Abstract
During the past decades, anticancer immunotherapy has evolved from a promising therapeutic option to a robust clinical reality. Many immunotherapeutic regimens are now approved by the US Food and Drug Administration and the European Medicines Agency for use in cancer patients, and many others are being investigated as standalone therapeutic interventions or combined with conventional treatments in clinical studies. Immunotherapies may be subdivided into “passive” and “active” based on their ability to engage the host immune system against cancer. Since the anticancer activity of most passive immunotherapeutics (including tumor-targeting monoclonal antibodies) also relies on the host immune system, this classification does not properly reflect the complexity of the drug-host-tumor interaction. Alternatively, anticancer immunotherapeutics can be classified according to their antigen specificity. While some immunotherapies specifically target one (or a few) defined tumor-associated antigen(s), others operate in a relatively non-specific manner and boost natural or therapy-elicited anticancer immune responses of unknown and often broad specificity. Here, we propose a critical, integrated classification of anticancer immunotherapies and discuss the clinical relevance of these approaches.
Keywords: adoptive cell transfer, checkpoint blockers, dendritic cell-based interventions, DNA-based vaccines, immunostimulatory cytokines, peptide-based vaccines, oncolytic viruses, Toll-like receptor agonists
INTRODUCTION
Our perception of cancer has changed dramatically during the past 3 decades. For instance, it has been appreciated that tumors are not a purely clonal disorder, although in some cases they do evolve from a single (pre-)malignant cell [1-3]. It is now clear that established neoplasms do not consist only of transformed cells, but contain an abundant and heterogeneous non-transformed component, including stromal, endothelial and immune cells [4-6]. We no longer consider the metabolism of cancer cells as completely distinct from that of their normal counterparts [7-9]. We have shown that the survival of transformed cells can critically depend on adaptive responses that per se are non-tumorigenic, establishing the concept of non-oncogene addiction [10, 11]. We discovered mechanisms other than intrinsic apoptosis that may be harnessed for therapeutic applications, such as several forms of regulated necrosis [12-14]. Finally, we obtained evidence indicating that the host immune system can recognize (and sometimes react against) (pre-)malignant cells as they transform, proliferate, evolve and respond to therapy, founding the theoretical grounds of anticancer immunosurveillance [15-17]. These conceptual shifts have profound therapeutic implications, some of which have already been translated into clinical realities. For instance, several anticancer agents that are now approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for use in cancer patients inhibit tumor-associated angiogenesis, perhaps the best characterized interaction between malignant and non-malignant components of the tumor microenvironment [18, 19].
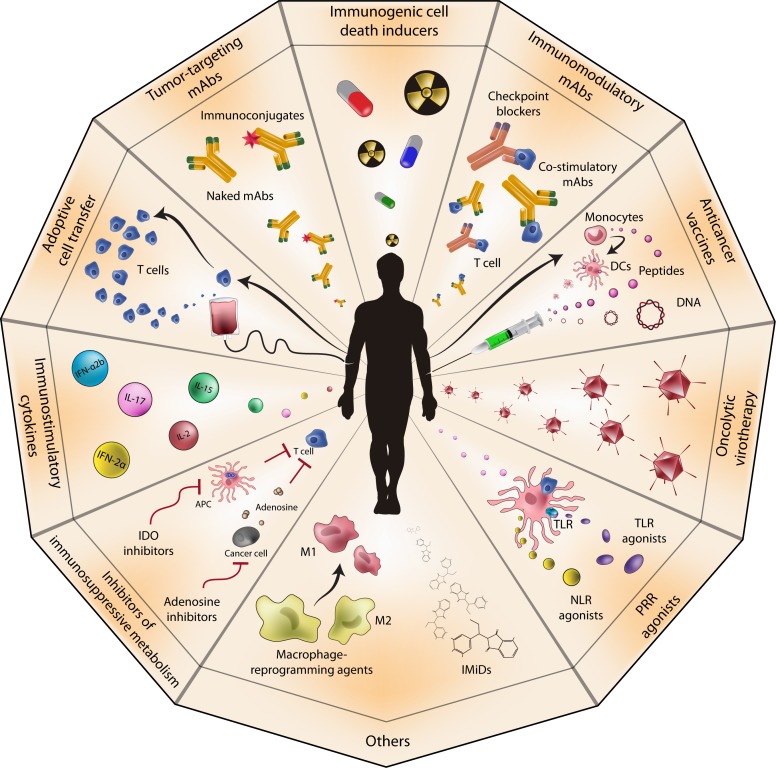
Over the last decade, great efforts have been dedicated to the development of interventions that mediate antineoplastic effects by initiating a novel or boosting an existing immune response against neoplastic cells (Table 1) [20-32]. This intense wave of preclinical and clinical investigation culminated with the approval of various immunotherapeutic interventions for use in humans (Table 2). In 2013, the extraordinary clinical success of immunotherapy was acknowledged by the Editors of Science Magazine with the designation of “Breakthrough of the Year” [33]. Nonetheless, we have just begun to unravel the therapeutic possibilities offered by anticancer immunotherapy. Clinical studies are being initiated at an ever accelerating pace to test the safety and efficacy of various immunotherapeutic regimens in cancer patients, either as standalone interventions or combined with other antineoplastic agents [34]. The hopes generated by this approach are immense, and several other forms of immunotherapy are expected to obtain regulatory approval within the next few years (Figure 1).
Table 1. Currently available anticancer immunotherapies.
| Paradigm | Licensed* |
|---|---|
| Tumor-targeting mAbs | YES |
| Adoptive cell transfer | NO |
| Oncolytic viruses | YES |
| DC-based interventions | YES |
| DNA-based vaccines | NO |
| Peptide-based vaccines | YES |
| Immunostimulatory cytokines | YES |
| Immunomodulatory mAbs | YES |
| Inhibitors of immunosuppressive metabolism | NO |
| PRR agonists | YES |
| ICD inducers | YES |
| Others | YES |
Abbreviations. ICD, immunogenic cell death; DC, dendritic cell; mAb, monoclonal antibody; PRR, pattern recognition receptor.
in one of its forms for use in cancer patients, by the US Food and Drug Administration or equivalent regulatory agency worldwide.
Table 2. Anticancer immunotherapeutics currently approved by regulatory agencies worldwide.
| Paradigm | Agent | Indication(s) | Year* | Proposed mechanism of action |
|---|---|---|---|---|
| Dendritic cell-based immunotherapies | Sipuleucel-T | Prostate carcinoma | 2010 | Priming of a PAP-specific immune response |
| Immunogenic cell death inducers | Bleomycin | Multiple hematological and solid tumors |
<1995 | DNA-damaging agent |
| Bortezomib | Mantle cell lymphoma Multiple myeloma |
2003 | Proteasomal inhibitor | |
| Cyclophosphamide | Multiple hematological and solid tumors |
<1995 | Alkylating agent | |
| Doxorubicin | Multiple hematological and solid tumors |
<1995 | DNA-intercalating agent | |
| Epirubicin | Breast carcinoma | 1999 | DNA-intercalating agent | |
| Mitoxantrone | Acute myeloid leukemia Prostate carcinoma |
<1995 | DNA-intercalating agent | |
| Oxaliplatin | Colorectal carcinoma | 2002 | DNA-damaging agent | |
| Photodynamic therapy | Multiple hematological and solid tumors |
1996 | Induction of oxidative stress with damage to (intra)cellular membranes | |
| Radiation therapy | Multiple hematological and solid tumors |
<1995 | DNA-damaging agent and oxidative stress inducer | |
| Immunostimulatory cytokines | IL-2 | Melanoma Renal cell carcinoma |
<1995 | Non-specific immunostimulation |
| IFN-α2a | Chronic myeloid leukemia Hairy cell leukemia Melanoma |
1999 | Non-specific immunostimulation | |
| IFN-α2b | Multiple hematological and solid tumors |
<1995 | Non-specific immunostimulation | |
| Immunomodulatory mAbs | Ipilimumab | Melanoma | 2011 | Blockage of CTLA4-dependent immunological checkpoints |
| Nivolumab | Melanoma | 2014 | Blockage of PDCD1-dependent immunological checkpoints | |
| Pembrolizumab | Melanoma | 2014 | Blockage of PDCD1-dependent immunological checkpoints | |
| Oncolytic viruses | Oncorine H101 | Head and neck cancer | 2005 | Selective lysis of malignant cells |
| Peptide-based vaccines | Vitespen | Renal cell carcinoma | 2008 | Activation of a tumor-specific immune response |
| PRR agonists | Bacillus Calmette-Guérin | Non-invasive bladder transitional cell carcinoma |
<1995 | TLR2/TLR4 agonist |
| Imiquimod | Actinic keratosis Condylomata acuminata Superficial basal cell carcinoma |
1997 | TLR7 agonist | |
| Mifamurtide | Osteosarcoma | 2009 | NOD2 agonist | |
| Monophosphoryl lipid A | Prevention of HPV-associated cervical carcinoma | 2009 | TLR2/TLR4 agonist | |
| Picibanil | Gastric carcinoma Head and neck cancer Lung carcinoma Thyroid carcinoma |
<1995 | TLR2/TLR4 agonist | |
| Tumor-targeting mAbs | Alemtuzumab | Chronic lymphocytic leukemia | 2001 | Selective recognition/opsonization of CD52+ neoplastic cells |
| Bevacizumab | Colorectal carcinoma Glioblastoma multiforme Cervical carcinoma Lung carcinoma Renal cell carcinoma |
2004 | VEGFA neutralization | |
| Brentuximab vedotin | Anaplastic large cell lymphoma Hodgkin's lymphoma |
2011 | Selective delivery of MMAE to CD30+ neoplastic cells | |
| Blinatumumab | Acute lymphoblastic leukemia | 2014 | CD3- and CD19-specific BiTE | |
| Catumaxomab | Malignant ascites in patients with EPCAM+ cancer |
2009 | CD3- and EPCAM-specific BiTE | |
| Cetuximab | Head and neck cancer Colorectal carcinoma |
2004 | Inhibition of EGFR signaling | |
| Denosumab | Breast carcinoma Prostate carcinoma Bone giant cell tumors |
2011 | Inhibition of RANKL signaling | |
| Gemtuzumab ozogamicin | Acute myeloid leukemia | 2000 | Selective delivery of calicheamicin to CD33+ neoplastic cells | |
| Ibritumomab tiuxetan | Non-Hodgkin lymphoma | 2002 | Selective delivery of 90Y or 111In to CD20+ neoplastic cells | |
| Panitumumab | Colorectal carcinoma | 2006 | Inhibition of EGFR signaling | |
| Pertuzumab | Breast carcinoma | 2012 | Inhibition of HER2 signaling | |
| Obinutuzumab | Chronic lymphocytic leukemia | 2013 | Selective recognition/opsonization of CD20+ neoplastic cells | |
| Ofatumumab | Chronic lymphocytic leukemia | 2009 | Selective recognition/opsonization of CD20+ neoplastic cells | |
| Ramucirumab | Gastric or gastroesophageal junction adenocarcinoma |
2014 | Inhibition of KDR signaling | |
| Rituximab | Chronic lymphocytic leukemia Non-Hodgkin lymphoma |
1997 | Selective recognition/opsonization of CD20+ neoplastic cells | |
| Siltuximab | Multicentric Castleman's disease | 2014 | IL-6 neutralization | |
| Tositumomab | Non-Hodgkin lymphoma | 2003 | Selective recognition/opsonization of, or selective delivery of 90Y or 111In to, CD20+ neoplastic cells | |
| Trastuzumab | Breast carcinoma Gastric or gastroesophageal junction adenocarcinoma |
1998 | Selective recognition/opsonization of, or selective delivery of mertansine to, HER2+ cancer cells | |
| Others | Lenalidomide | Mantle cell lymphoma Myelodysplastic syndrome Multiple myeloma |
2005 | IKZF degradation and immunomodulation |
| Pomalidomide | Multiple myeloma | 2013 | IKZF degradation and immunomodulation | |
| Thalidomide | Multiple myeloma | 2006 | IKZF degradation and immunomodulation | |
| Trabectedin | Soft tissue sarcoma Ovarian carcinoma |
2007 | Reprogramming of tumor-associated macrophages |
Abbreviations: ACPP, acid phosphatase, prostate; BiTE, Bispecific T-cell engager; CTLA4, cytotoxic T lymphocyte-associated protein 4; EGFR, epidermal growth factor receptor; EPCAM, epithelial cell adhesion molecule; HPV, human papillomavirus; IL, interleukin; IKZF, IKAROS family zinc finger; KDR, kinase insert domain receptor; mAb, monoclonal antibody; MMAE, monomethyl auristatin E; NOD2, nucleotide-binding oligomerization domain containing 2; PDCD1, programmed cell death 1; PRR, pattern recognition receptor; RANKL, Receptor activator of NF-κB ligand; TLR, Toll-like receptor; VEGFA, vascular endothelial growth factor A.
year of first approval.
Figure 1. Anticancer immunotherapy.
Several anticancer immunotherapeutics have been developed during the last three decades, including tumor-targeting and immunomodulatory monoclonal antibodies (mAbs); dendritic cell (DC)-, peptide- and DNA-based anticancer vaccines; oncolytic viruses; pattern recognition receptor (PRR) agonists; immunostimulatory cytokines; immunogenic cell death inducers; inhibitors of immunosuppressive metabolism; and adoptive cell transfer. 1MT, 1-methyltryptophan; APC, antigen-presenting cell; IDO, indoleamine 2,3-dioxigenase; IFN, interferon; IL, interleukin; IMiD, immunomodulatory drug; NLR, NOD-like receptor; TLR, Toll-like receptor.
Anticancer immunotherapies are generally classified as “passive” or “active” based on their ability to (re-)activate the host immune system against malignant cells [35]. From this standpoint, tumor-targeting monoclonal antibodies (mAbs) and adoptively transferred T cells (among other approaches) are considered passive forms of immunotherapy, as they are endowed with intrinsic antineoplastic activity [23, 24, 36, 37]. Conversely, anticancer vaccines and checkpoint inhibitors exert anticancer effects only upon the engagement of the host immune system, constituting clear examples of active immunotherapy [22, 27, 28, 32, 38]. An alternative classification of immunotherapeutic anticancer regimens is based on antigen-specificity. Thus, while tumor-targeting mAbs are widely considered antigen-specific interventions, immunostimulatory cytokines or checkpoint blockers activate anticancer immune responses of unknown (and generally broad) specificity [27, 39-42]. Herein, we critically revise these classifications while discussing the clinical relevance of various forms of anticancer immunotherapy.
Passive immunotherapy
Tumor-targeting mAbs
Tumor-targeting mAbs are the best-characterized form of anticancer immunotherapy, and perhaps the most widely employed in the clinic [43-46]. The expression “tumor-targeting” refers to mAbs that (1) specifically alter the signaling functions of receptors expressed on the surface of malignant cells [47-49]; (2) bind to, and hence neutralize, trophic signals produced by malignant cells or by stromal components of neoplastic lesions [50, 51]; (3) selectively recognize cancer cells based on the expression of a “tumor-associated antigen” (TAA), i.e., an antigen specifically (or at least predominantly) expressed by transformed cells but not (or at least less so) by their non-malignant counterparts [30, 52]. Tumor-targeting mAbs exist in at least 5 functionally distinct variants. First, naked mAbs that inhibit signaling pathways required for the survival or progression of neoplastic cells, but not of their non-malignant counterparts, such as the epidermal growth factor receptor (EGFR)-specific mAb cetuximab, which is approved by the US FDA for the treatment of head and neck cancer (HNC) and colorectal carcinoma (CRC) [47, 48, 53]. Second, naked mAbs that activate potentially lethal receptors expressed on the surface of malignant cells, but not of their non-transformed counterparts, such as tigatuzumab (CS-1008), a mAb specific for tumor necrosis factor receptor superfamily, member 10B, (TNFRSF10B, best known as TRAILR2 or DR5) that is currently under clinical development [49, 54]. Third, immune conjugates, i.e., TAA-specific mAbs coupled to toxins or radionuclides, such as gemtuzumab ozogamicin, an anti-CD33 calicheamicin conjugate currently approved for use in acute myeloid leukemia patients [55, 56]. Fourth, naked TAA-specific mAbs that opsonize cancer cells and hence activate antibody-dependent cell-mediated cytotoxicity (ADCC) [44, 57-59], antibody-dependent cellular phagocytosis [60], and complement-dependent cytotoxicity [61], such as the CD20-specific mAb rituximab, which is currently approved for the treatment of chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma [62, 63]. Fifth, so-called “bispecific T-cell engagers” (BiTEs), i.e., chimeric proteins consisting of two single-chain variable fragments from distinct mAbs, one targeting a TAA and one specific for a T-cell surface antigen (e.g., blinatumomab, a CD19- and CD3 BiTE recently approved for the therapy of Philadelphia chromosome-negative precursor B-cell acute lymphoblastic leukemia) [64-69].
The therapeutic activity of opsonizing mAbs and BiTEs clearly relies on the host immune system, implying that these molecules should be considered active immunotherapeutics. Conversely, tumor-targeting mAbs of the first two classes are endowed with intrinsic antineoplastic activity, and have been considered for a long time as passive forms of immunotherapy. However, growing evidence indicates that the actual antineoplastic potential of these molecules does not simply reflect their direct tumor-inhibitory activity, but also involves (at least to some degree) the activation of an anticancer immune response. For instance, cetuximab does not only inhibit EGFR signaling [53], but also promotes ADCC [70], and mediates immunostimulatory effects [71, 72]. Similarly, bevacizumab, a vascular endothelial growth factor A (VEGFA)-neutralizing mAb approved for the treatment of glioblastoma multiforme, CRC, as well as cervical carcinoma, renal cell carcinoma (RCC) and lung carcinoma, not only exerts anti-angiogenic effects [50, 73], but also boosts tumor infiltration by B and T lymphocytes, [74, 75], while inhibiting CD4+CD25+FOXP3+ regulatory T cells (Tregs) [76]. Moreover, polymorphisms in the genes coding for the receptors mainly responsible for ADCC, i.e., Fc fragment of IgG, low affinity IIa, receptor (FCGR2A, also known as CD32) and FCGR3A (also known as CD16a), have been shown to influence the response of cancer patients to most tumor-targeting mAbs [77]. Thus, it is possible (although not formally demonstrated) that tumor-targeting mAbs operate as active immunotherapeutics. Irrespective of this possibility, 18 distinct tumor-targeting mAbs are currently approved by the US FDA for use in cancer patients (source http://www.fda.gov) [45, 46], demonstrating the extraordinary success of this immunotherapeutic paradigm.
Adoptive cell transfer
The term “adoptive cell transfer” (ACT) refers to a particular variant of cell-based anticancer immunotherapy that generally involves: (1) the collection of circulating or tumor-infiltrating lymphocytes; (2) their selection/modification/expansion/activation ex vivo; and (3) their (re-)administration to patients, most often after lymphodepleting pre-conditioning and in combination with immunostimulatory agents [23, 24, 78-80]. Other anticancer (immune)therapies involving the (re)infusion of living cells, such as hematopoietic stem cell transplantation (HSCT), conceptually differ from ACT. ACT involves the (re-)introduction of a cell population enriched in potentially tumor-reactive immune effectors [23, 24, 81]. HSCT is employed as a means to reconstitute a healthy, allogeneic (and hence potentially tumor-reactive) immune system in patients with hematological malignancies previously subjected to myelo- and lymphoablating treatments (which aim at eradicating the majority of neoplastic cells) [82]. Dendritic cell (DC)-based interventions should also be conceptually differentiated from ACT for two reasons. First, (re-)infused DCs are not endowed with intrinsic anticancer activity, but act as anticancer vaccines to elicit a tumor-targeting immune response [83, 84]. Second, DCs are not administered in the context of lympho/myeloablating chemo(radio)therapy [85-87].
Several strategies have been devised to improve the therapeutic potential of ACT [79, 80, 88]. For instance, genetic engineering has been employed to endow peripheral blood lymphocytes (PBLs) with features such as a unique antigen specificity [89], an increased proliferative potential and persistence in vivo [90-93], an improved secretory profile [91], an elevated tumor-infiltrating capacity [94, 95], and superior cytotoxicity [96]. The specificity of PBLs can be altered prior to (re-)infusion by genetically modifying them to express: (1) a TAA-specific T-cell receptor (TCR) [89, 97-99], or (2) a so-called “chimeric antigen receptor” (CAR), i.e., a transmembrane protein comprising the TAA-binding domain of an immunoglobulin linked to one or more immunostimulatory domains [100-106]. The latter approach is advantageous in that it renders T cells capable of recognizing (and hence potentially killing) TAA-expressing cells in an MHC-independent fashion. Several clinical trials have already demonstrated the therapeutic potential of CAR-expressing T cells, in particular (but not only) for patients affected by hematological malignancies [102, 107-111]. T cells expressing TAA-specific TCRs have also been shown to provide objective benefit to cancer patients [89, 97-99]. Conversely, in spite of promising preclinical findings [112-117], the adoptive transfer of purified natural killer (NK) cells to cancer patients has been associated with limited therapeutic activity [118-120]. To the best of our knowledge, the adoptive transfer of purified B lymphocytes has not yet been investigated in the clinic [121], possibly because B cells (or at least some subsets thereof) can exert potent immunosuppressive effects [122-125]. Of note, no ACT protocol is currently approved by the US FDA for use in cancer patients (source http://www.fda.gov).
Since (re-)infused T cells are endowed with intrinsic antineoplastic activity, ACT is generally considered as a passive form of immunotherapy. However, the survival, expansion, migration and cytotoxic activity of adoptively transferred T cells rely on several cytokines, some of which are supplied by the host immune system. Current ACT protocols involve indeed the administration of exogenous interleukins (ILs), including IL-2, IL-15 or IL-21 [126-130], but these stimulate a cytokine cascade in the host that sustains the survival and activity of adoptively transferred cells. Thus, ACT may not represent a bona fide paradigm of passive immunotherapy.
Oncolytic viruses
The term “oncolytic viruses” refers to non-pathogenic viral strains that specifically infect cancer cells, triggering their demise [131-133]. Oncolytic viruses must be conceptually differentiated from so-called “oncotropic viruses”, i.e., viruses that exhibit a preferential tropism for malignant cells but no (or very limited) cytotoxic activity [134, 135]. The antineoplastic potential of oncolytic viruses can be innate and simply originate from the so-called cytopathic effect, i.e., the lethal overload of cellular metabolism resulting from a productive viral infection [136, 137]. As an alternative, these viruses can mediate an oncolytic activity because of (endogenous or exogenous) gene products that are potentially lethal for the host cell, irrespective of their capacity to massively replicate and cause a cytopathic effect [131, 132]. Of note, genetic engineering has been successfully employed to endow oncolytic virus with various advantageous traits, including sequences coding for (1) enzymes that convert an innocuous pro-drug into a cytotoxic agent [138-143]; (2) proteins that (at least theoretically) trigger lethal signaling cascades in cancer cells only [144-146]; or (3) short-hairpin RNAs that target factors that are strictly required for the survival of transformed, but not normal cells [147, 148]. Of note, no oncolytic virus has been approved by the US FDA for use in cancer patients (source http://www.fda.gov). Conversely, a recombinant adenovirus (H101, commercialized under the name of Oncorine®) has been approved by the regulatory authorities of the People's Republic of China for the treatment of HNC (in combination with chemotherapy) as early as in November 2005 [149, 150].
As oncolytic viruses are endowed with intrinsic anticancer activity, they are generally viewed as passive immunotherapeutics. Moreover, several effectors of innate and adaptive immunity limit the efficacy of oncolytic therapy because they can neutralize viral particles before they reach neoplastic lesions [131, 132, 151]. This is particularly true for the mononuclear phagocytic system of the liver and spleen, which is able to sequester large amounts of oncolytic viruses upon injection [152, 153]; the complement system, to which oncolytic viruses are particularly sensitive [154, 155]; and neutralizing antibodies, which can exist in patients prior to oncolytic virotherapy owing to their exposure to naturally occurring variants of the viral strains commonly employed for this purpose [156, 157]. This being said, accumulating preclinical and clinical evidence indicates that the therapeutic activity of oncolytic viruses stems, for the most part, from their ability to elicit tumor-targeting immune responses as they promote the release of TAAs in an immunostimulatory context. In support of this notion, oncolytic viruses engineered to drive the expression of co-stimulatory receptors [158-160] or immunostimulatory cytokines/chemokines [161-165] reportedly mediate superior antineoplastic effects as compared to their unmodified counterparts [131, 132]. Thus, conventional oncolytic viruses also appear to be active, rather than passive, immunotherapeutics.
Active immunotherapy
DC-based immunotherapies
Throughout the past 2 decades, remarkable efforts have been invested in the development of anticancer immunotherapeutics based on (most often autologous) DCs [28, 166, 167]. This intense wave of preclinical and clinical investigation reflects the critical position occupied by DCs at the interface between innate and adaptive immunity, and the ability of some DC subsets to prime robust, therapeutically relevant anticancer immune responses [168]. Several forms of DC-based immunotherapy have been developed, most of which involve the isolation of patient- or donor-derived circulating monocytes and their amplification/differentiation ex vivo, invariably in the presence of agents that promote DC maturation, such as granulocyte macrophage colony-stimulating factor (GM-CSF) [28]. This is particularly important because immature DCs exert immunosuppressive, rather than immunostimulatory, functions [169-171]. Most often, autologous DCs are re-infused into cancer patients upon exposure to a source of TAAs, including (1) TAA-derived peptides [172-175]; (2) mRNAs coding for one or more specific TAAs [176]; (3) expression vectors coding for one or more specific TAAs [177-180]; (4) bulk cancer cell lysates (of either autologous or heterologous derivation) [181-186]; (5) or bulk cancer cell-derived mRNA [187-191]. As an alternative, DCs are allowed to fuse ex vivo with inactivated cancer cells, generating so-called dendritomes [192-197]. The rationale behind all these approaches is that DCs become loaded ex vivo with TAAs or TAA-coding molecules, hence becoming able to prime TAA-targeting immune responses upon reinfusion. Additional DC-based anticancer immunotherapies include the targeting of specific TAAs to DCs in vivo [169, 198-205], the use of DC-derived exosomes [206-208], and the (re-)administration of autologous or allogeneic DCs amplified, matured and optionally genetically modified ex vivo, but not loaded with TAAs [209-214]. In the former setting, TAAs are fused to mAbs, polypeptides or carbohydrates that selectively bind to DCs [169, 198-202, 215, 216], encapsulated in DC-targeting immunoliposomes [217, 218], or (3) encoded by DC-specific vectors [219-221]. In the latter scenarios, DCs or their exosomes are administered as a relatively non-specific immunostimulatory intervention [209-213]. Interestingly, one cellular product containing a significant proportion of (partially immature) DCs is currently licensed for use in cancer patients, namely sipuleucel-T (also known as Provenge®) (source http://www.fda.gov). Sipuleucel-T has been approved by the US FDA and the EMA for the therapy of asymptomatic or minimally symptomatic metastatic castration-refractory prostate cancer as early as in 2010 [222-224]. However, the manufacturer of sipuleucel-T, Dendreon Co. (Seattle, WA, US), filed for bankruptcy in November 2014 (source http://dealbook.nytimes.com/2014/11/10/dendreon-maker-of-prostate-cancer-drug-provenge-files-for-bankruptcy/?_r=0). This reflects the disadvantageous cost-benefit ratio of such a cellular therapy, whose preparation requires a relatively elevated quantity of each patient's peripheral blood mononuclear cells [25, 222, 223]. The safety and efficacy of many DC-based cellular preparations other than are sipuleucel-T are currently being investigated in clinical settings, with promising results [225].
Although DCs isolated from cancer patients have been shown to exert cytotoxic activity against malignant cells [226], DC-based immunotherapies mediate antineoplastic effects mainly because they engage the host immune system against malignant lesions [227, 228]. Thus, all forms of DC-based anticancer interventions constitute paradigms of active immunotherapy.
Peptide- and DNA-based anticancer vaccines
DCs and other antigen-presenting cells (APCs) are also targeted by peptide- and DNA-based anticancer vaccines [83, 84, 229-231]. In the former scenario, full-length recombinant TAAs or peptides thereof are administered to cancer patients, most often via the intramuscular, subcutaneous or intradermal route, together with one or more immunostimulatory agents commonly known as adjuvants (which potently promote DC maturation) [232-237]. The rationale behind this approach is that resident DCs (or other APCs) acquire the ability to present the TAA-derived epitopes while maturing, hence priming a robust TAA-specific immune response [32, 238, 239]. The mechanisms underlying the priming of anticancer immune responses by peptide-based vaccines, and hence their efficacy, depend (at least in part) on their size [38]. Thus, while short peptides (8-12 amino acids) are conceived to directly bind to MHC molecules expressed on the surface of APCs, synthetic long peptides (25-30 residues) must be taken up, processed and presented by APCs for eliciting an immune response [38]. Normally, the therapeutic activity of synthetic long peptides is superior to that of their short counterparts, especially when they include epitopes recognized by both cytotoxic and helper T cells or when conjugated to efficient adjuvants [38, 240, 241]. This said, some commonly used immunostimulants such as the so-called incomplete Freund's adjuvant (IFA) have recently been shown to limit the efficacy of peptide-based anticancer vaccination [242], calling for the use of alternative immunostimulants. A peculiar type of peptide-based vaccines is constituted by autologous tumor lysates complexed with immunostimulatory chaperones, most often members of the heat-shock protein (HSP) family [243]. This approach is advantageous in that it does not rely on a single TAA but (at least hypothetically) on all TAAs that bind to HSPs (including patient-specific neo-TAAs) [243]. However, generating anticancer vaccines on a personalized basis is associated with considerable costs [243].
DNA-based anticancer vaccines rely on TAA-coding constructs, be them naked or vectored (by viral particles, non-pathogenic bacteria or yeast cells) [32, 244-246]. DNA-based vaccines either become a source of such TAA (as it is the case for bacterial and yeast vectors) or transform APCs or muscular cells to do so (as it is the case for naked constructs and viral vectors) [32, 244-247]. Theoretically, and especially in the presence of adequate adjuvants, this prompts resident DCs or other APCs to prime a TAA-targeting immune response [32, 183, 248, 249]. A particularly interesting approach in this context is represented by so-called “oncolytic vaccines”, i.e., oncolytic viruses genetically altered to code for a TAA [250-252]. Promising results have also been obtained with DNA-based vaccines administered per os [253-256]. In this setting, live-attenuated bacteria expressing a full-length TAA are taken up by APCs in the intestinal mucosa, resulting in the priming of a robust, TAA-specific immune response in the so-called “mucosa-associated lymphoid tissue” [253-256].
Both peptide- and DNA-based vaccines have been associated with clinical activity in patients affected by various neoplasms [83, 84, 229-231, 257]. For instance, a peptide-based vaccine targeting the human papillomavirus type 16 (HPV-16) proteins E6 and E7 have been shown to promote complete, long-lasting responses in a significant fraction of patients with vulvar intraepithelial neoplasia [258]. Along similar lines, the administration of a multipeptide vaccine after single-dose cyclophosphamide (an immunogenic alkylating agent, see below) has been shown to prolong overall survival in a cohort of RCC patients [259]. No peptide- or DNA-based anticancer vaccine is currently approved by the US FDA and EMA for use in humans (sources http://www.fda.gov and http://www.ema.europa.eu/ema/). However, vitespen (Oncophage®), a heat shock protein 90kDa beta (Grp94), member 1 (HSP90B1)-based anticancer vaccine, has been approved in Russia for the treatment of RCC patients with intermediate risk of recurrence as early as in 2008 [257]. Moreover, three DNA-based anticancer vaccines have been licensed for veterinary use [260-263], one of which relies on a human TAA (i.e., tyrosinase) [263].
Similar to DC-based interventions, both peptide- and DNA-based anticancer vaccines mediate antineoplastic effects as they (re-)activate the host immune system against malignant cells, hence constituting active forms of anticancer immunotherapy.
Immunostimulatory cytokines
Taken as a family, cytokines regulate (via autocrine, paracrine or endocrine circuits) virtually all biological functions [264-267]. It is therefore not surprising that various attempts have been made to harness the biological potency of specific cytokines to elicit novel or reinvigorate pre-existent tumor-targeting immune responses [268-271]. The administration of most immunostimulatory cytokines to cancer patients as standalone therapeutic interventions, however, is generally associated with little, if any, clinical activity [272-275]. Thus, immunostimulatory cytokines are generally employed as adjuvants for other anticancer (immuno)therapeutics, either as recombinant molecules or encoded within expression vectors [276-284]. Notable exceptions include interferon (IFN)-α2b (also known as Intron A®), and IL-2 (also known as aldesleukin and Proleukin®), which mediate single agent therapeutic activity in patients affected by melanoma, a tumor type particularly sensitive to immunotherapy [274, 284]. IFN-α2b is currently approved by the US FDA and EMA for the therapy of hairy cell leukemia (HCL), AIDS-related Kaposi's sarcoma, follicular lymphoma, multiple myeloma, melanoma, external genital/perianal warts (condylomata acuminata) and cervical intraepithelial neoplasms (both as a recombinant, unmodified protein, and as a pegylated variant), while IL-2 is licensed for the treatment of metastatic forms of melanoma and RCC. Moreover, IFN-α2a (also known as Roferon-A®) is approved for use in subjects with HCL and chronic phase, Philadelphia chromosome-positive chronic myeloid leukemia, upon minimal pretreatment (within 1 year of diagnosis). In Europe, IFN-α2a is also licensed for the treatment of melanoma. Of note, GM-CSF (also known as molgramostim, sargramostim, Leukomax®, Mielogen® or Leukine®) and granulocyte colony-stimulating factor (G-CSF, also known as filgrastim, lenograstim or Neupogen®) are approved by the US FDA and EMA for use in humans, but not as part of anticancer regimens [285-288]. Nonetheless, GM-CSF has been shown to potentiate the clinical activity of several immunotherapeutics, including (but not limited to) peptide-based vaccines and immunomodulatory mAbs [259, 289]. Recombinant tumor necrosis factor α (TNFα) is also licensed by several regulatory agencies worldwide (but not by the US FDA), for the treatment of limb-threatening soft tissue sarcoma and melanoma [290-292]. However, in this setting TNFα is not employed as an immunostimulatory agent but administered in combination with melphalan (an alkylating agent) to increment the local concentration of the drug (and hence boost its cytotoxicity), and to promote the selective destruction of the tumor vasculature [293].
The antineoplastic activity of immunostimulatory cytokines is expected to depend on the host immune system, implying that they underlie a bona fide paradigm of active immunotherapy. However, the actual mode of action of immunostimulatory cytokines has not yet been fully explored. Moreover, some of these agents may promote a cytokine cascade with unwarranted, potentially lethal effects, and hence should be employed with caution.
Immunomodulatory mAbs
At odds with their tumor-targeting counterparts, immunomodulatory mAbs operate by interacting with (hence altering the function of) soluble or cellular components of the immune system [22, 294]. Thus, immunomodulatory mAbs are designed to elicit a novel or reinstate an existing anticancer immune response [27, 295, 296]. So far, this has been achieved through four general strategies: (1) the inhibition of immunosuppressive receptors expressed by activated T lymphocytes, such as cytotoxic T lymphocyte-associated protein 4 (CTLA4) [297-299] and programmed cell death 1 (PDCD1, best known as PD-1) [39, 42, 300, 301], or NK cells, like various members of the killer cell immunoglobulin-like receptor (KIR) family [302-304]; (2) the inhibition of the principal ligands of these receptors, such as the PD-1 ligand CD274 (best known as PD-L1 or B7-H1) [300, 305-307]; (3) the activation of co-stimulatory receptors expressed on the surface of immune effector cells [308] such as tumor necrosis factor receptor superfamily, member 4 (TNFRSF4, best known as OX40) [309-313], TNFRSF9 (best known as CD137 or 4-1BB) [58, 314, 315], and TNFRSF18 (best known as GITR) [316-318]; and (4) the neutralization of immunosuppressive factors released in the tumor microenvironment, such as transforming growth factor β1 (TGFβ1) [319, 320].
The first of these approaches, which is commonly referred to as “checkpoint blockade”, has been shown to induce robust and durable responses in cohorts of patients with a variety of solid tumors [39, 300, 321-327]. As it stands, no less than three checkpoint-blocking mAbs are currently approved by international regulatory agencies for use in humans (source http://www.fda.gov): (1) the anti-CTLA4 mAb ipilimumab (Yervoy™), which was licensed by the US FDA for use in individuals with unresectable or metastatic melanoma on 2011, March 25th [328-332]; the anti-PD-1 mAb pembrolizumab (Keytruda™), which received accelerated approval by the US FDA for the treatment of advanced or unresectable melanoma patients who fail to respond to other therapies on 2014, September 4th [333-338]; and nivolumab (Opvido™), another PD-1-targeting mAb licensed by the Japanese Ministry of Health and Welfare for use in humans on 2014, July 07th [339]. Based on the results of a recently completed Phase III clinical trial demonstrating that nivolumab significantly improves the progression-free and overall survival of patients with BRAFWT melanoma [340], the approval of this mAb by the US FDA is expected within the next few months. The safety and efficacy of ipilimumab, pembrolizumab, nivolumab and other checkpoint-blocking mAbs are being demonstrated in a steadily expanding panel of oncological indications [45, 46, 341, 342]. Of note, some co-stimulatory mAbs including urelumab and PF-0582566 (both of which target CD137) are also under clinical development, with promising results [46, 341]. Preclinical data suggest that combining checkpoint blockers with co-stimulatory mAb mediates superior antineoplastic effects [294, 343, 344]. At least in part, this reflects the ability of co-stimulatory mAbs to promote NK cell functions [58, 345, 346]. In line with this notion, a few clinical trials testing checkpoint blockers in combination with urelumab or lirilumab (a KIR-inhibiting mAb) have just been initiated (source http://www.clinicaltrials.gov).
Designed to (re-)activate the host immune system against malignant cells, immunomodulatory mAbs constitute an established and clinically promising paradigm of active immunotherapy. Interestingly, despite their non-specific mechanism of action, the clinical efficacy of immunomodulatory mAbs (and in particular checkpoint blockers) may be profoundly influenced by the panel of (neo-)TAAs specific to each neoplasm [347].
Inhibitors of immunosuppressive metabolism
Indoleamine 2,3-dioxigenase 1 (IDO1) catalyzes the first, rate-limiting step in the so-called “kynurenine pathway”, the catabolic cascade that converts L-tryptophan (Trp) into L-kynurenine (Kyn) [348]. Although this enzyme was initially believed to mediate immunostimulatory effects (partly because inflammatory cues including IFNγ promote its expression in cells of the innate immune system) [349, 350], IDO1 mediates robust immunosuppressive effects, in both physiological (e.g., tolerance during pregnancy) and pathological (mostly oncological) settings [351-356]. IDO1 has been proposed to inhibit both innate and adaptive immune responses (1) by depleting immune effector cells of Trp, resulting in irresponsiveness to immunological challenges [352, 353, 357-359]; (2) by favoring the accumulation of Kyn and some of its derivatives, which exert cytotoxic effects on immune effector cells while promoting the differentiation of Tregs [360-364]; or (3) through various indirect mechanisms mediated by IDO1-expressing DCs [124, 365-371]. Evidence accumulated during the last decade indicates that both 1-methyltryptophan (an inhibitor of IDO1 and IDO2) and genetic interventions targeting IDO1 mediate antineoplastic effects while eliciting novel or reinvigorating existent anticancer immune responses [372-375]. No IDO1 inhibitor is currently approved by the US FDA for use in humans (source http://www.fda.gov). However, the results of recent Phase I-II studies suggest that 1-methyl-D-tryptophan (an inhibitor of the IDO pathway also known as indoximod), other pharmacological blockers of IDO1 (such as INCB024360), and IDO1-targeting vaccines are well tolerated by cancer patients and mediate antineoplastic effects, at least in a subset of individuals [376-382].
Extracellular ATP mediates robust immunostimulatory functions as it recruits and activates APCs via purinergic receptor P2Y, G-protein coupled, 2 (P2RY2) and purinergic receptor P2X, ligand-gated ion channel, 7 (P2RX7), respectively [383-386]. On the contrary, the degradation products of ATP (notably AMP and adenosine), have a pronounced immunosuppressive activity upon binding to adenosine A2a receptor (ADORA2A) and ADORA2B [387-389]. Two enzymes operates sequentially to degrade extracellular ATP, ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1, best known as CD39), which converts ATP into ADP and AMP [390-392], and 5′-nucleotidase, ecto (NT5E, best known as CD73), which transforms AMP into adenosine [393, 394]. Some human neoplasms express increased amounts of CD39 and/or CD73, reflecting the evolutionary advantage conferred to cancer cells by the stimulation of adenosine receptors [395, 396]. Efforts have therefore been dedicated to the development of agents that would limit the extracellular availability of adenosine or inhibit adenosine receptors [392, 397]. Preclinical evidence indicates that CD39- or CD79-targeting agents (mostly mAbs) mediate antineoplastic effects as standalone interventions and improve the efficacy of other anticancer agents [397]. The clinical development of these agents, however, has not yet been initiated. Conversely, ADORA2A antagonists are currently being tested in late-stage clinical trials, but as a therapeutic option against Parkinsonism [397]. It will be interesting to determine the safety and efficacy of inhibitors of adenosine generation or signaling in cancer patients.
Although it remains unclear whether these agents truly operate by altering the microenvironmental availability of Trp and Kyn [398], the antineoplastic effects of IDO inhibitors critically rely on the host immune system, implying that this constitutes an instance of active anticancer immunotherapy [399]. This also applies to strategies aimed at limiting the extracellular availability of adenosine.
PRR agonists
Pattern recognition receptors (PRRs) are evolutionarily conserved proteins involved in the recognition of danger signals [400, 401]. PRRs include (but are not limited to) Toll-like receptors (TLRs) [402, 403] and nucleotide-binding oligomerization domain containing (NOD)-like receptors (NLRs) [404, 405]. TLRs are transmembrane enzymatically-inactive proteins expressed by most APCs, including monocytes, macrophages and DCs, as well as by some types of epithelial cells [402, 403]. NLRs are expressed by a variety of cell types, including various components of the innate and adaptive immune system [404, 405]. Taken together, PRRs sense a wide panel of danger signals, including exogenous “microbe-associated molecular patterns” (MAMPs) like bacterial lipopolysaccharide (LPS) or muramyl dipeptide (MDP), and endogenous “damage-associated molecular patterns” (DAMPs), like the non-histone nuclear protein high-mobility group box 1 (HMGB1) and mitochondrial DNA [406-410]. The activation of various PRRs ignites a signal transduction cascade with potent pro-inflammatory outcomes, including the activation of NF-κB [411-413], and the secretion of immunostimulatory cytokines, like type I IFNs and TNFα [413-415]. Moreover, PRR signaling favors the maturation of DCs as well as the activation of macrophages and NK cells [416]. Besides being critical for the response of the host to viral and bacterial challenges [402, 403], some PRRs play a key role in the (re)activation of anticancer immune responses by chemo-, radio- and immunotherapeutic interventions [15, 413, 417-422].
Thus, PRR agonists have spurred interest not only as adjuvants for conventional vaccines [423, 424], but also as immunotherapeutic interventions that may mediate antineoplastic effects per se or boost the therapeutic activity of other anticancer agents [34, 48, 425]. Three TLR agonists are approved by the US FDA for use in cancer patients: (1) the bacillus Calmette-Guérin (BCG), an attenuated variant of Mycobacterium bovis that presumably operates as a mixed TLR2/TLR4 agonist, which is currently used as a standalone immunotherapeutic agent in subjects with non-invasive transitional cell carcinoma of the bladder [426]; (2) monophosphoryl lipid A (MPL), a TLR2/TLR4-activating derivative of Salmonella minnesota LPS currently utilized as adjuvant in Cervarix®, a vaccine for the prevention of HPV-16 and -18 infection [427]; and (3) imiquimod, an imidazoquinoline derivative that triggers TLR7 signaling, currently employed for the treatment of actinic keratosis, superficial basal cell carcinoma and condylomata acuminata [422, 426]. Of note, picibanil (a lyophilized preparation of Streptococcus pyogenes that operates as a TLR2/TLR4 agonist has been licensed for use in cancer patients by the Japanese Ministry of Health and Welfare (but not by the US FDA) as early as in 1975 [428, 429]; while mifamurtide (a synthetic lipophilic glycopeptide that activates NOD2) has been approved by the EMA for the treatment of osteosarcoma in 2009 [430-432]. Moreover, the safety and efficacy of several other PRR agonists are currently being evaluated in clinical trials [433-435]. These molecules include agatolimod (CpG-7909, PF-3512676, Promune®), an unmethylated CpG oligodeoxynucleotide that activates TLR9 [436]; polyriboinosinic polyribocytidylic acid (polyI:C, Ampligen™, Rintatolimod), a synthetic double-strand RNA that signals via TLR3 [437]; and Hiltonol™, a particular formulation of polyI:C that involves carboxymethylcellulose and poly-L-lysine [48, 438].
Some malignant cells express PRRs [439-445], implying that PRR agonists may not be completely devoid of intrinsic tumor-modulating functions. Nonetheless, a large body of preclinical and clinical literature indicates that the antineoplastic effects of PRR agonists stem from their ability to engage the host immune system. Thus, PRR agonists constitute active immunotherapeutics.
Immunogenic cell death inducers
Some conventional chemotherapeutics, often employed at metronomic doses [446, 447], as well as some forms of radiation therapy, can kill malignant cells while stimulating them to release specific DAMPs in a spatiotemporally coordinated manner [15, 420, 448]. Such DAMPs bind to receptors expressed on the surface of APCs (including TLR4), and not only boost their ability to engulf particulate material (including TAAs and cancer cell debris) but also trigger their maturation/activation [15, 418, 448, 449]. As a result, APCs acquire the ability to elicit a cancer-specific immune response that (at least in mice) is associated with the development of immunological memory [15, 450]. We have dubbed such a functionally atypical form of apoptosis “immunogenic cell death” (ICD) [15]. Importantly, ICD inducers exert optimal antineoplastic effects in immunocompetent, but not in immunodeficient, mice [15, 451-454]. However, the ability of a specific stimulus to trigger ICD can be properly assessed only by means of vaccination experiments involving immunocompetent mice and syngeneic tumor models [15, 455]. As it stands, a few FDA-approved therapies have been shown to constitute bona fide ICD inducers, including: doxorubicin, mitoxantrone and epirubicin (three anthracyclines currently employed against various carcinomas) [186, 449], bleomycin (a glycopeptide antibiotic endowed with antineoplastic properties) [456], oxaliplatin (a platinum derivative generally used for the therapy of colorectal carcinoma) [453, 457], cyclophosphamide (an alkylating agent employed against neoplastic and autoimmune conditions) [458-460], specific forms of radiation therapy [419, 461-466], photodynamic therapy (an intervention that relies on the administration of a photosensitizing agent coupled to light irradiation) [448, 467, 468], and bortezomib (a proteasomal inhibitor used for the treatment of multiple myeloma) [469, 470].
These and other (hitherto experimental) ICD inducers have been viewed as conventional forms of anticancer therapy, exerting antineoplastic effects via cytostatic or cytotoxic mechanisms. However, accumulating evidence indicates that the full-blown therapeutic potential of these molecules relies on the host immune system [15, 471]. Thus, we propose to classify ICD inducers as a form of active anticancer immunotherapy.
Others
Other anticancer immunotherapies are approved by regulatory agencies worldwide for use in cancer patients or are currently being investigated for safety and efficacy in preclinical or clinical settings.
Lenalidomide (Revlimid®, also known as CC-5013) and pomalidomide (Pomalyst®, also known as CC-4047) are two derivatives of thalidomide (Thalomid®) originally developed in the 1990s to achieve improved potency in the absence of significant side effects [472]. Thalidomide was indeed marketed as an over-the-counter sedative, tranquilizer, and antiemetic for morning sickness in various countries in the late 1950s, but was rapidly withdrawn following a peak of infants born with malformation of the limbs [473]. In spite of its pronounced teratogenic activity, thalidomide raised renewed interest as an inhibitor of TNFα secretion in the 1990s [474], and was approved by the US FDA (under a strictly controlled distribution program) for the therapy of erythema nodosum leprosum (a complication of leprosy etiologically linked to TNFα) in 1998 [475]. The combination of thalidomide with dexamethasone (a glucocorticoid) rapidly turned out to mediate therapeutic effects in patients with hematological malignancies, eventually resulting in the approval by the US FDA of this regimen for the treatment of newly diagnosed multiple myeloma [476]. Alongside, lenalidomide (which retains some degree of teratogenicity) was licensed for use in patients with multiple myeloma (also in combination with dexamethasone) and low or intermediate-1 risk myelodysplastic syndromes that harbor 5q cytogenetic abnormalities (as a standalone intervention) [477-480]. Conversely, pomalidomide (which is devoid of teratogenic activity) has been approved for use in multiple myeloma patients only in 2013, when the approval of lenalidomide has been extended to mantle cell lymphoma (MCL) [481-483]. Although the effects of thalidomide, lenalidomide and pomalidomide, which are collectively referred to as “immunomodulatory drugs” (IMiDs), on the immune system have been characterized with increasing precision throughout the past two decades [484], the underlying molecular mechanisms remained obscure [485]. Recent findings indicate that the therapeutic activity of IMiDs depend, at least in part, on their ability to bind the E3 ubiquitin ligase cereblon (CRBN) and hence boost the proteasomal degradation of the B cell-specific transcription factors IKAROS family zinc finger 1 (IKZF1) and IKZF3 [486, 487]. Of note, CRBN, which is also involved in the teratogenic effects of thalidomide and lenalidomide [488], regulates the abundance of interferon regulatory factor 4, perhaps accounting for the immunomodulatory functions of IMiDs [489]. Although endowed with intrinsic antineoplastic activity, IMiDs should be considered active immunotherapeutics.
As they progress and respond to treatment, neoplastic lesions are infiltrated by a significant amount of lymphoid and myeloid cells, including CD8+ T lymphocytes, Tregs, tumor-associated macrophages (TAMs) and immunosuppressive B-cell populations [122-124, 490, 491]. Robust tumor infiltration by CD8+ T lymphocytes is generally associated with a good prognosis, especially when the intratumoral levels of Tregs are limited [124, 492]. Along similar lines, high intratumoral levels of TAMs with a “classically-activated” M1 phenotype (which exert tumoricidal functions, stimulate NK cells and secrete TH1-polarizing cytokines) generally correlate with improved disease outcome [491, 493]. The contrary holds true when the myeloid tumor infiltrate contains high levels of “alternatively-activated” M2 TAMs or specific B-cell subsets, which can secrete not only immunosuppressive cytokines like IL-10 and TGFβ1, but also angiogenic mediators such as VEGFA and enzymes that remodel the extracellular matrix [491, 493]. These observations prompted the development of immunotherapeutic regimens based on the depletion/inhibition of Tregs or B lymphocytes, as well as on the conversion of M2 TAMs to their M1 counterparts.
Denileukin diftitox (also known as Ontak®) is a recombinant variant of IL-2 fused to the diphtheria toxin [494]. Owing to its selective cytotoxicity for cells expressing IL-2 receptor α (IL2RA, best known as CD25), denileukin diftitox has been approved by the US FDA and EMA for the treatment of CD25+ cutaneous T-cell lymphoma in the early 2000s [494]. More recently, denileukin diftitox has been tested for its ability to improve the efficacy of various immunotherapies by efficiently depleting Tregs (which also express CD25) in patients affected by various neoplasms [495-497]. In some (but not all) these clinical settings, denileukin diftitox enhanced the efficacy of immunotherapy as it provoked a sizeable Treg depletion [496, 497]. However, denileukin diftitox has recently been ascribed with a number of immunosuppressive effects [498, 499]. This may explain why in some cases denileukin diftitox had no clinical activity [495], and casts doubts on the possibility to use such Treg-depleting agent as a routine anticancer immunotherapeutic. This said, several conventional antineoplastic agents commonly used in the clinic appear to deplete or inhibit Treg, which presumably contributes to their therapeutic activity (see below) [420, 421]. Along similar lines, at least part of the clinical activity of ibrutinib (PCI-32765), a small molecule inhibitor of bruton tyrosine kinase (BTK) recently approved by the US FDA for use in patients with MCL and CLL [500-502], may stem from its ability to target tumor-infiltrating B lymphocytes or myeloid cells [503]. A clinical trial testing this possibility in pancreatic cancer patients will soon be initiated (LC, personal communication).
Several immunotherapeutic agents exert antineoplastic effects by altering the relative proportion between M2 and M1 TAMs in favor of the latter [491]. These include: (1) tasquinimod, a second-generation orally active quinoline-3-carboxamide analog initially developed as an antiangiogenic agent [504, 505]; trabectedin (Yondelis®), a marine antineoplastic agent currently approved in Europe, Russia and South Korea for the treatment of soft tissue sarcoma and ovarian carcinoma [506, 507]; (3) inhibitors of chemokine (C-C motif) ligand 2/chemokine (C-C motif) receptor 2 (CCL2/CCR2) signaling [508]; (3) mAbs specific for chemokine (C-X-C motif) receptor 4 (CXCR4) [509]; and (4) small molecule inhibitors and mAbs that suppress colony stimulating factor 1/colony stimulating factor 1 receptor (CSF1/CSFR1) signaling [510-512]. With the single exception of trabectedin (which was not developed as an immunotherapeutic agent), none of these strategies is currently approved by the US FDA or EMA for use in humans (sources http://www.fda.gov and http://www.ema.europa.eu/ema/). However, several Phase II-III clinical trials are currently ongoing to establish the safety and efficacy of these active immunotherapeutic agents in patients with various solid tumors (source http://www.clinicaltrials.gov).
Additional, hitherto experimental immunotherapeutic regimens act by stimulating the host immune system to mount a novel (or unleash an existing) immune response against malignant cells. These include: (1) strategies for the depletion of circulating myeloid-derived suppressor cells (MDSCs), a blood-borne population of immature, immunosuppressive myeloid cells that generally accumulate in the course of tumor progression [513-516]; (2) mAbs that block CD47, one of the major antiphagocytic receptor expressed by malignant cells [517-519]; and (3) vaccines relying on the administration of cancer cell lines expressing immunostimulatory molecules (e.g., GM-CSF) upon inactivation or lysis [520].
CONCLUDING REMARKS
During the past three decades, immunotherapy has become a clinical reality [35, 78, 521], and an ever-increasing number of cancer patients are expected to receive, at some stage of their disease, an immunotherapeutic intervention [522, 523]. The observations presented above suggest that various immunotherapies previously classified as passive, including several (if not all) tumor-targeting mAbs, ACT and oncolytic viruses, may de facto constitute active forms of immunotherapy. Moreover, accumulating preclinical and clinical evidence indicates that therapeutically relevant anticancer immune responses invariably exhibit some degree of epitope spreading, i.e., they eventually target several TAAs even when they were initially directed against a single one [524, 525]. This is not surprising considering that malignant cells exhibit a high degree of genetic/genomic instability and hence are relatively prone to generate so-called “antigen loss variants” that would render TAA-specific immunotherapies completely ineffective with time [526-528]. Thus, even if immunotherapies that truly generate an anticancer response with a unique antigen specificity existed [529, 530], they presumably would not mediate clinically relevant, long-term immune responses. In turn, this casts some doubts on the practical utility of classifying immunotherapies into “antigen-specific” or “non-specific”.
Recently, great attention has been given to the immunostimulatory effects of conventional chemotherapeutics [420, 421, 531, 532]. Indeed, several compounds that have been successfully used in the clinic, including the nucleoside analogs gemcitabine (which is approved by the US FDA for the treatment of pancreatic, ovarian, breast and non-small cell carcinoma) [533, 534] and 5-fluorouracil (which is licensed for use in patients affected by various neoplasms) [535, 536] have off-target immunostimulatory effects, in particular when administered as low doses and according to metronomic schedules (while, similar to radiation therapy, they are generally immunosuppressive when given at high doses) [537, 538]. It is therefore tempting to speculate that most (if not all) anticancer agents that are truly beneficial to patients operate as active immunotherapeutics, stimulating the host immune system to mount an antigenically broad (and hence insensitive to antigen loss) response against malignant cells. In support of this notion, an ever increasing number of combinatorial immuno(chemo)therapeutic regimens is being designed and tested in clinical trials, with promising results [34]. This being said, only the adequate implementation of protocols to monitor immune system-related parameters among patients participating in clinical trials (immunomonitoring) will provide insights into this possibility [539-543]. Such protocols are inherently complex, calling for international efforts toward standardization [544]. Harmonized immunomonitoring procedures will undoubtedly guide the development of new (immuno)therapies, and facilitate the identification of novel prognostic or predictive biomarkers [544]. We are positive that the next clinical success of anticancer immunotherapy is just behind the door.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labelisée); Agence Nationale de la Recherche (ANR); Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). MPC is supported by Association for Cancer Research (AIRC). SG is supported by Cancer Vaccine Collaborative and Cancer Research Institute. MJS is supported by the National Health and Medical Research Council of Australia; the QIMR Berghofer Medical Research Institute; and the Susan G Komen Breast Cancer Foundation. FM is supported by a grant from the Italian Ministry of Health.
Abbreviations
- ACT
adoptive cell transfer
- ADCC
antibody-dependent cell-mediated cytotoxicity
- ADORA
adenosine receptor
- APC
antigen-presenting cell
- BiTE
bispecific T-cell engager
- CAR
chimeric antigen receptor
- CLL
chronic lymphocytic leukemia
- CRBN
cereblon
- CRC
colorectal carcinoma
- CTLA4
cytotoxic T lymphocyte-associated protein 4
- DAMP
damage-associated molecular pattern
- DC
dendritic cell
- EGFR
epidermal growth factor receptor
- EMA
European Medicines Agency
- FCGR2A
Fc fragment of IgG, low affinity IIa, receptor
- FCGR3A
Fc fragment of IgG, low affinity IIIa, receptor
- FDA
Food and Drug Administration
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HCL
hairy cell leukemia
- HNC
head and neck cancer
- HPV
human papillomavirus
- HSCT
hematopoietic stem cell transplantation
- HSP
heat shock protein
- ICD
immunogenic cell death
- IDO1
indoleamine 2,3-dioxigenase 1
- IFN
interferon
- IKZF
IKAROS family zinc finger
- IL
interleukin
- IMiD
immunomodulatory drug
- KIR
killer cell immunoglobulin-like receptor
- Kyn
L-kynurenine
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- MCL
mantle cell lymphoma
- NLR
NOD-like receptors
- NK
natural killer
- PBL
peripheral blood lymphocyte
- PRR
pattern recognition receptor
- RCC
renal cell carcinoma
- TAA
tumor-associated antigen
- TAM
tumor-associated macrophage
- TCR
T-cell receptor
- TGFß1
transforming growth factor ß1
- TLR
Toll-like receptor
- TNFa
tumor necrosis factor a
- TNFRSF
tumor necrosis factor receptor superfamily
- Treg
regulatory T cell
- Trp
tryptophan
- VEGFA
vascular endothelial growth factor A
REFERENCES
- 1.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 2.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 3.Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13:727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 4.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 5.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 6.Holzel M, Bovier A, Tuting T. Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nat Rev Cancer. 2013;13:365–376. doi: 10.1038/nrc3498. [DOI] [PubMed] [Google Scholar]
- 7.Wellen KE, Thompson CB. A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13:270–276. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- 8.Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12:829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 9.Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science. 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Cell. 2007;130:986–988. doi: 10.1016/j.cell.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, Baehrecke EH, Bazan NG, Bertrand MJ, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 15.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013 doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 17.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 19.Albini A, Tosetti F, Li VW, Noonan DM, Li WW. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 2012;9:498–509. doi: 10.1038/nrclinonc.2012.120. [DOI] [PubMed] [Google Scholar]
- 20.Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 21.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer. 2006;6:613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 22.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 23.Humphries C. Adoptive cell therapy: Honing that killer instinct. Nature. 2013;504:S13–15. doi: 10.1038/504S13a. [DOI] [PubMed] [Google Scholar]
- 24.Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive Immunotherapy for Cancer or Viruses. Annu Rev Immunol. 2014 doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13:525–541. doi: 10.1038/nrc3565. [DOI] [PubMed] [Google Scholar]
- 30.Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 31.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 32.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 33.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 34.Vacchelli E, Prada N, Kepp O, Galluzzi L. Current trends of anticancer immunochemotherapy. Oncoimmunology. 2013;2:e25396. doi: 10.4161/onci.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy--revisited. Nat Rev Drug Discov. 2011;10:591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- 36.Weiner LM. Building better magic bullets--improving unconjugated monoclonal antibody therapy for cancer. Nat Rev Cancer. 2007;7:701–706. doi: 10.1038/nrc2209. [DOI] [PubMed] [Google Scholar]
- 37.Strebhardt K, Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 38.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 39.Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012;1:1223–1225. doi: 10.4161/onci.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dronca RS, Dong H. Immunomodulatory Antibody Therapy of Cancer: The Closer the Better. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, Romaguera J, Hagemeister F, Fanale M, Samaniego F, Feng L, Baladandayuthapani V, Wang Z, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lipson EJ. Re-orienting the immune system: Durable tumor regression and successful re-induction therapy using anti-PD1 antibodies. Oncoimmunology. 2013;2:e23661. doi: 10.4161/onci.23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alkan SS. Monoclonal antibodies: the story of a discovery that revolutionized science and medicine. Nat Rev Immunol. 2004;4:153–156. doi: 10.1038/nri1265. [DOI] [PubMed] [Google Scholar]
- 44.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galluzzi L, Vacchelli E, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zucman-Rossi J, Zitvogel L, Kroemer G. Trial Watch: Monoclonal antibodies in cancer therapy. Oncoimmunology. 2012;1:28–37. doi: 10.4161/onci.1.1.17938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vacchelli E, Eggermont A, Galon J, Sautes-Fridman C, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Monoclonal antibodies in cancer therapy. Oncoimmunology. 2013;2:e22789. doi: 10.4161/onci.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiner LM, Belldegrun AS, Crawford J, Tolcher AW, Lockbaum P, Arends RH, Navale L, Amado RG, Schwab G, Figlin RA. Dose and schedule study of panitumumab monotherapy in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:502–508. doi: 10.1158/1078-0432.CCR-07-1509. [DOI] [PubMed] [Google Scholar]
- 48.Ming Lim C, Stephenson R, Salazar AM, Ferris RL. TLR3 agonists improve the immunostimulatory potential of cetuximab against EGFR head and neck cancer cells. Oncoimmunology. 2013;2:e24677. doi: 10.4161/onci.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaplan-Lefko PJ, Graves JD, Zoog SJ, Pan Y, Wall J, Branstetter DG, Moriguchi J, Coxon A, Huard JN, Xu R, Peach ML, Juan G, Kaufman S, et al. Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer Biol Ther. 2010;9:618–631. doi: 10.4161/cbt.9.8.11264. [DOI] [PubMed] [Google Scholar]
- 50.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 51.Michielsen AJ, Ryan EJ, O'Sullivan JN. Dendritic cell inhibition correlates with survival of colorectal cancer patients on bevacizumab treatment. Oncoimmunology. 2012;1:1445–1447. doi: 10.4161/onci.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavallo F, Calogero RA, Forni G. Are oncoantigens suitable targets for anti-tumour therapy? Nat Rev Cancer. 2007;7:707–713. doi: 10.1038/nrc2208. [DOI] [PubMed] [Google Scholar]
- 53.de La Motte Rouge T, Galluzzi L, Olaussen KA, Zermati Y, Tasdemir E, Robert T, Ripoche H, Lazar V, Dessen P, Harper F, Pierron G, Pinna G, Araujo N, et al. A novel epidermal growth factor receptor inhibitor promotes apoptosis in non-small cell lung cancer cells resistant to erlotinib. Cancer Res. 2007;67:6253–6262. doi: 10.1158/0008-5472.CAN-07-0538. [DOI] [PubMed] [Google Scholar]
- 54.Forero-Torres A, Infante JR, Waterhouse D, Wong L, Vickers S, Arrowsmith E, He AR, Hart L, Trent D, Wade J, Jin X, Wang Q, Austin T, et al. Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer. Cancer Med. 2013;2:925–932. doi: 10.1002/cam4.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hughes B. Antibody-drug conjugates for cancer: poised to deliver? Nat Rev Drug Discov. 2010;9:665–667. doi: 10.1038/nrd3270. [DOI] [PubMed] [Google Scholar]
- 56.Leal M, Sapra P, Hurvitz SA, Senter P, Wahl A, Schutten M, Shah DK, Haddish-Berhane N, Kabbarah O. Antibody-drug conjugates: an emerging modality for the treatment of cancer. Ann N Y Acad Sci. 2014;1321:41–54. doi: 10.1111/nyas.12499. [DOI] [PubMed] [Google Scholar]
- 57.Hubert P, Amigorena S. Antibody-dependent cell cytotoxicity in monoclonal antibody-mediated tumor immunotherapy. Oncoimmunology. 2012;1:103–105. doi: 10.4161/onci.1.1.17963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Houot R, Kohrt H, Levy R. Boosting antibody-dependant cellular cytotoxicity against tumor cells with a CD137 stimulatory antibody. Oncoimmunology. 2012;1:957–958. doi: 10.4161/onci.19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kute T, Stehle Jr JR, Ornelles D, Walker N, Delbono O, Vaughn JP. Understanding key assay parameters that affect measurements of trastuzumab-mediated ADCC against Her2 positive breast cancer cells. Oncoimmunology. 2012;1:810–821. doi: 10.4161/onci.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winiarska M, Glodkowska-Mrowka E, Bil J, Golab J. Molecular mechanisms of the antitumor effects of anti-CD20 antibodies. Front Biosci (Landmark Ed) 2011;16:277–306. doi: 10.2741/3688. [DOI] [PubMed] [Google Scholar]
- 61.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 62.Scott SD. Rituximab: a new therapeutic monoclonal antibody for non-Hodgkin's lymphoma. Cancer Pract. 1998;6:195–197. doi: 10.1046/j.1523-5394.1998.006003195.x. [DOI] [PubMed] [Google Scholar]
- 63.Jones B. Haematological cancer: rituximab maintenance improves the outcome of elderly patients with FL. Nat Rev Clin Oncol. 2013;10:607. doi: 10.1038/nrclinonc.2013.164. [DOI] [PubMed] [Google Scholar]
- 64.Armeanu-Ebinger S, Hoh A, Wenz J, Fuchs J. Targeting EpCAM (CD326) for immunotherapy in hepatoblastoma. Oncoimmunology. 2013;2:e22620. doi: 10.4161/onci.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Topp MS, Gokbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, Neumann SA, Horst HA, Raff T, Viardot A, Stelljes M, Schaich M, Kohne-Volland R, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120:5185–5187. doi: 10.1182/blood-2012-07-441030. [DOI] [PubMed] [Google Scholar]
- 66.Walter RB. Biting back: BiTE antibodies as a promising therapy for acute myeloid leukemia. Expert Rev Hematol. 2014;7:317–319. doi: 10.1586/17474086.2014.896190. [DOI] [PubMed] [Google Scholar]
- 67.Choi BD, Gedeon PC, Sanchez-Perez L, Bigner DD, Sampson JH. Regulatory T cells are redirected to kill glioblastoma by an EGFRvIII-targeted bispecific antibody. Oncoimmunology. 2013;2:e26757. doi: 10.4161/onci.26757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi BD, Pastan I, Bigner DD, Sampson JH. A novel bispecific antibody recruits T cells to eradicate tumors in the «immunologically privileged» central nervous system. Oncoimmunology. 2013;2:e23639. doi: 10.4161/onci.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffman LM, Gore L. Blinatumomab, a Bi-Specific Anti-CD19/CD3 BiTE((R)) Antibody for the Treatment of Acute Lymphoblastic Leukemia: Perspectives and Current Pediatric Applications. Front Oncol. 2014;4:63. doi: 10.3389/fonc.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawaguchi Y, Kono K, Mimura K, Sugai H, Akaike H, Fujii H. Cetuximab induce antibody-dependent cellular cytotoxicity against EGFR-expressing esophageal squamous cell carcinoma. Int J Cancer. 2007;120:781–787. doi: 10.1002/ijc.22370. [DOI] [PubMed] [Google Scholar]
- 71.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, Lopez-Albaitero A, Gibson SP, Gooding WE, Ferrone S, Ferris RL. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Derer S, Lohse S, Valerius T. EGFR expression levels affect the mode of action of EGFR-targeting monoclonal antibodies. Oncoimmunology. 2013;2:e24052. doi: 10.4161/onci.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mansfield AS, Nevala WK, Lieser EA, Leontovich AA, Markovic SN. The immunomodulatory effects of bevacizumab on systemic immunity in patients with metastatic melanoma. Oncoimmunology. 2013;2:e24436. doi: 10.4161/onci.24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manzoni M, Rovati B, Ronzoni M, Loupakis F, Mariucci S, Ricci V, Gattoni E, Salvatore L, Tinelli C, Villa E, Danova M. Immunological effects of bevacizumab-based treatment in metastatic colorectal cancer. Oncology. 2010;79:187–196. doi: 10.1159/000320609. [DOI] [PubMed] [Google Scholar]
- 75.Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N, Friedlander P, Flaherty KT, Murphy GF, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 77.Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol. 2013;6:1. doi: 10.1186/1756-8722-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vacchelli E, Eggermont A, Fridman WH, Galon J, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Adoptive cell transfer for anticancer immunotherapy. Oncoimmunology. 2013;2:e24238. doi: 10.4161/onci.24238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial Watch: Adoptive cell transfer immunotherapy. Oncoimmunology. 2012;1:306–315. doi: 10.4161/onci.19549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lerret NM, Marzo AL. Adoptive T-cell transfer combined with a single low dose of total body irradiation eradicates breast tumors. Oncoimmunology. 2013;2:e22731. doi: 10.4161/onci.22731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jenq RR, van den Brink MR. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 2010;10:213–221. doi: 10.1038/nrc2804. [DOI] [PubMed] [Google Scholar]
- 83.Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1:1111–1134. doi: 10.4161/onci.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vacchelli E, Vitale I, Eggermont A, Fridman WH, Fucikova J, Cremer I, Galon J, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2013;2:e25771. doi: 10.4161/onci.25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ueno H, Palucka AK, Banchereau J. The expanding family of dendritic cell subsets. Nat Biotechnol. 2010;28:813–815. doi: 10.1038/nbt0810-813. [DOI] [PubMed] [Google Scholar]
- 86.Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–478. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galluzzi L, Lugli E. Rejuvenated T cells attack old tumors. Oncoimmunology. 2013;2:e24103. doi: 10.4161/onci.24103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer--what clinicians need to know. Nat Rev Clin Oncol. 2011;8:577–585. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Merhavi-Shoham E, Haga-Friedman A, Cohen CJ. Genetically modulating T-cell function to target cancer. Semin Cancer Biol. 2012;22:14–22. doi: 10.1016/j.semcancer.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 91.Liu K, Rosenberg SA. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J Immunol. 2001;167:6356–6365. doi: 10.4049/jimmunol.167.11.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kalbasi A, Shrimali RK, Chinnasamy D, Rosenberg SA. Prevention of interleukin-2 withdrawal-induced apoptosis in lymphocytes retrovirally cotransduced with genes encoding an antitumor T-cell receptor and an antiapoptotic protein. J Immunother. 2010;33:672–683. doi: 10.1097/CJI.0b013e3181e475cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, Klebanoff CA, Johnson LA, Kerkar SP, Yang S, Muranski P, Palmer DC, Scott CD, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bellone M, Calcinotto A, Corti A. Won't you come on in? How to favor lymphocyte infiltration in tumors. Oncoimmunology. 2012;1:986–988. doi: 10.4161/onci.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kershaw MH, Teng MW, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5:928–940. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- 97.Ray S, Chhabra A, Chakraborty NG, Hegde U, Dorsky DI, Chodon T, von Euw E, Comin-Anduix B, Koya RC, Ribas A, Economou JS, Rosenberg SA, Mukherji B. MHC-I-restricted melanoma antigen specific TCR-engineered human CD4+ T cells exhibit multifunctional effector and helper responses, in vitro. Clin Immunol. 2010;136:338–347. doi: 10.1016/j.clim.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 99.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10:267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Long AH, Haso WM, Orentas RJ. Lessons learned from a highly-active CD22-specific chimeric antigen receptor. Oncoimmunology. 2013;2:e23621. doi: 10.4161/onci.23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spear P, Barber A, Sentman CL. Collaboration of chimeric antigen receptor (CAR)-expressing T cells and host T cells for optimal elimination of established ovarian tumors. Oncoimmunology. 2013;2:e23564. doi: 10.4161/onci.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Badoual C, Bastier PL, Roussel H, Mandavit M, Tartour E. An allogeneic NK cell line engineered to express chimeric antigen receptors: A novel strategy of cellular immunotherapy against cancer. Oncoimmunology. 2013;2:e27156. doi: 10.4161/onci.27156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boissel L, Betancur-Boissel M, Lu W, Krause DS, Van Etten RA, Wels WS, Klingemann H. Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology. 2013;2:e26527. doi: 10.4161/onci.26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, Olszewska M, Bernal Y, Pegram H, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, Wu MF, Gee AP, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, Kalos M, June CH. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pegram HJ, Jackson JT, Smyth MJ, Kershaw MH, Darcy PK. Adoptive transfer of gene-modified primary NK cells can specifically inhibit tumor progression in vivo. J Immunol. 2008;181:3449–3455. doi: 10.4049/jimmunol.181.5.3449. [DOI] [PubMed] [Google Scholar]
- 113.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 114.Okada K, Nannmark U, Vujanovic NL, Watkins S, Basse P, Herberman RB, Whiteside TL. Elimination of established liver metastases by human interleukin 2-activated natural killer cells after locoregional or systemic adoptive transfer. Cancer Res. 1996;56:1599–1608. [PubMed] [Google Scholar]
- 115.Besser MJ, Shoham T, Harari-Steinberg O, Zabari N, Ortenberg R, Yakirevitch A, Nagler A, Loewenthal R, Schachter J, Markel G. Development of allogeneic NK cell adoptive transfer therapy in metastatic melanoma patients: in vitro preclinical optimization studies. PLoS One. 2013;8:e57922. doi: 10.1371/journal.pone.0057922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Terme M, Fridman WH, Tartour E. NK cells from pleural effusions are potent antitumor effector cells. Eur J Immunol. 2013;43:331–334. doi: 10.1002/eji.201243264. [DOI] [PubMed] [Google Scholar]
- 117.Mattarollo SR, Smyth MJ. NKT cell adjuvants in therapeutic vaccines against hematological cancers. Oncoimmunology. 2013;2:e22615. doi: 10.4161/onci.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lister J, Rybka WB, Donnenberg AD, deMagalhaes-Silverman M, Pincus SM, Bloom EJ, Elder EM, Ball ED, Whiteside TL. Autologous peripheral blood stem cell transplantation and adoptive immunotherapy with activated natural killer cells in the immediate posttransplant period. Clin Cancer Res. 1995;1:607–614. [PubMed] [Google Scholar]
- 119.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17:6287–6297. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, Rigatos G, Papamichail M, Perez SA. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1781–1789. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Q, Lao X, Pan Q, Ning N, Yet J, Xu Y, Li S, Chang AE. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin Cancer Res. 2011;17:4987–4995. doi: 10.1158/1078-0432.CCR-11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 123.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Senovilla L, Vacchelli E, Galon J, Adjemian S, Eggermont A, Fridman WH, Sautes-Fridman C, Ma Y, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology. 2012;1:1323–1343. doi: 10.4161/onci.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fremd C, Schuetz F, Sohn C, Beckhove P, Domschke C. B cell-regulated immune responses in tumor models and cancer patients. Oncoimmunology. 2013;2:e25443. doi: 10.4161/onci.25443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mignot G, Ullrich E, Bonmort M, Menard C, Apetoh L, Taieb J, Bosisio D, Sozzani S, Ferrantini M, Schmitz J, Mack M, Ryffel B, Bulfone-Paus S, et al. The critical role of IL-15 in the antitumor effects mediated by the combination therapy imatinib and IL-2. J Immunol. 2008;180:6477–6483. doi: 10.4049/jimmunol.180.10.6477. [DOI] [PubMed] [Google Scholar]
- 127.Ullrich E, Bonmort M, Mignot G, Jacobs B, Bosisio D, Sozzani S, Jalil A, Louache F, Bulanova E, Geissman F, Ryffel B, Chaput N, Bulfone-Paus S, et al. Trans-presentation of IL-15 dictates IFN-producing killer dendritic cells effector functions. J Immunol. 2008;180:7887–7897. doi: 10.4049/jimmunol.180.12.7887. [DOI] [PubMed] [Google Scholar]
- 128.Besser MJ, Shapira-Frommer R, Itzhaki O, Treves AJ, Zippel DB, Levy D, Kubi A, Shoshani N, Zikich D, Ohayon Y, Ohayon D, Shalmon B, Markel G, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19:4792–4800. doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- 129.Santegoets SJ, Turksma AW, Powell Jr DJ, Hooijberg E, de Gruijl TD. IL-21 in cancer immunotherapy: At the right place at the right time. Oncoimmunology. 2013;2:e24522. doi: 10.4161/onci.24522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singh H, Figliola MJ, Dawson MJ, Huls H, Olivares S, Switzer K, Mi T, Maiti S, Kebriaei P, Lee DA, Champlin RE, Cooper LJ. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 2011;71:3516–3527. doi: 10.1158/0008-5472.CAN-10-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vaha-Koskela MJ, Heikkila JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Oncolytic viruses for cancer therapy. Oncoimmunology. 2013;2:e24612. doi: 10.4161/onci.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Makela AR, Matilainen H, White DJ, Ruoslahti E, Oker-Blom C. Enhanced baculovirus-mediated transduction of human cancer cells by tumor-homing peptides. J Virol. 2006;80:6603–6611. doi: 10.1128/JVI.00528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Singh P, Destito G, Schneemann A, Manchester M. Canine parvovirus-like particles, a novel nanomaterial for tumor targeting. J Nanobiotechnology. 2006;4:2. doi: 10.1186/1477-3155-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008;4:e1000018. doi: 10.1371/journal.ppat.1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boisgerault N, Guillerme JB, Pouliquen D, Mesel-Lemoine M, Achard C, Combredet C, Fonteneau JF, Tangy F, Gregoire M. Natural oncolytic activity of live-attenuated measles virus against human lung and colorectal adenocarcinomas. Biomed Res Int. 2013;2013:387362. doi: 10.1155/2013/387362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wildner O, Blaese RM, Morris JC. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999;59:410–413. [PubMed] [Google Scholar]
- 139.Tseng JC, Zanzonico PB, Levin B, Finn R, Larson SM, Meruelo D. Tumor-specific in vivo transfection with HSV-1 thymidine kinase gene using a Sindbis viral vector as a basis for prodrug ganciclovir activation and PET. J Nucl Med. 2006;47:1136–1143. [PubMed] [Google Scholar]
- 140.Foloppe J, Kintz J, Futin N, Findeli A, Cordier P, Schlesinger Y, Hoffmann C, Tosch C, Balloul JM, Erbs P. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008;15:1361–1371. doi: 10.1038/gt.2008.82. [DOI] [PubMed] [Google Scholar]
- 141.Liu Y, Deisseroth A. Oncolytic adenoviral vector carrying the cytosine deaminase gene for melanoma gene therapy. Cancer Gene Ther. 2006;13:845–855. doi: 10.1038/sj.cgt.7700962. [DOI] [PubMed] [Google Scholar]
- 142.Leveille S, Samuel S, Goulet ML, Hiscott J. Enhancing VSV oncolytic activity with an improved cytosine deaminase suicide gene strategy. Cancer Gene Ther. 2011;18:435–443. doi: 10.1038/cgt.2011.14. [DOI] [PubMed] [Google Scholar]
- 143.Lampe J, Bossow S, Weiland T, Smirnow I, Lehmann R, Neubert W, Bitzer M, Lauer UM. An armed oncolytic measles vaccine virus eliminates human hepatoma cells independently of apoptosis. Gene Ther. 2013;20:1033–1041. doi: 10.1038/gt.2013.28. [DOI] [PubMed] [Google Scholar]
- 144.Shinoura N, Yoshida Y, Asai A, Kirino T, Hamada H. Adenovirus-mediated transfer of p53 and Fas ligand drastically enhances apoptosis in gliomas. Cancer Gene Ther. 2000;7:732–738. doi: 10.1038/sj.cgt.7700160. [DOI] [PubMed] [Google Scholar]
- 145.Zhao L, Dong A, Gu J, Liu Z, Zhang Y, Zhang W, Wang Y, He L, Qian C, Qian Q, Liu X. The antitumor activity of TRAIL and IL-24 with replicating oncolytic adenovirus in colorectal cancer. Cancer Gene Ther. 2006;13:1011–1022. doi: 10.1038/sj.cgt.7700969. [DOI] [PubMed] [Google Scholar]
- 146.Zhu W, Zhang H, Shi Y, Song M, Zhu B, Wei L. Oncolytic adenovirus encoding tumor necrosis factor-related apoptosis inducing ligand (TRAIL) inhibits the growth and metastasis of triple-negative breast cancer. Cancer Biol Ther. 2013;14:1016–1023. doi: 10.4161/cbt.26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jiang G, Li J, Zeng Z, Xian L. Lentivirus-mediated gene therapy by suppressing survivin in BALB/c nude mice bearing oral squamous cell carcinoma. Cancer Biol Ther. 2006;5:435–440. doi: 10.4161/cbt.5.4.2542. [DOI] [PubMed] [Google Scholar]
- 148.Shen W, Tu JK, Wang XH, Fu ZX. Oncolytic adenovirus mediated Survivin RNA interference and 5-fluorouracil synergistically suppress the lymphatic metastasis of colorectal cancer. Oncol Rep. 2010;24:1285–1290. doi: 10.3892/or_00000984. [DOI] [PubMed] [Google Scholar]
- 149.Liang M. Clinical development of oncolytic viruses in China. Curr Pharm Biotechnol. 2012;13:1852–1857. doi: 10.2174/138920112800958760. [DOI] [PubMed] [Google Scholar]
- 150.Raty JK, Pikkarainen JT, Wirth T, Yla-Herttuala S. Gene therapy: the first approved gene-based medicines, molecular mechanisms and clinical indications. Curr Mol Pharmacol. 2008;1:13–23. doi: 10.2174/1874467210801010013. [DOI] [PubMed] [Google Scholar]
- 151.Alvarez-Breckenridge CA, Yu J, Caligiuri MA, Chiocca EA. Uncovering a novel mechanism whereby NK cells interfere with glioblastoma virotherapy. Oncoimmunology. 2013;2:e23658. doi: 10.4161/onci.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bernt KM, Ni S, Gaggar A, Li ZY, Shayakhmetov DM, Lieber A. The effect of sequestration by nontarget tissues on anti-tumor efficacy of systemically applied, conditionally replicating adenovirus vectors. Mol Ther. 2003;8:746–755. doi: 10.1016/j.ymthe.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 153.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 154.Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg FH, Louis DN, Chiocca EA. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pensiero MN, Wysocki CA, Nader K, Kikuchi GE. Development of amphotropic murine retrovirus vectors resistant to inactivation by human serum. Hum Gene Ther. 1996;7:1095–1101. doi: 10.1089/hum.1996.7.9-1095. [DOI] [PubMed] [Google Scholar]
- 156.Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V, Seymour LW. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 157.Massari I, Donnini A, Argentati K, Straino S, Mangoni A, Gaetano C, Viticchi C, Capogrossi M, Provinciali M. Age-dependent effects of repeated immunization with a first generation adenovirus vector on the immune response and transgene expression in young and old rats. Exp Gerontol. 2002;37:823–831. doi: 10.1016/s0531-5565(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 158.Diaconu I, Cerullo V, Hirvinen ML, Escutenaire S, Ugolini M, Pesonen SK, Bramante S, Parviainen S, Kanerva A, Loskog AS, Eliopoulos AG, Pesonen S, Hemminki A. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res. 2012;72:2327–2338. doi: 10.1158/0008-5472.CAN-11-2975. [DOI] [PubMed] [Google Scholar]
- 159.Pesonen S, Diaconu I, Kangasniemi L, Ranki T, Kanerva A, Pesonen SK, Gerdemann U, Leen AM, Kairemo K, Oksanen M, Haavisto E, Holm SL, Karioja-Kallio A, et al. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res. 2012;72:1621–1631. doi: 10.1158/0008-5472.CAN-11-3001. [DOI] [PubMed] [Google Scholar]
- 160.Gomes EM, Rodrigues MS, Phadke AP, Butcher LD, Starling C, Chen S, Chang D, Hernandez-Alcoceba R, Newman JT, Stone MJ, Tong AW. Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD154) transgene construct in human breast cancer cells. Clin Cancer Res. 2009;15:1317–1325. doi: 10.1158/1078-0432.CCR-08-1360. [DOI] [PubMed] [Google Scholar]
- 161.Choi IK, Li Y, Oh E, Kim J, Yun CO. Oncolytic adenovirus expressing IL-23 and p35 elicits IFN-gamma- and TNF-alpha-co-producing T cell-mediated antitumor immunity. PLoS One. 2013;8:e67512. doi: 10.1371/journal.pone.0067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, Burke J, Lencioni R, Hickman T, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kanerva A, Nokisalmi P, Diaconu I, Koski A, Cerullo V, Liikanen I, Tahtinen S, Oksanen M, Heiskanen R, Pesonen S, Joensuu T, Alanko T, Partanen K, et al. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res. 2013;19:2734–2744. doi: 10.1158/1078-0432.CCR-12-2546. [DOI] [PubMed] [Google Scholar]
- 164.Egilmez NK, Harden JL, Rowswell-Turner RB. Chemoimmunotherapy as long-term maintenance therapy for cancer. Oncoimmunology. 2012;1:563–565. doi: 10.4161/onci.19369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li J, O'Malley M, Sampath P, Kalinski P, Bartlett DL, Thorne SH. Expression of CCL19 from oncolytic vaccinia enhances immunotherapeutic potential while maintaining oncolytic activity. Neoplasia. 2012;14:1115–1121. doi: 10.1593/neo.121272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 167.Coosemans A, Vergote I, Van Gool SW. Dendritic cell-based immunotherapy in ovarian cancer. Oncoimmunology. 2013;2:e27059. doi: 10.4161/onci.27059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Linette GP, Carreno BM. Dendritic cell-based vaccines: Shining the spotlight on signal 3. Oncoimmunology. 2013;2:e26512. doi: 10.4161/onci.26512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, Lotze MT. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 173.Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 175.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zeis M, Siegel S, Wagner A, Schmitz M, Marget M, Kuhl-Burmeister R, Adamzik I, Kabelitz D, Dreger P, Schmitz N, Heiser A. Generation of cytotoxic responses in mice and human individuals against hematological malignancies using survivin-RNA-transfected dendritic cells. J Immunol. 2003;170:5391–5397. doi: 10.4049/jimmunol.170.11.5391. [DOI] [PubMed] [Google Scholar]
- 177.Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, Lee JH, Menander K, Chada S, Gabrilovich DI. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 178.Irvine AS, Trinder PK, Laughton DL, Ketteringham H, McDermott RH, Reid SC, Haines AM, Amir A, Husain R, Doshi R, Young LS, Mountain A. Efficient nonviral transfection of dendritic cells and their use for in vivo immunization. Nat Biotechnol. 2000;18:1273–1278. doi: 10.1038/82383. [DOI] [PubMed] [Google Scholar]
- 179.Manickan E, Kanangat S, Rouse RJ, Yu Z, Rouse BT. Enhancement of immune response to naked DNA vaccine by immunization with transfected dendritic cells. J Leukoc Biol. 1997;61:125–132. doi: 10.1002/jlb.61.2.125. [DOI] [PubMed] [Google Scholar]
- 180.Blalock LT, Landsberg J, Messmer M, Shi J, Pardee AD, Haskell R, Vujanovic L, Kirkwood JM, Butterfield LH. Human dendritic cells adenovirally-engineered to express three defined tumor antigens promote broad adaptive and innate immunity. Oncoimmunology. 2012;1:287–357. doi: 10.4161/onci.18628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kokhaei P, Rezvany MR, Virving L, Choudhury A, Rabbani H, Osterborg A, Mellstedt H. Dendritic cells loaded with apoptotic tumour cells induce a stronger T-cell response than dendritic cell-tumour hybrids in B-CLL. Leukemia. 2003;17:894–899. doi: 10.1038/sj.leu.2402913. [DOI] [PubMed] [Google Scholar]
- 183.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 184.Kandalaft LE, Powell DJ, Jr, Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, June CH, Coukos G. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2:e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fucikova J, Rozkova D, Ulcova H, Budinsky V, Sochorova K, Pokorna K, Bartunkova J, Spisek R. Poly I: C-activated dendritic cells that were generated in CellGro for use in cancer immunotherapy trials. J Transl Med. 2011;9:223. doi: 10.1186/1479-5876-9-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spisek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–4833. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- 187.Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000;60:1028–1034. [PubMed] [Google Scholar]
- 188.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997;186:1177–1182. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Garg NK, Dwivedi P, Prabha P, Tyagi RK. RNA pulsed dendritic cells: an approach for cancer immunotherapy. Vaccine. 2013;31:1141–1156. doi: 10.1016/j.vaccine.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 191.Schmidt T, Ziske C, Marten A, Endres S, Tiemann K, Schmitz V, Gorschluter M, Schneider C, Sauerbruch T, Schmidt-Wolf IG. Intratumoral immunization with tumor RNA-pulsed dendritic cells confers antitumor immunity in a C57BL/6 pancreatic murine tumor model. Cancer Res. 2003;63:8962–8967. [PubMed] [Google Scholar]
- 192.Celluzzi CM, Falo LD., Jr Physical interaction between dendritic cells and tumor cells results in an immunogen that induces protective and therapeutic tumor rejection. J Immunol. 1998;160:3081–3085. [PubMed] [Google Scholar]
- 193.Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol. 1998;161:5516–5524. [PubMed] [Google Scholar]
- 194.Orentas RJ, Schauer D, Bin Q, Johnson BD. Electrofusion of a weakly immunogenic neuroblastoma with dendritic cells produces a tumor vaccine. Cell Immunol. 2001;213:4–13. doi: 10.1006/cimm.2001.1864. [DOI] [PubMed] [Google Scholar]
- 195.Koido S, Homma S, Okamoto M, Namiki Y, Kan S, Takakura K, Kajihara M, Uchiyama K, Hara E, Ohkusa T, Gong J, Tajiri H. Improved immunogenicity of fusions between ethanol-treated cancer cells and dendritic cells exposed to dual TLR stimulation. Oncoimmunology. 2013;2:e25375. doi: 10.4161/onci.25375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Koido S, Homma S, Okamoto M, Namiki Y, Takakura K, Uchiyama K, Kajihara M, Arihiro S, Imazu H, Arakawa H, Kan S, Komita H, Ito M, et al. Fusions between dendritic cells and whole tumor cells as anticancer vaccines. Oncoimmunology. 2013;2:e24437. doi: 10.4161/onci.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Koido S, Homma S, Okamoto M, Namiki Y, Takakura K, Uchiyama K, Kajihara M, Arihiro S, Imazu H, Arakawa H, Kan S, Komita H, Kamata Y, et al. Strategies to improve the immunogenicity of anticancer vaccines based on dendritic cell/malignant cell fusions. Oncoimmunology. 2013;2:e25994. doi: 10.4161/onci.25994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Berraondo P, Nouze C, Preville X, Ladant D, Leclerc C. Eradication of large tumors in mice by a tritherapy targeting the innate, adaptive, and regulatory components of the immune system. Cancer Res. 2007;67:8847–8855. doi: 10.1158/0008-5472.CAN-07-0321. [DOI] [PubMed] [Google Scholar]
- 200.Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O'Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S, Reiter Y, Palucka AK, Zurawski G, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schreibelt G, Klinkenberg LJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M, Adema GJ, Brown GD, Figdor CG, de Vries IJ. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood. 2012;119:2284–2292. doi: 10.1182/blood-2011-08-373944. [DOI] [PubMed] [Google Scholar]
- 202.Tacken PJ, Ginter W, Berod L, Cruz LJ, Joosten B, Sparwasser T, Figdor CG, Cambi A. Targeting DC-SIGN via its neck region leads to prolonged antigen residence in early endosomes, delayed lysosomal degradation, and cross-presentation. Blood. 2011;118:4111–4119. doi: 10.1182/blood-2011-04-346957. [DOI] [PubMed] [Google Scholar]
- 203.Tacken PJ, Zeelenberg IS, Cruz LJ, van Hout-Kuijer MA, van de Glind G, Fokkink RG, Lambeck AJ, Figdor CG. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood. 2011;118:6836–6844. doi: 10.1182/blood-2011-07-367615. [DOI] [PubMed] [Google Scholar]
- 204.Wang B. Targeting dendritic cells in situ for breast cancer immunotherapy. Oncoimmunology. 2012;1:1398–1400. doi: 10.4161/onci.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Garcia-Vallejo JJ, Unger WW, Kalay H, van Kooyk Y. Glycan-based DC-SIGN targeting to enhance antigen cross-presentation in anticancer vaccines. Oncoimmunology. 2013;2:e23040. doi: 10.4161/onci.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 207.Viaud S, Thery C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N. Dendritic cell-derived exosomes for cancer immunotherapy: what's next? Cancer Res. 2010;70:1281–1285. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 208.Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1:1074–1083. doi: 10.4161/onci.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Satoh Y, Esche C, Gambotto A, Shurin GV, Yurkovetsky ZR, Robbins PD, Watkins SC, Todo S, Herberman RB, Lotze MT, Shurin MR. Local administration of IL-12-transfected dendritic cells induces antitumor immune responses to colon adenocarcinoma in the liver in mice. J Exp Ther Oncol. 2002;2:337–349. doi: 10.1046/j.1359-4117.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 210.Nishioka Y, Hirao M, Robbins PD, Lotze MT, Tahara H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res. 1999;59:4035–4041. [PubMed] [Google Scholar]
- 211.Endo H, Saito T, Kenjo A, Hoshino M, Terashima M, Sato T, Anazawa T, Kimura T, Tsuchiya T, Irisawa A, Ohira H, Hikichi T, Takagi T, et al. Phase I trial of preoperative intratumoral injection of immature dendritic cells and OK-432 for resectable pancreatic cancer patients. J Hepatobiliary Pancreat Sci. 2012;19:465–475. doi: 10.1007/s00534-011-0457-7. [DOI] [PubMed] [Google Scholar]
- 212.You CX, Shi M, Liu Y, Cao M, Luo R, Hermonat PL. AAV2/IL-12 gene delivery into dendritic cells (DC) enhances CTL stimulation above other IL-12 applications: Evidence for IL-12 intracrine activity in DC. Oncoimmunology. 2012;1:847–855. doi: 10.4161/onci.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bol KF, Tel J, de Vries IJ, Figdor CG. Naturally circulating dendritic cells to vaccinate cancer patients. Oncoimmunology. 2013;2:e23431. doi: 10.4161/onci.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Harfuddin Z, Kwajah S, Chong Nyi Sim A, Macary PA, Schwarz H. CD137L-stimulated dendritic cells are more potent than conventional dendritic cells at eliciting cytotoxic T-cell responses. Oncoimmunology. 2013;2:e26859. doi: 10.4161/onci.26859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, Figdor CG. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106:1278–1285. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- 216.Haicheur N, Bismuth E, Bosset S, Adotevi O, Warnier G, Lacabanne V, Regnault A, Desaymard C, Amigorena S, Ricciardi-Castagnoli P, Goud B, Fridman WH, Johannes L, et al. The B subunit of Shiga toxin fused to a tumor antigen elicits CTL and targets dendritic cells to allow MHC class I-restricted presentation of peptides derived from exogenous antigens. J Immunol. 2000;165:3301–3308. doi: 10.4049/jimmunol.165.6.3301. [DOI] [PubMed] [Google Scholar]
- 217.van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG. Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res. 2004;64:4357–4365. doi: 10.1158/0008-5472.CAN-04-0138. [DOI] [PubMed] [Google Scholar]
- 218.Badiee A, Davies N, McDonald K, Radford K, Michiue H, Hart D, Kato M. Enhanced delivery of immunoliposomes to human dendritic cells by targeting the multilectin receptor DEC-205. Vaccine. 2007;25:4757–4766. doi: 10.1016/j.vaccine.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 219.Yang L, Yang H, Rideout K, Cho T, Joo KI, Ziegler L, Elliot A, Walls A, Yu D, Baltimore D, Wang P. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol. 2008;26:326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hangalapura BN, Oosterhoff D, de Groot J, Boon L, Tuting T, van den Eertwegh AJ, Gerritsen WR, van Beusechem VW, Pereboev A, Curiel DT, Scheper RJ, de Gruijl TD. Potent antitumor immunity generated by a CD40-targeted adenoviral vaccine. Cancer Res. 2011;71:5827–5837. doi: 10.1158/0008-5472.CAN-11-0804. [DOI] [PubMed] [Google Scholar]
- 221.Korokhov N, de Gruijl TD, Aldrich WA, Triozzi PL, Banerjee PT, Gillies SD, Curiel TJ, Douglas JT, Scheper RJ, Curiel DT. High efficiency transduction of dendritic cells by adenoviral vectors targeted to DC-SIGN. Cancer Biol Ther. 2005;4:289–294. doi: 10.4161/cbt.4.3.1499. [DOI] [PubMed] [Google Scholar]
- 222.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 223.Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, Kantoff PW. Sipuleucel-T. Nat Rev Drug Discov. 2010;9:513–514. doi: 10.1038/nrd3220. [DOI] [PubMed] [Google Scholar]
- 224.Galluzzi L. New immunotherapeutic paradigms for castration-resistant prostate cancer. Oncoimmunology. 2013;2:e26084. doi: 10.4161/onci.26084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lakomy D, Janikashvili N, Fraszczak J, Trad M, Audia S, Samson M, Ciudad M, Vinit J, Vergely C, Caillot D, Foucher P, Lagrost L, Chouaib S, et al. Cytotoxic dendritic cells generated from cancer patients. J Immunol. 2011;187:2775–2782. doi: 10.4049/jimmunol.1004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 228.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Senovilla L, Vacchelli E, Garcia P, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: DNA vaccines for cancer therapy. Oncoimmunology. 2013;2:e23803. doi: 10.4161/onci.23803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Aranda F, Vacchelli E, Eggermont A, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Peptide vaccines in cancer therapy. Oncoimmunology. 2013;2:e26621. doi: 10.4161/onci.26621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Vacchelli E, Martins I, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Peptide vaccines in cancer therapy. Oncoimmunology. 2012;1:1557–1576. doi: 10.4161/onci.22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Aruga A. Vaccination of biliary tract cancer patients with four peptides derived from cancer-testis antigens. Oncoimmunology. 2013;2:e24882. doi: 10.4161/onci.24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ricupito A, Grioni M, Calcinotto A, Bellone M. Boosting anticancer vaccines: Too much of a good thing? Oncoimmunology. 2013;2:e25032. doi: 10.4161/onci.25032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hailemichael Y, Overwijk WW. Peptide-based anticancer vaccines: The making and unmaking of a T-cell graveyard. Oncoimmunology. 2013;2:e24743. doi: 10.4161/onci.24743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bijker MS, Melief CJ, Offringa R, van der Burg SH. Design and development of synthetic peptide vaccines: past, present and future. Expert Rev Vaccines. 2007;6:591–603. doi: 10.1586/14760584.6.4.591. [DOI] [PubMed] [Google Scholar]
- 236.Yaddanapudi K, Mitchell RA, Eaton JW. Cancer vaccines: Looking to the future. Oncoimmunology. 2013;2:e23403. doi: 10.4161/onci.23403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O'Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci U S A. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, Mellstedt H. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 239.Binder DC, Schreiber H. High-affinity peptide-based anticancer vaccination to overcome resistance to immunostimulatory antibodies. Oncoimmunology. 2013;2:e26704. doi: 10.4161/onci.26704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund's adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179:5033–5040. doi: 10.4049/jimmunol.179.8.5033. [DOI] [PubMed] [Google Scholar]
- 241.Zom GG, Khan S, Britten CM, Sommandas V, Camps MG, Loof NM, Budden CF, Meeuwenoord NJ, Filippov DV, van der Marel GA, Overkleeft HS, Melief CJ, Ossendorp F. Efficient induction of antitumor immunity by synthetic toll-like receptor ligand-peptide conjugates. Cancer Immunol Res. 2014;2:756–764. doi: 10.1158/2326-6066.CIR-13-0223. [DOI] [PubMed] [Google Scholar]
- 242.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, Liu C, Lou Y, Wang Z, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ciocca DR, Cayado-Gutierrez N, Maccioni M, Cuello-Carrion FD. Heat shock proteins (HSPs) based anti-cancer vaccines. Curr Mol Med. 2012;12:1183–1197. doi: 10.2174/156652412803306684. [DOI] [PubMed] [Google Scholar]
- 244.Fioretti D, Iurescia S, Fazio VM, Rinaldi M. DNA vaccines: developing new strategies against cancer. J Biomed Biotechnol. 2010;2010:174378. doi: 10.1155/2010/174378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239:62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 246.Stevenson FK, Ottensmeier CH, Rice J. DNA vaccines against cancer come of age. Curr Opin Immunol. 2010;22:264–270. doi: 10.1016/j.coi.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 247.Zhou Q, Buchholz CJ. Cell type specific gene delivery by lentiviral vectors: New options in immunotherapy. Oncoimmunology. 2013;2:e22566. doi: 10.4161/onci.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shirota H, Petrenko L, Hong C, Klinman DM. Potential of transfected muscle cells to contribute to DNA vaccine immunogenicity. J Immunol. 2007;179:329–336. doi: 10.4049/jimmunol.179.1.329. [DOI] [PubMed] [Google Scholar]
- 249.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 250.Pol JG, Zhang L, Bridle BW, Stephenson KB, Resseguier J, Hanson S, Chen L, Kazdhan N, Bramson JL, Stojdl DF, Wan Y, Lichty BD. Maraba virus as a potent oncolytic vaccine vector. Mol Ther. 2014;22:420–429. doi: 10.1038/mt.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bridle BW, Chen L, Lemay CG, Diallo JS, Pol J, Nguyen A, Capretta A, He R, Bramson JL, Bell JC, Lichty BD, Wan Y. HDAC inhibition suppresses primary immune responses, enhances secondary immune responses, and abrogates autoimmunity during tumor immunotherapy. Mol Ther. 2013;21:887–894. doi: 10.1038/mt.2012.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bridle BW, Clouthier D, Zhang L, Pol J, Chen L, Lichty BD, Bramson JL, Wan Y. Oncolytic vesicular stomatitis virus quantitatively and qualitatively improves primary CD8 T-cell responses to anticancer vaccines. Oncoimmunology. 2013;2:e26013. doi: 10.4161/onci.26013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bolhassani A, Zahedifard F. Therapeutic live vaccines as a potential anticancer strategy. Int J Cancer. 2012;131:1733–1743. doi: 10.1002/ijc.27640. [DOI] [PubMed] [Google Scholar]
- 254.Gardlik R, Fruehauf JH. Bacterial vectors and delivery systems in cancer therapy. IDrugs. 2010;13:701–706. [PubMed] [Google Scholar]
- 255.Moreno M, Kramer MG, Yim L, Chabalgoity JA. Salmonella as live trojan horse for vaccine development and cancer gene therapy. Curr Gene Ther. 2010;10:56–76. doi: 10.2174/156652310790945566. [DOI] [PubMed] [Google Scholar]
- 256.Toussaint B, Chauchet X, Wang Y, Polack B, Le Gouellec A. Live-attenuated bacteria as a cancer vaccine vector. Expert Rev Vaccines. 2013;12:1139–1154. doi: 10.1586/14760584.2013.836914. [DOI] [PubMed] [Google Scholar]
- 257.Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, Mulders P, Zielinski H, Hoos A, Teofilovici F, Isakov L, Flanigan R, Figlin R, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372:145–154. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 258.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 259.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 260.Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, Bowen R, Bunning ML. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75:4040–4047. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Anderson ED, Mourich DV, Leong JA. Gene expression in rainbow trout (Oncorhynchus mykiss) following intramuscular injection of DNA. Mol Mar Biol Biotechnol. 1996;5:105–113. [PubMed] [Google Scholar]
- 262.Anderson ED, Mourich DV, Fahrenkrug SC, LaPatra S, Shepherd J, Leong JA. Genetic immunization of rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis virus. Mol Mar Biol Biotechnol. 1996;5:114–122. [PubMed] [Google Scholar]
- 263.Bergman PJ, McKnight J, Novosad A, Charney S, Farrelly J, Craft D, Wulderk M, Jeffers Y, Sadelain M, Hohenhaus AE, Segal N, Gregor P, Engelhorn M, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. 2003;9:1284–1290. [PubMed] [Google Scholar]
- 264.Tato CM, Cua DJ. SnapShot: Cytokines I. Cell. 2008;132:324:324–e321. doi: 10.1016/j.cell.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 265.Tato CM, Cua DJ. SnapShot: Cytokines II. Cell. 2008;132:500. doi: 10.1016/j.cell.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 266.Tato CM, Cua DJ. SnapShot: Cytokines III. Cell. 2008;132:900. doi: 10.1016/j.cell.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 267.Tato CM, Cua DJ. SnapShot: Cytokines IV. Cell. 2008;132:1062:e1061–1062. doi: 10.1016/j.cell.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 268.Vacchelli E, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Immunostimulatory cytokines. Oncoimmunology. 2013;2:e24850. doi: 10.4161/onci.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Vacchelli E, Galluzzi L, Eggermont A, Galon J, Tartour E, Zitvogel L, Kroemer G. Trial Watch: Immunostimulatory cytokines. Oncoimmunology. 2012;1:493–506. doi: 10.4161/onci.20459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Papatriantafyllou M. Cytokines: true to their family name. Nat Rev Immunol. 2013;13:544–545. doi: 10.1038/nri3496. [DOI] [PubMed] [Google Scholar]
- 271.Chen P, Balachandran S. Development of interferon gamma-based immunocytokines targeting renal cancer. Oncoimmunology. 2013;2:e24964. doi: 10.4161/onci.24964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, Cheung K, Hesdorffer C, Kim-Schulze S, Kaufman HL. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–1177. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 274.Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L, Hwu P, Radvanyi L. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest. 2014;124:99–110. doi: 10.1172/JCI46266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Camisaschi C, Filipazzi P, Tazzari M, Casati C, Beretta V, Pilla L, Patuzzo R, Maurichi A, Cova A, Maio M, Chiarion-Sileni V, Tragni G, Santinami M, et al. Effects of cyclophosphamide and IL-2 on regulatory CD4+ T cell frequency and function in melanoma patients vaccinated with HLA-class I peptides: impact on the antigen-specific T cell response. Cancer Immunol Immunother. 2013;62:897–908. doi: 10.1007/s00262-013-1397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tomov B, Popov D, Tomova R, Vladov N, Den Otter W, Krastev Z. Therapeutic response of untreatable hepatocellular carcinoma after application of the immune modulators IL-2, BCG and melatonin. Anticancer Res. 2013;33:4531–4535. [PubMed] [Google Scholar]
- 277.Robertson MJ, Kline J, Struemper H, Koch KM, Bauman JW, Gardner OS, Murray SC, Germaschewski F, Weisenbach J, Jonak Z, Toso JF. A dose-escalation study of recombinant human interleukin-18 in combination with rituximab in patients with non-Hodgkin lymphoma. J Immunother. 2013;36:331–341. doi: 10.1097/CJI.0b013e31829d7e2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gorin NC, Isnard F, Garderet L, Ikhlef S, Corm S, Quesnel B, Legrand O, Cachanado M, Rousseau A, Laporte JP. Administration of alemtuzumab and G-CSF to adults with relapsed or refractory acute lymphoblastic leukemia: results of a phase II study. Eur J Haematol. 2013;91:315–321. doi: 10.1111/ejh.12154. [DOI] [PubMed] [Google Scholar]
- 279.Cheung IY, Hsu K, Cheung NK. Activation of peripheral-blood granulocytes is strongly correlated with patient outcome after immunotherapy with anti-GD2 monoclonal antibody and granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2012;30:426–432. doi: 10.1200/JCO.2011.37.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Cheung NK, Guo H, Hu J, Tassev DV, Cheung IY. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. Oncoimmunology. 2012;1:477–486. doi: 10.4161/onci.19864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Coker SA, Dandamudi UB, Beelen AP, Crosby NA, Fisher JL, Obrocea M, Ernstoff MS, Lewis LD. A phase I, dose-escalation study of cyclical weekly oral temozolomide and weekly PEG-interferon alpha-2b in patients with refractory or advanced solid tumours. J Chemother. 2013;25:362–368. doi: 10.1179/1973947813Y.0000000102. [DOI] [PubMed] [Google Scholar]
- 282.West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123:2604–2615. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Omori R, Eguchi J, Hiroishi K, Ishii S, Hiraide A, Sakaki M, Doi H, Kajiwara A, Ito T, Kogo M, Imawari M. Effects of interferon-alpha-transduced tumor cell vaccines and blockade of programmed cell death-1 on the growth of established tumors. Cancer Gene Ther. 2012;19:637–643. doi: 10.1038/cgt.2012.42. [DOI] [PubMed] [Google Scholar]
- 284.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Arellano M, Lonial S. Clinical uses of GM-CSF, a critical appraisal and update. Biologics. 2008;2:13–27. doi: 10.2147/btt.s1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Demirer T, Ayli M, Ozcan M, Gunel N, Haznedar R, Dagli M, Fen T, Genc Y, Dincer S, Arslan O, Gurman G, Demirer S, Ozet G, et al. Mobilization of peripheral blood stem cells with chemotherapy and recombinant human granulocyte colony-stimulating factor (rhG-CSF): a randomized evaluation of different doses of rhG-CSF. Br J Haematol. 2002;116:468–474. doi: 10.1046/j.1365-2141.2002.03264.x. [DOI] [PubMed] [Google Scholar]
- 287.Chan KK, Siu E, Krahn MD, Imrie K, Alibhai SM. Cost-utility analysis of primary prophylaxis versus secondary prophylaxis with granulocyte colony-stimulating factor in elderly patients with diffuse aggressive lymphoma receiving curative-intent chemotherapy. J Clin Oncol. 2012;30:1064–1071. doi: 10.1200/JCO.2011.36.8647. [DOI] [PubMed] [Google Scholar]
- 288.Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, Vekemans MC, Biemond B, Sonneveld P, Passweg J, Verdonck L, Legdeur MC, Theobald M, Jacky E, et al. Favorable effect of priming with granulocyte colony-stimulating factor in remission induction of acute myeloid leukemia restricted to dose escalation of cytarabine. Blood. 2012;119:5367–5373. doi: 10.1182/blood-2011-11-389841. [DOI] [PubMed] [Google Scholar]
- 289.Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, Leming P, Puzanov I, Shin D, Kirkwood JM. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312:1744–1753. doi: 10.1001/jama.2014.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Deroose JP, Eggermont AM, van Geel AN, de Wilt JH, Burger JW, Verhoef C. 20 years experience of TNF-based isolated limb perfusion for in-transit melanoma metastases: TNF dose matters. Ann Surg Oncol. 2012;19:627–635. doi: 10.1245/s10434-011-2030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Eggermont AM, Schraffordt Koops H, Lienard D, Kroon BB, van Geel AN, Hoekstra HJ, Lejeune FJ. Isolated limb perfusion with high-dose tumor necrosis factor-alpha in combination with interferon-gamma and melphalan for nonresectable extremity soft tissue sarcomas: a multicenter trial. J Clin Oncol. 1996;14:2653–2665. doi: 10.1200/JCO.1996.14.10.2653. [DOI] [PubMed] [Google Scholar]
- 292.Deroose JP, Eggermont AM, van Geel AN, Burger JW, den Bakker MA, de Wilt JH, Verhoef C. Long-term results of tumor necrosis factor alpha- and melphalan-based isolated limb perfusion in locally advanced extremity soft tissue sarcomas. J Clin Oncol. 2011;29:4036–4044. doi: 10.1200/JCO.2011.35.6618. [DOI] [PubMed] [Google Scholar]
- 293.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 294.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 295.Chawla A, Philips AV, Alatrash G, Mittendorf E. Immune checkpoints: A therapeutic target in triple negative breast cancer. Oncoimmunology. 2014;3:e28325. doi: 10.4161/onci.28325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Nowak AK. Immunological checkpoint inhibitors enter adolescence. Lancet Oncol. 2013;14:1035–1037. doi: 10.1016/S1470-2045(13)70401-7. [DOI] [PubMed] [Google Scholar]
- 297.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 298.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 299.Waitz R, Fasso M, Allison JP. CTLA-4 blockade synergizes with cryoablation to mediate tumor rejection. Oncoimmunology. 2012;1:544–546. doi: 10.4161/onci.19442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Munir S, Andersen GH, Svane IM, Andersen MH. The immune checkpoint regulator PD-L1 is a specific target for naturally occurring CD4 T cells. Oncoimmunology. 2013;2:e23991. doi: 10.4161/onci.23991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol. 2009;9:568–580. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Joncker NT, Raulet DH. Regulation of NK cell responsiveness to achieve self-tolerance and maximal responses to diseased target cells. Immunol Rev. 2008;224:85–97. doi: 10.1111/j.1600-065X.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Schalper KA. PD-L1 expression and tumor-infiltrating lymphocytes: Revisiting the antitumor immune response potential in breast cancer. Oncoimmunology. 2014;3:e29288. doi: 10.4161/onci.29288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 307.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 308.Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19:1044–1053. doi: 10.1158/1078-0432.CCR-12-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Adler AJ, Vella AT. Betting on improved cancer immunotherapy by doubling down on CD134 and CD137 co-stimulation. Oncoimmunology. 2013;2:e22837. doi: 10.4161/onci.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Withers DR, Gaspal FM, Bekiaris V, McConnell FM, Kim M, Anderson G, Lane PJ. OX40 and CD30 signals in CD4(+) T-cell effector and memory function: a distinct role for lymphoid tissue inducer cells in maintaining CD4(+) T-cell memory but not effector function. Immunol Rev. 2011;244:134–148. doi: 10.1111/j.1600-065X.2011.01057.x. [DOI] [PubMed] [Google Scholar]
- 311.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4:420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 313.Hombach AA, Heiders J, Foppe M, Chmielewski M, Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology. 2012;1:458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 315.Ye Q, Song DG, Powell Jr DJ. Finding a needle in a haystack: Activation-induced CD137 expression accurately identifies naturally occurring tumor-reactive T cells in cancer patients. Oncoimmunology. 2013;2:e27184. doi: 10.4161/onci.27184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 317.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 318.Chacon JA, Pilon-Thomas S, Sarnaik AA, Radvanyi LG. Continuous 4-1BB co-stimulatory signals for the optimal expansion of tumor-infiltrating lymphocytes for adoptive T-cell therapy. Oncoimmunology. 2013;2:e25581. doi: 10.4161/onci.25581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Schnurr M, Duewell P. Breaking tumor-induced immunosuppression with 5′-triphosphate siRNA silencing TGFbeta and activating RIG-I. Oncoimmunology. 2013;2:e24170. doi: 10.4161/onci.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–799. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Peng W, Lizee G, Hwu P. Blockade of the PD-1 pathway enhances the efficacy of adoptive cell therapy against cancer. Oncoimmunology. 2013;2:e22691. doi: 10.4161/onci.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Lu H, Cuillerot JM, Lynch TJ. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 323.Mavilio D, Lugli E. Inhibiting the inhibitors: Checkpoints blockade in solid tumors. Oncoimmunology. 2013;2:e26535. doi: 10.4161/onci.26535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, Pardoll DM, Brahmer JR, Topalian SL. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Cooper ZA, Frederick DT, Ahmed Z, Wargo JA. Combining checkpoint inhibitors and BRAF-targeted agents against metastatic melanoma. Oncoimmunology. 2013;2:e24320. doi: 10.4161/onci.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Trial watch: ipilimumab success in melanoma provides boost for cancer immunotherapy. Nat Rev Drug Discov. 2010;9:584. doi: 10.1038/nrd3245. [DOI] [PubMed] [Google Scholar]
- 330.Erdmann MK. Immunity unleashed in melanoma. Lancet Oncol. 2010;11:108–109. doi: 10.1016/S1470-2045(09)70400-0. [DOI] [PubMed] [Google Scholar]
- 331.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr, Grob JJ, Chesney J, Chin K, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 332.Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nat Rev Drug Discov. 2011;10:411–412. doi: 10.1038/nrd3463. [DOI] [PubMed] [Google Scholar]
- 333.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.PD-1 Inhibitor Approved for Melanoma. Cancer Discov. 2014;4:1249. doi: 10.1158/2159-8290.CD-NB2014-144. [DOI] [PubMed] [Google Scholar]
- 335.Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74:1973–1981. doi: 10.1007/s40265-014-0314-5. [DOI] [PubMed] [Google Scholar]
- 336.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, Patnaik A, Dronca R, Zarour H, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 337.Bagcchi S. Pembrolizumab for treatment of refractory melanoma. Lancet Oncol. 2014;15:e419. doi: 10.1016/s1470-2045(14)70348-1. [DOI] [PubMed] [Google Scholar]
- 338.Galluzzi L, Kroemer G, Eggermont A. Novel immune checkpoint blocker approved for the treatment of advanced melanoma. Oncoimmunology. 2014;3 doi: 10.4161/21624011.2014.967147. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Deeks ED. Nivolumab: a review of its use in patients with malignant melanoma. Drugs. 2014;74:1233–1239. doi: 10.1007/s40265-014-0234-4. [DOI] [PubMed] [Google Scholar]
- 340.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2014 doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 341.Aranda F, Vacchelli E, Eggermont A, Galon J, Fridman WH, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Immunostimulatory monoclonal antibodies in cancer therapy. Oncoimmunology. 2014;3:e27297. doi: 10.4161/onci.27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2014 doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Dai M, Yip YY, Hellstrom I, Hellstrom KE. Curing Mice with Large Tumors by Locally Delivering Combinations of Immunomodulatory Antibodies. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wei H, Zhao L, Hellstrom I, Hellstrom KE, Guo Y. Dual targeting of CD137 co-stimulatory and PD-1 co-inhibitory molecules for ovarian cancer immunotherapy. Oncoimmunology. 2014;3:e28248. doi: 10.4161/onci.28248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, Mueller A, Sagiv-Barfi I, Marabelle A, Lira R, Troutner E, Richards L, Rajapaska A, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest. 2014;124:2668–2682. doi: 10.1172/JCI73014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 346.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Colevas AD, Weng WK, Clarke MF, Carlson RW, Stockdale FE, Mollick JA, Chen L, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 347.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Vacchelli E, Aranda F, Eggermont A, Sautes-Fridman C, Tartour E, Kennedy EP, Platten M, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2014;3 doi: 10.4161/21624011.2014.957994. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Yoshida R, Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1978;75:3998–4000. doi: 10.1073/pnas.75.8.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 352.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 353.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 354.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA, 3rd, Kahler DJ, Pihkala J, Soler AP, Munn DH, Prendergast GC, Mellor AL. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A. 2008;105:17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP, Laury-Kleintop LD, Mandik-Nayak L, Metz R, Ostrand-Rosenberg S, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2:722–735. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Abe BT, Macian F. Uncovering the mechanisms that regulate tumor-induced T-cell anergy. Oncoimmunology. 2013;2:e22679. doi: 10.4161/onci.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Galluzzi L, Bravo-San Pedro JM, Kroemer G. Organelle-specific initiation of cell death. Nat Cell Biol. 2014;16:728–736. doi: 10.1038/ncb3005. [DOI] [PubMed] [Google Scholar]
- 359.Creelan BC, Antonia S, Bepler G, Garrett TJ, Simon GR, Soliman HH. Indoleamine 2,3-dioxygenase activity and clinical outcome following induction chemotherapy and concurrent chemoradiation in Stage III non-small cell lung cancer. Oncoimmunology. 2013;2:e23428. doi: 10.4161/onci.23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, Huang Y, Douek DC, Brenchley JM, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, Quintana FJ. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010;11:846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, Gupta R, Lee LY, Kidd BA, Robinson WH, Sobel RA, Selley ML, Steinman L. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 364.Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, Elliott GI, Kufareva I, Abagyan R, Broide DH, Lee J, Raz E. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci U S A. 2007;104:18619–18624. doi: 10.1073/pnas.0709261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 366.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 367.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 368.Fallarino F, Orabona C, Vacca C, Bianchi R, Gizzi S, Asselin-Paturel C, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Ligand and cytokine dependence of the immunosuppressive pathway of tryptophan catabolism in plasmacytoid dendritic cells. Int Immunol. 2005;17:1429–1438. doi: 10.1093/intimm/dxh321. [DOI] [PubMed] [Google Scholar]
- 369.Fallarino F, Asselin-Paturel C, Vacca C, Bianchi R, Gizzi S, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol. 2004;173:3748–3754. doi: 10.4049/jimmunol.173.6.3748. [DOI] [PubMed] [Google Scholar]
- 370.Conrad C, Gilliet M. Plasmacytoid dendritic cells and regulatory T cells in the tumor microenvironment: A dangerous liaison. Oncoimmunology. 2013;2:e23887. doi: 10.4161/onci.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Sisirak V, Faget J, Vey N, Blay JY, Menetrier-Caux C, Caux C, Bendriss-Vermare N. Plasmacytoid dendritic cells deficient in IFNalpha production promote the amplification of FOXP3 regulatory T cells and are associated with poor prognosis in breast cancer patients. Oncoimmunology. 2013;2:e22338. doi: 10.4161/onci.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 373.Manuel ER, Diamond DJ. A road less traveled paved by IDO silencing: Harnessing the antitumor activity of neutrophils. Oncoimmunology. 2013;2:e23322. doi: 10.4161/onci.23322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Zheng X, Koropatnick J, Chen D, Velenosi T, Ling H, Zhang X, Jiang N, Navarro B, Ichim TE, Urquhart B, Min W. Silencing IDO in dendritic cells: a novel approach to enhance cancer immunotherapy in a murine breast cancer model. Int J Cancer. 2013;132:967–977. doi: 10.1002/ijc.27710. [DOI] [PubMed] [Google Scholar]
- 375.Blache CA, Manuel ER, Kaltcheva TI, Wong AN, Ellenhorn JD, Blazar BR, Diamond DJ. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res. 2012;72:6447–6456. doi: 10.1158/0008-5472.CAN-12-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J, Zeyher C, Gouttefangeas C, Thomsen BM, Holm B, Thor Straten P, Mellemgaard A, Andersen MH, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res. 2014;20:221–232. doi: 10.1158/1078-0432.CCR-13-1560. [DOI] [PubMed] [Google Scholar]
- 377.Soliman HH, Minton SE, Ismail-Khan R, Han HS, Vahanian NN, Ramsey WJ, Kennedy E, Link CJ, Sullivan D, Antonia SJ. A phase 2 study of docetaxel in combination with indoximod in metastatic breast cancer. J Clin Oncol. 2014;32 abstr TPS3124. [Google Scholar]
- 378.Jackson E, Dees EC, Kauh JS, Harvey RD, Neuger A, Lush R, Antonia SJ, Minton SE, Ismail-Khan R, Han HS, Vahanian NN, Ramsey WJ, Link CJ, et al. A phase I study of indoximod in combination with docetaxel in metastatic solid tumors. J Clin Oncol. 2013;31 abstr 3026. [Google Scholar]
- 379.Soliman HH, Antonia SJ, Sullivan D, Vanahanian N, Link CJ. Overcoming tumor antigen anergy in human malignancies using the novel indoleamine 2,3-dioxygenase (IDO) enzyme inhibitor, 1-methyl-D-tryptophan (1MT) J Clin Oncol. 2009;27 abstr 3004. [Google Scholar]
- 380.Beatty GL, O'Dwyer PJ, Clark J, Shi JG, Newton RC, Schaub R, Maleski J, Leopold L, Gajewski T. Phase I study of the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of the oral inhibitor of indoleamine 2,3-dioxygenase (IDO1) INCB024360 in patients (pts) with advanced malignancies. J Clin Oncol. 2013;31 doi: 10.1158/1078-0432.CCR-16-2272. abstr 3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Gibney GT, Hamid O, Gangadhar TC, Lutzky J, Olszanski AJ, Gajewski T, Chmielowski B, Boasberg PD, Zhao Y, Newton RC, Scherle PA, Bowman J, Maleski J, et al. Preliminary results from a phase 1/2 study of INCB024360 combined with ipilimumab (ipi) in patients (pts) with melanoma. J Clin Oncol. 2014;32 abstr 3010. [Google Scholar]
- 382.Newton RC, Scherle PA, Bowman K, Liu X, Beatty GL, O'Dwyer PJ, Gajewski T, Bowman J, Schaub R, Leopold L. Pharmacodynamic assessment of INCB024360, an inhibitor of indoleamine 2,3-dioxygenase 1 (IDO1), in advanced cancer patients. J Clin Oncol. 2012;30 abstr 2500. [Google Scholar]
- 383.Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, Smyth MJ, Zitvogel L. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855–858. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 384.Cauwels A, Rogge E, Vandendriessche B, Shiva S, Brouckaert P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis. 2014;5:e1102. doi: 10.1038/cddis.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Ma Y, Adjemian S, Yang H, Catani JP, Hannani D, Martins I, Michaud M, Kepp O, Sukkurwala AQ, Vacchelli E, Galluzzi L, Zitvogel L, Kroemer G. ATP-dependent recruitment, survival and differentiation of dendritic cell precursors in the tumor bed after anticancer chemotherapy. Oncoimmunology. 2013;2:e24568. doi: 10.4161/onci.24568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Yamaguchi H, Maruyama T, Urade Y, Nagata S. Immunosuppression via adenosine receptor activation by adenosine monophosphate released from apoptotic cells. Elife. 2014;3:e02172. doi: 10.7554/eLife.02172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Sorrentino R, Pinto A, Morello S. The adenosinergic system in cancer: Key therapeutic target. Oncoimmunology. 2013;2:e22448. doi: 10.4161/onci.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Michaud M, Sukkurwala AQ, Martins I, Shen S, Zitvogel L, Kroemer G. Subversion of the chemotherapy-induced anticancer immune response by the ecto-ATPase CD39. Oncoimmunology. 2012;1:393–395. doi: 10.4161/onci.19070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene. 2013;32:1743–1751. doi: 10.1038/onc.2012.269. [DOI] [PubMed] [Google Scholar]
- 393.Salmi M, Jalkanen S. Host CD73 impairs anti-tumor immunity. Oncoimmunology. 2012;1:247–248. doi: 10.4161/onci.1.2.18310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Zhang B. CD73 promotes tumor growth and metastasis. Oncoimmunology. 2012;1:67–70. doi: 10.4161/onci.1.1.18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Aliagas E, Vidal A, Texido L, Ponce J, Condom E, Martin-Satue M. High expression of ecto-nucleotidases CD39 and CD73 in human endometrial tumors. Mediators Inflamm. 2014;2014:509027. doi: 10.1155/2014/509027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 397.Young A, Mittal D, Stagg J, Smyth MJ. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. 2014;4:879–888. doi: 10.1158/2159-8290.CD-14-0341. [DOI] [PubMed] [Google Scholar]
- 398.Metz R, Smith C, DuHadaway JB, Chandler P, Baban B, Merlo LM, Pigott E, Keough MP, Rust S, Mellor AL, Mandik-Nayak L, Muller AJ, Prendergast GC. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int Immunol. 2014;26:357–367. doi: 10.1093/intimm/dxt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 401.Delgado M, Singh S, De Haro S, Master S, Ponpuak M, Dinkins C, Ornatowski W, Vergne I, Deretic V. Autophagy and pattern recognition receptors in innate immunity. Immunol Rev. 2009;227:189–202. doi: 10.1111/j.1600-065X.2008.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 403.Hennessy EJ, Parker AE, O'Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 404.Saleh M. The machinery of Nod-like receptors: refining the paths to immunity and cell death. Immunol Rev. 2011;243:235–246. doi: 10.1111/j.1600-065X.2011.01045.x. [DOI] [PubMed] [Google Scholar]
- 405.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Garg AD, Martin S, Golab J, Agostinis P. Danger signalling during cancer cell death: origins, plasticity and regulation. Cell Death Differ. 2014;21:26–38. doi: 10.1038/cdd.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 408.Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780–788. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- 409.Spel L, Boelens JJ, Nierkens S, Boes M. Antitumor immune responses mediated by dendritic cells: How signals derived from dying cancer cells drive antigen cross-presentation. Oncoimmunology. 2013;2:e26403. doi: 10.4161/onci.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Yi Y, Zhou Z, Shu S, Fang Y, Twitty C, Hilton TL, Aung S, Urba WJ, Fox BA, Hu HM, Li Y. Autophagy-assisted antigen cross-presentation: Autophagosome as the argo of shared tumor-specific antigens and DAMPs. Oncoimmunology. 2012;1:976–978. doi: 10.4161/onci.20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 412.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 413.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 414.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 415.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 416.Brennan TV, Lin L, Huang X, Cardona DM, Li Z, Dredge K, Chao NJ, Yang Y. Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD after allogeneic stem cell transplantation. Blood. 2012;120:2899–2908. doi: 10.1182/blood-2011-07-368720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 418.Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, Sukkurwala AQ, Menger L, Zitvogel L, Kroemer G. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–69. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 419.Vacchelli E, Vitale I, Tartour E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Anticancer radioimmunotherapy. Oncoimmunology. 2013;2:e25595. doi: 10.4161/onci.25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 421.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 422.Demaria S, Vanpouille-Box C, Formenti SC, Adams S. The TLR7 agonist imiquimod as an adjuvant for radiotherapy-elicited in situ vaccination against breast cancer. Oncoimmunology. 2013;2:e25997. doi: 10.4161/onci.25997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Brito LA, O'Hagan DT. Designing and building the next generation of improved vaccine adjuvants. J Control Release. 2014;190:563–579. doi: 10.1016/j.jconrel.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 424.Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc Natl Acad Sci U S A. 2014;111:12294–12299. doi: 10.1073/pnas.1400478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Narayan R, Nguyen H, Bentow JJ, Moy L, Lee DK, Greger S, Haskell J, Vanchinathan V, Chang PL, Tsui S, Konishi T, Comin-Anduix B, Dauphine C, et al. Immunomodulation by imiquimod in patients with high-risk primary melanoma. J Invest Dermatol. 2012;132:163–169. doi: 10.1038/jid.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Hoffman ES, Smith RE, Renaud RC., Jr From the analyst's couch: TLR-targeted therapeutics. Nat Rev Drug Discov. 2005;4:879–880. doi: 10.1038/nrd1880. [DOI] [PubMed] [Google Scholar]
- 427.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 428.Okamoto H, Shoin S, Koshimura S, Shimizu R. Studies on the anticancer and streptolysin S-forming abilities of hemolytic streptococci. Jpn J Microbiol. 1967;11:323–326. doi: 10.1111/j.1348-0421.1967.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 429.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 430.Ando K, Mori K, Corradini N, Redini F, Heymann D. Mifamurtide for the treatment of nonmetastatic osteosarcoma. Expert Opin Pharmacother. 2011;12:285–292. doi: 10.1517/14656566.2011.543129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Murray JL, Kleinerman ES, Cunningham JE, Tatom JR, Andrejcio K, Lepe-Zuniga J, Lamki LM, Rosenblum MG, Frost H, Gutterman JU, et al. Phase I trial of liposomal muramyl tripeptide phosphatidylethanolamine in cancer patients. J Clin Oncol. 1989;7:1915–1925. doi: 10.1200/JCO.1989.7.12.1915. [DOI] [PubMed] [Google Scholar]
- 432.Fidler IJ, Fogler WE, Brownbill AF, Schumann G. Systemic activation of tumoricidal properties in mouse macrophages and inhibition of melanoma metastases by the oral administration of MTP-PE, a lipophilic muramyl dipeptide. J Immunol. 1987;138:4509–4514. [PubMed] [Google Scholar]
- 433.Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial Watch: Experimental Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2012;1:699–716. doi: 10.4161/onci.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Vacchelli E, Galluzzi L, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2012;1:894–907. doi: 10.4161/onci.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2013;2:e25238. doi: 10.4161/onci.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Murad YM, Clay TM, Lyerly HK, Morse MA. CPG-7909 (PF-3512676, ProMune): toll-like receptor-9 agonist in cancer therapy. Expert Opin Biol Ther. 2007;7:1257–1266. doi: 10.1517/14712598.7.8.1257. [DOI] [PubMed] [Google Scholar]
- 437.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 438.Levy HB, Baer G, Baron S, Buckler CE, Gibbs CJ, Iadarola MJ, London WT, Rice J. A modified polyriboinosinic-polyribocytidylic acid complex that induces interferon in primates. J Infect Dis. 1975;132:434–439. doi: 10.1093/infdis/132.4.434. [DOI] [PubMed] [Google Scholar]
- 439.Hagstrom J, Heikkila A, Siironen P, Louhimo J, Heiskanen I, Maenpaa H, Arola J, Haglund C. TLR-4 expression and decrease in chronic inflammation: indicators of aggressive follicular thyroid carcinoma. J Clin Pathol. 2012;65:333–338. doi: 10.1136/jclinpath-2011-200402. [DOI] [PubMed] [Google Scholar]
- 440.Kauppila JH, Mattila AE, Karttunen TJ, Salo T. Toll-like receptor 5 (TLR5) expression is a novel predictive marker for recurrence and survival in squamous cell carcinoma of the tongue. Br J Cancer. 2013;108:638–643. doi: 10.1038/bjc.2012.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Kauppila JH, Mattila AE, Karttunen TJ, Salo T. Toll-like receptor 5 and the emerging role of bacteria in carcinogenesis. Oncoimmunology. 2013;2:e23620. doi: 10.4161/onci.23620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Ahmed A, Redmond HP, Wang JH. Links between Toll-like receptor 4 and breast cancer. Oncoimmunology. 2013;2:e22945. doi: 10.4161/onci.22945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Conti L, Lanzardo S, Arigoni M, Antonazzo R, Radaelli E, Cantarella D, Calogero RA, Cavallo F. The noninflammatory role of high mobility group box 1/Toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. FASEB J. 2013;27:4731–4744. doi: 10.1096/fj.13-230201. [DOI] [PubMed] [Google Scholar]
- 444.Tye H, Kennedy CL, Najdovska M, McLeod L, McCormack W, Hughes N, Dev A, Sievert W, Ooi CH, Ishikawa TO, Oshima H, Bhathal PS, Parker AE, et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell. 2012;22:466–478. doi: 10.1016/j.ccr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 445.Cherfils-Vicini J, Platonova S, Gillard M, Laurans L, Validire P, Caliandro R, Magdeleinat P, Mami-Chouaib F, Dieu-Nosjean MC, Fridman WH, Damotte D, Sautes-Fridman C, Cremer I. Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J Clin Invest. 2010;120:1285–1297. doi: 10.1172/JCI36551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 447.Sheng Sow H, Mattarollo SR. Combining low-dose or metronomic chemotherapy with anticancer vaccines: A therapeutic opportunity for lymphomas. Oncoimmunology. 2013;2:e27058. doi: 10.4161/onci.27058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, Annaert W, Golab J, de Witte P, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Cirone M, Di Renzo L, Lotti LV, Conte V, Trivedi P, Santarelli R, Gonnella R, Frati L, Faggioni A. Activation of dendritic cells by tumor cell death. Oncoimmunology. 2012;1:1218–1219. doi: 10.4161/onci.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, Schlemmer F, Sulpice E, Locher C, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4:143ra199. doi: 10.1126/scitranslmed.3003807. [DOI] [PubMed] [Google Scholar]
- 452.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 453.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 454.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, Martins I, Schlemmer F, Michaud M, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–741. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 455.Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N, Bracci L, Breckpot K, Brough D, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3 doi: 10.4161/21624011.2014.955691. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Bugaut H, Bruchard M, Berger H, Derangere V, Odoul L, Euvrard R, Ladoire S, Chalmin F, Vegran F, Rebe C, Apetoh L, Ghiringhelli F, Mignot G. Bleomycin exerts ambivalent antitumor immune effect by triggering both immunogenic cell death and proliferation of regulatory T cells. PLoS One. 2013;8:e65181. doi: 10.1371/journal.pone.0065181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, Tesniere A, Zitvogel L, Kroemer G. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30:1147–1158. doi: 10.1038/onc.2010.500. [DOI] [PubMed] [Google Scholar]
- 458.Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, Sanchez M, Lorenzi S, D'Urso MT, Belardelli F, Gabriele L, Proietti E, Bracci L. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;71:768–778. doi: 10.1158/0008-5472.CAN-10-2788. [DOI] [PubMed] [Google Scholar]
- 459.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, Fend L, Hannani D, Aymeric L, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 460.Guerriero JL, Ditsworth D, Fan Y, Zhao F, Crawford HC, Zong WX. Chemotherapy induces tumor clearance independent of apoptosis. Cancer Res. 2008;68:9595–9600. doi: 10.1158/0008-5472.CAN-08-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Galluzzi L, Kepp O, Kroemer G. Immunogenic cell death in radiation therapy. Oncoimmunology. 2013;2:e26536. doi: 10.4161/onci.26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T, Kono K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72:3967–3976. doi: 10.1158/0008-5472.CAN-12-0851. [DOI] [PubMed] [Google Scholar]
- 463.Ko A, Kanehisa A, Martins I, Senovilla L, Chargari C, Dugue D, Marino G, Kepp O, Michaud M, Perfettini JL, Kroemer G, Deutsch E. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 2014;21:92–99. doi: 10.1038/cdd.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Schildkopf P, Frey B, Ott OJ, Rubner Y, Multhoff G, Sauer R, Fietkau R, Gaipl US. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother Oncol. 2011;101:109–115. doi: 10.1016/j.radonc.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 466.Hasumi K, Aoki Y, Wantanabe R, Mann DL. Clinical response of advanced cancer patients to cellular immunotherapy and intensity-modulated radiation therapy. Oncoimmunology. 2013;2:e26381. doi: 10.4161/onci.26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Galluzzi L, Kepp O, Kroemer G. Enlightening the impact of immunogenic cell death in photodynamic cancer therapy. EMBO J. 2012;31:1055–1057. doi: 10.1038/emboj.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Garg AD, Krysko DV, Vandenabeele P, Agostinis P. Hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol Immunother. 2012;61:215–221. doi: 10.1007/s00262-011-1184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Demaria S, Santori FR, Ng B, Liebes L, Formenti SC, Vukmanovic S. Select forms of tumor cell apoptosis induce dendritic cell maturation. J Leukoc Biol. 2005;77:361–368. doi: 10.1189/jlb.0804478. [DOI] [PubMed] [Google Scholar]
- 471.Vacchelli E, Galluzzi L, Rousseau V, Rigoni A, Tesniere A, Delahaye N, Schlemmer FD, Menger L, Sukkurwala AQ, Adjemian S, Martins I, Michaud M, Dunant A, et al. Loss-of-function alleles of P2RX7 and TLR4 fail to affect the response to chemotherapy in non-small cell lung cancer. Oncoimmunology. 2012;1:271–278. doi: 10.4161/onci.18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Muller GW, Corral LG, Shire MG, Wang H, Moreira A, Kaplan G, Stirling DI. Structural modifications of thalidomide produce analogs with enhanced tumor necrosis factor inhibitory activity. J Med Chem. 1996;39:3238–3240. doi: 10.1021/jm9603328. [DOI] [PubMed] [Google Scholar]
- 473.Curran WJ. The thalidomide tragedy in Germany: the end of a historic medicolegal trial. N Engl J Med. 1971;284:481–482. doi: 10.1056/NEJM197103042840906. [DOI] [PubMed] [Google Scholar]
- 474.Sarno EN, Grau GE, Vieira LM, Nery JA. Serum levels of tumour necrosis factor-alpha and interleukin-1 beta during leprosy reactional states. Clin Exp Immunol. 1991;84:103–108. [PMC free article] [PubMed] [Google Scholar]
- 475.Raje N, Anderson K. Thalidomide--a revival story. N Engl J Med. 1999;341:1606–1609. doi: 10.1056/NEJM199911183412110. [DOI] [PubMed] [Google Scholar]
- 476.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, Zeddis J, Barlogie B. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 477.Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, San Miguel J, Hellmann A, Facon T, Foa R, Corso A, Masliak Z, Olesnyckyj M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 478.Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV, Chanan-Khan AA, Lonial S, Yu Z, Patin J, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 479.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, Powell B, Greenberg P, Thomas D, Stone R, Reeder C, Wride K, Patin J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 480.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, Rimsza L, Heaton R, Knight R, Zeldis JB. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 481.San Miguel J, Weisel K, Moreau P, Lacy M, Song K, Delforge M, Karlin L, Goldschmidt H, Banos A, Oriol A, Alegre A, Chen C, Cavo M, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 482.Goy A, Sinha R, Williams ME, Kalayoglu Besisik S, Drach J, Ramchandren R, Zhang L, Cicero S, Fu T, Witzig TE. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31:3688–3695. doi: 10.1200/JCO.2013.49.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Wang M, Fayad L, Wagner-Bartak N, Zhang L, Hagemeister F, Neelapu SS, Samaniego F, McLaughlin P, Fanale M, Younes A, Cabanillas F, Fowler N, Newberry KJ, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13:716–723. doi: 10.1016/S1470-2045(12)70200-0. [DOI] [PubMed] [Google Scholar]
- 484.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 485.Semeraro M, Galluzzi L. Novel insights into the mechanism of action of lenalidomide. Oncoimmunology. 2014;3:e28386. doi: 10.4161/onci.28386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong KK, Bradner JE, Kaelin WG., Jr The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–309. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, Ciarlo C, Hartman E, Munshi N, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 489.Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, Karasawa S, Carmel G, Jackson P, Abbasian M, Mahmoudi A, Cathers B, Rychak E, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–2335. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology. 2013;2:e24720. doi: 10.4161/onci.24720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Senovilla L, Aranda F, Galluzzi L, Kroemer G. Impact of myeloid cells on the efficacy of anticancer chemotherapy. Curr Opin Immunol. 2014;30C:24–31. doi: 10.1016/j.coi.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 492.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 493.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Kadin ME, Vonderheid EC. Targeted therapies: Denileukin diftitox--a step towards a ‘magic bullet’ for CTCL. Nat Rev Clin Oncol. 2010;7:430–432. doi: 10.1038/nrclinonc.2010.105. [DOI] [PubMed] [Google Scholar]
- 495.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Yamada Y, Aoyama A, Tocco G, Boskovic S, Nadazdin O, Alessandrini A, Madsen JC, Cosimi AB, Benichou G, Kawai T. Differential effects of denileukin diftitox IL-2 immunotoxin on NK and regulatory T cells in nonhuman primates. J Immunol. 2012;188:6063–6070. doi: 10.4049/jimmunol.1200656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Baur AS, Lutz MB, Schierer S, Beltrame L, Theiner G, Zinser E, Ostalecki C, Heidkamp G, Haendle I, Erdmann M, Wiesinger M, Leisgang W, Gross S, et al. Denileukin diftitox (ONTAK) induces a tolerogenic phenotype in dendritic cells and stimulates survival of resting Treg. Blood. 2013;122:2185–2194. doi: 10.1182/blood-2012-09-456988. [DOI] [PubMed] [Google Scholar]
- 500.Burger JA, Keating MJ, Wierda WG, Hartmann E, Hoellenriegel J, Rosin NY, de Weerdt I, Jeyakumar G, Ferrajoli A, Cardenas-Turanzas M, Lerner S, Jorgensen JL, Nogueras-Gonzalez GM, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–1099. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.O'Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, Grant B, Richards DA, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, Barrientos JC, Chmielowska E, Radford J, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, Ma Y, Wiesen JF, Wong MH, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Dalrymple SL, Becker RE, Isaacs JT. The quinoline-3-carboxamide anti-angiogenic agent, tasquinimod, enhances the anti-prostate cancer efficacy of androgen ablation and taxotere without effecting serum PSA directly in human xenografts. Prostate. 2007;67:790–797. doi: 10.1002/pros.20573. [DOI] [PubMed] [Google Scholar]
- 505.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 506.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F, Nebuloni M, van Rooijen N, Mortarini R, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 507.Allavena P, Germano G, Belgiovine C, D'Incalci M, Mantovani A. Trabectedin: A drug from the sea that strikes tumor-associated macrophages. Oncoimmunology. 2013;2:e24614. doi: 10.4161/onci.24614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, Wang-Gillam A, Eberlein TJ, Denardo DG, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C, Cao F, Niekro W, Kempe T, Henning KA, Cohen LJ, Korman AJ, Cardarelli PM. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res. 2013;19:357–366. doi: 10.1158/1078-0432.CCR-12-2333. [DOI] [PubMed] [Google Scholar]
- 510.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R Blockade Reprograms Tumor-Infiltrating Macrophages and Improves Response to T-cell Checkpoint Immunotherapy in Pancreatic Cancer Models. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, Johnson M, Lusis AJ, Cohen DA, Iruela-Arispe ML, Wu L. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–1471. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Ghansah T. A novel strategy for modulation of MDSC to enhance cancer immunotherapy. Oncoimmunology. 2012;1:984–985. doi: 10.4161/onci.20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–918. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Abrams SI, Waight JD. Identification of a G-CSF-Granulocytic MDSC axis that promotes tumor progression. Oncoimmunology. 2012;1:550–551. doi: 10.4161/onci.19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra267. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Weiskopf K, Ring AM, Schnorr PJ, Volkmer JP, Volkmer AK, Weissman IL, Garcia KC. Improving macrophage responses to therapeutic antibodies by molecular engineering of SIRPalpha variants. Oncoimmunology. 2013;2:e25773. doi: 10.4161/onci.25773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, Ozkan E, Fernhoff NB, van de Rijn M, Weissman IL, Garcia KC. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA, Weiskopf K, Miyanishi M, Weissman IL. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110:11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Smith BD, Kasamon YL, Kowalski J, Gocke C, Murphy K, Miller CB, Garrett-Mayer E, Tsai HL, Qin L, Chia C, Biedrzycki B, Harding TC, Tu GH, et al. K562/GM-CSF immunotherapy reduces tumor burden in chronic myeloid leukemia patients with residual disease on imatinib mesylate. Clin Cancer Res. 2010;16:338–347. doi: 10.1158/1078-0432.CCR-09-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Characiejus D. Cancer immunotherapy: Benefit and harm? Oncoimmunology. 2012;1:232–233. doi: 10.4161/onci.1.2.18183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Agur Z, Vuk-Pavlovic S. Personalizing immunotherapy: Balancing predictability and precision. Oncoimmunology. 2012;1:1169–1171. doi: 10.4161/onci.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Dranoff G. Immunotherapy at large: Balancing tumor immunity and inflammatory pathology. Nat Med. 2013;19:1100–1101. doi: 10.1038/nm.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Mittendorf EA, Gurney JM, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Vaccination with a HER2/neu peptide induces intra- and inter-antigenic epitope spreading in patients with early stage breast cancer. Surgery. 2006;139:407–418. doi: 10.1016/j.surg.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 525.Hardwick N, Chain B. Epitope spreading contributes to effective immunotherapy in metastatic melanoma patients. Immunotherapy. 2011;3:731–733. doi: 10.2217/imt.11.62. [DOI] [PubMed] [Google Scholar]
- 526.Olson BM, McNeel DG. Antigen loss and tumor-mediated immunosuppression facilitate tumor recurrence. Expert Rev Vaccines. 2012;11:1315–1317. doi: 10.1586/erv.12.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Monjazeb AM, Zamora AE, Grossenbacher SK, Mirsoian A, Sckisel GD, Murphy WJ. Immunoediting and antigen loss: overcoming the achilles heel of immunotherapy with antigen non-specific therapies. Front Oncol. 2013;3:197. doi: 10.3389/fonc.2013.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Maletzki C, Stier S, Linnebacher M. Microsatellite instability in hematological malignancies: Hypermutation vs. immune control-who is challenging who? Oncoimmunology. 2013;2:e25419. doi: 10.4161/onci.25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Kroemer G, Galluzzi L, Zitvogel L. Immunological effects of chemotherapy in spontaneous breast cancers. Oncoimmunology. 2013;2:e27158. doi: 10.4161/onci.27158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Kepp O, Galluzzi L, Kroemer G. Immune effectors required for the therapeutic activity of vorinostat. Oncoimmunology. 2013;2:e27157. doi: 10.4161/onci.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Ghiringhelli F, Apetoh L. The interplay between the immune system and chemotherapy: emerging methods for optimizing therapy. Expert Rev Clin Immunol. 2013 doi: 10.1586/1744666X.2014.865520. [DOI] [PubMed] [Google Scholar]
- 534.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 535.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 536.Ghiringhelli F, Bruchard M, Apetoh L. Immune effects of 5-fluorouracil: Ambivalence matters. Oncoimmunology. 2013;2:e23139. doi: 10.4161/onci.23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Malvicini M, Piccioni F, Bayo J, Fiore E, Atorrasagasti C, Alaniz L, Garcia M, Aquino JB, Gidekel M, Matar P, Mazzolini G. Chemoimmunotherapy for advanced gastrointestinal carcinomas: A successful combination of gene therapy and cyclophosphamide. Oncoimmunology. 2012;1:1626–1628. doi: 10.4161/onci.21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Foy KC, Miller MJ, Moldovan N, Bozanovic T, Carson Iii WE, Kaumaya PT. Immunotherapy with HER-2 and VEGF peptide mimics plus metronomic paclitaxel causes superior antineoplastic effects in transplantable and transgenic mouse models of human breast cancer. Oncoimmunology. 2012;1:1004–1016. doi: 10.4161/onci.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Kadota K, Nitadori JI, Adusumilli PS. Prognostic value of the immune microenvironment in lung adenocarcinoma. Oncoimmunology. 2013;2:e24036. doi: 10.4161/onci.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Escors D, Liechtenstein T, Perez-Janices N, Schwarze J, Dufait I, Goyvaerts C, Lanna A, Arce F, Blanco-Luquin I, Kochan G, Guerrero-Setas D, Breckpot K. Assessing T-cell responses in anticancer immunotherapy: Dendritic cells or myeloid-derived suppressor cells? Oncoimmunology. 2013;2:e26148. doi: 10.4161/onci.26148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Bindea G, Mlecnik B, Angell HK, Galon J. The immune landscape of human tumors: Implications for cancer immunotherapy. Oncoimmunology. 2014;3:e27456. doi: 10.4161/onci.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Malyguine A, Umansky V, Kotlan B, Aptsiauri N, Shurin MR. Conference overview: Cancer Immunotherapy and Immunomonitoring (CITIM): moving forward. J Immunotoxicol. 2012;9:231–235. doi: 10.3109/1547691X.2012.686930. [DOI] [PubMed] [Google Scholar]
- 543.Popadic D, Anegon I, Baeten D, Eibel H, Giese T, Marits P, Martinez-Caceres E, Mascart F, Nestle F, Pujol-Borrell R, Savic E, Scheibenbogen C, Seliger B, et al. Predictive immunomonitoring -- the COST ENTIRE initiative. Clin Immunol. 2013;147:23–26. doi: 10.1016/j.clim.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 544.van der Burg SH, Kalos M, Gouttefangeas C, Janetzki S, Ottensmeier C, Welters MJ, Romero P, Britten CM, Hoos A. Harmonization of immune biomarker assays for clinical studies. Sci Transl Med. 2011;3:108ps144. doi: 10.1126/scitranslmed.3002785. [DOI] [PubMed] [Google Scholar]