Abstract
Purpose
Tricuspid regurgitation contributes to right ventricular failure (RVF) and is associated with worse clinical outcomes in patients undergoing Left Ventricular Assist Device (LVAD). However, whether tricuspid valve repair (TVR) at the time of LVAD implantation improves outcomes is not clear.
Methods
We identified all patients undergoing initial implantation of a long-term continuous flow LVAD at our institution from 3/2006 to 8/2011. We assessed the impact of TVR on survival and incidence of RVF using Kaplan-Meier curves and proportional hazards regression adjusted for age, gender, baseline tricuspid regurgitation, RV function, MELD score, albumin, and indication (bridge vs. destination).
Results
A total of 101 patients were included in the analysis, of which 14 patients underwent TVR concomitant LVAD. All TVR patients had moderate or severe baseline regurgitation. Crude survival was not different between groups. In multivariable models adjusted for confounding factors, TVR showed a significant association with improved survival (HR=0.1, p=0.049). Adjusted models showed no difference in RVF.
Conclusions
In this cohort of patients TVR at the time of LVAD implantation appears associated with better survival. Additional larger studies are needed to verify the effect of TVR at the time of LVAD implantation, and whether it should be utilized more frequently.
Keywords: Tricuspid Valve Insufficiency, Cardiac Valve Annuloplasty, Heart Failure, Heart, Artificial
INTRODUCTION
The use of continuous flow left ventricular assist devices (LVADs) as bridge and destination therapy for patients with end stage heart failure is growing rapidly as overall outcomes improve. 1-3Despite the positive effects of LVADs on patient mortality, acute and chronic right ventricular failure after LVAD implantation remains a major postoperative complication.4 In one report examining implantation of continuous flow LVADs as bridge therapy, 22% of the patients experienced post implant RV dysfunction.5 Severe RV dysfunction often results in the need for mechanical RV support or prolonged inotropic support.5 Tricuspid regurgitation (TR) is common in patients with RV dilation and dysfunction (almost 50%), and significant pre-operative TR has been associated with longer post-LVAD inotropic support, longer hospital stays and a trend towards greater mortality.6,7 LVAD implantation itself may cause an acute increase in TR, possibly by volume over loading the RV.8
Given the risk associated with TR and the crucial importance of RV function after LVAD,6,9 tricuspid valve repair (TVR) is often considered concomitantly with LVAD implantation. The decision to perform concomitant TVR is obvious in extreme cases; however, many patients have moderate or less severe TR, and importantly the actual assessment of TR severity can vary over time. Other considerations include the concern that TR may worsen after LVAD, as well as the incremental increase in surgical risk by adding to the procedure complexity. Indeed, there is data to suggest that additional procedures performed at the time of LVAD implantation were associated with worse outcomes,10 but also data indicating that TVR may be beneficial in the setting of LVAD placement. In order to better illuminate the effect of TVR on post-LVAD outcomes, we assessed the association of concurrent TVR during LVAD implantation on the incidence of right heart failure and overall post-LVAD patient mortality.
METHODS
Subjects
The records of all patients who underwent implantation of long term continuous flow LVADS (HeartMate II or HeartWare HVAD) between March 2006 and August 2011 at our institution were reviewed. Clinical data from all available sources were retrospectively reviewed and collected. Operative notes were reviewed to determine if patient underwent TVR at the time of LVAD. All LVAD implantations were performed on cardiopulmonary bypass and without cardioplegic arrest (unless concomitant aortic valve repair replacement was indicated). Tricuspid valve repair was performed with an annuloplasty band (MC3 Edwards ring) in all cases. Baseline characteristics including demographics, pre-operative TR severity, RV function, mean pulmonary artery pressure (mPAP) and MELD scores were noted, and outcomes assessed. The primary endpoint of study was survival, and the secondary endpoint of interest was incident of RVF after the procedure. RVF was defined as placement of right-sided mechanical support or intravenous inotrope infusion for greater than 2 weeks. The decision to perform TVR for each patient was based on assessment by the operating surgeon at the time of LVAD implantation.
Echocardiography
Preoperative echocardiograms were reviewed and compared to echocardiograms in the immediate post-operative period. A trans-esophageal echocardiogram was also performed during the procedure. All echocardiograms were performed by using standard clinical protocols and interpreted by staff cardiologists at Henry Ford Hospital. The severity of TR and right ventricular dysfunction before LVAD implantation was based on a graded scale of 0 to 3 for the following: none or trace (0), mild (1), moderate (2), and severe (3).
Statistical Analysis
Categorical data are presented as counts and proportions. Continuous variables are expressed as a mean ± standard deviation (SD). Variables were compared using Student t tests or Chi-square tests, as appropriate. Kaplan-Meier survival curves and proportional hazards regression modeling were used to compare survival times between groups using all subjects. A secondary analysis of proportional hazards regression was performed restricted to subjects with baseline TR greater than mild. Logistic regression was used to compare the incidence of RVF between groups. Multivariable models were adjusted for baseline characteristics previously demonstrated to be associated with survival or specified as important potential confounders. These covariates were age, gender, baseline tricuspid regurgitation, RV function, creatinine, albumin, and indication (bridge vs. destination). For all analyses, a p value of <0.05 was considered statistically significant. All analyses were performed in SAS version 9.1.3 (SAS Institute, Cary, North Carolina).
RESULTS
101 patients with chronic heart failure underwent implantation of a CF-LVAD at our institution during the study period, of which 14 underwent concomitant TVR. Table 1 shows baseline characteristics for the two groups. All patients who underwent TVR at the time of LVAD implantation had moderate or severe TR at baseline. Forty patients in the LVAD alone group had moderate or severe TR (n = 3 with severe). Seven patients in the TVR group (50%) had at least moderately reduced RV function before surgery, compared to 36 patients (41.4%) in the LVAD alone group. The mean age for LVAD patients undergoing TVR was 54± 15.3 years with 10 (10%) males and 4 (4.%) females, while those who did not undergo TVR had a mean age of 53.2 ± 11.2 , with 65 (64%) males and 22 (22%) females. Devices were implanted as a bridge to transplantation (BTT) in 62 patients and as destination therapy (DT) in 39 patients. Pre-operative serum creatinine, bilirubin, MELD scores, and mean PAP were similar between the groups. Interestingly SGPT differed between groups (p=0.005) but SGOT did not. The LVAD implant was complicated by reoperation in 17 patients (19.5%) from the no-TVR group, compared to 2 patients (14.3%) in the TVR group (all except one for bleeding). There was one incidence of device malfunction that required re-operation in the no-TVR group.
Table 1.
Baseline Clinical Characteristics of LVAD Patients
| Variable | LVAD Alone | TVR |
|---|---|---|
| Numbers | 87 | 14 |
| Mean Age (Years) | 53.2 ± 11.2 | 54 ± 15.3 |
| Gender | ||
| Male | 65 (64.4%)2 | 10 (9.9%)2 |
| Female | 22 (21.8%)2 | 4 (4.0%)2 |
| Race | ||
| Caucasian | 53 (52.5%)2 | 10 (9.9%)2 |
| African American | 34 (33.7%)2 | 4 (4.0%)2 |
| Indication | ||
| BTT % (N) | 61% ( | 64 %( |
| DT % (N) | 39% () | 36 % ( ) |
| Etiology of HF | ||
| Ischemic CM | 32 (31.7%)2 | 2 (2.0%)2 |
| NIDCM | 55 (54.5%)2 | 12 (11.9%)2 |
| RV EF at baseline (echo) | ||
| Normal/mildly reduced | ||
| Moderately reduced | ||
| Severely reduced | ||
| Tricuspid Regurgitation | ||
| None/Mild | ||
| Moderate/Severe | ||
| BSA | 2.02 ± 0.271 | 1.91 ± 0.271 |
| BMI | 28.74 ± | 25.74 ± |
| Albumin | 3.28± 0.471 | 3.041± 0.641 |
| DM | 41 (40.6%)2 | 5 (5.0%)2 |
| HTN | 78 (77.2%)2 | 12 (11.9%)2 |
| Creatinine | 1.34 ± 0.461 | 1.61 ± 0.661 |
| COPD* | 16 (15.8%)2 | 3 (3.0%)2 |
| Mechanical ventilation | 13 (12.9%)2 | 5 (5.0%)2 |
| Reoperation | 17 (16.8%)2 | 2 (2.0%)2 |
| SGOT | 37.90 ± | 32.71 ± |
| SGPT | 46.82 ± | 21.00 ± |
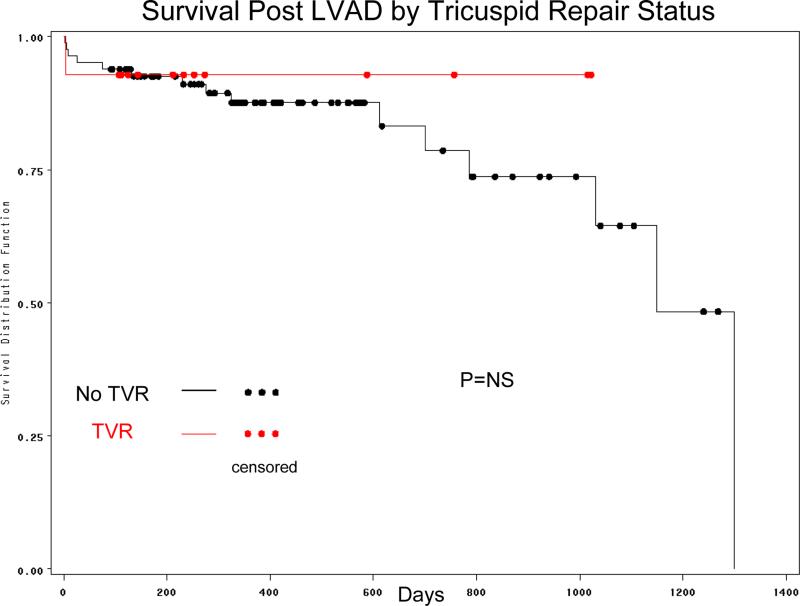
Among patients undergoing LVAD without TVR, 76 (87.4%) survived till discharged, while this was13 (92.9%) in the TVR group (Table 2). Kaplan-Mejer survival curves are shown in Figure 1 and did not differ statistically (p=NS). After adjustment for possible confounders (age, gender, indication, TVR, baseline RV function, baseline albumin and baseline MELD score) and established predictors of survival post LVAD, TVR showed a significant association with improved survival (HR=0.1, p=0.049). A sub-group analysis was performed just on patients with moderate or severe tricuspid regurgitation prior to LVAD implantation (n=54). This was essentially consistent with the primary analysis revealing protective association (HR for TVR 0.064) of borderline statistical significance (p=0.065).
Table 2.
Outcomes of patients undergoing LVAD with TVR vs. those without TVR.
| Variable | LVAD (no TVR) | LVAD + TVR |
|---|---|---|
| Re-operation Required | 17 (19.54%) | 2 (14.29%) |
| Survived to discharge | 76 (87.36%) | 13 (92.86%) |
| Length of stay (post-implant) | ||
| RVF post LVAD | 12 (13.80%) | 4 (28.57%) |
| Median Survival (days) | ||
Figure 1.
Comparison of Kaplan Meier survival between patients who underwent TVR versus those who did not undergo TVR.
In terms of perioperative RVF, 12 patients (13.8%) in the no-TVR group had RVF post-implant, while 4 patients (28.6%) in the TVR group suffered this, trending towards more RVF in these high-risk subjects (OR=1.77, p=0.43). However, after adjustment for potential confounders this trend reversed (OR=0.68, p=0.7), particularly among patients who survived to hospital discharge (OR=0.29, p=0.28).
DISCUSSION
In our study there is a statistically significant higher in survival for the TVR group after adjusting for confounders, though no effect on post-operative RVF was seen. Our results could be viewed as consistent with previous studies that have shown no increase in mortality with concomitant TVR at the time of LVAD implantation,11 and suggest a possible benefit.12
Most patients undergoing LVAD implantation have some right heart dysfunction in addition to left heart failure. TR is a common finding in patients with heart failure,13 and the incidence of pre-operative TR in LVAD patients was reported to be as high as 50%.14 Piacentino et al. demonstrated that TR is not reduced immediately after LVAD implantation and is in fact, associated with adverse clinical outcomes post-operatively. While some investigators have argued that as LV failure resolves after LVAD implantation the accompanying decrease in LA pressure and pulmonary congestion decreases RV afterload and improving tricuspid insufficiency15 there is little evidence that simply reducing RV afterload resolves TR.16 In addition, increased flow from the LVAD can lead to increased preload returning to the RV, negatively affect RV geometry, and may thus exacerbate tricuspid insufficiency and RVF.
Traditionally, there was some reluctance to repair TR in patients with advanced cardiomyopathy to avoid the acute increase in RV afterload that can occur after TVR. Concern existed that this may lead to acutely increased RV dysfunction as the incompetent tricuspid valve could serve as a ‘pop-off’ valve, decreasing RV afterload. However, Krishnan et al questioned the pop-off hypothesis and demonstrated no increase in peri-operative mortality, and similar post-operative outcomes in patients who underwent TVR and those who did not.17 In other studies as well, concomitant tricuspid procedures, in contrast to aortic procedures, have not been associated with an increase in early post-operative mortality.10 Some more recent studies have demonstrated varying results pointing to at least as good 11 or improved14,18 clinical outcomes in patients who underwent concomitant TVR with LVAD implantation, with which our data agree. It is worth noting that our findings of better survival were not mediated via reduced RVF in the perioperative setting. This could be interpreted as showing inconsistency of the data, but alternatively it is possible that the benefit of TVR occurs over a longer period of time. Our existing data do not provide a window into long term incidence of RVF.
Our study has additional limitations worth noting when interpreting the data. This is a modest sized, retrospective review. Thus all potential selection bias can never be eliminated, though we have tried to mitigate this by adjusting for known confounders. The number of patients that underwent repair were relatively small, though our cohort size appears typical of single center LVAD studies. As a result the statistical power of the analysis is limited. However, the main effect size was large, indicating that while there is certainly noise and the HR is overestimated, there is also likely an important signal of possible effect. Regardless, our findings should be interpreted quite conservatively, and further studies with more patients and longer follow-up are needed.
Conclusions
Our results show that concomitant TVR with LVAD implantation in patients with moderate or severe TR appears associated with improved survival and trends towards less RVF. Additional larger observational studies are required to further describe this association, and ultimately a randomized study of subjects with mild to moderate TR would be needed to establish the utility of concomitant TVR at the time of LVAD implantation.
Acknowledgments
Dr. Lanfear's time is supported in part by grants from the National Heart, Lung and Blood Institute (K23HL085124, R01HL103871).
Footnotes
Conflicts of Interest:
RB- Thoratec (Research) Heartware (Research)
CW- Thoratec (Research)
JM- Thoratec (Research) Heartware (Research)
DL- Thoratec (Research and Speaking) Heartware (Research).
REFERENCES
- 1.John R, Kamdar F, Liao K, Colvin-Adams M, Boyle A, Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008 Oct;86(4):1227–1234. doi: 10.1016/j.athoracsur.2008.06.030. discussion 1234-1225. [DOI] [PubMed] [Google Scholar]
- 2.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007 Aug 30;357(9):885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009 Dec 3;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 4.Dang NC, Topkara VK, Mercando M, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. 2006 Jan;25(1):1–6. doi: 10.1016/j.healun.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010 May;139(5):1316–1324. doi: 10.1016/j.jtcvs.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Piacentino V, 3rd, Williams ML, Depp T, et al. Impact of tricuspid valve regurgitation in patients treated with implantable left ventricular assist devices. Ann Thorac Surg. 2011 May;91(5):1342–1346. doi: 10.1016/j.athoracsur.2011.01.053. discussion 1346-1347. [DOI] [PubMed] [Google Scholar]
- 7.Potapov EV, Stepanenko A, Dandel M, et al. Tricuspid incompetence and geometry of the right ventricle as predictors of right ventricular function after implantation of a left ventricular assist device. J Heart Lung Transplant. 2008 Dec;27(12):1275–1281. doi: 10.1016/j.healun.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Holman WL, Bourge RC, Fan P, Kirklin JK, Pacifico AD, Nanda NC. Influence of longer term left ventricular assist device support on valvular regurgitation. ASAIO J. 1994 Jul-Sep;40(3):M454–459. doi: 10.1097/00002480-199407000-00041. [DOI] [PubMed] [Google Scholar]
- 9.Kukucka M, Stepanenko A, Potapov E, Krabatsch T, Kuppe H, Habazettl H. Impact of tricuspid valve annulus dilation on mid-term survival after implantation of a left ventricular assist device. Journakl Heart Lung Transplant. 2012 Sep;31(9):967–971. doi: 10.1016/j.healun.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Pal JD, Klodell CT, John R, et al. Low operative mortality with implantation of a continuous-flow left ventricular assist device and impact of concurrent cardiac procedures. Circulation. 2009 Sep 15;120(11 Suppl):S215–219. doi: 10.1161/CIRCULATIONAHA.108.844274. [DOI] [PubMed] [Google Scholar]
- 11.Deo SV, Hasin T, Altarabsheh SE, et al. Concomitant tricuspid valve repair or replacement during left ventricular assist device implant demonstrates comparable outcomes in the long term. J Card Surg. 2012 Nov;27(6):760–766. doi: 10.1111/jocs.12020. [DOI] [PubMed] [Google Scholar]
- 12.Piacentino V, 3rd, Ganapathi AM, Stafford-Smith M, et al. Utility of concomitant tricuspid valve procedures for patients undergoing implantation of a continuous-flow left ventricular device. J Thorac Cardiovasc Surg. 2012 Nov;144(5):1217–1221. doi: 10.1016/j.jtcvs.2012.07.064. [DOI] [PubMed] [Google Scholar]
- 13.Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002 Sep;144(3):524–529. doi: 10.1067/mhj.2002.123575. [DOI] [PubMed] [Google Scholar]
- 14.Piacentino V, 3rd, Troupes CD, Ganapathi AM, et al. Clinical impact of concomitant tricuspid valve procedures during left ventricular assist device implantation. Ann Thorac Surg. 2011 Oct;92(4):1414–1418. doi: 10.1016/j.athoracsur.2011.05.084. discussion 1418-1419. [DOI] [PubMed] [Google Scholar]
- 15.Rao V, Slater JP, Edwards NM, Naka Y, Oz MC. Surgical management of valvular disease in patients requiring left ventricular assist device support. Ann Thorac Surg. 2001 May;71(5):1448–1453. doi: 10.1016/s0003-4975(01)02479-1. [DOI] [PubMed] [Google Scholar]
- 16.Song H, Kim MJ, Chung CH, et al. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart. 2009 Jun;95(11):931–936. doi: 10.1136/hrt.2008.152793. [DOI] [PubMed] [Google Scholar]
- 17.Krishan K, Nair A, Pinney S, Adams DH, Anyanwu AC. Liberal use of tricuspid-valve annuloplasty during left-ventricular assist device implantation. Eur J Cardiothorac Surg. 2012 Jan;41(1):213–217. doi: 10.1016/j.ejcts.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeed D, Kidambi T, Shalli S, et al. Tricuspid valve repair with left ventricular assist device implantation: is it warranted? J Heart Lung Transplant. 2011 May;30(5):530–535. doi: 10.1016/j.healun.2010.12.002. [DOI] [PubMed] [Google Scholar]