Abstract
The aim of this study was to document anti-inflammatory properties of a dried fermentate derived from Saccharomyces cerevisiae (EpiCor®), hereafter referred to as dried fermentate in vitro using cell-based bioassays, and in vivo using a skin irritation model in healthy humans. In vitro testing involved parallel assessment of primary human polymorphonuclear (PMN) cell formation of reactive oxygen species (ROS) and migration toward the inflammatory mediator Leukotriene B4. In vivo evaluation used a single-blind placebo-controlled design, where dermal histamine-induced inflammation was used as a model for the complex intercellular signals involved in the initiation, escalation, and resolution of the inflammatory response. Microvascular blood perfusion was evaluated using noninvasive laser Doppler probes applied to the inner forearms of 12 healthy human subjects, where parallel sites were treated with either dried fermentate or saline (placebo). Subjective scores of dermal irritation were also collected. Treatment of PMN cells in vitro resulted in reduced ROS formation and migratory activity toward Leukotriene B4. Clinical results demonstrated significantly reduced microvascular inflammatory responses to histamine-induced skin inflammation, and significantly reduced subjective scores of irritation at the inflamed sites treated with dried fermentate compared with the sites treated with placebo (P<.05). Treatment of inflammatory cells in vitro with dried fermentate resulted in reduced inflammatory responses. This was confirmed in vivo, suggesting that the dried fermentate facilitates the resolution of inflammatory responses. The effects using a topical skin model suggest that similar events may happen when the dried fermentate is introduced across mucosal membranes after consumption.
Key Words: : reactive oxygen species, polymorphonuclear, migration, laser Doppler, histamine, blood perfusion, leukotriene B4
Introduction
Inflammation is an important and necessary component of the innate immune system. As an initial response to harmful stimuli such as infection or injury, inflammation plays a key role in the body's response to pathogens and cellular damage leading to the elimination of pathogens and the recruitment of cells (macrophages and neutrophils) to sites of injury to remove infected/damaged cells. This acute inflammation is an important part of a healthy response to infection or injury. Conversely, chronic inflammation, that is, inflammation that is not properly resolved and persists after the initial insult has been removed, can be harmful to the organism. The absence of inflammation resolution has been linked to a number of chronic diseases including cancer, metabolic syndrome, arthritis, and atherosclerosis.1
Yeasts were traditionally used in the processes of bread making and food fermentation dating back thousands of years. Long before the advent of “baker's” or “brewer's” yeast the initial use of yeast relied on wild isolates composed of many different strains. Recently, individual yeast species of the Saccharomyces genus including Saccharomyces cerevisiae and the probiotic Saccharomyces boulardii have been shown to have beneficial effects on the immune system when consumed directly.2,3
Among the known immunomodulatory compounds from S. cerevisiae, various cell wall compounds have been studied. The primary compound of the inner layer of the cell wall is beta-1–3-glucan, interspersed with some beta-1–6-glucan, whereas the outer part of the wall is mostly composed of mannans.4 Glucans are present in many plants and all fungi. Beta-glucans are known immunomodulators.5–7 Glucans typically mediate innate immune activating properties, such as natural killer cell and macrophage activation, and beta-glucans are generally considered pro-inflammatory in nature.8,9
In contrast to pure beta-glucans and yeast cell wall preparations, complex fungal-derived products present a highly complex profile of bioactive compounds to the immune system. The dried fermentate tested in this study (EpiCor®) does not consist solely of yeast cells or yeast biomass. The product is based on a proprietary anaerobic fermentation process using baker's yeast (S. cerevisiae). After the cells have been stressed from the fermentation, the whole liquid fermentate is dried, resulting in a product that is high in yeast metabolites, including vitamins, polyphenols, sterols, and phospholipids. Bioactive components include the nutrient/vitamin profile, cell wall components, and stress-induced defense metabolites.
The dried fermentate has previously been shown to activate natural killer cells in vitro10 and provide immune support against cold, flu, and allergies in clinical studies.11–13 This may in part be accounted for by the documented rapid effects on immune status within 1–2 h after consumption.14 In addition to safety documentation,15 evidence is accumulating to document other facets of the dried fermentate's bioactivities, pointing to more complex immune regulatory mechanisms of action, including anti-inflammatory effects.16 Other aspects associated with the dried fermentate consumption include beneficial effects on gut health.17,18
The aim of this study was to expand the understanding of the anti-inflammatory properties of this dried yeast fermentate by documenting specific effects on controlled inflammatory insults in vitro and in vivo.
Methods
Reagents
The following buffers and reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA): Phosphate-buffered saline (PBS), hydrogen peroxide 30% solution, RPMI-1640 culture medium, Histopaque 1077 and 1119, fibronectin 0.1% from bovine plasma, and dimethyl sulfoxide (DMSO) 99.9%. The following reagents were obtained from Life Technologies/Molecular Probes (Eugene, OR, USA): 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) and CyQuant®.
Dried fermentate
The dried fermentate EpiCor was provided by the manufacturer (Embria Health Sciences, Ankeny, IA, USA) as a powder comprised of the heat-inactivated yeast S. cerevisiae and the fermentation medium in which it grew. For both the in vitro testing in cell-based bioassays and for the skin testing in humans, the dried fermentate was prepared by taking 0.5 g powder into 5 mL physiological saline. The mixture was allowed to sit for 1 h under gentle agitation, after which solids were removed by centrifugation and filtration using a 0.22 μm cellulose acetate syringe filter.
Purification of primary human polymorphonuclear cells
Healthy human volunteers between the ages of 18 and 50 years served as blood donors upon written informed consent, as approved by the Sky Lakes Medical Center Institutional Review Board (FWA2603). Freshly drawn peripheral venous blood samples in sodium heparin were layered onto a double-gradient of Histopaque 1119 and 1077, and centrifuged for 25 min at 500 g. The lower polymorphonuclear (PMN) cell layers were harvested using sterile transfer pipettes into new vials, and washed twice with 10 mL PBS without calcium or magnesium and centrifuged at 500 g for 10 min.
Reactive oxygen species production by PMN cells
The intracellular level of reactive oxygen species (ROS) in activated PMN cells pretreated with the dried fermentate was evaluated as follows. PMN cells at 106/mL were incubated at 37°C, 5% CO2 for 20 min, either treated with test product over a range of 10-fold serial dilutions (1, 0.1, 0.01, 0.001, and 0.0001 g/L) or untreated (control). The precursor dye CM-H2DCF-DA, which upon exposure to free radicals is converted to the brightly green fluorescent DCF-DA, was prepared by adding 0.18 mL DMSO to a 0.05 mg aliquot of DCF-DA. A final working solution of dichlorofluorescein diacetate (DCF-DA) was then prepared by adding 0.01 mL stock to 10 mL PBS. The PMN cells were washed thrice in PBS and then resuspended in the DCF-DA working solution and incubated for 1 h at 37°C. All samples, excluding the untreated control samples, were then exposed to 2 mM H2O2 for 45 min to induce ROS production. The untreated cells that were not exposed to H2O2 served as negative control samples, to document that cells treated with H2O2 showed a significant increase in DCF-DA fluorescence (assay readout). Samples were washed twice in PBS to remove the peroxide, and transferred to vials for flow cytometry. Samples were kept dark on an ice bath until flow cytometry acquisition (FacsCalibur; Becton-Dickinson, San Jose, CA, USA), and all samples were acquired within 2 h after laboratory processing. Data were collected in triplicate and experiments performed thrice using cells derived from three different donors. The relative amount of ROS formation in PMN cells was determined by green fluorescence intensity.
PMN cell migration toward the inflammatory mediator Leukotriene B4
The PMN cell is a highly active and migratory cell type. The effect on PMN cell migration toward the inflammatory chemoattractant Leukotriene B4 (LTB4) was tested, as described previously.19,20 Cells were incubated with 10-fold serial dilutions of test product for 10 min prior to applying to the top chamber of a 3.0 μm pore size trans-well migration plate (Millipore, Billerica, MA, USA), which had been previously coated with 50 μg/mL fibronectin for a period of 30 min. Either RPMI 1640 (control wells) or LTB4 diluted in RPMI 1640 (10 nM) were added to the appropriate bottom chamber wells of the trans-well migration plate. Fifty microliters of cells (1×106/mL) were plated in the top chambers, and the top chamber plate was then lowered into the bottom plate and incubated at 37°C, 5% CO2 for 18 h. Fluorescent CyQuant staining was performed to quantify the relative number of cells that migrated to the bottom chambers. Fluorescence intensity was quantified in a Tecan Spectrafluor fluorescence plate reader. Samples were assayed in quadruplicate and experiments repeated thrice with cells from three different blood donors.
Human clinical trial: histamine skin test with laser Doppler assessment
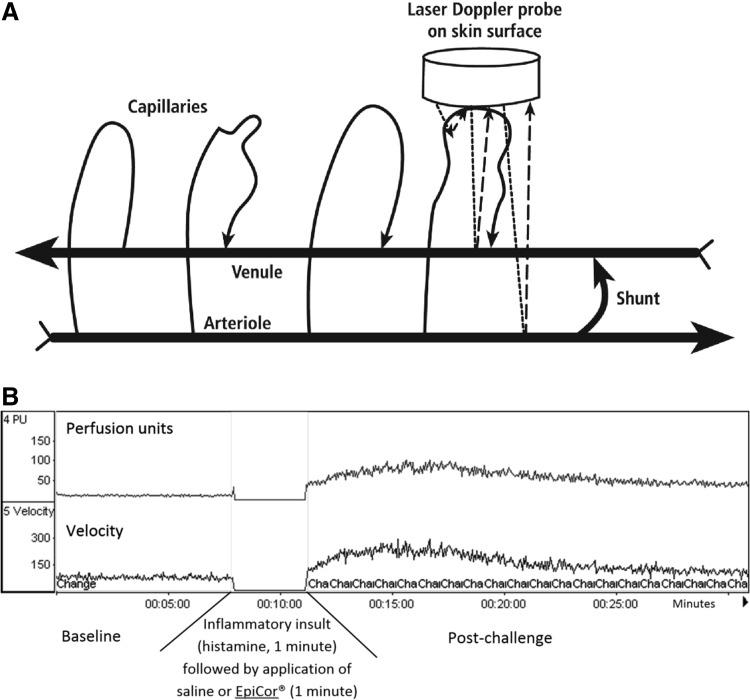
A human clinical study was conducted using a single-blind, placebo-controlled, crossover study involving 12 volunteers (Table 1). All volunteers were in good health, and none of them were on antidepressant, diabetic, or allergy medication. The following procedures were performed after informed consent, as approved by Sky Lakes Medical Center's Institutional Review Board (FWA2603). The purpose of this procedure was to evaluate the resolution of a histamine-induced inflammatory response, when the dried fermentate solution was applied topically after the histamine response had been initiated. The test procedure was performed in a manner similar to allergy skin testing, except that allergy skin testing relies on visual inspection of the sites, whereas the high sensitivity of the laser Doppler method was perfomed by assessing the microvascular blood perfusion using a PeriFlux 5000 (PeriMed Las Vegas NV), and allowed the use of much lower doses of histamine to provoke a measurable response. The laser Doppler probes send out a fine laser beam at 780 nm wavelength, and when the light hits moving red blood cells in the microvasculature (capillaries, arterioles, venules, and shunts between those), the light undergoes a change in wavelength (Doppler shift), while light hitting static objects is unchanged. The magnitude and frequency distribution of these changes in wavelength are directly related to the number and velocity of the blood cells in the sample volume. The information is picked up by a returning fiber, converted into an electronic signal, and analyzed (Fig. 2A).
Table 1.
Skin Perfusion and Irritation Scores After a Histamine-Induced Inflammatory Response
| Volunteer No. | Gender | Age | Irritation score (saline) | Irritation score (dried fermentate)a |
|---|---|---|---|---|
| Volunteer 1 | M | 49 | 100 | 26 |
| Volunteer 2 | M | 24 | 100 | 74 |
| Volunteer 3 | F | 46 | 100 | 30 |
| Volunteer 4 | F | 45 | 100 | 100b |
| Volunteer 5 | F | 20 | 100 | 9 |
| Volunteer 6 | F | 19 | 100 | 29 |
| Volunteer 7 | M | 32 | 100 | 69 |
| Volunteer 8 | M | 23 | 100 | 56 |
| Volunteer 9 | M | 22 | 100 | 12 |
| Volunteer 10 | F | 22 | 100 | 86 |
| Volunteer 11 | F | 24 | 100 | 55 |
| Volunteer 12 | F | 19 | 100 | 60 |
| Mean | 28.75 | 100 | 46 | |
| Standard error | 3.28 | 7.84 |
After completion of the laser Doppler evaluation, volunteers were asked to score the irritation (itchiness and burning) for the two skin sites on the forearms. They were asked to identify the most irritated site, and then with that set to “100” they were asked to score the less itchy site from 0 to 100. The volunteers were blinded to the treatments (saline versus dried fermentate).
This volunteer was later found to have a mild food sensitivity to yeast. It is therefore not surprising that similar irritation scores were given for both arms. This volunteer did show reduced blood perfusion on the dried fermentate-treated site, suggesting that the dried fermentate helped accelerate the resolution of the inflammatory response also in her case.
FIG. 2.
Diagram showing the principles and data generated from laser Doppler evaluation of microvascular blood perfusion. (A) Noninvasive application of a laser Doppler probe on the skin surface sends laser light into the skin. Data on reflected laser light of the same wavelength were not collected by the probe, however, laser light of a different wavelength, as a result of a Doppler shift, is collected and quantified as a measure of relative microvascular blood perfusion and red blood cell velocity. The Doppler shift only happens when laser light hits moving red blood cells. (B) Representative example of the laser Doppler data collected during a test run for this study: Baseline blood perfusion is evaluated for a minimum of 5 min, after which the probe is gently removed from its positioning on the skin. Ten microliters of a dilute solution of histamine (0.4 mg/mL), is applied to the site, and a small prick made using a Hollister–Stier lancet. After 1 min, a cotton tip is used to remove the histamine by gentle blotting without rubbing the skin site. Then, 10 microliters of either the dried fermentate (dose 0.1 g/mL) or saline is applied, the Doppler probe is re-applied to the site, and data were collected to measure the magnitude of the microvascular effects of the inflammatory insult.
The procedure for topical skin inflammation was as follows: On each healthy subject, a skin site was identified on the inner forearms where no visible major blood vessels were visible under the skin. The sites were marked and a ring of double-sided tape was applied to hold the laser Doppler probes in place, to allow for removal and reattachment of the probes without applying pressure or otherwise disturbing the microvascular structure under each site. A baseline reading of the microvascular blood perfusion was performed for a minimum of 10 min (Fig. 2B). Inflammation was induced on each skin site by applying 0.01 mL of a dilute solution of histamine (0.4 mg/mL), and performing a scratch using a sterile Hollister-Stier lancet. After 1 min, the histamine solution was removed and 0.01 mL dried fermentate (0.1 g/mL) was applied to the site. After 1 min, the dried fermentate solution was removed and the laser Doppler probes gently reapplied to the ring of double-sided tape without causing further irritation to the sites. The laser Doppler data collection continued for an additional 10 min. Probes were removed, and each subject was asked to score the level of itching on each skin site using a 100 mm Visual Analogue Scale. The procedure was simultaneously performed on left and right forearm, where the dried fermentate solution was tested on one site and saline applied as a placebo control on the other site; study participants were unaware of which site received saline and which site received the dried fermentate solution.
Statistical analysis
Statistical analysis was performed on the in vitro bioassay data using the independent two-tailed t-test. Analysis of the human clinical data, where placebo and dried fermentate treatments happened simultaneously on two different skin sites, was performed as “within-subject” analysis using the paired two-tailed t-test.
Results
Reduction of free radical formation and chemotaxis by PMN cells
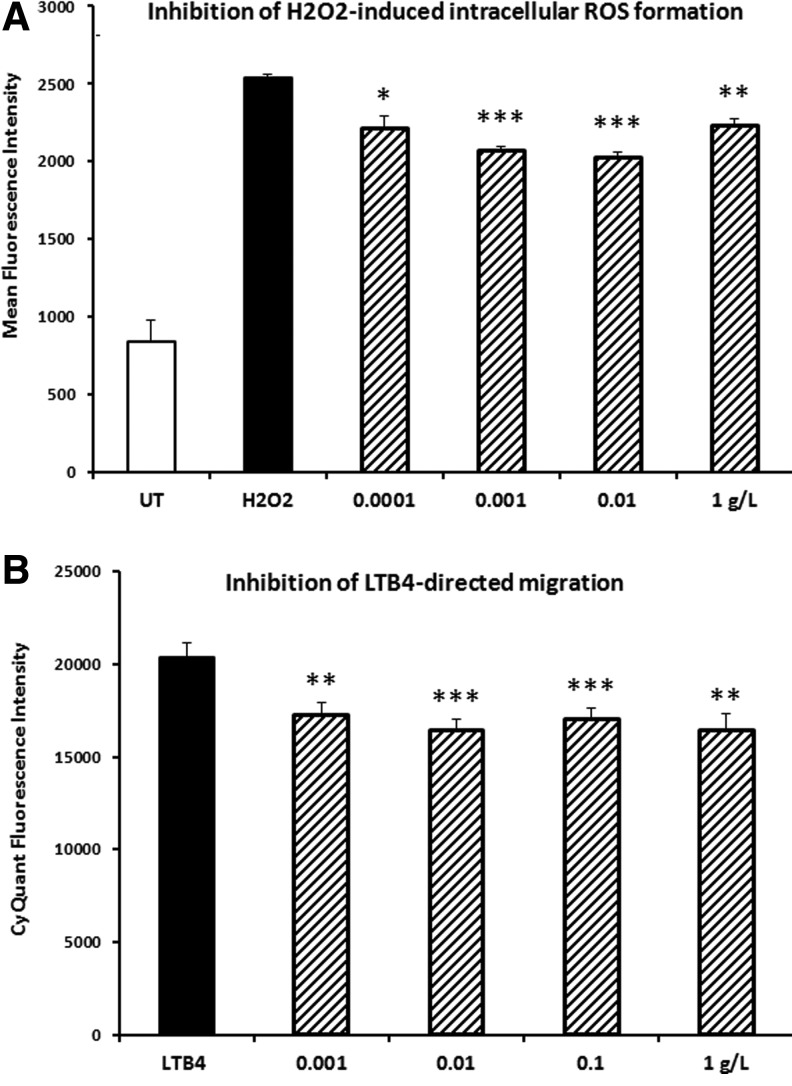
The PMN cell type is abundant in the blood circulation, and typically comprises 50–70% of all circulating white blood cells. The cells are short-lived and capable of rapid engagement in pro-inflammatory immune defense mechanisms, such as production of free radicals for antimicrobial defense and for pro-inflammatory intercellular signaling, and chemotactic migration toward inflammatory mediators as part of the cellular recruitment into inflammatory tissue sites. A reduction in ROS was observed in PMN cells under oxidative stress when cells were pretreated with the dried fermentate (Fig. 1A). This reduction in ROS formation was statistically significant over a wide dose range. Pretreatment of PMN cells with the dried fermentate resulted in a reduction in migration toward the inflammatory chemokine LTB4 (Fig. 1B). This reduction in PMN cell migration was statistically significant over a wide dose range.
FIG. 1.
Anti-inflammatory effects were seen when polymorphonuclear cells were pretreated with the dried fermentate in vitro before the cells were tested in two different inflammation models. (A) Formation of reactive oxygen species (ROS) by polymorphonuclear cells in vitro. (B) Migration in response to the pro-inflammatory chemotactic mediator Leukotriene B4 (LTB4). Black bars show the inflammatory reactions by cells that were exposed to inflammatory stimuli without pretreatment with the dried fermentate (untreated: UT), and hatched bars show data from cells treated with the dried fermentate at the indicated doses in vitro before being assayed for the inflammatory responses. Each data point is based on triplicate measures (A) or quadruplicate measures (B). Levels of statistical significance shown by asterisks (*P<.05, **P<.01, and ***P<.001).
Accelerated resolution of an inflammatory insult in vivo
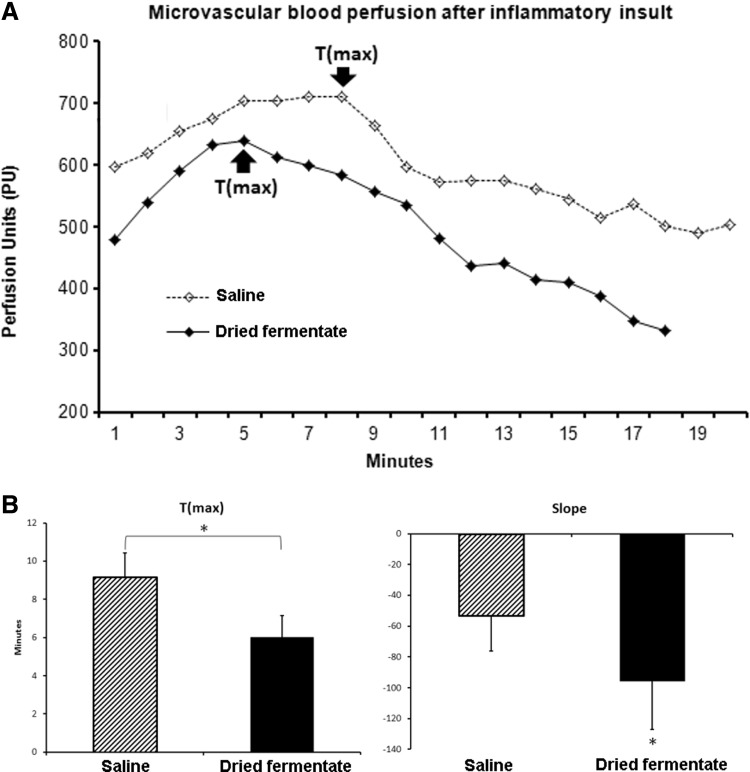
As an in vivo model for the complex cascades involved in an acute inflammatory reaction, histamine-induced skin inflammation was chosen. One of several end points in this inflammatory cascade involving crosstalk between mast cells, endothelial cells, and recruitment of blood PMN cells into tissue, is an increased blood perfusion locally in the inflamed site. Another important end point is the time where the inflammatory cascade peaks and begins to resolve. The blood perfusion was measured using noninvasive laser Doppler probes attached on the surface of each skin site (placebo, dried fermentate), where baseline data and postchallenge data were collected (Fig. 2). The laser Doppler data were analyzed for the following parameters: the time to maximum blood perfusion (Tmax, Fig. 3A), and the slope of the curve generated during the resolution phase over time (Slope) as a measure of the speed of resolution (Fig. 3B).
FIG. 3.
Laser Doppler perfusion on two skin sites on the forearm. Both sites were treated with histamine and one site subsequently treated with the dried fermentate, while the other site was treated with saline. The treatments were blinded to the subject. (A) Blood perfusion on each site from a representative study participant. The number of minutes it took for the histamine-induced increase in blood perfusion to plateau and start to decline was identified as the Time to max (Tmax) for each site (saline versus dried fermentate). (B) T(max) and the slope for the subsequent reduction in blood perfusion was averaged for all study participants. The average and standard deviation are shown for T(max) and slope. The reduced T(max) and steeper slope for the sites treated with the dried fermentate were significantly lower than the T(max) for the sites treated with saline (*P<.05).
Among the 12 subjects, the observed average Tmax on the site treated with the dried fermentate was significantly shorter than the Tmax seen for the site treated with saline (P<.05) (Fig. 3A). In addition, the slope of the curve after Tmax, that is, during the resolution phase, was also significantly reduced compared with the saline-treated site (P<.05) (Fig. 3B), indicating that the increased blood perfusion caused by the histamine challenge resolved slower in the sites treated with saline, and that treatment of the inflamed site with the dried fermentate resulted in a faster process of inflammation resolution.
Discussion
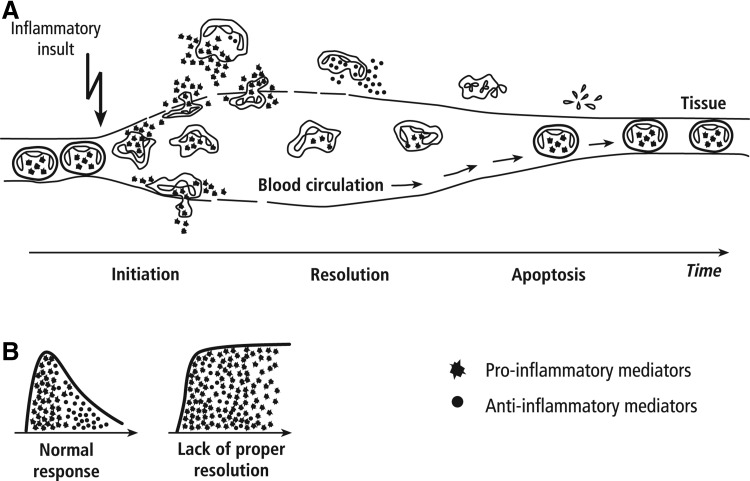
The resolution of acute inflammation is necessary for inflamed tissues to return to homeostasis (Fig. 4). PMN cells are a major player in the process of orchestrating a rapid inflammatory response. Interestingly, these same cells are responsible for the resolution of inflammation through the production of lipid mediators such as lipoxins, resolvins, protectins, and maresins.21,22 The in vitro data presented here regarding the reduced inflammatory responses by PMN cells in the presence of the dried fermentate may relate to either the induction phase or the resolution phase, or a combination of both. Previous work has also demonstrated the anti-inflammatory effects of this dried fermentate in animal models.16 Recent work has documented that the dried fermentate retains anti-inflammatory properties after in vitro digestion, as shown by the reduction in interleukin-8 and tumor necrosis factor-alpha production by Caco-2/THP-1 co-cultures exposed to the dried fermentate from the dynamic gastrointestinal simulator SHIME®.18 In the SHIME model, the dried fermentate also showed significant prebiotic potential, including modulation of the microbiota. The fermentate promoted probiotic bacterial strains capable of production of short-chain fatty acids, particularly butyrate, which was produced at higher levels than in the presence of the known prebiotics inulin and fructooligosaccharide.
FIG. 4.
Diagram showing the initiation and resolution of the inflammatory response. (A) Circulating polymorphonuclear cells are shown as they adhere to the vessel wall, extravasate, produce pro-inflammatory compounds, produce anti-inflammatory compounds, and finally undergo apoptosis. (B) The normal wave of production of pro- versus anti-inflammatory compounds is shown to the left. To the right is shown an inflammatory response with inappropriate resolution, and prolonged production of pro-inflammatory compounds.
The results from the present work provide further clinical support for the anti-inflammatory properties of the dried fermentate. The topical application of the dried fermentate in the histamine-induced skin inflammation model may allow some correlations to be made to effects after consumption, where the dried fermentate is brought into direct contact with the gastrointestinal mucosa. Since the dried fermentate was added a full minute after the histamine-induction of inflammation had occurred, this experimental model did not aim at exploring whether the dried fermentate prevented the initial events of the inflammatory response; rather the model aimed at documenting the dried fermentate's effects on the resolution phase. The histamine-induced changes in blood perfusion showed that the increase reached a maximum (Tmax) earlier, and showed a more rapid return to baseline blood perfusion (Slope) when the dried fermentate was added, compared to the site on the opposite forearm treated with saline. Therefore, the data point to an acceleration of the resolution of the inflammation response. The microanatomy of the skin and vascular beds underlying each test site, along with the density of mast cells in each area, must be considered an unknown variable that directly affects the magnitude of the increased blood perfusion after histamine treatment.
The skin model used for the data presented here may have broader relevance, for example, the effect the dried fermentate may have in the gut after being consumed. This could indicate a promising role for the dried fermentate consumption in protecting and maintaining a healthy gut environment through reduction of inflammatory reactions. For this exploratory study, a skin model was proposed for convenience, as events observed in the skin after topical application of the dried fermentate likely can be extrapolated to events in the gut mucosa after the dried fermentate is ingested. Since an anti-inflammatory effect was observed by application of this fermentate directly to the skin, it could also imply that similar effects occur along the gastrointestinal mucosal surfaces after consumption of the fermentate where bioactive compounds come into contact with immune cells along the gut lining. This direct mechanism would be in addition to the anti-inflammatory effect that happens when the product is fermented by probiotic gut bacteria, in part due to increased butyrate production.18
An unbalanced immune activation, in response to pathogens from the gut lumen, has been implicated as a risk factor for many chronic inflammatory conditions, including irritable bowel disease, ulcerative colitis, and Crohn's disease. It has been a contributing factor in obesity, heart disease, Alzheimer disease, autoimmune diseases such as rheumatoid arthritis and psoriasis, and has been implicated in neoplasia.23,24 There is a growing interest in unique foods and nutritional supplements that combine vitamins, antioxidants, and immunomodulating compounds for use in nutritional strategies to reduce inflammation and support various aspects of immune function.25
Furthermore, this dried fermentate inhibited ROS formation and chemotactic migration toward the inflammatory mediator Leukotriene B4 in human PMN cells. As several ROS species play roles in cell signaling, it is likely that the dried fermentate is capable of reducing the background noise of chronic inflammation, thereby increasing the capacity for maintaining balanced immune responses. Based on these studies, this product has the potential to act as an immune modulator while at the same time helping to reduce gastrointestinal and, possibly, systemic inflammation. The data presented here on the test product can be seen as support for further clinical work to determine the possible effects of product consumption on chronic inflammatory disorders.
In conclusion, the data shown here provide support from human clinical data, suggesting that the dried fermentate's anti-inflammatory effects include specific mechanisms to promote the resolution of inflammation.
Acknowledgments
This project was sponsored by Embria Health Sciences LLC and performed at NIS Labs, an independent contract research lab specializing in natural products research.
Author Disclosure Statement
G.S.J., S.G.C., and K.F.B. are employees of NIS Labs, and have no financial interest in the subject matter. SGR and LRE are employees of Embria Health Sciences, the sponsor of the study.
References
- 1.Laveti D, Kumar M, Hemalatha R, Sistla R, Naidu VG, Talla V, Verma V, Kaur N, Nagpal R: Anti-inflammatory treatments for chronic diseases: a review. Inflamm Allergy Drug Targets 2013;12:349–361 [DOI] [PubMed] [Google Scholar]
- 2.Salim HM, Kang HK, Akter N, Kim DW, Kim JH, Kim MJ, Na JC, Jong HB, Choi HC, Suh OS, Kim WK: Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poult Sci 2013;92:2084–2090 [DOI] [PubMed] [Google Scholar]
- 3.Martins FS, Vieira AT, Elian SD, Arantes RM, Tiago FC, Sousa LP, Araújo HR, Pimenta PF, Bonjardim CA, Nicoli JR, Teixeira MM: Inhibition of tissue inflammation and bacterial translocation as one of the protective mechanisms of Saccharomyces boulardii against Salmonella infection in mice. Microbes Infect 2013;15:270–279 [DOI] [PubMed] [Google Scholar]
- 4.Aguilar-Uscanga B, François JM: A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett Appl Microbiol 2003;37:268–274 [DOI] [PubMed] [Google Scholar]
- 5.Plat J, Mensink RP: Food components and immune function. Curr Opin Lipidol 2005;16:31–37 [DOI] [PubMed] [Google Scholar]
- 6.Pelizon AC, Kaneno R, Soares AM, Meira DA, Sartori A: Downmodulation of lymphoproliferation and interferon-gamma production by beta-glucan derived from Saccharomyces cerevisiae. Mem Inst Oswaldo Cruz 2003;98:1083–1087 [DOI] [PubMed] [Google Scholar]
- 7.Kournikakis B, Mandeville R, Brousseau P, Ostroff G: Anthrax protective effects of yeast beta 1,3 glucans. Med Gen Med 2003;5:1. [PubMed] [Google Scholar]
- 8.Cassone A, Marconi P, Bistoni F: Cell wall of Candida albicans and host response. Crit Rev Microbiol 1987;15:87–95 [DOI] [PubMed] [Google Scholar]
- 9.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan ASH, Magee AS, Danielson ME, Weiss A, Vasilakos JP, Underhill DM: Activation of the innate immune receptor Dectin-1 upon formation of a “phagocytic synapse.” Nature 2011;472:471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen GS, Hart AN, Schauss AG: An antiinflammatory immunogen from yeast culture induces activation and alters chemokine receptor expression on human natural killer cells and B lymphocytes in vitro. Nutr Res 2007;27:327–335 [DOI] [PubMed] [Google Scholar]
- 11.Moyad MA, Robinson LE, Zawada ET, Jr, Kittelsrud JM, Chen DG, Reeves SG, Weaver SE: Effects of a modified yeast supplement on cold/flu symptoms. Urol Nurs 2008;28:50–55 [PubMed] [Google Scholar]
- 12.Moyad MA, Robinson LE, Kittelsrud JM, Reeves SG, Weaver SE, Guzman AI, Bubak ME: Immunogenic yeast-based fermentation product reduces allergic rhinitis-induced nasal congestion: a randomized, double-blind, placebo-controlled trial. Adv Ther 2009;26:795–804 [DOI] [PubMed] [Google Scholar]
- 13.Moyad MA, Robinson LE, Zawada ET, Kittelsrud J, Chen DG, Reeves SG, Weaver S: Immunogenic yeast-based fermentate for cold/flu-like symptoms in nonvaccinated individuals. J Altern Complement Med 2010;16:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen GS, Redman KA, Benson KF, Carter SG, Mizner MA, Reeves SG, Robinson LE: Antioxidant bioavailability and rapid immune-modulating effects after consumption of a single acute dose of a high-metabolite yeast immunogen: Results of a Placebo-Controlled Double-Blinded Crossover Pilot Study. J Med Food 2011;14:1002–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schauss AG, Glavits R, Endres JR, Jensen GS, Clewell A: Safety Evaluation of a Proprietary Food-Grade, Dried Fermentate Preparation of Saccharomyces cerevisiae. Intl J Toxicol 2012;31:34–45 [DOI] [PubMed] [Google Scholar]
- 16.Evans M, Reeves S, Robinson LE: A dried yeast fermentate prevents and reduces inflammation in two separate experimental immune models. Evid Based Complement Alternat Med 2012;2012:973041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen GS, Redman KA, Benson KF, Carter SG, Mitzner MA, Reeves S, Robinson L: A Double-Blind Placebo-Controlled, Randomized Pilot Study: consumption of a high-metabolite immunogen from yeast culture has beneficial effects on erythrocyte health and mucosal immune protection in healthy subjects. Open Nutr J 2008;2:68–75 [Google Scholar]
- 18.Possemiers S, Pinheiro I, Verhelst A, Van den Abbeele P, Maignien L, Laukens D, Reeves SG, Robinson LE, Raas T, Schneider Y-J, Van de Wiele T, Marzorati M: A dried yeast fermentate selectively modulates both the luminal and mucosal gut microbiota, enhances butyrate production and protects against inflammation, as studied in an integrated in vitro approach. J Agric Food Chem 2013;61:9380–9392 [DOI] [PubMed] [Google Scholar]
- 19.Honzel D, Carter SG, Redman KA, Schauss AG, Endres JR, Jensen GS: Comparison of chemical and cell-based antioxidant methods for evaluation of foods and natural products: generating multifaceted data by parallel testing using erythrocytes and polymorphonuclear cells. J Agric Food Chem 2008;56:8319–8325 [DOI] [PubMed] [Google Scholar]
- 20.Benson KF, Redman KA, Carter SG, Keller D, Farmer S, Endres JR, Jensen GS: Probiotic metabolites from Bacillus coagulans GanedenBC30™ support maturation of antigen-presenting cells in vitro. World J Gastroenterol 2012;18:1875–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alessandri AL, Sousa LP, Lucas CD, Rossi AG, Pinho V, Teixeira MM: Resolution of inflammation: mechanisms and opportunity for drug development. Pharmacol Ther 2013;139:189–212 [DOI] [PubMed] [Google Scholar]
- 22.Spite M, Serhan CN: Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res 2010;107:1170–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kontogianni MD, Zampelas A, Tsigos C: Nutrition and inflammatory load. Ann NY Acad Sci 2006;1083:214–238 [DOI] [PubMed] [Google Scholar]
- 24.Schottenfeld D, Beebe-Dimmer J: Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin 2006;56:69–83 [DOI] [PubMed] [Google Scholar]
- 25.Tapsell LC, Hemphill I, Cobiac L, Patch CS, Sullivan DR, Fenech M, Roodenrys S, Keogh JB, Clifton PM, Williams PG, Fazio VA, Inge KE: Health benefits of herbs and spices: the past, the present, the future. Med J Aust 2006;185:S4–S24 [DOI] [PubMed] [Google Scholar]