Abstract
Objectives
Despite growing research interest in spirituality and health, and recommendations on the importance of spiritual care in advanced cancer and palliative care, relationships between spiritual belief and psychological health near death remain unclear. We investigated (i) relationships between strength of spiritual beliefs and anxiety and depression, intake of psychotropic/analgesic medications and survival in patients with advanced disease; and (ii) whether the strength of spiritual belief changes as death approaches.
Methods
We conducted a prospective cohort study of 170 patients receiving palliative care at home, 97% of whom had a diagnosis of advanced cancer. Data on strength of spiritual beliefs (Beliefs and Values Scale [BVS]), anxiety and depression (Hospital Anxiety and Depression Scale [HADS]), psychotropic/analgesic medications, daily functioning, global health and social support were collected at recruitment then 3 and 10 weeks later. Mortality data were collected up to 34 months after the first patient was recruited.
Results
Regression analysis showed a slight increase in strength of spiritual belief over time approaching statistical significance (+0.16 BVS points per week, 95% CI [−0.01, 0.33], p = 0.073). Belief was unrelated to anxiety and depression (−0.15 points decrease in HADS for 10 points increased in BVS (95% CI [−0.57, 0.27], p = 0.49) or consumption of psychotropic medication). There was a non-significant trend for decreasing analgesic prescription with increasing belief. Mortality was higher over 6 months in participants with lower belief at recruitment.
Conclusion
Results suggest that although religious and spiritual beliefs might increase marginally as death approaches, they do not affect levels of anxiety or depression in patients with advanced cancer. © 2013 The Authors. Psycho-Oncology published by John Wiley & Sons, Ltd.
Keywords: cancer, oncology, end-of-life, spirituality, religion, wellbeing
Introduction
Oncologists' interest in palliative care has been stimulated by recent evidence of its positive effect on outcomes in some patient groups 1–3. Recent guidelines on quality standards in palliative care from the UK National Institute for Clinical Excellence stress the importance of providing spiritual care according to individual need and preference 4. It may be viewed as a component of active rehabilitation in advanced disease 5. Religious and spiritual beliefs may strengthen a person's resilience to depression or anxiety, at least in part by providing meaning and purpose in their experiences.
However, evidence for the impact of spiritual beliefs on psychological and physical health is mixed. Whereas some studies report that beliefs correlate with less depression and anxiety 6–8, greater quality of life 9, less perceived pain 10 and increased survival 11, others suggest the reverse or no direct effects 12–14. Much work has focused on chronic illness, increasingly relevant in cancer, and stronger beliefs may augment illness adjustment 15–17. Our recent Cochrane review of interventions with a definite spiritual or transcendent component found some evidence of effectiveness in improving wellbeing 18. Fewer studies have measured change in belief, especially in advanced illness 7. A lack of consistent findings may reflect difficulties conceptualising and measuring spirituality 19–23, the limitations of cross-sectional designs and inadequate adjustment for confounding influences 21. Spiritual measures may have doubtful psychometric properties or commingle spiritual beliefs with psychological variables 24.
Depression and anxiety are common in people treated for advanced, progressive disease who face the end of their lives 25,26. We report a prospective study of the impact of the strength of spiritual beliefs on anxiety and depression in cancer patients receiving specialist palliative care in the UK by using a measure of spirituality which does not conflate spirituality with psychological variables: the Beliefs and Values Scale (BVS) 27. The BVS is based on detailed qualitative work and centres on a multidimensional and inclusive conceptualisation of spirituality, which reflects the definition of spirituality agreed upon by the European Association of Palliative Care taskforce for Spirituality in Palliative Care:
Spirituality is the dynamic dimension of human life that relates to the way persons (individual and community) experience, express and/or seek meaning, purpose and transcendence, and the way they connect to the moment, to self, to others, to nature, to the significant and/or the sacred 28.
We acknowledge the importance of making clear how spirituality is or is not defined in medical research and health service provision and further point readers towards the more detailed conceptualisation of spirituality published by King and Koenig 29.
In this study, we aimed to investigate the hypotheses that (i) spiritual beliefs become stronger towards the end of life, and that stronger spiritual beliefs are associated with (ii) less anxiety and depression, (iii) lower intake of psychotropic and analgesic medications, and (iv) increased survival in patients with advanced illness.
Method
Design
A prospective cohort study was conducted over 10 weeks.
Sample and recruitment
Patients older than 18 years, receiving specialist palliative care at home and able to give written informed consent were eligible for inclusion in the study. Patients unable to understand English or whose death was considered imminent were excluded. Patients were recruited through nine specialist palliative care teams across London over 26 months (August 2007–September 2009). Eligible patients were identified by palliative care teams who screened their clinical case loads. Those eligible were contacted first by the palliative care teams, and the names of those agreeing to being contacted were forwarded to the research team. Researchers then contacted those interested to explain the study in more detail and arrange a baseline visit. Patients were seen by a researcher either at home or in a hospice, and informed consent was taken at first interview. In order to address the difficulty of conducting empirical research in palliative care settings, regular research briefing and education sessions were held with local palliative care teams to emphasise the value of research and address individual concerns.
Ethics
A favourable opinion was received from the Essex 1 Research Ethics Committee (8 May 2007, ref 07/Q0301/3).
Procedure
Patients were assessed at baseline and after 3 and 10 weeks by one of two researchers, both graduates in psychology and experienced in conducting interviews with vulnerable groups on sensitive research topics (F. O./H. L.). Researchers met regularly with clinical members of the research team for debriefing and supervision. Patients were given the opportunity to complete measures by themselves or with researchers who read out questions verbatim and recorded responses. At each time-point, data were collected on our main variables, namely spiritual beliefs and psychological status, as well as other factors including daily function, somatic symptoms, physical health status and information on all prescribed medication, including analgesic and psychotropic drugs. At baseline, patients were also asked to complete questionnaires on basic demographic data and social support. In order to test whether a response shift occurred during follow-up, a random half of participants were asked to rate the BVS again according to how they felt at baseline (the so-called then-test).
Measures
Demographic questionnaire
Information on age, sex, religious affiliation and practice, education, socioeconomic status, ethnicity, marital status, diagnosis and duration of illness was collected at recruitment.
Medical Outcomes Study social support scale
Patients' level of social support was measured at recruitment using the Medical Outcomes Study social support scale 30.
Beliefs and Values Scale
The BVS 27 is a multidimensional, validated and reliable measure of spiritual belief, irrespective of religious belief or practice. It comprises 20 statements, which were developed from detailed qualitative research with a diverse sample of ill and healthy people. Each statement in the scale has a response in a 5-point Likert format, all of which sum to a total score for strength of belief from 0 to 80. A higher score indicates stronger belief. The scale was specifically developed to facilitate a distinction between religious and spiritual belief. Whereas some items refer to more traditional religious concepts, for example, ‘I believe there is a God’, others refer to broadly conceived spiritual concepts such as ‘I feel most at one with the world when surrounded by nature’. In order not to conflate belief and health or wellbeing, the scale does not contain items on coping or wellbeing.
Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) is a short questionnaire to assess anxiety and depression that is widely used in healthcare research and practice 31–35. It comprises 14 items, giving a total score between 0 (no anxiety or depression) and 42. It has well-established psychometric properties and is quick and easy to complete by people with poor health.
National Comprehensive Cancer Network distress thermometer
The National Comprehensive Cancer Network distress thermometer is a simple measure of overall psychological distress ranging from 0 (no distress) to 10 (extreme distress), developed in the USA and validated for use in a UK population 36.
EQ-5D
The EQ-5D is a well-standardised, sensitive measure of daily functioning 37. It is composed of five questions that combine a score of utility. In addition, participants are also asked to rate their overall health state on a 100-point visual analogue scale.
Karnofsky Performance Status scale
The Karnofsky Performance Status scale 38, a 10-point scale completed by the researcher, was used to measure likely disease progression and global performance status. Researchers were given guidance on using the measure by the clinical leads on the study (L. J./A. T.).
Prescribed medication
Patients were asked to report use of analgesic and psychotropic medications at each assessment. Patients who were unsure which medications they were taking were asked to produce medication bottles or prescription lists, and details were recorded.
Survival
Mortality data on all patients were collected for 34 months after the first patient was recruited.
Statistical methods
A statistical analysis plan was developed before the data were explored. We used descriptive statistics to report on the cohort at each assessment point. Participant characteristics were compared with level of belief by dichotomizing at the median BVS score. All subsequent analyses were based on imputed data. By taking into account measured predictors of missingness, multiple imputation predicts missing data to reduce the risk of bias, whilst preserving the uncertainty surrounding the imputed values. Multiple data imputations were conducted for each specific analysis using variables predicting missing data, as well as those included in each analysis model and any strongly correlated with them. We conducted multiple imputations by chained equation using the ice package in stata 39 to generate 30 sets of imputed data. We obtained combined estimates using Rubin's rules 40.
Change of belief over time, the relation between BVS and HADS, and the relation between BVS and reported consumption of medication were analysed using generalised estimating equation (GEE), which relaxes the assumptions of independent observations in the analysis of repeated measurements 41. GEE were fitted using exchangeable correlation matrices and robust standard errors. We also explored baseline predictors of change in belief using linear regressions adjusted for baseline belief.
In order to investigate a possible change in internal standards over time when participants completed the BVS (response shift), we compared the BVS score at recruitment and its retrospective scoring at week 10 (then-test) 42. The degree of response shift is estimated from the mean difference between the baseline and then-test scores.
For the survival analysis, BVS was divided into terciles (decided a priori to have informative group sizes) and compared using the logrank test. As the impact of a low belief seemed to differ before and after 6 months, we conducted a further analysis on the basis of the findings, not on an a priori hypothesis. In this post hoc analysis, we fitted a Cox proportional hazard model with two separate coefficients.
The sensitivity of our results to imputation was examined by repeating the analyses in the observed data. All analyses were performed using stata release 11 43. All tests were two sided and considered significant at the 5% level.
Power and sample size
In developing the protocol, we assumed that the main analysis would be a multivariable regression in which we would include up to 10 predictor variables. We used a statistical rule of thumb that 15–20 participants would be required for each variable adjusted for in the model, which meant that we needed to obtain data on between 150 and 200 patients.
Results
Our descriptive and survival analyses are based on observed data, whereas GEE models are derived from multiply imputed data.
Recruitment and attrition
Palliative care professionals approached 494 eligible patients, of whom 302 were referred to the research team. Of these, 132 (44%) did not participate (see Figure 1 for reasons). Therefore, 170 (34%) of the 494 patients approached by palliative care professionals were seen at baseline. Of these, 137 (81%) completed the 3-week assessment and 113 (67%) the 10-week assessment (Figure 1). Participants dropping out were older (mean age 69 years vs. 64, p = 0.03), had poorer health at baseline (mean Karnofsky score 66 vs. 72, p = 0.007; mean EQ-5D visual analogue score 48 vs. 59, p < 0.001) and were more likely to have been prescribed steroids (31% vs. 11%, p = 0.001).
Figure 1.
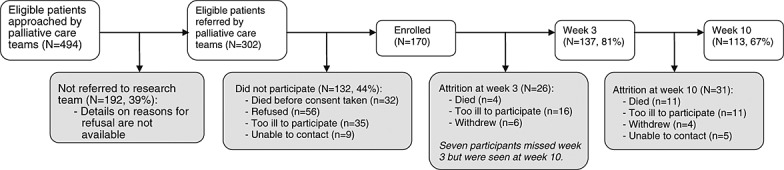
Recruitment and attrition
Population characteristics at recruitment
The majority of participants were women (62%) and of white ethnicity (85%) (Table 1). Mean age was 66 years (SD 13.8, range 22 to 96), and whereas 60% identified themselves as Christian, 28% did not observe a religion. Lung and breast cancer were the most frequent diagnoses. Reflecting clinical caseload, four patients had an advanced disease other than cancer. Thirty-nine per cent of participants reported use of psychotropic medication, 45% analgesics and 19% steroids.
Table 1.
Participants characteristics overall and by level of belief
| Characteristic | Overall (n = 170)a | BVS < 54 (n = 85) | BVS ≥ 54 (n = 84) | p-valueb |
|---|---|---|---|---|
| Sex | ||||
| Female | 106 (62) | 46 (54) | 60 (71) | 0.026 |
| Age | ||||
| Mean (SD) (years) | 66.2 (13.8) | 65.6 (15.2) | 66.6 (12.3) | 0.631 |
| Marital status | ||||
| Married/living with partner | 71 (42) | 35 (41) | 35 (42) | 1.00 |
| Ethnicity | ||||
| White | 145 (85) | 82 (96) | 62 (74) | <0.001 |
| Black | 16 (10) | 2 (2) | 14 (17) | |
| Other | 9 (5) | 1 (1) | 8 (10) | |
| Education (n = 169)c | ||||
| No qualification | 63 (37) | 27 (32) | 35 (42) | 0.080 |
| GCSE/A-level/higher education | 47 (28) | 26 (31) | 21 (25) | |
| Degree | 42 (25) | 26 (31) | 16 (19) | |
| Other | 17 (10) | 5 (6) | 12 (14) | |
| Employment status (n = 169) | ||||
| Employed | 16 (9) | 12 (14) | 4 (5) | 0.207 |
| Unemployed | 22 (13) | 11 (13) | 11 (13) | |
| Retired | 115 (68) | 53 (63) | 61 (73) | |
| On sick leave | 16 (9) | 8 (10) | 8 (10) | |
| Religion (n = 169) | ||||
| Does not observe a religion | 47 (28) | 45 (54) | 2 (2) | <0.001 |
| Christianity—Protestant | 52 (31) | 16 (19) | 35 (42) | |
| Christianity—Roman Catholic | 35 (21) | 9 (11) | 26 (31) | |
| Christianity—other | 14 (8) | 4 (5) | 10 (12) | |
| Judaism | 10 (6) | 7 (8) | 3 (4) | |
| Other | 11 (7) | 3 (4) | 8 (10) | |
| Tumour site (n = 168) | ||||
| Lung | 35 (21) | 21 (25) | 14 (17) | 0.609 |
| Upper gastrointestinal | 29 (17) | 10 (12) | 19 (23) | |
| Breast | 24 (14) | 11 (13) | 13 (16) | |
| Genito-urinary | 22 (13) | 12 (14) | 9 (11) | |
| Colorectal | 20 (12) | 12 (14) | 8 (10) | |
| Gynaecological | 12 (7) | 5 (6) | 7 (8) | |
| Haematological | 10 (6) | 4 (5) | 6 (7) | |
| Central nervous system | 6 (4) | 3 (4) | 3 (4) | |
| Other cancer | 6 (4) | 3 (4) | 3 (4) | |
| Non cancer | 4 (2) | 3 (4) | 1 (1) | |
| Time from diagnosis (n = 168) | ||||
| Median (IQR) (months) | 17 (6–42) | 18 (7–41) | 15 (6–42) | 0.631 |
Frequency (%), mean (SD) or median (IQR). BVS, Beliefs and Values Scale; GCSE, General Certificate of Secondary Education; IQR, interquartile range.
Including one participant with missing total BVS score.
p-values for differences by BVS group from chi-square, t-test (age) or Mann–Whitney (time from diagnosis) tests.
n reported when different from 170.
At recruitment, the median BVS score was 54 and the median HADS score was 10 (Table 2). Twenty-seven per cent of participants scored above the threshold of 8 on the HADS depression subscale, indicating moderate or severe depressive symptoms. Thirty-four per cent scored above 8 on the HADS anxiety subscale, indicating moderate or severe anxiety. After dividing participants into two groups according to the median BVS score, level of belief was significantly related to gender, ethnicity and religious denomination (Table 1).
Table 2.
Questionnaires score at each follow-up (median scores and interquartile ranges)
| Questionnaire | Recruitment (n = 170) | Week 3 (n = 137) | Week 10 (n = 113) |
|---|---|---|---|
| BVS | 54 (36–68) | 56 (40–68) | 58 (42–71) |
| HADS | 10 (7–15) | 11 (7–15.5) | 11.5 (6–15.5) |
| Distress | 3 (1–5) | 4 (1–6) | 4 (1–6) |
| EQ-5 VAS | 50 (40–70) | 60 (40–70) | 50 (40–70) |
| EQ-5 score | 0.66 (0.26–0.76) | 0.69 (0.27–0.80) | 0.62 (0.19–0.81) |
| Karnofsky | 70 (60–80) | 70 (60–80) | 70 (60–80) |
BVS, Beliefs and Values Scale; HADS, Hospital Anxiety and Depression Scale; VAS, visual analogue scale.
Change in belief
The GEE model using imputed data showed a slight but non-significant increase in BVS score of 0.16 points per week on average (95% CI [−0.01, 0.33], p = 0.073). At week 10, 57 patients completed the retrospective assessment of their belief at recruitment. The mean response shift over 10 weeks was 0.15 (−1.73, 2.02, p = 0.88), suggesting stable personal internal standards when rating beliefs. No baseline variable was predictive of change in belief over 10 weeks (p-values range 0.22 to 0.94), with the exception of distress, which was associated with a greater increase in belief. For each additional point on the distress thermometer (indicating greater distress) at baseline, the change in BVS score over 10 weeks was greater by 0.67 points (stronger belief) (95% CI [0.10, 1.23], p = 0.022).
Belief and psychological status
The relation between BVS and HADS was not significant (for each additional 10 points on the BVS, the HADS score varied by −0.16 (95% CI [−0.60, 0.29], p = 0.49)), indicating no relationship between belief and psychological status over 10 weeks. Adjustment for age, sex, duration of illness, social support, physical functioning and use of steroids, psychotropic medication and analgesics made no difference to this result (Table 3).
Table 3.
Adjusted relationship between BVS and HADS
| Coefficient | 95% CI | Significance | |
|---|---|---|---|
| Factor of interest | |||
| BVS score (10 units) | −0.15 | [−0.57, 0.27] | 0.49 |
| Adjustment covariates | |||
| Time (weeks) | 0.02 | [−0.10, 0.15] | 0.71 |
| Gender (male) | 0.89 | [−1.08, 2.85] | 0.38 |
| Age (years) | −0.05 | [−0.11, 0.01] | 0.13 |
| Duration of illness (log (days)) | −0.57 | [−1.34, 0.20] | 0.14 |
| MOS Social Support Survey | −0.85 | [−1.89, 0.19] | 0.11 |
| Steroid use | −0.92 | [−3.23, 1.38] | 0.43 |
| Psychotropic use | 0.93 | [−1.23, 3.09] | 0.40 |
| Analgesic use | 0.41 | [−1.49, 2.32] | 0.67 |
| Karnofsky Performance Status score | −0.11 | [−0.18, −0.05] | 0.001 |
From generalised estimating equation model with HADS as dependent variable, on imputed data (n = 170 × 3). BVS, Beliefs and Values Scale; HADS, Hospital Anxiety and Depression Scale; MOS, Medical Outcomes Study.
In an exploratory analysis, the HADS was also not associated with either of the two principal factors of the BVS (religious and non-religious spirituality). We also explored the relationship between individual items of the BVS and the HADS; results are reported in Supplementary Table 1. Although none were statistically significant, the strongest associations with less psychological distress were in agreement with seven traditional statements on religious beliefs (items 3, 5, 7, 13, 14, 17 and 20—Supplementary Table 1).
Belief and drug prescription
No relationship was found between BVS scores and psychotropic medication, either unadjusted (odds ratio (OR) for each 10 points increase in BVS = 0.95, 95% CI [0.79, 1.12], p = 0.52) or after adjustment for gender, age, duration of illness, social support and Karnofsky score (OR = 0.94, 95% CI [0.78, 1.13], p = 0.49). There was a non-significant trend for lower analgesic consumption in those with higher BVS scores (OR = 0.91, 95% CI [0.80, 1.03], p = 0.13, adjusted OR = 0.90 95% CI [0.80, 1.03], p = 0.13).
Belief and survival
Long-term survival was very similar for the three belief groups (BVS score categorised into terciles) (logrank test, p = 0.81) (Figure 2).
Figure 2.
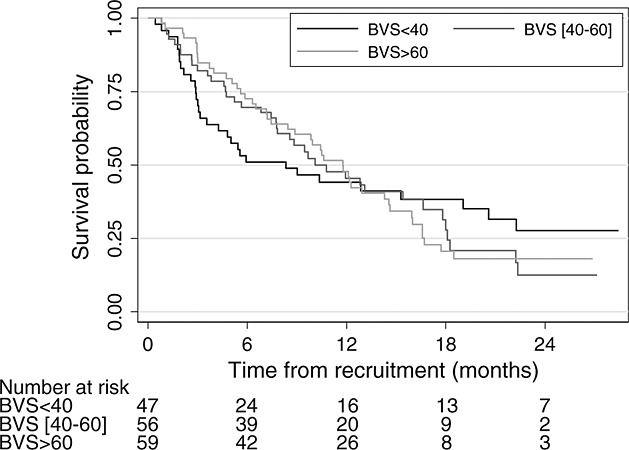
Kaplan Meir survival estimates from time of recruitment to death, by level of belief
However, the effect of low belief (BVS score < 40—the lower tercile) on mortality seemed to differ before and after 6 months. We therefore fitted a Cox proportional hazards model, allowing for the effect of low belief to change over time. The unadjusted HR in the first 6 months was 2.19 (95% CI [1.30, 3.70], p = 0.003), indicating that those with a BVS score below 40 had twice the mortality as those with a BVS score over 40. The effect remained after adjustment for age, gender, type of diagnosis (upper gastrointestinal or lung cancer vs. other), time from diagnosis and Karnofsky score (HR = 2.45, 95% CI [1.42, 4.22], p = 0.001). After 6 months, the mortality rate was lower for patients with low belief, but the difference was not significant (unadjusted HR = 0.57, 95% CI [0.27, 1.20], p = 0.136, adjusted HR = 0.60, 95% CI [0.28, 1.28], p = 0.187).
Sensitivity to data imputation
As described earlier, 33 and 57 participants did not attend week 3 and week 10 visits, respectively, giving an overall proportion of missing data of 18%. There was little difference in our findings in the complete case analysis. For example, the unadjusted coefficient for the relationship between a 10 points' change on BVS and HADS was −0.18 (p = 0.394). However, some results close to significance on imputed data were significant on complete case data; this was the case for change in belief (coefficient 0.15, 95% CI [0.01, 0.30], p = 0.036) and for the relation between belief and analgesic use (adjusted OR 0.90, 95% CI [0.81, 0.99], p = 0.031).
Discussion
Main findings
Over 97% of participants had advanced cancer, and the strength of their spiritual beliefs increased slightly but non-significantly over 10 weeks. We found no relationship between strength of belief and anxiety or depression either at recruitment or during follow-up. Nor was belief associated with use of psychotropic medication. However, there was a trend for decreasing analgesic prescription with increasing belief, but again this was non-significant. In a post hoc analysis, we observed higher mortality over 6 months in participants with lower belief scores.
Relevance of our findings
Our findings challenge the suggestion that stronger spiritual beliefs are associated with less anxiety and depression near the end of life. The stress experienced as death approaches may be so overwhelming that it overwhelms any psychological advantage available to well people with spiritual beliefs. However, this explanation is not supported by data from a national psychiatric morbidity study in the UK 44 or a recent prospective study in a large European population, both of which suggest that spiritual belief is not associated with markers of psychological wellbeing such as anxiety and depression in well people either 45.
Many studies reporting positive associations have used instruments to measure religious and spiritual belief that contain questions assessing positive character traits or good mental health, for example, optimism, peacefulness, harmony and general wellbeing 24. Thus, religion and spirituality are conflated with psychological outcomes, and it is not surprising that research using such measures reports positive associations. The BVS avoids this pitfall by limiting itself to the nature and strength of belief. It also has relevance to people who are not involved in organised religion.
Our sample population was made up almost entirely of people with cancer. Although for some the illness is short, others may endure repeated relapse and recurrence, and adjustment may vary according to experience and type of cancer 46–48.
We observed a small but non-significant increase in strength of spiritual belief over 10 weeks. A response-shift analysis confirmed that this was not caused by a change of internal standards (recalibration) on completing the BVS. Although this indicates that patients may increase their belief as death approaches, we acknowledge that our research may have increased patients' propensity to reflect about spiritual matters and thus altered the natural course of their faith or beliefs.
We hypothesised that palliative care patients with stronger spiritual beliefs might experience less psychological distress and have less need for psychotropic or analgesic medication. Although our finding that belief and wellbeing were not related challenged this assumption, we observed a slightly lower prescription of analgesics in patients with stronger beliefs. However, this was non-significant.
We also observed higher mortality in the 6 months following recruitment for patients with lower beliefs; however, this difference was not sustained. Despite the significance of this post hoc finding, there is no clear explanation for the change of effect at 6 months, and further investigation is needed. In a systematic review of observational cohort studies, Chida et al. 11 reported that religiosity/spirituality was not associated with survival in diseased populations (combined HR = 0.98, 95% CI [0.94, 1.01], p = 0.19), whereas it was associated in healthy populations (combined HR for mortality = 0.82, 95% CI [0.76–0.87], p < 0.001). In accounting for this discrepancy, the authors suggested that once diseases are established, identified and under treatment, religiosity/spirituality may not affect outcome.
Implications of our findings
Our finding that spiritual and religious beliefs are not associated with psychological status as death approaches does not negate the potential value of including a spiritual element in palliative care. Spiritual care is about being open to discussing difficult existential issues that patients or their families may raise. It may well be that spiritual or religious beliefs and values impact on outcomes other than those we examined here. Furthermore, religious practice is a dimension we did not examine. However, the suggestion that stronger spiritual beliefs are linked to less anxiety and depression 49 is not supported here.
Limitations
Our overall response of 34% may appear relatively low, but it is similar to that reported in much palliative care research, particularly prospective designs 50. We do not have sufficient information to compare participants with non-participants, and there are potential recruitment biases, including staff ’protecting’ sicker patients and self-selection through interest in the study topic. In particular, there may be an under-representation of patients with weaker spiritual or religious beliefs. This possibility is supported by our finding that mean BVS scores in this study were higher than in the populations on which the questionnaire was validated 27. The study population may not be representative of all palliative care patients, and caution is required in generalising the findings. The risk of bias by attrition, an important concern in longitudinal studies in palliative care 51, was limited by the use of multiple imputations. Our sample size offered acceptable power to test our hypotheses; the use of repeated measurement and multiple imputations of missing data enhanced that power. Borderline significant results must be interpreted with caution, however, taking into account the increased risk of chance findings on multiple analyses. Performance status was assessed by non-clinicians, which may have introduced inaccuracy in ranking. Finally, self-report data on medications may be inaccurate.
Conclusion
Our results suggest that stronger spiritual beliefs do not mitigate anxiety and depression in people with advanced cancer, but we cannot judge if spirituality is irrelevant to other aspects of wellbeing, or whether more complex processes near death are occurring, which limit any possible measurable advantage.
Acknowledgments
This study was supported in part by a research grant from Cancer Research UK (M. King—C1432/A8254) and from funding by Marie Curie Cancer Care. It was included in the National Cancer Research Network research portfolio. We thank all the patients who gave their time voluntarily to the study; Kelly Barnes for contributing to the grant submission for funding; and Sarah Davis and Andrea Beetison (who was funded by the North London Cancer Network) and staff at the Barnet, Enfield, Haringey, Pembridge, Royal Free Hospital, UCLH, West Essex, Whittington Palliative Care Teams and St Joseph's Hospice, Hackney, for their support in recruitment. We also thank members of the study steering group, in particular, Lallita Carballo, Mel Francis, Eve Garrard, Katherine Hopkins, Ruth Sack, Peter Speck, Liz Thomas and Rachael Williams.
Conflict of interest
None.
Supporting Information
Supporting info item
References
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30:394–400. doi: 10.1200/JCO.2011.35.7996. [DOI] [PubMed] [Google Scholar]
- Von Roenn JH, Temel J. The integration of palliative care and oncology: the evidence. Oncology (Williston Park) 2011;25:1258–5. [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (NICE) 2011. Quality standard for end of life care in adults http://www.nice.org.uk/media/EE7/57/EoLCFinalQS.pdf (accessed 13 March 2013)
- Jones L, Fitzgerald G, Leurent B, et al. Rehabilitation in advanced, progressive, recurrent cancer: a randomized controlled trial. J Pain Symptom Manage. 2012 doi: 10.1016/j.jpainsymman.2012.08.017. ; doi: 10.1016/j.jpainsymman.2012.08.017 (accessed 13 March 2013) [DOI] [PubMed] [Google Scholar]
- Kandasamy A, Chaturvedi SK, Desai G. Spirituality, distress, depression, anxiety, and quality of life in patients with advanced cancer. Indian J Cancer. 2011;48:55–59. doi: 10.4103/0019-509X.75828. [DOI] [PubMed] [Google Scholar]
- Yanez B, Edmondson D, Stanton AL, et al. Facets of spirituality as predictors of adjustment to cancer: relative contributions of having faith and finding meaning. J Consult Clin Psychol. 2009;77:730–741. doi: 10.1037/a0015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CJ, Rosenfeld B, Breitbart W, Galietta M. Spirituality, religion, and depression in the terminally ill. Psychosomatics. 2002;43:213–220. doi: 10.1176/appi.psy.43.3.213. [DOI] [PubMed] [Google Scholar]
- Krupski TL, Kwan L, Fink A, Sonn GA, Maliski S, Litwin MS. Spirituality influences health related quality of life in men with prostate cancer. Psycho-Oncology. 2006;15:121–131. doi: 10.1002/pon.929. [DOI] [PubMed] [Google Scholar]
- Wiech K, Farias M, Kahane G, Shackel N, Tiede W, Tracey I. An fMRI study measuring analgesia enhanced by religion as a belief system. Pain. 2008;139:467–476. doi: 10.1016/j.pain.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A, Powell LH. Religiosity/spirituality and mortality. Psychother Psychosom. 2009;78:81–90. doi: 10.1159/000190791. [DOI] [PubMed] [Google Scholar]
- Mystakidou K, Tsilika E, Parpa E, et al. Exploring the relationships between depression, hopelessness, cognitive status, pain, and spirituality in patients with advanced cancer. Arch Psychiatr Nurs. 2007;21:150–161. doi: 10.1016/j.apnu.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Park CL. Religiousness/spirituality and health: a meaning systems perspective. J Behav Med. 2007;30:319–328. doi: 10.1007/s10865-007-9111-x. [DOI] [PubMed] [Google Scholar]
- Sherman AC, Simonton S, Latif U, Spohn R, Tricot G. Religious struggle and religious comfort in response to illness: health outcomes among stem cell transplant patients. J Behav Med. 2005;28:359–367. doi: 10.1007/s10865-005-9006-7. [DOI] [PubMed] [Google Scholar]
- McClain-Jacobson C, Rosenfeld B, Kosinski A, Pessin H, Cimino JE, Breitbart W. Belief in an afterlife, spiritual well-being and end-of-life despair in patients with advanced cancer. Gen Hosp Psychiatry. 2004;26:484–486. doi: 10.1016/j.genhosppsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Gall TL, de Renart RM M, Boonstra B. Religious resources in long-term adjustment to breast cancer. J Psychosoc Oncol. 2000;18:21–37. [Google Scholar]
- Biegler K, Cohen L, Scott S, et al. The role of religion and spirituality in psychological distress prior to surgery for urologic cancer. Integr Cancer Ther. 2011 doi: 10.1177/1534735411416456. ; doi: 10.1177/1534735411416456 (accessed 13 March 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candy B, Jones L, Varagunam M, Speck P, Tookman A, King M. Spiritual and religious interventions for well-being of adults in the terminal phase of disease. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD007544.pub2. ; doi: 10.1002/14651858.CD007544.pub2 (accessed 13 March 2013) [DOI] [PubMed] [Google Scholar]
- Shreve-Neiger AK, Edelstein BA. Religion and anxiety: a critical review of the literature. Clin Psychol Rev. 2004;24:379–397. doi: 10.1016/j.cpr.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Stefanek M, McDonald PG, Hess SA. Religion, spirituality and cancer: current status and methodological challenges. Psycho-Oncology. 2005;14:450–463. doi: 10.1002/pon.861. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Bagiella E, Powell T. Religion, spirituality, and medicine. Lancet. 1999;353:664–667. doi: 10.1016/s0140-6736(98)07376-0. [DOI] [PubMed] [Google Scholar]
- Thune-Boyle IC, Stygall JA, Keshtgar MR, Newman SP. Do religious/spiritual coping strategies affect illness adjustment in patients with cancer? A systematic review of the literature. Soc Sci Med. 2006;63:151–164. doi: 10.1016/j.socscimed.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Schreiber JA, Brockopp DY. Twenty-five years later—What do we know about religion/spirituality and psychological well-being among breast cancer survivors? A systematic review. J Cancer Surviv. 2012;6:82–94. doi: 10.1007/s11764-011-0193-7. [DOI] [PubMed] [Google Scholar]
- Koenig HG. Concerns about measuring “spirituality” in research. J Nerv Ment Dis. 2008;196:349–355. doi: 10.1097/NMD.0b013e31816ff796. [DOI] [PubMed] [Google Scholar]
- Potash M, Breitbart W. Affective disorders in advanced cancer. Hematol Oncol Clin North Am. 2002;16:671–700. doi: 10.1016/s0889-8588(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Tremblay A, Breitbart W. Psychiatric dimensions of palliative care. Neurol Clin. 2001;19:949–967. doi: 10.1016/s0733-8619(05)70055-4. [DOI] [PubMed] [Google Scholar]
- King M, Jones L, Barnes K, et al. Measuring spiritual belief: development and standardization of a Beliefs and Values Scale. Psychol Med. 2006;36:417–425. doi: 10.1017/S003329170500629X. [DOI] [PubMed] [Google Scholar]
- Van de Geer DCL, Wulp M. Spiritual care in palliative care: working towards an EAPC Task Force. EJPC. 2011;18:86–89. [Google Scholar]
- King MB, Koenig HG. Conceptualising spirituality for medical research and health service provision. BMC Health Serv Res. 2009;9:116. doi: 10.1186/1472-6963-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS Social Support Survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Hill KM, Muers MF. Palliative care for patients with non-malignant end stage respiratory disease. Thorax. 2000;55:979–981. doi: 10.1136/thorax.55.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Fevre P, Devereux J, Smith S, Lawrie SM, Cornbleet M. Screening for psychiatric illness in the palliative care inpatient setting: a comparison between the Hospital Anxiety and Depression Scale and the General Health Questionnaire-12. Palliat Med. 1999;13:399–407. doi: 10.1191/026921699671260095. [DOI] [PubMed] [Google Scholar]
- Olssøn I, Mykletun A, Dahl AA. The Hospital Anxiety and Depression Rating Scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry. 2005;5 doi: 10.1186/1471-244X-5-46. . doi: 10.1186/1471-244X-5-46. (accessed 13 March 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Zullig KJ, Ward RM, Horn T. The association between perceived spirituality, religiosity, and life satisfaction: the mediating role of self-rated health. Soc Indic Res. 2006;79:255–274. [Google Scholar]
- Gessler S, Low J, Daniells E, et al. Screening for distress in cancer patients: is the distress thermometer a valid measure in the UK and does it measure change over time? A prospective validation study. Psycho-Oncology. 2008;17:538–547. doi: 10.1002/pon.1273. [DOI] [PubMed] [Google Scholar]
- The EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Schag CC, Heinrich RL, Ganz PA. Karnofsky Performance Status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- Royston P. Multiple imputation of missing values: further update of ice, with an emphasis on interval censoring. Stata Journal. 2007;7(4):445–464. [Google Scholar]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons; 1987. [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- Schwartz CE, Sprangers MAG. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med. 1999;48:1531–1548. doi: 10.1016/s0277-9536(99)00047-7. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software: release 9. College Station, TX: StataCorp LP; 2005. [Google Scholar]
- King M, Marston L, McManus S, Brugha T, Meltzer H, Bebbington P. Religion, spirituality and mental health: results from a national study of English households. Br J Psychiatry. 2012;202:68–73. doi: 10.1192/bjp.bp.112.112003. [DOI] [PubMed] [Google Scholar]
- Leurent B, Nazareth I, Bellon-Saameno J, et al. Spiritual and religious beliefs as risk factors for the onset of major depression: an international cohort study. Psychol Med. 2013 doi: 10.1017/S0033291712003066. doi: 10.1017/S0033291712003066 (accessed 13 March 2013) [DOI] [PubMed] [Google Scholar]
- Hodgkinson K, Butow P, Hunt GE, Pendlebury S, Hobbs KM, Wain G. Breast cancer survivors' supportive care needs 2–10 years after diagnosis. Support Care Cancer. 2007;15:515–523. doi: 10.1007/s00520-006-0170-2. [DOI] [PubMed] [Google Scholar]
- McGrath P. Reflections on serious illness as spiritual journey by survivors of haematological malignancies. Eur J Cancer Care (Engl) 2004;13:227–237. doi: 10.1111/j.1365-2354.2004.00457.x. [DOI] [PubMed] [Google Scholar]
- Mellon S, Northouse LL, Weiss LK. A population-based study of the quality of life of cancer survivors and their family caregivers. Cancer Nurs. 2006;29:120–131. doi: 10.1097/00002820-200603000-00007. [DOI] [PubMed] [Google Scholar]
- Koenig H, Carson VB. Handbook of Religion and Health. 2nd. USA: Oxford University Press; 2012. [Google Scholar]
- Rinck GC, van den Bos GA, Kleijnen J, de Haes HJ, Schade E, Veenhof CH. Methodologic issues in effectiveness research on palliative cancer care: a systematic review. J Clin Oncol. 1997;15:1697–1707. doi: 10.1200/JCO.1997.15.4.1697. [DOI] [PubMed] [Google Scholar]
- Jordhoy MS, Kaasa S, Fayers P, Ovreness T, Underland G, Ahlner-Elmqvist M. Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliat Med. 1999;13:299–310. doi: 10.1191/026921699668963873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


