Abstract
Growing evidence indicates that normative pubertal maturation is associated with increased threat reactivity, and this developmental shift has been implicated in the increased rates of adolescent affective disorders. However, the neural mechanisms involved in this pubertal increase in threat reactivity remain unknown. Research in adults indicates that testosterone transiently decreases amygdala–orbitofrontal cortex (OFC) coupling. Consequently, we hypothesized that increased pubertal testosterone disrupts amygdala–OFC coupling, which may contribute to developmental increases in threat reactivity in some adolescents. Hypotheses were tested in a longitudinal study by examining the impact of testosterone on functional connectivity. Findings were consistent with hypotheses and advance our understanding of normative pubertal changes in neural systems instantiating affect/motivation. Finally, potential novel insights into the neurodevelopmental pathways that may contribute to adolescent vulnerability to behavioral and emotional problems are discussed.
Keywords: puberty, testosterone, amygdale, OFC, threat, connectivity
A hallmark of adolescent development is intensification in several aspects of affective (emotional/motivational) processing, particularly in social contexts (Blakemore, 2008; Crone and Dahl, 2012). This intensification occurs not only in the domain of positively valenced signals (e.g. sensation seeking/reward processing; Blakemore and Robbins, 2012) but also with negatively valenced signals (e.g. threat processing, Quevedo et al., 2009). Moreover, pubertal shifts in affective systems are thought to be a key factor contributing to the increase in difficulty with regulating behavior and emotion that emerges during adolescence (Crone and Dahl, 2012). In the extreme, adolescent difficulties with behavior and emotion contribute to increased morbidity and mortality including accidents due to reckless behavior and increased rates of anxiety and depressive disorders that emerge during this maturational period (Paus et al., 2008; Steinberg, 2010). As a result, the short- and long-term health and economic consequences of affect intensification during this period appear to be enormous (Dahl, 2004). Thus, it is crucially important to advance our understanding of normative changes in affective processes and their related neural mechanisms during this developmental window.
One key set of affective processes that appears to intensify during adolescence are those involved in reactivity to threat (e.g. increased fear-potentiated startle; Quevedo et al., 2009). Emerging evidence suggests that increased reactivity in the amygdala, a subcortical structure involved in identifying salient stimuli (Pessoa and Adolphs, 2010), forms a crucial component in the neural circuitry by which threat processing intensifies in puberty (Moore et al., 2012; Spielberg et al., 2014). However, there is little understanding of the neural mechanisms by which pubertal maturation might intensify threat-related activity in amygdala (Scherf et al., 2013).
We hypothesize that one important contribution to these normative neurodevelopmental changes involves the heavy interconnections between orbitofrontal cortex (OFC) and amygdala (Cohen et al., 2008), which are thought to involve top-down modulation of amygdala value representations (Dolan, 2007). Thus, decoupling of amygdala and OFC may lead to increased amygdala reactivity, and extant evidence supports this hypothesis. For example, disrupting amygdala–OFC connectivity in rhesus monkeys impairs responsivity to change in the value of reinforcers (Baxter et al., 2000). Additionally, several studies in humans have found that successful downregulation of emotion in response to negatively valenced (including threat-related) stimuli was associated with both decreased amygdala activation and greater amygdala–OFC coupling, suggesting that OFC downregulates amygdala activity to dampen negative emotion (Ochsner et al., 2002; Banks et al., 2007; Lee et al., 2012). Although there is variation in the particular regions involved, central/centromedial OFC (areas 11l, 13l and 13m) appears to be the most consistent area found to be coupled with amygdala.
Mounting evidence in adults indicates that testosterone decreases coupling between amygdala and central/centromedial OFC, providing one mechanism by which puberty may precipitate greater amygdala reactivity to threat. For example, higher levels of endogenous testosterone in men have been linked to decreased amygdala–OFC coupling (Volman et al., 2011), and similar decoupling has been observed after single-dose administration of testosterone to women (van Wingen et al., 2010; Bos et al., 2012a). However, no study to date has examined the impact of testosterone on amygdala–OFC coupling during adolescence—a developmental period in which testosterone undergoes dramatic increases (2-fold in females, 18-fold in males). Thus, the testosterone-related decoupling seen in adults may also contribute to the increased amygdala reactivity observed during pubertal maturation, and testing this hypothesis in a normative sample was the focus of the present study.
LONGITUDINAL DEVELOPMENTAL STUDY
The present study examined the theory that pubertal increases in testosterone are related to decreases in threat-related OFC modulation of amygdala. To test this proposal, functional magnetic resonance imaging (fMRI) data were collected in the same participants at two time points (2 years apart, mean ages 11.9 and 14.0 years) while adolescents viewed social stimuli associated with potential threat (i.e. faces with anger or fear expressions). We chose this particular developmental period because of our specific focus on pubertal maturation (for which there is maximal variance during this time). To assess the impact of testosterone on amygdala–OFC coupling, longitudinal changes in testosterone were correlated with variation over time in condition-dependent amygdala–OFC connectivity.
RESULTS
Participants performed a well-established social threat-processing task (Hariri et al., 2000), during which they indicated which of two stimuli matched a target stimulus. The task contrast of interest compared a threat face condition with a neutral control condition (geometric shape/neutral face). Initial analyses tested whether behavior and neural activation/connectivity changed across time (collection time points were two years apart). The analyses of main interest assessed whether change in testosterone over time moderated change in behavior/neural activation/connectivity.
Testosterone
Testosterone increased over time in boys (Time 1 mean = 166.5, s.d. = 139.5; Time 2 mean = 401.3, s.d. = 125.0; t = 13.2, P < 0.01) and girls (Time 1 mean = 44.7, s.d. = 12.6; Time 2 mean = 57.9, s.d. = 14.5; t = 5.6, P < 0.01). Individually, testosterone increased over time in all participants, except one girl who maintained the same level.
Behavioral data
Accuracy did not differ by Time [F(1,35) = 0.01, P = 0.87], Condition [F(1,35) = 0.60, P = 0.44] or Time × Condition interaction [F(1,35) = 0.00, P = 0.98]. Similarly, there was no main effect of testosterone [F(1,34) = 0.76, P = 0.39], nor did testosterone moderate Time [F(1,34) = 0.51, P = 0.48), Condition [F(1,34) = 0.05, P = 0.82] or Time × Condition interaction [F(1,34) = 0.54, P = 0.47].
Reaction time (RT) differed by Time [F(1,35) = 9.77, P < 0.01], with Time 1 RT greater than Time 2. RT also differed by Condition [F(1,35) = 67.82, P < 0.01], with threat faces greater than neutral control shapes. There was no interaction between these factors [F(1,35) = 0.50, P = 0.49]. Similarly, there was no main effect of testosterone [F(1,34) = 2.66, P = 0.11], nor did testosterone moderate Time [F(1,34) = 2.71, P = 0.11], Condition [F(1,34) = 2.79, P = 0.10] or Time × Condition interaction [F(1,34) = 0.32, P = 0.58].
Main effects of task
The threat face–neutral shape main effect was significant in two clusters (Table 1). No significant changes over time in task main effect emerged.
Table 1.
Task main effects averaged across time
| Region | Cluster size | Maximum z-value | Cluster P-value | Location |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| R OFC/IFG/MFG (BA 9/11/45/46/47) | 1948 | 5.36 | 0.003 | 54 | 40 | 14 |
| B amygdala/hippocampus/STG/fusiform/lingual/cuneus/inferior and middle occipital (BA 17/18/19/20/30/36/37/38) | 17 644 | 8.12 | >0.001 | 20 | −4 | −14 |
Note: Main effect for the threat face–neutral shape contrast; R, right; B, bilateral; OFC, orbitofrontal cortex; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; STG, superior temporal gyrus; BA, Brodmann’s area; Cluster size, number of voxels in cluster; Location, coordinates of the maximum z-value (in Montreal Neurological Institute (MNI)152 space).
Psychophysiological interaction connectivity with amygdala
Psychophysiological interaction (PPI) connectivity was examined, with left/right amygdala time series as the physiological predictor and threat face–neutral shape as the task predictor. Both mean connectivity and change in connectivity across time were examined.
Five clusters emerged in which there was a significant PPI effect averaged across time for left amygdala (see Table 2). Three clusters (bilateral lingual/cuneus/inferior/middle occipital and right and left fusiform/inferior occipital) exhibited a positive PPI effect (more positive coupling with amygdala during threat than neutral), whereas two clusters (in posterior cingulate and left middle-temporal gyrus) exhibited a negative PPI effect (more positive coupling during neutral than threat). Similar clusters were found for right amygdala, except for the absence of the middle temporal gyrus cluster. No significant differences across time in PPI emerged.
Table 2.
PPI connectivity with amygdala averaged across time
| Region | Cluster size | Max z-value | Cluster P-value | Location |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Coupling with left amygdala | ||||||
| B lingual/cuneus/inferior and middle occipital (BA 17/18/19) | 1216 | 4.66 | < 0.001 | −22 | −96 | 22 |
| R fusiform/inferior occipital (BA 19/37) | 625 | 4.64 | < 0.001 | 36 | −50 | −20 |
| L fusiform/inferior occipital (BA 19/37) | 535 | 4.36 | < 0.001 | −40 | −76 | −10 |
| PCC (BA 23/29/30/31) | 441 | −4.53 | 0.001 | 0 | −42 | 22 |
| L MTG (BA 21/22) | 226 | −3.95 | 0.04 | −62 | −20 | −8 |
| Coupling with right amygdala | ||||||
| B fusiform/lingual/cuneus/inferior and middle occipital (BA 17/18/19/37) | 2600 | 4.77 | < 0.001 | −26 | −82 | −8 |
| R fusiform/inferior occipital (BA 37) | 348 | 4.06 | 0.006 | 34 | −46 | −20 |
| PCC (BA 23/29/30/31) | 619 | −4.89 | >0.001 | 0 | −42 | 22 |
Note: Task contrast in PPI was threat face–neutral shape; R, right; L, left; B, bilateral; PCC, posterior cingulate cortex; MTG, middle temporal gyrus; BA, Brodmann’s area; Cluster size, number of voxels in cluster; Location, coordinates of the maximum z-value (in MNI152 space).
Impact of testosterone on PPI connectivity with amygdala
To test our hypothesis that threat-induced amygdala–OFC connectivity is inversely related to change in testosterone, we examined the degree to which change over time in testosterone moderated change over time in connectivity between these regions.
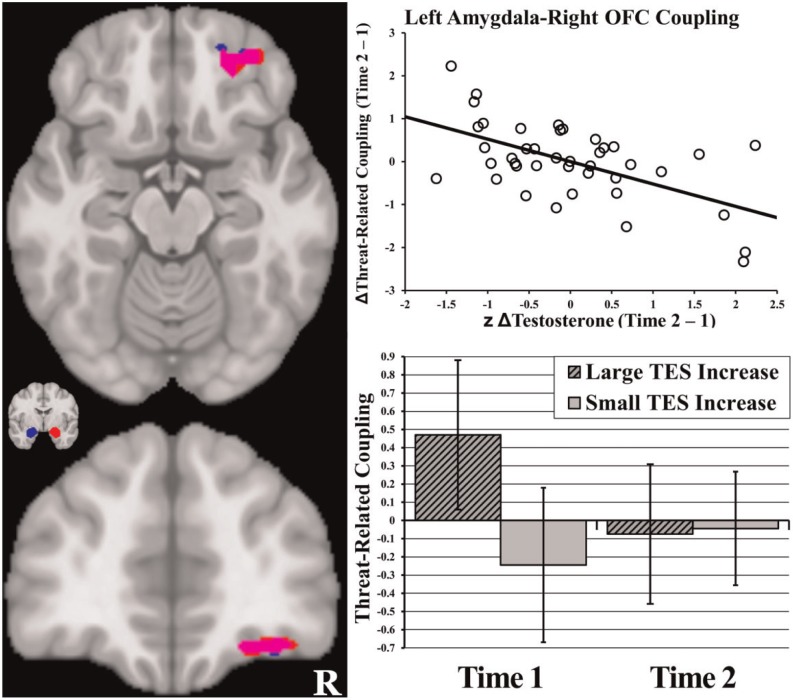
In line with hypotheses, increased testosterone over time was associated with decreased connectivity between left amygdala and a cluster in right centromedial OFC during the threat face condition (Figure 1 and Table 3). A similar relationship was found with connectivity between right amygdala and an overlapping cluster in right centromedial OFC (Figure 1 and Table 3). As illustrated in Figure 1, adolescents with a large increase in testosterone exhibited positive amygdala–right OFC connectivity at Time 1, which became decoupled at Time 2.
Fig. 1.
Decreased threat-related coupling with amygdala over time in participants with large increases in testosterone. Note: OFC = orbitofrontal cortex. The left images display two views of regions of OFC that exhibited decreased threat-related coupling with left (blue) and right (red; overlap = purple) amygdala in individuals with large increases in testosterone over time. Also pictured is a representative coronal slice that shows the average location of the amygdala seed regions. The scatterplot on the top right illustrates the relationship between change in testosterone over time (x-axis, z-scored within gender) and change in threat-related left amygdala–OFC coupling (y-axis, mean change in coupling β-value, converted to z-scores). The bar graph in the bottom right illustrates this relationship broken down by time point and by size of testosterone increase (determined by median split). Error bars represent 95% confidence intervals. The graphs for the coupling with right amygdala exhibited extremely similar patterns.
Table 3.
Orbitofrontal regions exhibiting testosterone-dependent changes in coupling with amygdala
| Region | Cluster size | Max z-value | Cluster P-value | Location |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Coupling with left amygdala | ||||||
| R OFC (BA 11) | 52 | −3.71 | 0.04 | 34 | 48 | −16 |
| Coupling with right amygdala | ||||||
| R OFC (BA 11) | 69 | −3.77 | 0.01 | 34 | 48 | −16 |
Note: Task contrast in PPI was threat face–neutral shape; R, right; OFC, orbitofrontal cortex; BA, Brodmann’s area; Cluster size, number of voxels in cluster; Location, coordinates of the maximum z-value (in MNI152 space).
This pattern is also present when examining connectivity only during the threat face condition (vs fixation). Similarly, an overlapping cluster emerges for left amygdala when using the neutral face condition as a baseline [cluster P = 0.04, voxel n = 25, maximum z = 3.65, (x, y, z) = 24, 46, −16]. Thus, this effect does not depend on using shape as a baseline.
Findings remain significant when covarying out Δage, mean testosterone and testosterone at Time 1, indicating that findings are not due simply to development over time or baseline/final testosterone level. Thus, present findings support the hypothesis that surges in testosterone during adolescence decouple amygdala and OFC, which may lead to reduced top-down regulation of amygdala by OFC.
To ensure that findings were not driven by participants who had only a small change in testosterone, we reexamined these relationships only in participants with a large increase in testosterone. Importantly, the left amygdala finding remained highly significant. The right amygdala finding became marginal. Because the effect size remained consistent, the marginality of significance is most likely because of decreased power due to a smaller sample size.
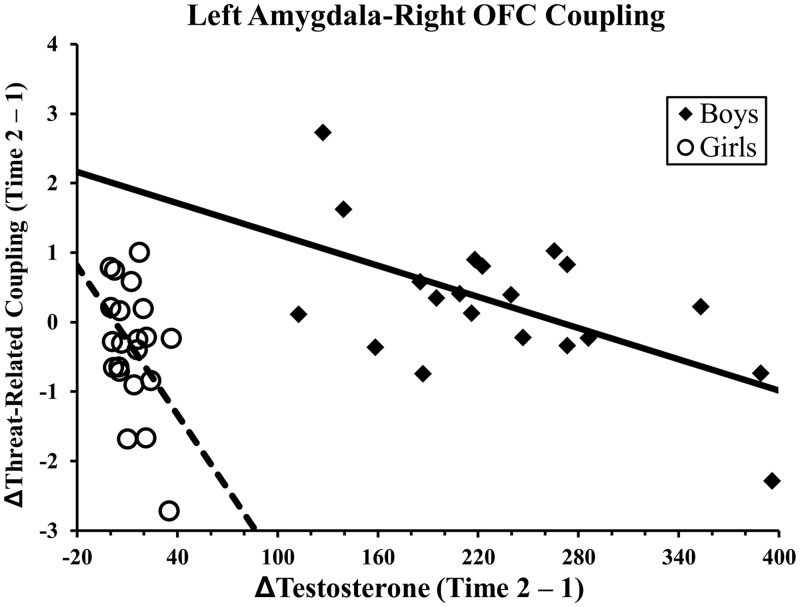
To determine whether findings were driven by one gender, the impact of testosterone on coupling was examined within gender. As depicted in Figure 2, the relationship between left amygdala and OFC was significant in both girls (β = −0.41, P = 0.05, ΔR = 0.16) and boys (β = −0.67, P < 0.01, ΔR = 0.49). Similarly, the relationship between right amygdala and OFC was significant in both girls (β = −0.47, P = 0.03, ΔR = 0.21) and boys (β = −0.47, P = 0.04, ΔR = 0.22).
Fig. 2.
Within gender threat-related coupling between left amygdala and OFC. Note: OFC = orbitofrontal cortex. The scatterplot illustrates the relationship between change in testosterone over time (x-axis) and change in threat-related left amygdala–OFC coupling (y-axis, mean change in coupling β-value, converted to z-scores) plotted separately for each gender (boys = filled diamonds and a solid line; girls = open circles and a dotted line).
Relationship between changes in coupling and approach/withdrawal temperament
The present study focused on a carefully screened normative sample, and we did not pose any hypotheses related to individual differences in coupling and clinical measures of affective changes (e.g. anxiety). However, as a preliminary exploration of this dimension, we examined the relationship between (change over time in) coupling and a normative measure of approach/withdrawal temperament in those participants with a large increase in testosterone. Decreased coupling over time was associated with increased levels of withdrawal temperament (P = 0.02), consistent with an increase in threat sensitivity.
DISCUSSION
The goal of this study was to examine the interactive biological mechanisms—neural and hormonal—by which normative pubertal development gives rise to increased amygdala reactivity to threat. Our findings are consistent with the theory that pubertal surges in testosterone contribute to increased amygdala reactivity to threat via decreased coupling between amygdala and OFC. Specifically, we demonstrate for the first time that adolescents with large increases in testosterone over 2 years of pubertal maturation show large reductions in positive coupling between these regions 2 years later. Crucially, this pattern of relative decoupling was observed only in the threat condition and was independent of the baseline condition used, suggesting that these findings are specific to threat processing. Exploratory analyses also found that decreased amygdala–OFC coupling over time was associated with increased withdrawal temperament, consistent with the proposal that this change in coupling is associated with an increase in threat sensitivity. Finally, the region of OFC observed in the present study is consistent with that found previously in adults to be associated with both testosterone-related reductions in coupling and top-down control of amygdala during emotion regulation (Ochsner et al., 2002; Lee et al., 2012), suggesting that the particular mechanism at play may be a reduction in top-down biasing of amygdala by OFC.
These findings have implications for understanding the normal development of emotion regulation during adolescence. Specifically, research suggests that successful downregulation of negative emotion is dependent, in part, on intact amygdala–OFC coupling (Ochsner et al., 2002; Lee et al., 2012). Accordingly, the disruption in coupling observed in the present study suggests that regulating negative emotion may become more difficult as puberty progresses.
With regard to the particular psychological processes impacted by reduced coupling, research suggests that intact amygdala–OFC coupling is particularly important for overriding past value associations. For example, recent work indicates that OFC is involved in calculating value on the fly in novel situations that require the integration of valuation information from several sources (Jones et al., 2012). In other words, OFC is required when an individual must infer the value of a stimulus—not based on direct past experience of the particular situation currently faced—but by mapping together contingency and value information from different sources to estimate value. When this value differs from cached values (e.g. represented in amygdala), OFC is thought to override such values through top-down modulation. Although speculative, it is possible that the reduced coupling observed in the present study has a particularly strong impact when adolescents engage with stimuli that are novel and/or change in value over time. For example, the value attached to social relationships—both in general and with specific individuals—shows a high degree of variability in adolescence (Gardner and Steinberg, 2005). The reduced amygdala–OFC coupling observed in the present study may thus contribute to greater social volatility—at precisely the time during which social relationships, particularly with peers, become increasingly salient (Brown, 2004).
Increasing evidence indicates that testosterone plays a key modulatory role in processes involved in navigating social interactions, particularly when social status can be gained or lost. Recent reviews have concluded that testosterone promotes the search for, and maintenance of, social status and alters the appraisal of motivationally relevant social stimuli (Carney et al., 2010; Bos et al., 2012b). This role for testosterone is consistent with research on rodents and non-human primates, wherein testosterone acts to enhance approach behavior in threatening social situations (Fernandez-Guasti and Martinez-Mota, 2005; Lacreuse et al., 2010).
Our findings suggest a potential neural mechanism by which testosterone increases the pursuit of social status. Specifically, it may be that disrupting top-down OFC regulation renders amygdala more reactive toward threats to social status and salient social cues in general, given that amygdala is particularly reactive to social stimuli (Adolphs et al., 1998). This is not to imply that testosterone’s impact on the pursuit of social status (e.g. during adolescence) reflects a lack of ‘control’. Rather, it is possible that reducing amygdala–OFC coupling increases the motivational salience of social status, making high status a more valued goal. Accordingly, present findings suggest the possibility that changes in coupling impact the type of goals chosen rather than the ability to regulate the pursuit of goals per se.
Although the focus of the present study is on threat processing, it should be noted that both amygdala and OFC are also involved in the processing of appetitive stimuli (Baxter and Murray, 2002). Thus, future research should examine a modulatory role of testosterone on amygdala–OFC coupling in the context of potential reward to determine whether similar decreases in coupling are observed.
Adolescent psychopathology and maladaptive behavior
Although the present study examined only a normative sample of adolescents, and thus can extend inferences only to this population, present findings provide a new direction in which to investigate the neural mechanisms involved in adolescent psychopathology and maladaptive behavior. In particular, progress toward understanding how puberty contributes to the development of behavioral and emotional problems has been challenged by an apparent paradox: adolescence is associated with increases in both risk taking and anxiety. Although it is reasonable for intensification in systems commonly associated with threat avoidance to lead to greater anxiety, it is rather counterintuitive that risk taking increases over the same interval. Indeed, initial attempts to explain increased risk taking in adolescence posited that reactivity to threat decreases during this time (Ernst et al., 2006), a position contradicted by recent research (Quevedo et al., 2009).
Attempting to resolve this discrepancy, Spielberg et al. (2014) proposed that puberty is associated in some adolescents with an increase in the tendency to experience some aspects of threat as exciting/thrilling, increasing the likelihood of approaching (vs avoiding) potential threats. The findings of this study were consistent with this theory and further indicated that this effect was present only in adolescents who also exhibited increased threat-related activation in nucleus accumbens (NAcc). Interestingly, amygdala activation was associated with higher levels of sensation seeking and decreased symptoms of anxiety disorders only in participants with increased NAcc. Thus, initial evidence indicates that pubertal increases in threat reactivity may contribute to the emergence of both anxiety disorders and risk taking during this period. Thus, although very preliminary, present findings suggest that testosterone-related reductions in amygdala–OFC coupling may form one link in the neurodevelopmental pathway by which puberty leads to increased anxiety and risk taking. Specifically, it is possible that disrupting top-down OFC control may leave amygdala free to react more strongly to potential threat and salient stimuli more generally.
Strengths and limitations
The present study benefits from a number of strengths, including a longitudinal design, which allows for more powerful and accurate tests of developmental change and remains uncommon in the developmental neuroscience literature. Additionally, the design allowed for disentanglement of puberty and age effects, which are often confounded. Puberty and age were de-confounded in the present study because participants were sampled such that there was variance in the level of pubertal development at the two time points, but all participants had the same change in age (2 years). Therefore, there was a large amount of between-participant variance in how much pubertal status changed, whereas there was only minimal between-participant variance in age change. Thus, change in testosterone across time was uncorrelated with change in age in the present design. In addition, the present study examined connectivity specifically during a threat condition, using two neutral control conditions as baseline comparisons. Several previous studies (e.g. van Wingen et al., 2010) examined connectivity across the entire paradigm, leaving unclear whether reduced coupling occurs differentially, or at all, in threat contexts.
Findings extend previous research on the impact of testosterone on connectivity, which focused solely on adults using either administrations of testosterone or examination of individual differences in endogenous testosterone. Although extremely useful, several methodological issues limit the utility of these studies, particularly for understanding the impact of testosterone during puberty. For example, artificial administration of testosterone does not capture the consequence of naturalistic surges in testosterone, especially over months/years and during a period of brain development. In addition, the duration and level of the testosterone increase in adult studies are brief and relatively modest; in contrast, testosterone levels at puberty typically double in girls and increase 18-fold in males. Similarly, examining individual differences in endogenous testosterone does not address the impact that increases in testosterone have on connectivity. Most important with regard to development, testosterone is likely to have differential effects in adolescents, given the unique neural and environmental context during this period. The present study addressed these disadvantages by investigating the impact of naturalistic increases in testosterone on amygdala–OFC coupling during this key developmental period.
Interestingly, the observed effects were present for both boys and girls, even though increases in testosterone were substantially higher in boys. When examined within gender (see Figure 2), it appears that effects depend on a relative change rather than absolute change in testosterone. This may be due to a greater sensitivity in girls or another as yet unknown mechanism.
As with any study, there are several interpretational limitations that must be considered. First, although there is evidence that OFC exerts top-down control over amygdala, the analyses used in the present study are correlational and cannot determine direction/causality. Thus, it is possible that present findings are due to amygdala exerting control over OFC and/or a third brain region influencing both regions. Future research employing methods with finer time resolution (e.g. magnetoencephalography source localization) would help to determine the direction of present effects.
Second, it is possible that findings were driven by differences in fMRI data quality at the two time points (which may differentially impact the connectivity effect size). Although this can never be ruled out, there are several reasons why it is unlikely for this confound to be driving the observed effects. First, because we use a neutral baseline, differences in data quality would have to impact the threat condition to a greater degree. In addition, these (condition-differential) differences in data quality would have to scale with differences testosterone to be driving present findings. Finally, we investigated three potential sources of degradation in data quality. First, we visually examined fMRI coverage in OFC and amygdala between time points and across participants and did not observe noticeable differences. Second, we calculated signal to noise ratio in OFC and found no differences across time, by condition or scaling with testosterone. Third, we found that head motion did not differ significantly between time points. Therefore, although differences in data quality remain a potential confound (as they do in any longitudinal/individual differences study), we believe that it is relatively unlikely to be driving the effects observed in the present study.
A final limitation is that the present study only examined development from pre-/early to mid-adolescence. Thus, we are unable to investigate developmental changes occurring from mid to late adolescence/early adulthood. Because significant development in motivational, affective and social processes continues to occur during this later time period, effects observed in the present study may be exacerbated and/or distinct effects may emerge. Although an interesting direction for future research, we do not believe that this complexity undermines the inferences of the present study.
Although not a limitation per se, it should be noted that the study used a carefully screened normative sample free from clinical anxiety, affective disorders and pathological risk taking. Therefore, present findings cannot speak directly to the development of pathology in these realms. However, we believe that the study provides a deeper understanding of normative developmental changes that help to frame new hypotheses that can be tested in clinical samples.
In spite of these limitations, the present study reports novel findings that deepen our understanding of the neurodevelopmental mechanisms that confer vulnerability for adolescent psychopathology and maladaptive behavior. Specifically, we demonstrate that pubertal surges in testosterone over a 2 year period were associated with concurrent decreases in threat-related amygdala–OFC coupling. Overall, the present study offers evidence of a novel link in adolescent neurodevelopmental pathways—pathways that may result in psychopathology and maladaptive behavior.
ONLINE METHODS
Participants
Adolescents were part of a study of pubertal influences on normal affective development and were recruited from the community through advertisements, flyers and phone lists. Adolescents were free of current and lifetime psychiatric disorders, did not have braces and had no history of head injury, serious medical illness or psychotropic medication/alcohol/illicit drug use. Each participant and a parent/guardian provided informed assent/consent according to the guidelines of the University of Pittsburgh Institutional Review Board.
fMRI and testosterone data were collected at two time points, ∼2 years apart (mean = 2.1, s.d. = 0.2). Data were collected from 61 participants at both time points. Data for 19 participants were not used due to motion ≥ 5 mm (n = 8), poor amygdala segmentations (n = 4) or poor registrations (n = 7) at one or both time points. Data from one participant were removed because of motion-related fMRI artifact. In the final sample (n = 41, 51% female), mean ages were 11.5 years (s.d. = 0.7) for females and 12.4 years (s.d. = 0.6) for males at Time 1 and 13.6 years (s.d. = 0.7) for females and 14.4 years (s.d. = 0.6) for males at Time 2. Females were purposefully sampled to be younger based on epidemiologic findings that girls in the USA undergo puberty earlier than boys (Wu et al., 2002).
Testosterone
Circulating levels of testosterone were assessed in the morning through bloodspot sampling using a minimally invasive finger-stick procedure (Worthman and Stallings, 1997). Testosterone assays were a modification of a commercially available serum/plasma radioimmunoassay kit (Pantex, Santa Monica, CA). No participants were below the minimal detectable dose sensitivity criterion (14.2 ng/dl for males and 14.0 ng/dl for females). Inter-assay coefficients of variation were acceptable. To model testosterone increases over time, a change predictor was created by subtracting testosterone at Time 1 from Time 2. All participants demonstrated an increase in testosterone over time, except for one girl who maintained the same level. To remove gender-related differences in the mean and variance of testosterone scores, the change predictor was z-scored separately within gender. Pubertal development was also assessed via Tanner staging based on a physical examination conducted by a nurse. Testosterone level was highly correlated with Tanner stage at Time 1 (r = 0.63, P < 0.001) and less so at Time 2 (r = 0.31, P = 0.05) (the reduction appears to be due to a ceiling effect in Tanner stage).
Temperament questionnaire
To measure approach/withdrawal temperament, the Revised Dimensions of Temperament Survey (DOTS; Windle and Lerner, 1986) was administered to adolescents at each time point. Only the seven-item approach/withdrawal subscale was examined in the present study.
Threat processing paradigm
The threat processing paradigm was a block-design face-processing task used in dozens of studies of threat processing (Hariri et al., 2000). The paradigm contained four blocks of a face-processing task interleaved with five blocks of a neutral control task. During the face task, participants viewed a trio of faces and indicated which of two faces was identical to the target face. Each block contained six novel exemplars of one type of facial expression (angry, fearful or neutral) presented for 2 s each (three images per gender). Affect type was consistent within block, and blocks of threat faces (angry + fearful) alternated with blocks of neutral faces (each two blocks) with order counterbalanced across participants/time points. All face stimuli were derived from a standard set of pictures of facial affect (http://www.macbrain.org/resources.htm). During the neutral control condition, participants viewed a set of three geometric shapes (circles, vertical ellipses and horizontal ellipses) and selected one of the two shapes (bottom) that matched the target shape (top). Each neutral control block consisted of six different images, each of which was presented twice in a pseudorandom order for 2 s. Within each block, interstimulus interval varied 2–6 s.
Behavior analysis
Accuracy and mean RT were calculated for each participant by time point and condition. Repeated-measures analyses of variance (ANOVAs) were used, with Time and Condition as within-participant factors and change in testosterone as a between-participant continuous predictor.
fMRI data acquisition
Participants were scanned using a Siemens 3T Allegra scanner. Blood Oxygen Level Dependent data were acquired with a gradient echo-planar imaging sequence covering 34 axial slices (3 mm thick, interleaved collection, Time to Repeat/Time to Echo = 2000/25 ms, field of view = 20 cm, matrix = 64 × 64). A 192-slice MPRAGE was acquired (spatial resolution 1 mm × 1 mm × 1.54 mm).
fMRI data processing
fMRI processing was carried out using FSL tools. Functional data for each participant were motion corrected, temporally filtered with a non-linear high-pass filter that attenuated frequencies <1/150 Hz, spatially smoothed using a 3D Gaussian kernel (full width half max = 5 mm), corrected for slice-timing differences and intensity-normalized. To remove motion-related variance, independent component analysis was carried out for each data set using MELODIC. Components that reflected variance due to motion (i.e. rings around the brain, activation in ventricles) were identified and removed.
Functional data were registered to the structural scan using boundary-based registration and then warped into stereotaxic space (the non-linear MNI152 template standard with FSL) using FMRIB's Nonlinear Registration Tool (FNIRT). Analyses were performed on the processed functional time series of each participant using FMRIB's Improved Linear Model (FILM). The three task conditions were entered as predictors after being convolved with a gamma function. Two contrasts were created: (i) Threat Face – Neutral Shape and (ii) Threat Face – Neutral Face.
A fixed effects ANOVA was conducted (via fMRI Expert Analysis Tool (FEAT)), using each participant’s Time 1 and Time 2 data, that modeled both changes in task effect over time (Time 2–Time 1) and the mean task effect across time. The resultant β maps were carried up to the group level, where the mean for each effect was calculated. Two-tailed t-tests were conducted on the β’s for the predictor of interest and converted to z-scores to determine the significance of the β’s. Gaussian random field theory was used to correct for multiple comparisons (via cluster) with an individual voxel-level threshold of z ≥ 2.81 and an overall error rate of P ≤ 0.05. A mask of gray matter was used to constrain the number of voxels under consideration.
PPI connectivity analyses
Left and right amygdalae were segmented in each structural scan via FIRST. The inverse of the functional→structural transform was applied to the segmented masks to convert them into functional space. The mean (across voxels in each mask) for each time point was extracted to create amygdalae time series seed predictors. To model brain-wide signal fluctuations that could confound estimates of connectivity, the mean across all intra-cerebral voxels was calculated for each time point.
PPI connectivity analyses were performed on the processed time series of each participant using FILM. Two sets of analyses were conducted, one in which the shape condition was used as the baseline for the task predictor and one in which the neutral face condition was used as the baseline. Seven predictors were entered in each analysis: (i) amygdala time series, (ii) a predictor modeling the mean difference between the threat face and neutral condition (coded as 1 during the threat condition, −1 during the neutral condition 0 at all other times), (iii) the PPI connectivity term (the product of amygdala time series and the condition difference predictor) and (iv–vii) four nuisance predictors that modeled the variance associated with the sum of the threat face condition and appropriate neutral condition (to model variance shared among these conditions), the neutral face (or shape) condition and mean ventricle and white matter time series. Before creating the PPI connectivity term, condition predictors were convolved with a gamma function.
A fixed effects ANOVA was computed, using each participant’s Time 1 and Time 2 PPI β maps, which modeled both changes in connectivity over time (Time 2–Time 1) and mean connectivity across time. The mean and difference connectivity maps were then used as dependent variables in group-level regressions (separately for right/left amygdala) in FMRIB's Local Analysis of Mixed Effects (FLAME). First, means across group were calculated to determine both mean (across time) connectivity and change over time in connectivity. Next, to test the main hypotheses of interest, change in testosterone over time (Time 2–Time 1) was entered as a predictor. To remove variance due to receipt of different counterbalancing orders at the two time points, a nuisance covariate was included modeling consistency of counterbalancing orders. Because several participants were left handed, a handedness covariate was included. Thresholding and correction for multiple comparisons is described above. Because the present study focused on OFC, a mask of OFC gray matter was used.
Because the present study used a normative sample, we did not expect to find relationships between changes in coupling and clinical measures (e.g. anxiety). However, as a preliminary analysis, we examined the correlation between change over time in coupling and change over time in the approach/withdrawal DOTS subscale in those participants with a large change in testosterone over time.
Acknowledgments
R.E.D., E.E.F., C.D.L., and N.D.R. designed the experiments; R.E.D., E.E.F., C.D.L., C.M.W., and N.D.R. performed aspects of the experiments; J.M.S. performed analyses, with the assistance of T.M.O.; J.M.S. and R.E.D wrote the manuscript; all authors provided critical revisions of the manuscript.
This work was supported by the National Institute on Drug Abuse (R01 DA018910).
REFERENCES
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–4. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2(4):303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Review Neuroscience. 2002;3(7):563–73. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. Journal of Neuroscience. 2000;20(11):4311–19. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Review Neuroscience. 2008;9(4):267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Robbins TW. Decision-making in the adolescent brain. Nature Neuroscience. 2012;15(9):1184–91. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Bos PA, Hermans EJ, Ramsey NF, van Honk J. The neural mechanisms by which testosterone acts on interpersonal trust'. Neuroimage. 2012a;61(3):730–7. doi: 10.1016/j.neuroimage.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthe RM, van Honk J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Frontiers in Neuroendocrinology. 2012b;33(1):17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Brown BB. Adolescents’ relationships with peers. Handbook of Adolescent Psychology. 2004;2:363–94. [Google Scholar]
- Carney DR, Cuddy AJ, Yap AJ. Power posing: brief nonverbal displays affect neuroendocrine levels and risk tolerance. Psychological Science. 2010;21(10):1363–8. doi: 10.1177/0956797610383437. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Weber B. Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. Neuroimage. 2008;39(3):1396–407. doi: 10.1016/j.neuroimage.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Review Neuroscience. 2012;13(9):636–50. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2007;362(1481):787–99. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30(8):762–70. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41(4):625–35. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Jones JL, Esber GR, McDannald MA, et al. Orbitofrontal cortex supports behavior and learning using inferred but not cached values. Science. 2012;338(6109):953–6. doi: 10.1126/science.1227489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, King HM, Kurdziel LB, et al. Testosterone may increase selective attention to threat in young male macaques. Hormones and Behavior. 2010;58(5):854–63. doi: 10.1016/j.yhbeh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage. 2012;62(3):1575–81. doi: 10.1016/j.neuroimage.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WE, III, Pfeifer JH, Masten CL, Mazziotta JC, Iacoboni M, Dapretto M. Facing puberty: associations between pubertal development and neural responses to affective facial displays. Social Cognitive and Affective Neuroscience. 2012;7(1):35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11(11):773–83. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: effects on the psychophysiology of defensive and appetitive motivation. Development and Psychopathology. 2009;21(1):27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Smyth JM, Delgado MR. The amygdala: an agent of change in adolescent neural networks. Hormones and Behavior. 2013;64(2):298–313. doi: 10.1016/j.yhbeh.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Olino TM, Forbes EE, Dahl RE. Exciting fear in adolescence: does pubertal development alter threat processing. Developmental Cognitive Neuroscience. 2014;8:86–95. doi: 10.1016/j.dcn.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52(3):216–24. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- van Wingen G, Mattern C, Verkes RJ, Buitelaar J, Fernandez G. Testosterone reduces amygdala-orbitofrontal cortex coupling. Psychoneuroendocrinology. 2010;35(1):105–13. doi: 10.1016/j.psyneuen.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Volman I, Toni I, Verhagen L, Roelofs K. Endogenous testosterone modulates prefrontal-amygdala connectivity during social emotional behavior. Cerebral Cortex. 2011;21(10):2282–90. doi: 10.1093/cercor/bhr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Lerner RM. Reassessing the dimensions of temperamental individuality across the life span: the Revised Dimensions of Temperament Survey (DOTS-R) Journal of Adolescent Research. 1986;1(2):213–29. [Google Scholar]
- Worthman CM, Stallings JF. Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. American Journal of Physical Anthropology. 1997;104(1):1–21. doi: 10.1002/(SICI)1096-8644(199709)104:1<1::AID-AJPA1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Wu T, Mendola P, Buck GM. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics. 2002;110(4):752–7. doi: 10.1542/peds.110.4.752. [DOI] [PubMed] [Google Scholar]