Abstract
A vast body of research showed that social exclusion can trigger aggression. However, the neural mechanisms involved in regulating aggressive responses to social exclusion are still largely unknown. Transcranial direct current stimulation (tDCS) modulates the excitability of a target region. Building on studies suggesting that activity in the right ventrolateral pre-frontal cortex (rVLPFC) might aid the regulation or inhibition of social exclusion-related distress, we hypothesized that non-invasive brain polarization through tDCS over the rVLPFC would reduce behavioral aggression following social exclusion. Participants were socially excluded or included while they received tDCS or sham stimulation to the rVLPFC. Next, they received an opportunity to aggress. Excluded participants demonstrated cognitive awareness of their inclusionary status, yet tDCS (but not sham stimulation) reduced their behavioral aggression. Excluded participants who received tDCS stimulation were no more aggressive than included participants. tDCS stimulation did not influence socially included participants’ aggression. Our findings provide the first causal test for the role of rVLPFC in modulating aggressive responses to social exclusion. Our findings suggest that modulating activity in a brain area (i.e. the rVLPFC) implicated in self-control and emotion regulation can break the link between social exclusion and aggression.
Keywords: aggression, social exclusion, emotion regulation, social pain, transcranial direct current stimulation (tDCS)
INTRODUCTION
Malloy didn't speak to them as they went by the boiler. They drew into themselves and no one could foresee how they would come out of the cloud. For there are two possible reactions to social ostracism—either a man emerges determined to be better, purer, and kindlier or he goes bad, challenges the world and does even worse things.
—John Steinbeck, Cannery Row (1945)
As Steinbeck quote suggests, when people are socially ostracized, excluded or rejected by others, they can either try to gain acceptance by behaving prosocially or lash out at others by behaving aggressively. Lashing out at others seems paradoxical, because it would invite further social rejection (Twenge et al., 2001; Buckley et al., 2004). Yet, rejected people often choose this course of action (Twenge et al., 2001; Buckley et al., 2004; MacDonald and Leary, 2005; Leary et al., 2006; DeWall et al., 2009; Riva et al., 2011; for a review, see DeWall and Bushman, 2011).
Social rejection increases aggression both inside and outside the laboratory. In one laboratory experiment (Twenge et al., 2001), for example, participants were informed after a group interaction that nobody wanted to work with them (i.e. excluded) or that everybody wanted to work with them (i.e. included). Excluded participants behaved more aggressively against members of their group, which took the form of blasting them with unpleasant and prolonged noise. In an analysis of news reports involving 15 US school shooters (Leary et al., 2003), all but two of the shooters had been socially excluded, such as by a girlfriend or by peers. Social exclusion was a better predictor of school shootings than several other risk factors (e.g. evidence of a psychological disorder, interest in guns, bombs or explosives, fascination with death). The potential for exclusion to elicit such violence necessitates the understanding of neural mechanisms that are involved in regulating (or suppressing) aggressive responses that typically follow social rejection. What might suppress aggressive responses to social exclusion? The next section explores one possibility, namely activation in a brain region associated with regulation of negative emotions.
The regulatory function of right ventrolateral pre-frontal cortex
The right ventrolateral pre-frontal cortex (rVLPFC) is a potential candidate for a neural mechanism that may weaken the link between social exclusion and aggression. According to recent theories (Cohen et al., 2012), rVLPFC is the neural region commonly recruited across different forms of self-control. For example, the rVLPFC is involved in motor control (Chikazoe et al., 2009), risk-taking behavior (Ernst et al., 2002), control over immediate temptations (McClure et al., 2004) and emotional control (Kim and Hamann, 2007; see also Wager et al., 2008). More specifically, several brain imaging studies suggest that the rVLPFC might be directly involved in the regulation or suppression of negative emotions elicited by a wide array of stimuli (Lieberman et al., 2004; Ochsner and Gross, 2005; Wager et al., 2008; Berkman and Lieberman, 2009; Cohen et al., 2012). One study found that rVLPFC activity correlated with reduced negative emotional experience during reappraisal of aversive images (Wager et al., 2008). In that study, the degree of activity in rVLPFC and correlated with self-reported negative emotions, indicating not only that this region is active when people try to regulate their negative emotions but also that rVLPFC activity relates directly with the amount of negative emotions an individual is able to regulate (see also Cohen et al., 2012).
Focusing on neural responses to social exclusion, past research has shown that the rVLPFC is often activated when people experience threats to social belongingness, and serves also to inhibit the emotional distress of such a threatening event. Specifically, studies have found an inverse association between activation of rVLPFC and (i) activation of brain regions associated with distress elicited by social rejection [e.g. dorsal anterior cingulate cortex (dACC)] and (ii) self-reported social distress. These findings suggest that the rVLPFC might be involved in down-regulating the emotional distress caused by threats to social belongingness (Eisenberger et al., 2003, 2007; Onoda et al., 2009). Similarly, other studies have shown increased activation of the rVLPFC when social exclusion is accompanied by social support (Onoda et al., 2010). In addition, studies investigating individual differences in social pain perception have found that people low in rejection sensitivity display higher levels of rVLPFC activation when excluded than do people high in rejection sensitivity (Kross et al., 2007; Onoda et al., 2009). Therefore, in addition to being broadly involved in emotion regulation, the rVLPFC plays a key role in regulating the pain of social rejection.
What implications might these findings have for behaviors that often accompany social exclusion? One possibility is that the regulation of social pain by the rVLPFC may reduce subsequent aggression. Indeed, activation of the dACC during social rejection, a likely indicator of social pain, was associated with subsequent aggression but only when participants did not possess the executive ability to regulate the pain (Chester et al., 2013). Conversely, participants with greater regulatory abilities showed a negative association between dACC activation and aggression. These findings suggest that top-down, inhibitory functions may be able to break the link between social exclusion and aggression by negating the ability of social pain to translate into aggression. The rVLPFC might serve just such a regulatory or inhibitory role.
Neuromodulation via transcranial direct current stimulation
Transcranial direct current stimulation (tDCS) is a top-down modulatory approach that involves attaching two electrodes to the scalp and applying a weak electrical current from the positively charged cathode to the negatively charged anode. Although the neurophysiological mechanisms underlying the modulation effects of tDCS are not fully understood (Utz et al., 2010), there is evidence that anodal stimulation increases the excitability of cortical neurons beneath the electrode, whereas cathodal stimulation has an opposite effect (Nitsche and Paulus, 2000). This technique therefore allows researchers to modulate the pattern of brain responses instead of simply observing them, which allows for causal inferences to be made.
Using tDCS, we recently found a causal relationship between rVLPFC activity and pain regulation (Riva et al., 2012). We argue that given the regulatory function of rVLPFC during social exclusion, increasing the cortical excitability of this region might also reduce the typical aggressive response that often follows social exclusion. We hypothesized that if rVLPFC stimulation buffers against the hurt feelings associated with social rejection, then rVLPFC stimulation could also buffer against the aggressive behavior associated with social rejection.
Overview
In this study, we tested whether non-invasive brain polarization through anodal tDCS over the rVLPFC could decrease aggression resulting from social exclusion. Our hypothesis is based on prior research suggesting that the rVLPFC is involved in regulation of several domains, including—broadly—regulation of negative emotions (Lieberman et al., 2004; Ochsner and Gross, 2005; Wager et al., 2008; Berkman and Lieberman, 2009; Cohen et al., 2012) and—more specifically—regulation of negative emotions resulting from social exclusion (Eisenberger et al., 2003; Onoda et al., 2010, Riva et al., 2012).
We predicted that the increased cortical excitability resulting from anodal tDCS stimulation over rVLPFC could potentiate the regulatory function of this cortical region (Nitsche and Paulus, 2000), thus reducing people’s tendency to react aggressively after being socially excluded.
METHOD
Participants
Participants were 80 healthy university students (79% female; Mage = 23.06, s.d. = 4.36) who received 10€ ($13). Participants with a prior or existing history of neurological disease, psychiatric disorder, epilepsy, head injury or any communication impairment were excluded.
Procedure
A few weeks before the experiment proper, participants completed a measure of individual differences in trait anger on the internet (i.e. State-Trait Anger Scale; Spielberger and Sydeman, 1994). We included this measure to control for individual differences in aggressiveness.
In the experiment proper, participants were tested individually. They were told the researchers were studying the effect of brain stimulation on the relationship between mental visualization and taste testing. After informed consent was obtained, participants rated how much they liked five tastes (i.e. salty, sweet, bitter, hot and spicy and sour; 1 = not at all to 10 = extremely).
Next, participants were randomly assigned to receive either anodal tDCS or sham stimulation over the rVLPFC. Stimulation was applied using a constant current regulator via sponge-soaked electrodes (DC-STIMULATOR, NeuroConn GmbH, Germany). The 25 cm2 anodal electrode was placed over F6 (MNI coordinates: 58, 30, 8; Onoda et al., 2010), consistent with the international 10–20 system for electroencephalogram (EEG) electrode placement. The 35 cm2 reference (cathodal) electrode was placed over the contralateral supraorbital area. We used these electrode sizes and placements for three reasons. First, using two differently sized electrodes is the standard procedure to increase the current underneath the target electrode (Nitsche et al., 2008). Second, the small distance between the two electrodes should cause a large amount of current to be shunted through the scalp, thus further increasing the focality of the stimulation (Datta et al., 2008; Bikson et al., 2010). Third, the placement of the cathode over the supraorbital area should reduce the amount of electrical stimulation because the distance between the cortical surface and the scalp is increased, and because air is present in the sinus cavities.
A constant current of 1.5 mA intensity was applied for 20 min, leading to a current density of 0.06 mA/cm2 for the stimulation electrode and 0.04 mA/cm2 for the reference electrode. For sham stimulation, the electrodes were placed in the same position, but the stimulator was turned on for 30 s only (Gandiga et al., 2006) and the current intensity was gradually increased at the beginning of the session (8 s of ‘ramp up’) and decreased at the end of the session (5 s of ‘ramp down’) to mimic the itching sensation of the real stimulation. In the following 1157 s the stimulation was off but the monitor of the device kept showing the impedence control. Thus, all participants believed they received stimulation for 20 min.
Five minutes before the end of the tDCS or sham stimulation, participants played a virtual online ball-tossing game called ‘Cyberball’ (Williams et al., 2000). Participants were told that they would engage in a ball-throwing game with two other players, ostensibly real participants, for the purposes of exercising their mental visualization abilities. Participants were told that they should visualize all aspects of the game, the players and the location. In actuality, the two computer characters were pre-programmed agents randomly assigned to either include or exclude the real participant from the game. In the social exclusion condition, after a few throws, the two computer players stopped throwing the ball to the participant for the remainder of the game. In the inclusion condition, the computer players threw the ball to the actual participant for ∼10 of the 30 total tosses (Williams et al., 2000). As a manipulation check, participants were asked how often (0–100%) they received the ball.
Aggression was measured using the well-validated hot-sauce paradigm (Lieberman et al., 1999). The experimenter gave the participants a copy of the taste-test questionnaire with the taste preferences of one of the other Cyberball players, which contained the value ‘2’ for ‘Hot and Spicy’ foods. Participants were told that a random generator in the computer would assign them to allocate vs taste one of the foods listed. The experimenter pressed a button on the computer and the role that was ostensibly randomly assigned to the participant appeared on the screen. In reality, all the participants were assigned to allocate hot sauce to their ostensible partner by putting the amount they wanted their partner to taste in a plastic cup. They were told their partner had to eat the entire amount in the cup and they would not know who gave him or her the hot sauce. The amount of hot sauce allocated was weighed using a digital scale that was accurate to 0.10 g. Giving hot sauce to someone who does not like to eat spicy food is a face-valid measure of aggression that is easily quantifiable and ecologically valid (Lieberman et al., 1999). A debriefing followed. During the debriefing, participants were asked whether they perceived any physical sensation from the electrodes.
RESULTS
Preliminary analysis
Age and gender differences
A 2 (social inclusion vs exclusion) × 2 (anodal vs sham stimulation) between-subjects ANOVA found that excluded vs included participants did not differ in terms of age, F(1,74) = 1.23, P = 0.27. Similarly, the mean age of those who received anodal stimulation was not different from that of those who received sham stimulation, F(1,74) = 0.23, P = 0.64. Moreover, the number of males and females did not differ across experimental conditions, χ2(3) = 4.20, P = 0.24. Thus, the data from males and females were combined for subsequent analyses.
Trait anger
A 2 (social inclusion vs exclusion) × 2 (anodal vs sham stimulation) between-subjects ANOVA found that trait anger scores did not differ between excluded and included participants, F(1,74) = 1.29, P = 0.26. Similarly, trait anger scores did not differ between those who received anodal stimulation and sham stimulation, F(1,74) = 0.66, P = 0.42.
Physical sensation from electrodes
In line with previous research (Nitsche et al., 2008), we found that very few participants (i.e. 5 of 80, or 6%) reported experiencing physical sensation from the electrodes. Crucially, a Pearson chi-square test showed that self-reported physical sensation did not vary across experimental conditions, χ2(3) = 2.28, P = 0.51.
Exclusion manipulation check
A 2 (social inclusion vs exclusion) × 2 (anodal vs sham stimulation) between-subjects ANOVA found that excluded participants reported receiving fewer tosses (11.20%) than did included participants (28.82%), F(1,76) = 61.99, P < 0.001, d = 1.73. Crucially, percentage of throws was not affected by the tDCS manipulation [Interaction: F(1,75) = 0.23, P > 0.63, ηp2 = 0.00], suggesting that participants in the tDCS and sham stimulation groups were equally cognitively aware of their inclusionary status during the game.
Primary analyses
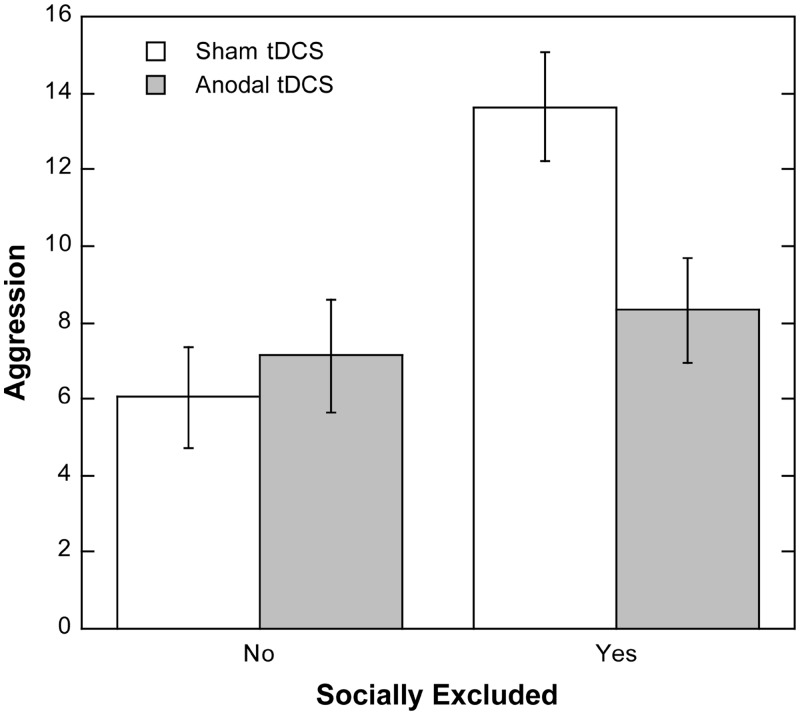
A 2 × 2 ANOVA found that excluded participants behaved more aggressively (M = 10.84, s.d. = 8.41) than did included participants (M = 6.53, s.d. = 3.52), F(1,76) = 9.87, P < 0.002, d = 0.72. This finding replicates numerous studies showing that social exclusion can increase aggression. Although it was in the predicted direction and not trivial in size, the main effect for stimulation type was not significant, F(1,76) = 2.30, P < 0.14, d = 0.35. Most important, the predicted interaction effect was significant, F(1,76) = 5.26, P < 0.025, ηp2 = 0.065. As can be seen in Figure 1, socially excluded participants given anodal stimulation over the rVLPFC were less aggressive than those given sham stimulation, F(1,76) = 7.28, P < 0.009, d = 0.62. Among socially included participants, no aggression differences emerged between the anodal and sham stimulation, F(1,76) = 0.30, P < .59, d = 0.12. Crucially, excluded participants who received anodal stimulation were no more aggressive than included participants, F(1,76) = 1.22, P < 0.28, d = 0.41 (see Figure 1).
Fig. 1.
Hot sauce allocation (in grams) for excluded and included participants given anodal or sham stimulation. Capped vertical bars denote 1 SE.
DISCUSSION
Domestic violence, school shootings and workers’ disruptive reactions following their discharge represent a few examples of the strong link between social rejection and aggressive behavior. Past research has shown that when people are rejected, ostracized or humiliated (e.g. when they are unable to fulfill the ‘need to belong,’; see Baumeister and Leary, 1995), they often behave aggressively against those who exclude them (Twenge et al., 2001; Buckley et al., 2004; MacDonald and Leary, 2005; Leary et al., 2006; DeWall et al., 2009; Riva et al., 2011), and they sometimes even aggress against innocent targets (e.g. Twenge et al., 2001; DeWall et al., 2010).
Previous research has also identified the rVLPFC as a critical region involved in emotion regulation (Lieberman et al., 2004; Ochsner and Gross, 2005; Wager et al., 2008; Berkman and Lieberman, 2009; Cohen et al., 2012). Furthermore, previous research has shown that anodal tDCS over the rVLPFC can decrease pain following social exclusion (Riva et al., 2012).
The present experiment sought to test the possible modulatory role of the rVLPFC on the link between social exclusion and aggression. We replicated the well-documented finding that social exclusion triggers behavioral aggression. Specifically, excluded participants gave a greater amount of hot sauce to an interaction partner who hated spicy foods than did included participants. More important, we found that increasing the cortical excitability of the rVLPFC reduced the relationship between social exclusion and aggression. Specifically, we found that those who were excluded but received tDCS stimulation over the rVLPFC were no more aggressive than included participants. Thus, aggression decreased when brain stimulation (vs sham stimulation) was applied over the rVLPFC following social exclusion.
The results of the present study thus provide evidence for the causal role of rVLPFC in emotion regulation. The type of montage used in the present study aimed at obtaining a stronger current underneath our target electrode. However ‘target’ and ‘reference’ are conventional terms for the electrodes, and we cannot rule out that a weaker neuromodulatory effect was present underneath the cathode. It is therefore possible that a cathodal-inhibitory effect over the left pre-frontal regions (lPFC) affected the observed results. Thus, future research should test whether decreasing the cortical excitability (through cathodal stimulation) of the lPFC is necessary to produce the behavioral effect we found.
To our knowledge, this is the first research to show that neural stimulation can disrupt the link between social exclusion and aggression. Our study supports and extends previous correlational research suggesting the modulatory role of rVLPFC in a variety of domains, including control over immediate temptations (McClure et al., 2004) and emotional control (Kim and Hamann, 2007; Wager et al., 2008). More generally, we showed how brain stimulation techniques (i.e. tDCS) have the potential to make a unique contribution to the field of social neuroscience applied to aggression research. Indeed, these techniques (e.g. tDCS, TMS) have an exclusive capacity—compared with brain imaging techniques—to modulate distinct components of the neural system and allow the measurement of observable behavioral changes.
Providing evidence for the causal role of the rVLPFC activity in modulating aggressive responses to social exclusion, our experiment contributes to the growing literature focused on understanding the neural and psychological underpinnings of the rejection–aggression link (Chester et al., 2013).
Limitations and future research
A possible limitation of tDCS is its low spatial resolution. However, tDCS effects largely come from the cortical area beneath the electrode (see Zaghi et al., 2010). Computer-based modeling studies show that the direct functional effects of tDCS are restricted to the area under the active electrode (Miranda et al., 2006). Nevertheless, future research should test for the feasibility of adopting other stimulation techniques, such as repetitive transcranial magnetic stimulation (rTMS) over the rVLPFC, thus providing more detailed information of the effect we found.
As to the underlying mechanism, rVLPFC stimulation is known to numb the pain of social rejection (Riva et al., 2012), and this might make rejected people less likely to aggressively lash out against others (see MacDonald and Leary, 2005). Another possibility is that stimulating the rVLPFC reduces the negative emotions people experience during social exclusion (see Riva et al., 2011). Therefore, future studies should test the possibility that the perception of social distress and/or negative emotions mediate the effect of tDCS on the link between social rejection and aggression.
We recognize that other mechanisms may play a role in the effect of tDCS stimulation on aggression. For instance, previous research has linked greater right than left frontal cortical activity to avoidance motivation or inhibition, whereas greater left than right frontal cortical activity has been linked to approach motivation (such as aggression; see Harmon-Jones et al., 2010). Accordingly, a recent study found that anodal stimulation over lPFC increased the anger–aggression link (Hortensius et al., 2012). It is thus possible that increasing the cortical excitability through anodal tDCS stimulation over right PFC counteracts the approach motivation that precipitates aggressive behavior. Therefore, we recognize that a challenge for future research is to identify potential mediating variables of the modulatory effect of tDCS on aggression.
CONCLUSION
The present research provides critical knowledge in understanding the role of a neural structure (i.e. the rVLPFC) that seems to be critically involved in regulating aggressive responses that typically follow social rejection. As Steinbeck insightfully observed in 1945, ‘there are two possible reactions to social ostracism—either a man emerges determined to be better, purer, and kindlier or he goes bad, challenges the world and does even worse things’. The present research shows that people are less likely go bad or do even worse things, such as aggress against others, following stimulation to the portion of the brain that regulates negative emotions and the pain of social rejection.
REFERENCES
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. Using neuroscience to broaden emotion regulation: theoretical and methodological considerations. Social and Personality Psychology Compass. 2009;3:475–93. doi: 10.1111/j.1751-9004.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Datta A, Rahman A, Scaturro J. Electrode montages for tDCS and weak transcranial electrical stimulation: role of “return” electrode’s position and size. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2010;121:1976. doi: 10.1016/j.clinph.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley KE, Winkel RE, Leary MR. Reactions to acceptance and rejection: effects of level and sequence of relational evaluation. Journal of Experimental Social Psychology. 2004;40:14–28. [Google Scholar]
- Chester DS, Eisenberger NI, Pond RS, Richman SB, Bushman BJ, DeWall CN. The interactive effect of social pain and executive functioning on aggression: an fMRI experiment. Social Cognitive and Affective Neuroscience. 2013;9:699–704. doi: 10.1093/scan/nst038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, et al. Functional dissociation in right inferiorfrontal cortex during performance of go/no-go task. Cerebral Cortex. 2009;19:146–52. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Berkman ET, Lieberman MD. Intentional and incidental self-control in ventrolateral PFC. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. 2013. 2nd edn, New York: Oxford University Press, pp. 417–40. [Google Scholar]
- Datta A, Elwassif M, Battaglia F, Bikson M. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. Journal of Neural Engineering. 2008;5:163. doi: 10.1088/1741-2560/5/2/007. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Bushman BJ. Social acceptance and rejection: the sweet and the bitter. Current Directions in Psychological Science. 2011;20:256–60. [Google Scholar]
- DeWall CN, Twenge JM, Bushman BJ, Im C, Williams KD. A little acceptance goes a long way: applying social impact theory to the rejection-aggression link. Social Psychological and Personality Science. 2010;1:168–74. [Google Scholar]
- DeWall CN, Twenge JM, Gitter SA, Baumeister RF. It's the thought that counts: the role of hostile cognition in shaping aggressive responses to social exclusion. Journal of Personality and Social Psychology. 2009;96:45. doi: 10.1037/a0013196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–12. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, et al. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–91. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology. 2006;117:845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hortensius R, Schutter DJ, Harmon-Jones E. When anger leads to aggression: induction of relative left frontal cortical activity with transcranial direct current stimulation increases the anger–aggression relationship. Social Cognitive and Affective Neuroscience. 2012;7:342–7. doi: 10.1093/scan/nsr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biological Psychology. 2010;84:451–62. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19:776–98. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. Journal of Cognitive Neuroscience. 2007;19:945–56. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- Leary MR, Kowalski RM, Smith L, Phillips S. Teasing, rejection, and violence: case studies of the school shootings. Aggressive Behavior. 2003;29:202–14. [Google Scholar]
- Leary MR, Twenge JM, Quinlivan E. Interpersonal rejection as a determinant of anger and aggression. Personality and Social Psychology Review. 2006;10:111–32. doi: 10.1207/s15327957pspr1002_2. [DOI] [PubMed] [Google Scholar]
- Lieberman JD, Solomon S, Greenberg J, McGregor HA. A hot new way to measure aggression: hot sauce allocation. Aggressive Behavior. 1999;25:331–48. [Google Scholar]
- Lieberman MD, Jarcho JM, Berman S, et al. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22:447–55. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin. 2005;131:202. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clinical Neurophysiology. 2006;117:1623–9. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimulation. 2008;1:206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology. 2000;527:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima KI, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience. 2009;4:443–54. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, et al. Does low self-esteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Social Cognitive and Affective Neuroscience. 2010;5:385–91. doi: 10.1093/scan/nsq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva P, Romero Lauro LJR, DeWall CN, Bushman BJ. Buffer the pain away stimulating the right ventrolateral prefrontal cortex reduces pain following social exclusion. Psychological Science. 2012;23:1473–5. doi: 10.1177/0956797612450894. [DOI] [PubMed] [Google Scholar]
- Riva P, Wirth JH, Williams KD. The consequences of pain: the social and physical pain overlap on psychological responses. European Journal of Social Psychology. 2011;41:681–7. [Google Scholar]
- Spielberger CD, Sydeman SJ. State-trait anxiety inventory and state-trait anger expression inventory. In: Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcome Assessment. Hillsdale, NJ: Lawrence Erlbaum Associates; 1994. pp. 292–321. [Google Scholar]
- Twenge JM, Baumeister RF, Tice DM, Stucke TS. If you can’t join them, eat them: effects of social exclusion on aggressive behavior. Journal of Personality and Social Psychology. 2001;81:1058–69. doi: 10.1037//0022-3514.81.6.1058. [DOI] [PubMed] [Google Scholar]
- Utz KS, Dimova V, Oppenländer K, Kerkhoff G. Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology—a review of current data and future implications. Neuropsychologia. 2010;48:2789–10. doi: 10.1016/j.neuropsychologia.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD, Cheung CK, Choi W. Cyberostracism: effects of being ignored over the internet. Journal of Personality and Social Psychology. 2000;79:748. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Zaghi S, Acar M, Hultgren B, Boggio PS, Fregni F. Noninvasive brain stimulation with low-intensity electrical currents: putative mechanisms of action for direct and alternating current stimulation. The Neuroscientist. 2010;16:285–307. doi: 10.1177/1073858409336227. [DOI] [PubMed] [Google Scholar]