Abstract
Stressful life events increase vulnerability to problematic alcohol use, and they may do this by disrupting reward-related neural circuitry. This is particularly relevant for adolescents because alcohol use rises sharply after mid-adolescence and alcohol abuse peaks at age 20. Adolescents also report more stressors compared with children, and neural reward circuitry may be especially vulnerable to stressors during adolescence because of prefrontal cortex remodeling. Using a large sample of male participants in a longitudinal functional magnetic resonance imaging study (N = 157), we evaluated whether cumulative stressful life events between the ages of 15 and 18 were associated with reward-related brain function and problematic alcohol use at age 20 years. Higher cumulative stressful life events during adolescence were associated with decreased response in the medial prefrontal cortex (mPFC) during monetary reward anticipation and following the receipt of monetary rewards. Stress-related decreases in mPFC response during reward anticipation and following rewarding outcomes were associated with the severity of alcohol dependence. Furthermore, mPFC response mediated the association between stressful life events and later symptoms of alcohol dependence. These data are consistent with neurobiological models of addiction that propose that stressors during adolescence increase risk for problematic alcohol use by disrupting reward circuit function.
Keywords: stressful life events, reward, fMRI, adolescence, alcohol dependence
Alcohol use disorders are a leading cause of global disease burden, with 1.9% of individuals worldwide experiencing alcohol use-related disability at any given time (World Health Organization, 2008). Problematic alcohol use is especially common in men, who are more than twice as likely as women to experience substance abuse or dependence (Kessler et al., 2005; Hasin et al., 2007). Furthermore, of the individuals who meet criteria for alcohol use disorders during their lifetime, half experience their first episode of alcohol abuse or dependence between mid-adolescence and the mid-twenties (Kessler et al., 2005). The hazard rate for alcohol abuse or dependence peaks at age 20 (Hasin et al., 2007). This makes late adolescence a key developmental phase to examine risk factors and mechanisms of problematic drinking.
Stress is a consistent predictor of increased alcohol use and alcohol use disorders, and stressors during adolescence may be particularly detrimental. Prospective data in adolescents and young adults demonstrate that stressful life events predict increased alcohol use (Newcomb and Harlow, 1986; Wills, 1986; Wills et al., 1996). Epidemiological data indicate that exposure to adversity before the age of 18 is associated with the incidence and persistence of alcohol use disorders (Lloyd and Turner, 2008; Green et al., 2010; McLaughlin et al., 2010). Complementing these data, studies in animals indicate that early-life stressors, such as maternal separation or foot shock, result in increased alcohol consumption (Sinha, 2001). Furthermore, research in both humans (Larson and Ham, 1993; Ge et al., 1994) and rodents (Spear, 2000; Lupien et al., 2009) indicates that adolescents are more susceptible to the effects of stressors than children or adults. Notably, common adolescent stressors, such as trouble at school or with parents, may have a greater impact on emotional functioning than more distal major events (Rowlison and Felner, 1988; Compas et al., 1989). Although these studies demonstrate that stressful life events during adolescence, especially the accumulation of common stressors, may increase risk for alcohol use and alcohol use disorders, the neural mechanisms linking stress to problematic substance use are not adequately understood.
One mechanism by which stress may confer vulnerability to problematic alcohol use is by disrupting neural response to rewards. In their prominent neurobiological model of addiction, Koob and Le Moul (1997, 2005) propose that stressful life events decrease neural response to normally rewarding cues, such as monetary or social rewards, and thereby sensitize individuals to the reinforcing properties of drugs. Complimenting this model, neurodevelopmental theories of psychopathology posit that reward systems are especially vulnerable to the effects of stressors during childhood and adolescence as the brain continues to mature (Forbes and Dahl, 2005; Nelson et al., 2005; Andersen and Teicher, 2008; Davey et al., 2008; Arnsten and Rubia, 2012; Spear, 2013). During adolescence, stressors may differentially impact reward function in late-developing regions of the prefrontal cortex (PFC), particularly the dorsomedial and orbitofrontal PFC, which go through a period of rapid neurodevelopment between the early teens and the mid-twenties (Gogtay et al., 2004; Tamnes et al., 2010; Mills et al., 2012). These regions of the PFC are involved in higher-order executive functions (e.g. incentive appraisal, goal representation, emotion regulation, evaluation of social cues, self-reflection; Frith and Frith, 2001; Mitchell et al., 2005; Etkin et al., 2011).
Because of inhibitory projections from the PFC to subcortical regions, stress-related disruption of medial and orbitofrontal PFC function may result in disinhibition of stimulus–reward associations—a core function of the amygdala (Baxter and Murray, 2002)—and incentive salience or reward ‘wanting’—a core function of the ventral striatum (Berridge and Robinson, 1998). In the context of alcohol use, heightened stimulus–reward associations and motivation to pursue rewards may promote stronger associations between alcohol-related cues and hedonia, as well as increased alcohol-directed behavior (Koob and Le Moal, 1997; 2005). Consistent with this model, research in adolescent male rats found that repeated social defeat—a potent stressor—decreases dopamine activity in the medial PFC (mPFC) and heightens drug-seeking behavior (Burke et al., 2012). The increase in stressors and stress reactivity coupled with PFC plasticity during adolescence may help explain the coincident uptick in substance use disorders during this period.
Research linking psychosocial stress to disruptions in brain reward circuitry and problematic alcohol use is limited. However, neuroimaging studies have explored the relationship between stress and brain reward function. For example, response to monetary rewards in the mPFC, orbitofrontal cortex (OFC) and ventral striatum is disrupted by acute laboratory stressors such as watching aversive films or submerging one’s hand in ice water (Ossewaarde et al., 2011; Porcelli et al., 2012). There are also two neuroimaging studies that report a link between early-life stressors and brain reward function: Dillon et al. (2009) found that adults with a history of childhood maltreatment had decreased responses to monetary rewards in the globus pallidus compared with control participants; Mehta et al. (2010) found that adolescent adoptees who experienced global deprivation in Romanian institutions during early childhood had decreased striatal response during anticipation of monetary rewards compared with non-institutionalized non-adopted adolescents. There is also evidence that alcohol use disorders are associated with disrupted reward circuit function: Wrase et al. (2007) demonstrated that alcoholics relative to healthy individuals had decreased neural response during anticipation of monetary rewards in the ventral striatum, but increased striatal response to alcohol-related cues. These studies provide preliminary evidence for the relationship between stressful life events, reward circuit function and addiction. However, additional research is needed to evaluate whether reward circuit disruption is a mechanism by which adolescent life stress increases risk for problematic alcohol use.
The present study examines the association between cumulative stressful life events during late adolescence and brain reward function and problematic alcohol use at age 20 in a large community sample of young men. Common adolescent stressors (e.g. arguments with a family member, academic or social problems) were assessed annually from ages 15 through 18. At age 20, participants completed an assessment of problematic alcohol use and underwent functional magnetic resonance imaging (fMRI) during a monetary reward task to assess neural response during reward anticipation and following rewarding outcomes. We anticipated that (i) higher levels of cumulative life stress during adolescence would be associated with decreased mPFC, OFC, ventral striatum and amygdala response during reward anticipation at age 20, (ii) stress-related decreases in neural response during reward anticipation would also be associated with higher levels of problematic alcohol use at age 20 and (iii) neural response to reward would mediate the association between stressful life events and problematic alcohol use. We expected stressful life events and alcohol dependence to be more strongly associated with neural response during reward anticipation than following rewarding outcomes because neurobiological models of problematic substance use (e.g. Koob and Le Moal, 1997) and previous studies linking stress to reward circuit function (e.g. Mehta et al. 2010) have focused on reward anticipation, or ‘wanting’, rather than response to rewarding outcomes, or ‘liking’.
METHOD
Participants
Participants were 186 boys from the Pittsburgh Mother and Child Project (PMCP), an ongoing study of child risk and resilience for psychopathology (Shaw et al., 2003). The original PMCP sample included 310 low-income families with infant boys. Families were recruited from a government nutrition program in the Pittsburgh metropolitan area. Child and family assessments were initially completed when the boys were 12 or 18 months old and follow-up assessments were conducted at ages 2, 3.5, 5, 5.5, 6, 8, 10, 11, 12, 15, 16, 17, 18 and 20 years. Mean per capita family income at the initial assessment was $12 565 per year (s.d. = $7690) with a mean Hollingshead (1975) socioeconomic status of 23.32 (s.d. = 9.29), indicating that the sample was predominantly working class. The informed consent process conformed with the Declaration of Helsinki and university research review committee approval and oversight.
At age 20, the sample retention rate was 83% (N = 258), and 186 participants were eligible and able to complete the fMRI scan. Of these young men, 29 were excluded from fMRI analyses for this study. Reasons for exclusion included failure to complete any adolescent life stress assessments (n = 8), <80% response rate on the reward task (n = 6), not understanding the reward task (e.g. consistently responding at inappropriate times; n = 4), <80% ventral striatum coverage (n = 5), poor-quality scan (n = 1), psychosis (n = 1), severe autism (n = 1) and marijuana use immediately before the scan (n = 2). This left data from 157 participants with stressful life events available for analyses. Of these 157 participants, five did not complete the problematic alcohol use assessment. Therefore, analyses relating neural response to reward to problematic alcohol use were based on 152 participants.
Questionnaires
Adolescent stressful life events were assessed when participants were 15, 16, 17 and 18 years of age. Participants rated the 1 year incidence (0 or 1) and distress (0, 1 or 2) associated with 30 common negative events from two life event inventories: the Life Event Questionnaire for Adolescents (LEQ; Masten et al., 1994) and the Interpersonal Problem Situations Inventory for Urban Adolescents (IPSI; Farrell et al.,1998). The LEQ assesses common adolescent stressors such as injury or death in the family, poor performance in school, difficult financial circumstances and arguments with family members. The IPSI measures perceived injustice from adults and bullying from peers. Items from the LEQ and IPSI are non-overlapping. A cumulative stressful life event score was calculated by summing the LEQ and IPSI incidence and distress ratings across ages 15 through 18 years. Cumulative scores could range from 0 to 468, with higher scores indicating more stressful life events.
Alcohol dependence was assessed when participants were 20 years of age using the Alcohol Dependence Scale (ADS; Skinner and Allen, 1982). The ADS includes 25 items that assess alcohol-related problems (e.g. withdrawal, tolerance, compulsive drinking) over the past 6 months. Each item is scored ‘0’ for ‘no’ and ‘1’ for ‘yes’. Total scores range from 0 to 25, with higher scores indicating more severe symptoms.
Tobacco use, childhood neighborhood disadvantage and caregiver education were also assessed and included as covariates in all regression analyses. Tobacco use was included as a covariate because it is associated with decreased reward-circuit function (Rose et al., 2013). It was assessed at age 20 using a single item from the Alcohol and Drug Consumption Questionnaire (Cahalan et al.,1969). Neighborhood disadvantage was included as a covariate to determine whether life stress would predict reward-related blood-oxygen-level-dependent (BOLD) response after controlling for childhood demographic factors that can increase exposure to adversity (Vanderbilt-Adriance and Shaw, 2008). Childhood neighborhood disadvantage was ascertained using PMCP demographic data collected at ages 17 months through 15 years, as described by Vanderbilt-Adriance and Shaw (2008), and mean neighborhood disadvantage across all assessment points was used for analysis. Maternal education in years when the participants were 17 was included as a covariate to control for socioeconomic status. In addition, past-year stressful life events were assessed at age 20 using the Life Event Survey (LES; Sarason et al., 1978). Scores on the LES were used as a covariate in secondary analyses to determine whether the effects of adolescent life stress on neural response to reward were sustained when accounting for life stress at the time of the scan.
Scores on LEQ-IPSI, ADS and LES were square-root transformed before analysis to approximate a normal distribution. Additional description of the measures, including methods for imputation of missing data and alpha coefficients for each measure, is provided as Supplementary Material.
Reward task
Participants performed a reward-guessing task during fMRI acquisition. This task was designed to index brain activation during anticipation of monetary incentives and following feedback about monetary gain and loss. Previous studies show that this task reliably elicits activation in neural reward circuitry (Forbes et al., 2009, 2010). Participants were instructed to guess whether the value of a visually presented card, with possible value from 1 to 9, would be greater than or less than 5. Each trial began with the presentation of a blank card. Participants had 4 s to guess the value of the card via button press. The type of trial (gain or loss) was then displayed for 6 s using an image with hands shuffling cards with an upward facing yellow arrow to indicate potential reward trials and a downward facing yellow arrow to indicate potential loss trials. This incentive anticipation interval was followed by an outcome interval, in which the ‘actual’ value of the card was displayed for 500 ms, feedback on the trial outcome was displayed for 500 ms (a green upward-facing arrow for win or a yellow circle for a no-change outcome on win trials, a red downward-facing arrow for loss or a yellow circle for a no-change outcome on loss trials), and then a crosshair was displayed for 9 s. The final 3 s of the crosshair served as a baseline condition. There were 24 trials, 20 s each administered over a single 8 min run. Trials were presented in pseudorandom order, and outcomes were predetermined with a balanced number of trial types (12 possible win, 12 possible loss; 6 win, 6 loss and 12 no-change outcomes). Analyses focused on the 12 possible win trials and the 6 win outcomes. Based on the effect sizes in a previous study (Casement et al., 2014), this number of trials had >95% power to elicit a significant BOLD response in each of our regions of interest. Participants were told that they would receive their winnings after the scan ($1 per win outcome and 50 cents per loss); in fact, all participants received $10.
Magnetic resonance imaging acquisition, processing and analysis
Neuroimaging was conducted on a Siemens 3.0 Tesla TIM Trio scanner. BOLD functional images were acquired using a gradient echo planar imaging (EPI) sequence that included 39 axial slices (3.1 mm wide) beginning at the cerebral vertex and extending across the entire cerebrum and most of the cerebellum (repetition time (TR)/echo time (TE) = 2000/28 ms, field of view (FOV) = 20 cm, matrix = 64 × 64). A reference EPI scan was acquired before fMRI data collection to visually inspect for artifacts (e.g. ghosting) and ensure adequate signal across the entire volume. In addition, a 160-slice high-resolution sagittally acquired T1-weighted anatomical image was collected for coregistration and normalization of functional images (TR/TE = 2300/2.98 ms, FOV = 20 cm, matrix 256 × 240). Preprocessing and analysis of imaging data were conducted using Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Preprocessing included segmentation of the anatomical scan and functional image realignment, coregistration, normalization and smoothing (details in Supplementary Material).
Second-level random effects models were used to estimate reward responsivity while accounting for scan-to-scan and between-participant variability. For each participant, condition effects were calculated at each voxel using t-tests for two contrasts: reward anticipation > baseline and reward outcome > baseline. Reward anticipation was defined as the 12 potential-win intervals after the guessing condition (6 s each). Reward outcome was defined as the intervals that included number presentation, arrow feedback and the first 6 s of the crosshair during the six win-outcome trials (7 s each). The last 3 s of all 24 trials served as the baseline condition. Analysis of imaging data focused on four pre-specified regions of interest (ROIs): the mPFC, striatum, OFC and amygdala. AlphaSim (http://afni.nimh.nih.gov/afni/) cluster extent thresholds were calculated a priori to determine the minimum cluster size necessary to maintain a corrected P < 0.05 across all four ROIs. Further description of each ROI and the AlphaSim thresholds is provided as Supplementary Material.
Regression analyses were performed in SPM to determine whether cumulative negative life events were associated with neural response during reward anticipation and reward outcome across participants. All regression analyses included a dichotomous index of tobacco use (daily, <daily), mean neighborhood disadvantage and caregiver education level (in years) as covariates. Additional regression analyses were conducted in SPM with LES scores included as a fourth covariate. Unstandardized regression coefficients, standard errors (SE), and confidence intervals (CI) were computed in SPSS. To accomplish this, functional masks from SPM regressions of reward response on cumulative life stress were saved and used as functional ROIs for t-tests of BOLD response during reward anticipation and reward outcome. Beta values for the average BOLD response within each functional mask were extracted using the ‘eigenvariate’ tool in SPM, and regressions were performed in SPSS using the covariates described above.
To link disruptions in neural response to reward to problematic alcohol use, conjunction analysis (Nichols et al., 2005; see Supplementary Material) was used to determine whether ADS scores were associated with reward anticipation and reward outcome in regions that were also associated with adolescent life stress. ADS scores were individually entered as predictors of reward anticipation response and reward outcome response. Results were masked for regions in which there was a significant relationship between cumulative stressful life events and reward-related BOLD response. Conjunction analyses used the same covariates as regressions of reward response on cumulative negative life events.
Finally, for each region that was significantly associated with both stressful life events and problematic alcohol use, mediation analyses were used to examine whether neural response during reward anticipation or reward outcome accounted for a significant portion of the association between stressful life events and ADS scores. To accomplish this, a second set of functional masks was created, based on significant clusters yielded by regressions of BOLD response on problematic alcohol use. These functional masks were saved and used as functional ROIs for t-tests of BOLD response during reward anticipation and reward outcome. Average BOLD response beta values across each significant cluster were extracted from these results and tested as a mediator of the relationship between stressful life events and problematic alcohol use in SPSS using the same covariates that were applied in SPM. Mediation analyses were implemented using the bootstrap method with the SPSS PROCESS macro (Hayes, 2013; see Supplementary Material for further description).
RESULTS
Participant characteristics
Table 1 describes the characteristics of the sample when they underwent neuroimaging at age 20. Two-tailed Pearson’s correlation indicated that higher levels of cumulative stressful life events during adolescence were associated with more problematic alcohol use at age 20 (r = 0.18, P = 0.03). The association between cumulative stressful life events and problematic alcohol use was not significant when adjusting for stressful life events at age 20 (r = 0.09, P = 0.26). Two-tailed Pearson’s correlation indicated that higher levels of stressful life events at age 20 were associated with more problematic alcohol use (r = 0.25, P = 0.002).
Table 1.
Participant characteristics at time of scan (N = 157)
| Characteristic | Percentage |
|---|---|
| Race | |
| Black | 37 |
| White | 54 |
| Other or mixed race | 9 |
| Highest level of school completed | |
| Below grade 12 | 11 |
| Grade 12 or GED | 50 |
| Some college | 36 |
| Associate’s degree or trade school | 3 |
| Primary caregiver’s highest level of school completed | |
| Below grade 12 | 4 |
| Grade 12 or GED | 34 |
| Some college or trade school | 50 |
| Completed 4 years of college | 9 |
| Completed graduate or professional school | 3 |
| ADS score > 8 | 17 |
| Mean (s.d.) | |
| Age | 19.52 (0.51) |
| Age of first significant alcohol use (≥10×/year) | 16.84 (1.56) |
| Stressful life events score, ages 15-18 | 43.69 (26.44) |
| Age 15 | 11.16 (7.79) |
| Age 16 | 9.93 (7.65) |
| Age 17 | 13.93 (10.59) |
| Age 18 | 8.67 (6.40) |
| LES score, age 20 | 8.82 (9.51) |
| ADS score | 4.91 (4.39) |
| Mean neighborhood disadvantage | 0.20 (0.67) |
Note: Scores above 8 on the ADS indicate likely alcohol dependence. Scores above 0 for mean neighborhood disadvantage indicate moderate to high risk. GED, General Educational Development test of high-school level academic skills.
Reward-related BOLD response
Whole-brain and ROI results for within-subject t-tests of BOLD response during reward anticipation (reward anticipation > baseline) and reward outcome (reward outcome > baseline) are available as Supplementary Data (Supplementary Tables S1 and S2). These results confirm that all four regions of interest (i.e. mPFC, ventral striatum, OFC and amygdala; see Supplementary Material) had increased response during reward anticipation and reward outcome compared with baseline. Whole-brain results are presented to describe regions that were responsive to reward outside of our pre-defined ROIs (e.g. occipital and parietal cortex).
Association between stressful life events and reward-related BOLD response
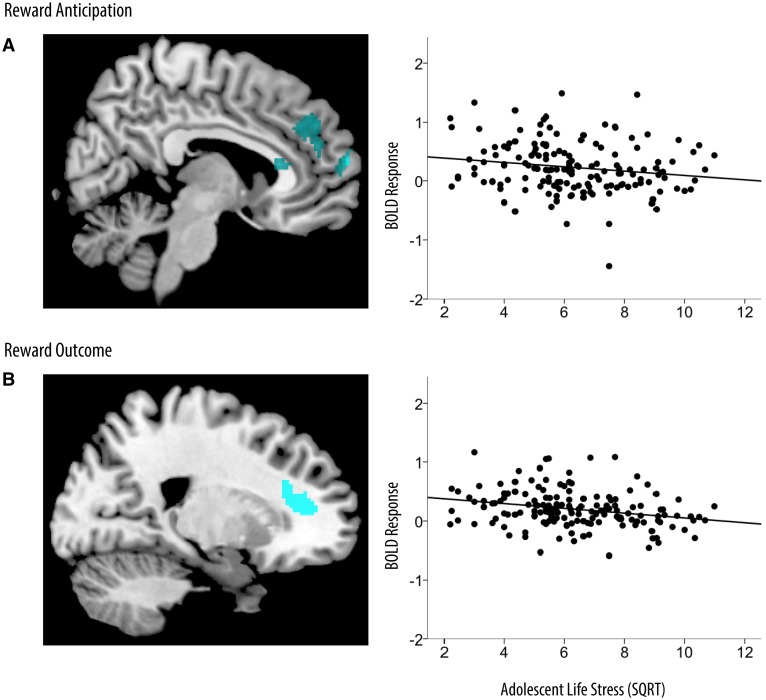
During reward anticipation, higher cumulative life stress during adolescence was associated with reduced response in the mPFC at age 20 (b = −0.04, SE b = 0.02, 95% CI: −0.08, −0.01, P = 0.01; Table 2; Figure 1). This association was maintained when stressful life events at age 20 were accounted for (b = −0.04, SE b = 0.02, 95% CI: −0.07, −0.01, P = 0.01). Following rewarding outcomes, higher cumulative life stress was associated with reduced response in the mPFC (b = −0.05, SE b = 0.01, 95% CI: −0.08, −0.03, P < 0.001; Table 2; Figure 1). This association was not statistically significant when stressful life events at age 20 were accounted for.
Table 2.
Cumulative stressful life events as a predictor of reward-related BOLD response
| Condition | MNI coordinates |
Cluster size | t (df = 152) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Reward anticipation | |||||
| mPFC (BA 10) | 6 | 64 | 14 | 369 | 3.53 |
| mPFC (BAs 24, 32) | −14 | 34 | 38 | 1117 | 3.17 |
| Reward outcome | |||||
| mPFC (BAs 24, 32) | 20 | 34 | 10 | 261 | 3.67 |
| With past-year negative life events included as a covariate (df = 151) | |||||
| Reward anticipation | |||||
| mPFC (BAs 10, 32) | −14 | 32 | 38 | 671 | 3.38 |
Note: ‘Tobacco use’, ‘neighborhood disadvantage’ and ‘caregiver education’ were included in these analyses as covariates. Alpha Sim corrected P < 0.05 for all contrasts. BA, Brodmann Area; BOLD, blood-oxygen-level-dependent; MNI, Montreal Neurological Institute.
Fig. 1.
Cumulative stressful life events as a predictor of reward-related BOLD response. Brain images and scatterplots depict significant associations between stressful life events and BOLD response in the mPFC during reward anticipation (A) and reward outcome (B). Scatterplots depict average BOLD response across clusters in the mPFC during reward anticipation (MNI coordinates: 6, 64, 14; R2 = −0.21) and reward outcome (MNI coordinates: 20, 34, 10; R2 = −0.34). SQRT, square-root transformation.
Association between reward-related BOLD response and severity of problematic alcohol use in regions that were associated with stressful life events
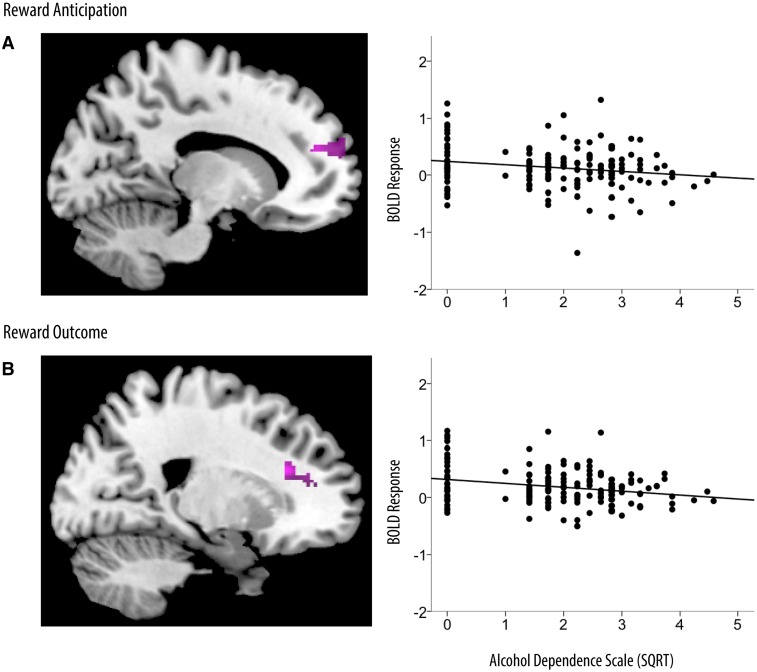
During reward anticipation, higher levels of problematic alcohol use were associated with reduced response in the mPFC (b = −0.07, SE b = 0.03, 95% CI: −0.12, −0.02, P = 0.009; Table 3; Figure 2). This association was maintained after adjusting for cumulative stressful life events (b = −0.55, SE b = 0.25, 95% CI: −1.06, −0.06, P = 0.03), stressful life events at age 20 (b = −0.07, SE b = 0.03, 95% CI: −0.12, −0.01, P = 0.02) and both cumulative stressors and stressful life events at age 20 (b = −0.54, SE b = 0.24, 95% CI: −1.01, −0.07, P = 0.03). Following rewarding outcomes, higher levels of alcohol dependence were also associated with reduced response in the mPFC (b = −0.09, SE b = 0.02, 95% CI: −0.14, −0.05, P < 0.001; Table 3; Figure 2). This association was maintained after adjusting for cumulative stressful life events (b = −0.83, SE b = 0.29, 95% CI: −1.40, −0.26, P = 0.005).
Table 3.
Alcohol dependence at age 20 as a predictor of reward-related BOLD response
| Condition | MNI Coordinates |
Cluster size | t (df = 147) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Reward Anticipation | |||||
| mPFC (BA 32) | 16 | 48 | 20 | 142 | 3.71 |
| mPFC (BA 10) | −14 | 46 | 20 | 236 | 3.13 |
| mPFC (BA 24) | 0 | 28 | 16 | 86 | 3.08 |
| mPFC (BA 32) | −6 | 46 | 20 | 84 | 2.79 |
| Reward outcome | |||||
| mPFC (BA 32) | 20 | 28 | 22 | 129 | 4.62 |
| With past-year negative life events included as a covariate (df = 146) | |||||
| Reward anticipation | |||||
| mPFC (BA 32) | 12 | 48 | 22 | 66 | 3.12 |
| mPFC (BAs 10, 32) | −8 | 48 | 22 | 85 | 3.06 |
Note: Alpha Sim corrected P < 0.05 for all contrasts. Results were masked for regions in which there was a significant relationship between cumulative stressful life events and reward-related BOLD response. ‘Tobacco use’, ‘neighborhood disadvantage’ and ‘caregiver education’ were included in these analyses as covariates. BA, Brodmann Area; BOLD, blood-oxygen-level-dependent; MNI, Montreal Neurological Institute.
Fig. 2.
Alcohol dependence as a predictor of reward-related BOLD response. Results were masked for regions in which there was a significant relationship between stressful life events and reward-related BOLD response. Brain images and scatterplots depict significant associations between alcohol dependence and BOLD response in the mPFC during reward anticipation (A) and reward outcome (B). Scatterplots depict average BOLD response across clusters in the mPFC during reward anticipation (MNI coordinates: 16, 48, 20; R2 = −0.23) and reward outcome (MNI coordinates: 20, 28, 22; R2 = −0.33). SQRT, square-root transformation.
Bootstrap tests of mediation indicated that BOLD response in the mPFC during reward anticipation (b = 0.02, SE b = 0.01, 95% CI: 0.002, 0.06, P < 0.05) and following rewarding outcomes (b = 0.04, SE b = 0.02, 95% CI: 0.009, 0.08, P < 0.05) significantly mediated the association between cumulative stressful life events and problematic alcohol use. The direct effect of cumulative stressful life events on problematic alcohol use was no longer significant when the model included mPFC response during reward anticipation (b = 0.07, SE b = 0.05, 95% CI: −0.02, 0.16, P = 0.15) or following rewarding outcomes (b = 0.05, SE b = 0.05, 95% CI: −0.04, 0.15, P = 0.25).
Neural response during reward anticipation remained a significant mediator of the relationship between cumulative adolescent stressful life events and problematic alcohol use when past-year stressful life events were accounted for (b = 0.03, SE b = 0.01, 95% CI: 0.004, 0.063, P < 0.05). The direct effect of cumulative stressful life events on problematic alcohol use was no longer significant when mPFC response during reward anticipation was included in the model and past-year stress was included as a covariate (b = 0.02, SE b = 0.05, 95% CI: −0.08, 0.12, P = 0.65).
DISCUSSION
This study provides the first evidence that cumulative life stress during late adolescence is associated with blunted mPFC response to reward in early adult men. Previous neuroimaging research showed that acute laboratory stressors were associated with decreased neural response in the mPFC (Ossewaarde et al., 2011), and early childhood maltreatment and deprivation were associated with decreased neural response in the striatum (Dillon et al., 2009; Mehta et al., 2010), during anticipation of monetary rewards. The current study indicates that very common adolescent stressors, such as arguments with family or social and academic problems in school, are also associated with decreased neural response to reward in the mPFC during reward anticipation and following rewarding outcomes. This was true even when neighborhood disadvantage and caregiver education were accounted for, suggesting that life stress during late adolescence independently contributes to reward-related brain function and cannot be simply attributed to neighborhood demographics or family socioeconomic status in childhood. The association between cumulative stressful life events and mPFC response during reward anticipation was also maintained after adjusting for concurrent life stress at the time of the scan. Previous studies have found significant associations between stressful life events and neural response during reward anticipation but not following rewarding outcomes (i.e. Dillon et al., 2009; Mehta et al., 2010; Ossewaarde et al., 2011). In combination, these results suggest that stressors may have a larger effect on circuits that support reward anticipation than circuits that support evaluation of received reward.
Our data are consistent with the hypothesis that stress-related alterations in brain reward function may be causally related to problematic alcohol use. Blunted mPFC function during reward anticipation and following rewarding outcomes was associated with more symptoms of alcohol dependence. Furthermore, mPFC response to rewards statistically mediated the relationship between stressful life events and problematic alcohol use. One interpretation of these results is that cumulative life stress during adolescence may dampen neural control mechanisms that help regulate alcohol-motivated behavior. Another possibility is that cumulative stress during adolescence diminishes mPFC response to normally rewarding events and thereby enhances the perceived benefits of alcohol use. Either interpretation is consistent with Koob and Le Moul’s (1997) proposal that stressors progressively result in a dysregulated reward response system and sensitize individuals to the hedonic properties of addictive drugs. In fact, Koob and Le Moul (2005) specifically implicated hypoactivation of the PFC in response to persistent aversive states (such as stressors or drug withdrawal) as a vulnerability for negative reinforcement from drug use.
Adolescent stressful life events were not associated with neural response to reward in the striatum, OFC or amygdala. In contrast, Dillon et al. (2009) found associations between childhood maltreatment and decreased reward responsiveness in the putamen and globus pallidus in young adults, and Mehta et al. (2010) found associations between global deprivation during early childhood and decreased striatal response to monetary rewards in mid-adolescence. Neither Dillon et al. or Mehta et al. found associations between early life stress and reward-related mPFC function. Neurodevelopmental models of psychopathology posit that the PFC, particularly the dorsomedial PFC, is especially vulnerable to the effects of stressors during adolescence relative to childhood or later adulthood because early adolescence through the mid-twenties is a period of dramatic PFC maturation (Forbes and Dahl, 2005; Nelson et al., 2005; Andersen and Teicher, 2008; Davey et al., 2008; Arnsten and Rubia, 2012; Spear, 2013). Furthermore, structural neuroimaging studies in humans (Andersen et al., 2008) and experimental studies in animals (Leussis et al., 2008) indicate that adolescent stressors affect PFC volume but not the volume of earlier-maturing regions (e.g. hippocampus, caudate). Collectively, these studies suggest that the developmental timing of stressors may be an important moderator of reward circuit function. However, between-study differences in stress–reward associations could also reflect the influence of different types of stressors (e.g. maltreatment vs common stressful life events), samples (samples of mixed-gender and socioeconomic status vs men from predominantly low-income families) or reward tasks (monetary incentive delay task vs monetary guessing task). Comparisons of different types of stressors or reward tasks within a sample, or of the same types of stressors and reward tasks at different developmental periods, could further delineate the neural circuitry and stages of neurodevelopment involved in stress-related brain reward function.
Further research in adolescent samples could clarify the relationship between stress-related reward circuit function and problematic use of alcohol and other drugs (e.g. tobacco, marijuana). For example, because a mediational model assumes that stressful life events precede and predict reward circuit dysfunction, and reward circuit dysfunction precedes and predicts alcohol dependence, additional longitudinal data are needed to conduct a true test of mediation and to elucidate the roles of these factors in the development of alcohol use problems over time. We cannot rule out the possibility that blunted reward circuit function predated our assessment of stressful life events from ages 15 through 18. However, given that the average age of first significant alcohol use (>10×/year) was 16.84, almost 3 years into the four annual assessments of stressful life events, stressful life events likely preceded the onset of alcohol dependence for the majority of our sample. Moreover, experimental studies in humans (Ossewaarde et al., 2011; Porcelli et al., 2012) and animals (Sinha, 2001; Pryce et al., 2005) indicate that stressful life events can play a causal role in brain reward function. We would hypothesize, given our pattern of findings, that the influence of stressful life events on neural reward circuitry at sensitive points in brain development leads to a cascade of events involving the neural response to alcohol and other drugs, initiating the process of addiction in those who are vulnerable.
Another limitation of the present study is the relatively brief interval between conditions in the Reward Guessing Task. BOLD response to visual stimuli peaks roughly 5 s after stimulus onset and takes a similar length of time to return to baseline (Huettel and McCarthy, 2000). Furthermore, a 6 s interval between conditions only allows for 90% of expected signal to the second condition (e.g. the reward outcome condition in our experiment; Huettel and McCarthy, 2000). Given that the reward outcome stimulus occurred 6 s after the reward anticipation stimulus in this task design, BOLD response during the reward outcome interval likely reflects some degree of signal recovery from reward anticipation and may be slightly attenuated. Notably, the main conclusion of this study—that neural reward processing mediates the association between life stress and problematic alcohol use—was true for both reward anticipation and reward outcome. We report the reward outcome results despite the short interval between conditions because previous research indicates that these processes are neurally distinct (e.g. wanting vs liking; Berridge and Robinson, 1998), and neural response to reward anticipation and rewarding outcomes has been assessed separately in other literature on psychosocial stress and disruptions in reward circuit function (e.g. Dillon et al., 2009; Mehta et al., 2010).
It is particularly important to understand the role of stressors on reward circuit function during adolescence and young adulthood because the onset of alcohol use disorders peaks during this developmental phase (Kessler et al., 2005; Hasin et al., 2007). This study provides the strongest neuroimaging evidence to date of the putative links between adolescent stressors, brain reward function and problematic alcohol use. It is also the first study to report a relationship between common adolescent stressors, such as arguments with family or problems in school, and reward circuit function and problematic alcohol use in early adulthood. If common life stressors during late adolescence increase risk for mental illness by disrupting brain response to rewards, then interventions for adolescents that boost stress regulation and reward responsiveness (e.g. mindfulness training, behavioral activation, sleep extension) may counteract the detrimental impact of these stressors. Applying these interventions in adolescents, particularly stressed adolescents, could help preserve reward function in the mPFC and other reward circuitry, and decrease the prevalence of alcohol use disorders. This application of our current findings would be an exciting area for further research.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers R01-MH050907 to D.S.S., R01-DA026222 to D.S.S., and R01-MH093605 to E.E.F.]. The study sponsor had no role in the design, analysis or interpretation of the study data, the writing of the report, or the decision to submit the manuscript for publication.
The authors would like to gratefully acknowledge the staff of the Pittsburgh Mother Child Project for their years of support and the study families who made this research possible. The authors would also like to thank Ella Vanderbilt-Adriance for sharing her data on neighborhood disadvantage, and Michele Bertocci and Eric Rodriguez for calculating AlphaSim thresholds for our fMRI analyses.
REFERENCES
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences. 2008;31(4):183–91. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of Neuropsychiatry and Clinical Neurosciences. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(4):356–67. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3(7):563–73. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Burke AR, Forster GL, Novick AM, Roberts CL, Watt MJ. Effects of adolescent social defeat on adult amphetamine-induced locomotion and corticoaccumbal dopamine release in male rats. Neuropharmacology. 2012;67:359–69. doi: 10.1016/j.neuropharm.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices: a national study of drinking behavior and attitudes. Monographs of the Rutgers Center of Alcohol Studies. 1969;6:260. [Google Scholar]
- Casement MD, Guyer AE, Hipwell A, et al. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental Cognitive Neuroscience. 2014;8:18–27. doi: 10.1016/j.dcn.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Howell DC, Phares V, Williams RA, Giunta CT. Risk factors for emotional/behavioral problems in young adolescents: a prospective analysis of adolescent and parental stress and symptoms. Journal of Consulting and Clinical Psychology. 1989;57(6):732–40. doi: 10.1037//0022-006x.57.6.732. [DOI] [PubMed] [Google Scholar]
- Davey CG, Yücel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Reviews. 2008;32(1):1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Dillon D, Holmes A, Birk J, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry. 2009;66:206–13. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell AD, Ampy LA, Meyer AL. Identification and assessment of problematic interpersonal situations for urban adolescents. Journal of Clinical Child Psychology. 1998;27(3):293–305. doi: 10.1207/s15374424jccp2703_6. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17(03):827–50. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Olino TM, Ryan ND, et al. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cognitive, Affective, and Behavioral Neuroscience. 2010;10(1):107–18. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith C. The biological basis of social interaction. Current Directions in Psychological Science. 2001;10(5):151–5. [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, Simons RL. Trajectories of stressful life events and depressive symptoms during adolescence. Developmental Psychology. 1994;30(4):467–83. [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences USA. 2004;101(21):8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;67(2):113–23. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64(7):830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Huettel SA, McCarthy G. Evidence for a refractory period in the hemodynamic response to visual stimuli as measured by MRI. Neuroimage. 2000;11(5):547–53. doi: 10.1006/nimg.2000.0553. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nature Neuroscience. 2005;8(11):1442–4. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Larson R, Ham M. Stress and “storm and stress” in early adolescence: the relationship of negative events with dysphoric affect. Developmental Psychology. 1993;29(1):130–140. [Google Scholar]
- Leussis MP, Lawson K, Stone K, Andersen SL. The enduring effects of an adolescent social stressor on synaptic density, part II: poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synapse. 2008;62(3):185–92. doi: 10.1002/syn.20483. [DOI] [PubMed] [Google Scholar]
- Lloyd DA, Turner RJ. Cumulative lifetime adversities and alcohol dependence in adolescence and young adulthood. Drug and Alcohol Dependence. 2008;93(3):217–26. doi: 10.1016/j.drugalcdep.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Masten AS, Neemann J, Andenas S. Life events and adjustment in adolescents: the significance of event independence, desirability, and chronicity. Journal of Research on Adolescence. 1994;4(1):71–97. [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: associations with persistence of DSM-IV disorders. Archives of General Psychiatry. 2010;67(2):124–32. doi: 10.1001/archgenpsychiatry.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SCR, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience. 2010;22(10):2316–25. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Mills KL, Lalonde F, Clasen LS, Giedd JN, Blakemore S-J. Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience. 2012;9:123–31. doi: 10.1093/scan/nss113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17(8):1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–74. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Newcomb MD, Harlow LL. Life events and substance use among adolescents: mediating effects of perceived loss of control and meaninglessness in life. Journal of Personality and Social Psychology. 1986;51(3):564–77. doi: 10.1037//0022-3514.51.3.564. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L, Qin S, Van Marle HJF, van Wingen GA, Fernández G, Hermans EJ. Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage. 2011;55(1):345–52. doi: 10.1016/j.neuroimage.2010.11.068. [DOI] [PubMed] [Google Scholar]
- Porcelli AJ, Lewis AH, Delgado MR. Acute stress influences neural circuits of reward processing. Frontiers in Neuroscience. 2012;6(157):1–9. doi: 10.3389/fnins.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Rüedi-Bettschen D, Dettling AC, et al. Long-term effects of early-life environmental manipulations in rodents and primates: potential animal models in depression research. Neuroscience and Biobehavioral Reviews. 2005;29(4-5):649–74. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Ross TJ, Salmeron BJ, Lee M. Acute nicotine differentially impacts anticipatory valence- and magnitude-related striatal activity. Biological Psychiatry. 2013;73:280–8. doi: 10.1016/j.biopsych.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlison RT, Felner RD. Major life events, hassles, and adaptation in adolescence: confounding in the conceptualization and measurement of life stress and adjustment revisited. Journal of Personality and Social Psychology. 1988;55(3):432–44. doi: 10.1037//0022-3514.55.3.432. [DOI] [PubMed] [Google Scholar]
- Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. Journal of Consulting and Clinical Psychology. 1978;46(5):932–46. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Gilliom M, Ingoldsby EM, Nagin DS. Trajectories leading to school-age conduct problems. Developmental Psychology. 2003;39(2):189–200. doi: 10.1037//0012-1649.39.2.189. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158(4):343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. Journal of Abnormal Psychology. 1982;91(3):199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurodevelopment. Journal of Adolescent Health. 2013;52(S2):S7–S13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex. 2010;20(3):534–48. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Vanderbilt-Adriance E, Shaw DS. Protective factors and the development of resilience in the context of neighborhood disadvantage. Journal of Abnormal Child Psychology. 2008;36(6):887–901. doi: 10.1007/s10802-008-9220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA. Stress and coping in early adolescence: relationships to substance use in urban school samples. Health Psychology. 1986;5(6):503–29. doi: 10.1037//0278-6133.5.6.503. [DOI] [PubMed] [Google Scholar]
- Wills TA, McNamara G, Vaccaro D, Hirky AE. Escalated substance use: a longitudinal grouping analysis from early to middle adolescence. Journal of Abnormal Psychology. 1996;105(2):166–80. doi: 10.1037//0021-843x.105.2.166. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The Global Burden of Disease. World Health Organization; 2008. pp. 1–160. [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, et al. Dysfunction of reward procesing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–94. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.