 models articulated in final occlusion and stabilized with bite registration material are required.
A virtual splint planning session is then planned. The session starts by verification of patient information. This is followed by orientation of a superimposed soft-tissue profile obtained from the CT scan or digital clinical photographs. The position of key anatomic landmark, including anterior nasal spine, A point, maxillary midline, canine tip, pogonian, B point, and maxillary and mandibular first molar mesiobuccal cusp position, is identified in 3 dimensions. These measurements may be compared to those obtained during clinical examination to ensure correct orientation and positioning of the different structures in the virtual model.
Virtual surgery starts by setting the maxillary position. This includes differential impaction for correction of any cant and advancement or setback for correction of anteroposterior deformity. The software is able to identify areas of bony overlap and interferences and assign numerical values in millimeters to such overlap to guide surgical reduction of bone on different sides of the maxillary osteotomy intraoperatively (Fig. 6). The new position of the maxilla is viewed in frontal, profile, and worm’s-eye view positions. The effect of the planned surgical movement on the 3-dimensional position of the aforementioned anatomical landmarks is evaluated. At this point, adjustment of the position and angulation of the maxillomandibular complex is possible to allow for precise control of key anatomical landmarks, like ANS, and thus achieving the most desirable aesthetic outcome (Fig. 7).
Fig. 6.
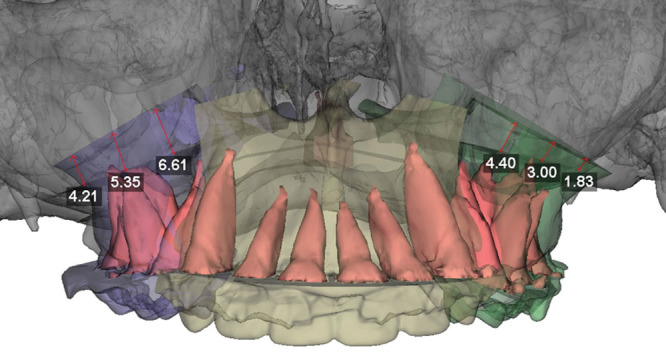
VSP maxillary movement with bony overlap.
Fig. 7.
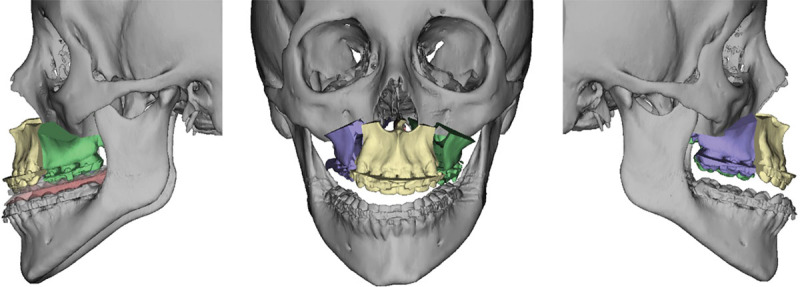
Three-piece LeFort I for intermediate position.
This is followed by simulation of the mandibular surgery. First, information from hand-articulated casts is used to position the distal segment of the mandible in final occlusion against the maxillary teeth. The relationship of the proximal segments to the distal segment of the mandible is then visualized and any gaps or overlaps are identified (Fig. 8). This allows the surgeon to anticipate any required modification or bone grafting of the segments intraoperatively to ensure a stable fixation. The symmetry and final position of the mandible are evaluated from a worm’s-eye view and frontal and profile views (Fig. 9). Similarly, the final position of the pogonion is evaluated to ensure an optimum aesthetic outcome. Once the virtual surgery is completed, the medical modeling company then fabricates stereolithographic intermediate and final occlusal splints to be used during orthognathic surgery.
Fig. 8.
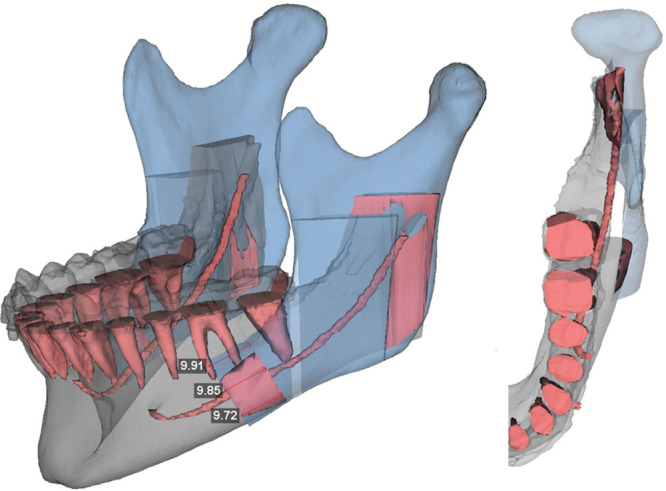
VSP mandibular movement with bony overlap.
Fig. 9.
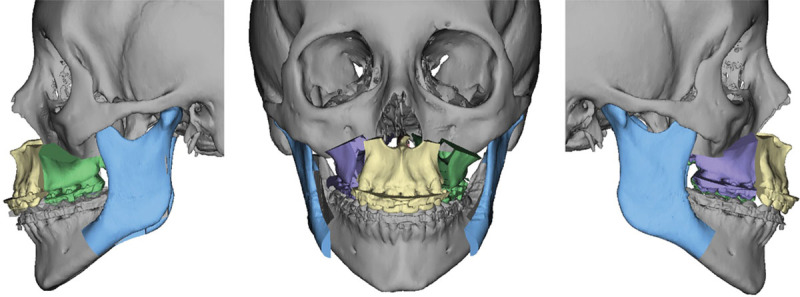
Three-piece LeFort I and BSSO for final position.
DISCUSSION
Orthognathic surgery is a multidisciplinary field that requires extensive experience and knowledge in dental craniomaxillofacial relationships and deformities. The traditional method of clinical quantitative analysis, cephalometric and radiographic analysis, analytical model analysis, and model surgery for the production of intermediate and final splints used in double-jaw surgery has withstood the test of time. This process, however, is extremely time-consuming. Without having a multidisciplinary team and a highly effective and efficient system in place, it becomes very difficult for the solo private practitioner to continue to provide orthognathic surgical care, especially in light of the significant insurance limitations as it relates to compensation for the surgical procedure, not to mention the lack of compensation for the presurgical workup and splint fabrication. This inevitably has led many clinicians away from practicing orthognathic surgery as their time could be more cost-effective doing other procedures. By contrast, at large academic institutions that have access to oral surgery, plastic surgery, and craniofacial residents and fellows, the traditional route has still proven to be highly effective and educational.
VSP has challenged the current state of presurgical orthognathic preparation and workups. What has yet to be determined is whether the application and feasibility of virtual model surgery is at a point where it will eliminate the need for traditional model surgery in both the private and academic setting. Certainly, VSP and medical modeling are significant time-saving tools that are proving to be highly accurate in terms of imaging, quantitative analysis, and predictability of aesthetic outcomes from the planned surgical movements on key components of the maxillofacial and mandibular skeleton and their overlying soft-tissue components.23 VSP splints, which have been found to be as accurate as acrylic splints in a 2003 study, actually allow surgeons to eliminate the use of acrylic splints that (1) can often have warping issues leading to poor intraoperative fits and (2) are obtained from model surgery stones that undergo an nonnegligible amount of deterioration and blunting of the occlusal surfaces from overuse and manipulation of the models to obtain splints.24 Not only are the splints highly reliable in their construction and accuracy but also surgeons are reporting results from CAD/CAM surgery within 2 mm of predicted maxillary and mandibular positions when comparing the planned and postoperative outcomes.10
Although there are many obvious advantages to VSP, there are still significant limitations. The ability of cone beam and/or medical CTs to capture the occlusal surfaces of the maxilla and mandible is nonexistent. Currently, treating physicians must complete the initial steps of traditional model surgery of taking dental impressions in the office and pouring model stones for acquisition of occlusal surfaces. These are subsequently sent to a technician assisting in the virtual model surgery who laser scans the surfaces which are then integrated into the CT images. Another contentious area VSP relates to is multisegment LeFort I osteotomies. At this time, companies providing VSP products advise against virtual segmenting of the maxilla as it is not Food and Drug Administration approved. The current recommendations are for the clinician to take an impression of the maxilla, pour the impression in stone, and subsequently perform the 3-piece LeFort I osteotomy. The segments are then repositioned and affixed into 1 piece and placed into final occlusion with the mandibular model and sent to the company for registration of the desired final bite. This effectively encompasses all of the time-consuming traditional methods of model surgery excluding face-bow transfer, mounting of the model, and production of the intermediate and final splints. This is important to consider as many patients treated in a craniofacial center are cleft patients who by virtue of the cleft undergo multisegment LeFort procedures.
CONCLUSIONS
Through the advent and surgical advances of orthognathic surgical planning, it seems inevitable that in the near future VSP and medical modeling will eliminate analytical model surgery and traditional presurgical workup. The question now is whether or not that time has come and if, in fact, the days and hours spent in the dental laboratory performing model surgery and fabricating acrylic splints are behind us. With regard to jaw surgery and the use of VSP, it is best to delineate which surgeries are best served by traditional model surgery versus 3D surgical planning. To better understand this, see Figure 10 for a proposed orthognathic surgical algorithm.
Fig. 10.
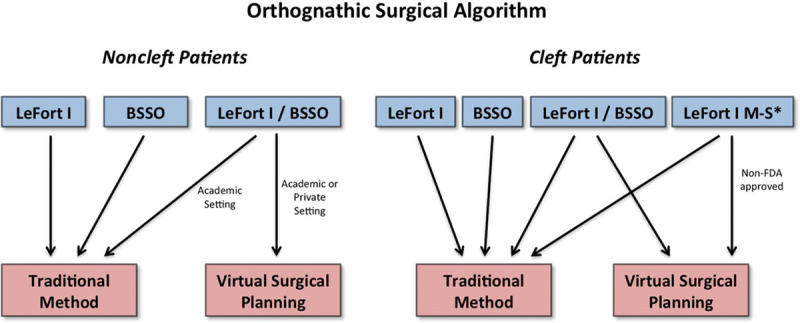
Proposed algorithm for tradition model surgery versus virtual surgical planning.
For single-jaw surgery, it is our opinion that traditional model surgery in a reasonably supported environment still reigns superior to VSP. An experienced craniomaxillofacial surgeon who is facile at taking dental impressions, pouring the stones, and mounting maxillary and mandibular stones on a simple hinge articulator can set the final occlusion and fabricate any acrylic splint in well under an hour. A surgeon can cut that time investment to 15 minutes by having an assistant obtain the impressions and pour the models. The simplicity, minimal time commitment, and cost savings in preoperative preparation for single-jaw surgery dictate the practice of traditional model surgery in this scenario. This is also valid for straightforward bilateral sagittal split osteotomies surgical procedures.
The true application and potential superiority of VSP lies in the double-jaw procedures, where a LeFort I and a BSSO are necessary. In the traditional route, this would require the clinician to take an impression of the upper and lower jaw, obtain a face-bow transfer, pour plaster stone models, mount the maxilla on the mandible per the face bow, perform a LeFort I osteotomy to fabricate an intermediate splint on a semiadjustable articulator, and finally mount the surgically modified maxilla onto the mandible for the desired occlusion to fabricate the final splint. Even if the practitioner is experienced, this is extremely time-consuming and prohibitive in a solo practice in terms of compensation. It is in this setting that virtual planning and medical modeling prove to be the method of choice.
In larger academic institutions with residents and fellows, the traditional method is not time nor cost prohibitive and found to be highly educational and informative. It teaches various principles to the novice surgeon who will actually perform the operation on models to fabricate the intermediate and final splint. It allows the novice surgeon to have an outstanding spatial relationship of the 3-dimensional movements necessary to perform successful jaw surgery, which will facilitate their true intraoperative experience. As stated, however, this requires institutional organization and support. On the other hand, in a world becoming more and more dependent on technology, the accuracy of VSP and its educational possibilities cannot be overlooked. It is, however, important to note the limitation in VSP osteotomies which should be addressed:
Currently, the Food and Drug Administration has not approved VSP for multiple-piece LeFort planning. This may or may not discourage practitioners from using the program as it brings into account risk mitigation.
The inability of VSP to incorporate occlusal surfaces into the CT images; thus, the practitioner still must take dental impressions and pour stone models to submit to VSP technicians so they may scan the occlusal surfaces of the teeth.
The requirement of having to use an intermediary technician to facilitate VSP.
A splint fabrication time lag from completion of VSP to splint delivery.
Splint production by an outside laboratory as opposed to an in-house 3D printer or milling device.
Cost restriction of VSP includes an uncovered insurance benefit. Cost range to the patient or the practitioner is $800–$1200.
Lack of long-term follow-up data documenting the efficacy of VSP.
When these limitations are mitigated, craniofacial centers treating cleft and syndromic patients with complex dentofacial abnormalities will be in a position where VSP replaces traditional model surgery in its entirety.
In summary, it is our opinion that virtual model surgery will clearly displace and replace traditional model surgery. The obstacles that need to be overcome for this to happen are not difficult, and this will likely happen in the near future. At some point, VSP will be cost and time effective in the private and academic setting and practitioners providing orthognathic surgical care will no longer have to endure the tedious application of analytical model surgery. With all of this being said, however, it seems appropriate to end on a quote from Hausamen5 in a 2001 article: “(one) has to realize that every step forward, every allegedly new development as well as our entire knowledge and technical know-how in medicine are only temporary; they have only a transitory character; they are certain to change.”
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was waived at the discretion of the Editor-in-Chief.
REFERENCES
- 1.Erickson KL, Bell WH, Goldsmith DH. Analytical model surgery. In: Bell WH, editor. In: Modern Practice in Orthognathic and Reconstructive Surgery. Philadelphia, PA: Saunders; 1992. pp. 154–216. [Google Scholar]
- 2.Bell WH, Jacobs JD, Quejada JG. Simultaneous repositioning of the maxilla, mandible, and chin. Treatment planning and analysis of soft tissues. Am J Orthod. 1986;89:28–50. doi: 10.1016/0002-9416(86)90110-7. [DOI] [PubMed] [Google Scholar]
- 3.Levine JP, Patel A, Saadeh PB, et al. Computer-aided design and manufacturing in craniomaxillofacial surgery: the new state of the art. J Craniofac Surg. 2012;23:288–293. doi: 10.1097/SCS.0b013e318241ba92. [DOI] [PubMed] [Google Scholar]
- 4.Steinhäuser EW. [Retrospective view of the development of malocclusion surgery and prospects]. Mund Kiefer Gesichtschir. 2003;7:371–379. doi: 10.1007/s10006-003-0509-5. [DOI] [PubMed] [Google Scholar]
- 5.Hausamen JE. The scientific development of maxillofacial surgery in the 20th century and an outlook into the future. J Craniomaxillofac Surg. 2001;29:2–21. doi: 10.1054/jcms.2000.0174. [DOI] [PubMed] [Google Scholar]
- 6.Proffit WR, White RP., Jr. Who needs surgical-orthodontic treatment? Int J Adult Orthodon Orthognath Surg. 1990;5:81–89. [PubMed] [Google Scholar]
- 7.Bell RB. Computer planning and intraoperative navigation in cranio-maxillofacial surgery. Oral Maxillofac Surg Clin North Am. 2010;22:135–156. doi: 10.1016/j.coms.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Bell RB. Computer planning and intraoperative navigation in orthognathic surgery. J Oral Maxillofac Surg. 2011;69:592–605. doi: 10.1016/j.joms.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Gateno J, Xia JJ, Teichgraeber JF, et al. Clinical feasibility of computer-aided surgical simulation (CASS) in the treatment of complex cranio-maxillofacial deformities. J Oral Maxillofac Surg. 2007;65:728–734. doi: 10.1016/j.joms.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Gelesko S, Markiewicz MR, Weimer K, et al. Computer-aided orthognathic surgery. Atlas Oral Maxillofac Surg Clin North Am. 2012;20:107–118. doi: 10.1016/j.cxom.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Xia J, Ip HH, Samman N, et al. Computer-assisted three-dimensional surgical planning and simulation: 3D virtual osteotomy. Int J Oral Maxillofac Surg. 2000;29:11–17. [PubMed] [Google Scholar]
- 12.Polley JW, Figueroa AA. Orthognathic positioning system: intraoperative system to transfer virtual surgical plan to operating field during orthognathic surgery. J Oral Maxillofac Surg. 2013;71:911–920. doi: 10.1016/j.joms.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Bell WH, Mannai C, Luhr HG. Art and science of the Le Fort I down fracture. Int J Adult Orthodon Orthognath Surg. 1988;3:23–52. [PubMed] [Google Scholar]
- 14.Epker BN, Turvey T, Fish LC. Indications for simultaneous mobilization of the maxilla and mandible for the correction of dentofacial deformities. Oral Surg Oral Med Oral Pathol. 1982;54:369–381. doi: 10.1016/0030-4220(82)90381-4. [DOI] [PubMed] [Google Scholar]
- 15.Bell WH, Jacobs JD. Tridimensional planning for surgical/orthodontic treatment of mandibular excess. Am J Orthod. 1981;80:263–288. doi: 10.1016/0002-9416(81)90290-6. [DOI] [PubMed] [Google Scholar]
- 16.Park N, Posnick JC. Accuracy of analytic model planning in bimaxillary surgery. Int J Oral Maxillofac Surg. 2013;42:807–813. doi: 10.1016/j.ijom.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Marko JV. Simple hinge and semiadjustable articulators in orthognathic surgery. Am J Orthod Dentofacial Orthop. 1986;90:37–44. doi: 10.1016/0889-5406(86)90025-9. [DOI] [PubMed] [Google Scholar]
- 18.Henry CH. Modified Boley gauge for use as a reference plane in orthognathic surgery. J Oral Maxillofac Surg. 1990;48:535–539. doi: 10.1016/0278-2391(90)90250-6. [DOI] [PubMed] [Google Scholar]
- 19.Perkins SJ, Newhouse RF, Bach DE. A modified Boley gauge for accurate measurement during maxillary osteotomies. J Oral Maxillofac Surg. 1992;50:1018–1019. doi: 10.1016/0278-2391(92)90067-a. [DOI] [PubMed] [Google Scholar]
- 20.Ellis E., III Bimaxillary surgery using an intermediate splint to position the maxilla. J Oral Maxillofac Surg. 1999;57:53–56. doi: 10.1016/s0278-2391(99)90633-x. [DOI] [PubMed] [Google Scholar]
- 21.Swennen GR, Mollemans W, Schutyser F. Three-dimensional treatment planning of orthognathic surgery in the era of virtual imaging. J Oral Maxillofac Surg. 2009;67:2080–2092. doi: 10.1016/j.joms.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Xia JJ, Gateno J, Teichgraeber JF. New clinical protocol to evaluate craniomaxillofacial deformity and plan surgical correction. J Oral Maxillofac Surg. 2009;67:2093–2106. doi: 10.1016/j.joms.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchetti C, Bianchi A, Muyldermans L, et al. Validation of new soft tissue software in orthognathic surgery planning. Int J Oral Maxillofac Surg. 2011;40:26–32. doi: 10.1016/j.ijom.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Gateno J, Xia J, Teichgraeber JF, et al. The precision of computer-generated surgical splints. J Oral Maxillofac Surg. 2003;61:814–817. doi: 10.1016/s0278-2391(03)00240-4. [DOI] [PubMed] [Google Scholar]


