Abstract
A heightened sense of self-esteem is associated with a reduced risk for several types of affective and psychiatric disorders, including depression, anxiety and eating disorders. However, little is known about how brain systems integrate self-referential processing and positive evaluation to give rise to these feelings. To address this, we combined diffusion tensor imaging (DTI) and functional magnetic resonance imaging (fMRI) to test how frontostriatal connectivity reflects long-term trait and short-term state aspects of self-esteem. Using DTI, we found individual variability in white matter structural integrity between the medial prefrontal cortex and the ventral striatum was related to trait measures of self-esteem, reflecting long-term stability of self-esteem maintenance. Using fMRI, we found that functional connectivity of these regions during positive self-evaluation was related to current feelings of self-esteem, reflecting short-term state self-esteem. These results provide convergent anatomical and functional evidence that self-esteem is related to the connectivity of frontostriatal circuits and suggest that feelings of self-worth may emerge from neural systems integrating information about the self with positive affect and reward. This information could potentially inform the etiology of diminished self-esteem underlying multiple psychiatric conditions and inform future studies of evaluative self-referential processing.
Keywords: self-esteem, connectivity, medial prefrontal cortex, ventral striatum, diffusion tensor imaging, functional magnetic resonance imaging
Maintaining a positive sense of self is considered essential for mental health and well-being. Decades of research in psychology have revealed that individuals with high self-esteem are less prone to affective disorders such as depression (Abramson et al., 1989; Butler et al., 1994), anxiety (Greenberg et al., 1992) and eating disorders (Heatherton and Baumeister, 1991; Vohs et al., 2001) and show a greater amount of positive affect and enhanced initiative in the face of challenges that promote general happiness (Baumeister et al., 2003). Additionally, patients with greater self-esteem and more positive self-regard tend to be more responsive to treatment for their conditions (Roberts et al., 1999; Ciesla and Roberts, 2002). Moreover, high self-esteem has the potential to contribute to the resiliency of an individual’s mental health even when the information on which a person bases this attitude is objectively inaccurate or favorably inflated (Lewinsohn et al., 1980). This suggests that the mere feeling of positive self-esteem may endogenously contribute to psychological well-being independently of externally driven influences and thus reflect neurocognitive processes within the brain.
Though the clinical relevance and popular appeal of self-esteem are well established, systematic studies of the neural systems that give rise to self-esteem are conspicuously sparse in the neuroimaging literature (Mitchell, 2009). Previous work has shown that self-esteem modulates neural responses to social feedback in the dorsal anterior cingulate cortex (dACC), dorsal medial prefrontal cortex (dMPFC), posterior superior temporal sulcus (pSTS) and anterior insula (Eisenberger et al., 2011) as well as the ventral anterior cingulate cortex (vACC) and MPFC (Somerville et al., 2010). Though not investigating self-esteem explicitly, other work has explored the neural basis of self-evaluation processes related to self-esteem function. Researchers have found that activity within the orbitofrontal cortex (OFC) and dACC are negatively correlated with the degree to which people view themselves as above average compared with their peers (Beer and Hughes, 2010), and that social-evaluative threats increase neural activity in the dorsolateral prefrontal cortex, OFC, MPFC, insula and amygdala when making self-relevant trait judgments (Hughes and Beer, 2013). Interestingly, researchers have also shown that dMPFC and ventral medial prefrontal cortex (vMPFC) are differentially activated depending on the level of certainty and personal value the traits have in relation to the self (D’Argembeau et al., 2012).
Despite the relevance of these studies, questions remain regarding the representation of self-esteem in the brain. Some of this work provides insight into how self-esteem impacts reactions to social rejection, but it is difficult to draw conclusions about the more general role of neural systems underlying self-esteem outside the context of social feedback. Other studies add to an important growing literature in evaluative self-referential cognition but do not explore the relationship of self-esteem to these processes directly. Moreover, despite the multifaceted nature of the self, none of these studies explored self-esteem or evaluative self-referential processing from a distributed network perspective. This approach could be particularly important given the evidence that the phylogenetic expansion of the human prefrontal cortex may have been driven by an increase in anatomical connections rather than cortical gray matter volume (Schoenemann et al., 2005) over evolutionary history. Thus, one of the primary reasons for the fragmented literature on brain systems underlying self-esteem may be that the phenomenon of self-esteem does not exist as the function of any individual brain region, but rather as the integration of information from multiple regions working together. By definition, the processes that give rise to self-esteem must incorporate evaluative processing with information about the self, and these processes may be reflected in the underlying neural connectivity.
Though the nature of the self-concept is inherently complex, researchers studying the neural basis of self-referential processing have reached a surprisingly high degree of consensus: self-referential processing is most consistently associated with activity in the MPFC. Across numerous laboratories using a variety of different paradigms, the MPFC shows greater activity to representations of the self than that of other people or general semantic information and has a linear increase in activation as information becomes more self-relevant (Wagner et al., 2012). Indeed, a recent meta-analysis of 107 published neuroimaging studies of self-referential processing corroborates the notion that MPFC activity underlies cognitive processes associated with the self compared with baseline activity as well as activity associated with processing information about others (Denny et al., 2012). Because feelings of self-esteem must draw information from one’s self-representation, it stands to reason that the MPFC may be recruited when processing information during self-evaluation.
Although the MPFC appears to support the role of self-representation, evaluative cognition is most associated with activity in another brain area. The ventral striatum, which is part of the mesolimbic dopaminergic pathway, is involved in hedonic motivation and reward. This region is also critical for being able to recognize and maintain positive affect during evaluative processing and has been linked to mental health outcomes. Previous research has shown that clinically depressed patients are unable to maintain sustained ventral striatal activity when attempting to generate positive affect, and failure to engage these systems may contribute to anhedonic symptoms of depression (Heller et al., 2013). Moreover, researchers have linked dopaminergic striatal function to thoughts of self-superiority but have acknowledged that these functions are likely supported by additional frontostriatal circuits not examined in their studies (Yamada et al., 2013). This leaves open the possibility that the MPFC connectivity is contributing to evaluative self-referential processing in ways not currently described. Taken together, connectivity of the ventral striatum and MPFC may contribute to self-esteem by integrating self-representation with feelings of positive affect and reward.
Self-esteem, however, is a multifaceted construct characterized by stable maintenance of positive self-worth as well as momentary feelings of positive self-evaluation. As such, psychologists have designed measures to independently gauge levels of long-term ‘trait’ self-esteem as well as short-term ‘state’ self-esteem (Heatherton and Wyland, 2003). The most popular measure of global (i.e. domain general) trait self-esteem in the literature is the Rosenberg Self-Esteem Scale (Rosenberg, 1965). Unfortunately, an often overlooked issue with this scale is that individuals tend to respond systematically to positively and negatively valenced items regardless of their content, indicating the measure may reflect a response set (Carmines and Zeller, 1974). Other measures of global trait self-esteem, such as the Revised Janis and Field Feelings of Inadequacy Scale (Fleming and Courtney, 1984), avoid these issues and are also commonly used in the literature. State self-esteem is most commonly measured using the State Self-Esteem Scale (Heatherton and Polivy, 1991). This scale is a psychometrically validated measure designed to tap into momentary fluctuations in feelings of self-esteem that are independent of general mood (Bagozzi and Heatherton, 1994) and sensitive to situational manipulation. Overall, trait and state measures of self-esteem tend to be related to one another but operate on separate timescales.
Because trait and state self-esteem are related but temporally distinct psychological constructs, they may be represented by different aspects of the brain. Structural and functional neuroimaging methods offer complimentary means by which to infer both stable and transient connectivity between brain regions. Diffusion tensor imaging (DTI) is a structural magnetic resonance technique designed to assay the stable structural integrity and anatomical connectivity of white matter tracts in the brain, which may better characterize long-term trait self-esteem measures. By contrast, functional magnetic resonance imaging (fMRI) offers a method by which to measure momentary functional connectivity induced by particular events, which may better characterize the transitory nature of state self-esteem. The current study capitalizes on this distinction by using both techniques to investigate individual differences in self-esteem. Using a multimodal approach, it is possible to provide a more compressive investigation of how frontostriatal connectivity contributes to both trait and state aspects self-esteem.
METHODS
Participants
A total of 48 subjects between the ages of 18 and 19 years (28 female) were recruited from the local Dartmouth community. All subjects completed behavioral questionnaires and high-resolution anatomical and DTI scans. A subset of 43 of these subjects also completed the fMRI portion of the experiment. Of these individuals, three subjects were removed because of head movement >3 mm (corresponding to the voxel size) during at least one run of the task, and two others were removed because of incorrectly following task instructions (i.e. did not respond or used the same response for the entire duration of the experiment) leaving 38 subjects (22 female) in the fMRI analyses. All subjects were screened to be right-handed and self-reported no current or history of psychiatric or neurological conditions. Subjects gave informed consent in accordance with the guidelines set by the Committee for the Protection of Human Subjects at Dartmouth College and received course credit or were paid for their participation.
Image acquisition
Magnetic resonance imaging was conducted with a Philips Achieva 3.0 Tesla scanner using a 32-channel phased array coil. Structural images were acquired using a T1-weighted MP-RAGE protocol (220 sagittal slices; TR: 8.176 ms; TE: 3.72 ms; flip angle: 8°; 1 mm isotropic voxels). Diffusion-weighted images were collected using 70 contiguous 2 mm thick axial slices with 32 diffusion directions (91 ms TE, 8848 TR, 1000 s/mm2 b-value, 240 mm FOV, 90° flip angle, 1.875 mm × 1.875 mm × 2 mm voxel size). Two diffusion scans were acquired per subject. Functional images were acquired using a T2*-weighted echo-planar sequence (TR: 2000 ms; TE: 35 ms; flip angle: 90°). For each participant, two runs of 151 whole-brain volumes (35 axial slices per whole-brain volume, 3 mm isotropic voxels) were collected.
Procedure
Each subject’s trait and state self-esteem was assessed outside the scanner. Trait self-esteem was measured using the Janis and Field Feelings of Inadequacy Scale (Fleming and Courtney, 1984), which asked participants to report general self-evaluative feelings over the course of the previous year. This scale was also identified as having robust psychometric properties and was evaluated as the best trait measure of self-esteem (Blascovich and Tomaka, 1991). State self-esteem was measured using State Self-Esteem Scale (Heatherton and Polivy, 1991), which asked participants to report current feelings of self-evaluative attitudes at that moment.
The fMRI procedure was an evaluative self-referential processing paradigm adapted from Moran et al. (2006) using an event-related design. During this task, subjects were asked to make self-relevance judgments to a series of 200 trait adjectives. These words were selected from a list normed for valence (Anderson, 1968) such that half of the presented words were positively valenced (e.g. ‘nice’, ‘competent’), whereas the other half were negatively valenced (e.g. ‘boring’, ‘lazy’). Each word was presented for 1250 ms in white print on a black background followed by a fixation cross for 750 ms. Intertrial intervals consisting of a fixation cross for 2000 ms were pseudo-randomly interspersed to introduce jitter into the fMRI time series. Subjects responded via button box using the scale 1 (‘not at all like me’) through 4 (‘most like me’). Words rated as 1 or 2 were collapsed into traits low in self-relevance, whereas words rated as 3 or 4 were collapsed into traits high in self-relevance. Trials were then sorted into four conditions based on the valence of each trait and the subject’s self-relevance responses to those traits and were used for task regressors in the fMRI time series.
Image analyses
Diffusion tensor imaging
Across modalities and behavioral measures, all data used in the final analysis met the assumption normality for correlational analysis using formal tests as well as visualization (e.g. QQ plots). DTI scans were visually inspected for quality to ensure there were no gross distortions or registration misalignments. DTI data were analyzed using the Diffusion toolbox in FSL (Behrens et al., 2003). Standard preprocessing included brain extraction, eddy current correction and motion correction. We used probabilistic tractography to delineated white matter tracts between the MPFC and bilateral ventral striatum defined anatomically using an automated subcortical segmentation tool, FIRST (Patenaude et al., 2011). A dual-fiber model was implemented with BEDPOSTx (Behrens et al., 2007) to account for crossing fiber uncertainty in the diffusion imaging signal. Using two-mask seeding, 5000 probabilistic tract streamlines were taken at each voxel. This method allowed resulting tractography maps to only include streamlines passing through both seed masks. These results were then normalized to MNI standard space using non-linear registration in FNIRT. To ensure tracts were consistent among subjects, registered tractography results were then binarized and overlaid with all other subjects, and thresholding was set at the group level such that there was a 50% tract probability across subjects in standard space. We quantified individual differences in white matter integrity using first-orientation partial volume fractions (PVF) by overlaying tractography results onto each subject’s PVF images and averaging across voxels. Importantly, these PVF measures are analogous to fractional anisotropy measures (used most commonly in the diffusion imaging literature) but are more conservative and attempt to account for crossing fiber uncertainty by inferring signal from only a single fiber orientation at a time (Jbabdi et al., 2010). These values were then used for the structural connectivity correlational analyses with the self-esteem measures.
Functional magnetic resonance imaging
The fMRI data were analyzed using fMRI Expert Analysis Tool in FSL (Smith et al., 2004). Preprocessing of the fMRI data followed a standard procedure. First, all slices were interpolated to a common time point (i.e. slice-time correction) to correct for differences in slice acquisition. Next, images were smoothed using a Gaussian kernel of 6 mm FWHM, mean-based intensity normalization of all volumes by the same factor and high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 80.0 s). Time-series statistical analyses were carried out using local autocorrelation correction. A two-step normalization process was performed using linear registrations in FLIRT by aligning functional data to each subject’s anatomical scan before registering it to the MNI template.
A psychophysiological interaction (PPI) analysis (Friston et al., 1997) was conducted to assess task-induced MPFC and ventral striatum functional connectivity. Mean time-series signal values were extracted from a 6 mm diameter seed region in the MPFC (MNI: x = 10, y = 52, z = 2) independently defined from peak coordinates of activation to self-relevant stimuli previously found in Kelley et al. (2002), reproduced in Moran et al. (2006) and overlapping with significant areas of self-referential processing from the meta-analysis by Denny et al. (2012). Following the standard PPI protocol in FSL (O’Reilly et al., 2012), these values were used as the physiological regressor in the general linear model along with the mean-centered regressors from each of the four task conditions and their interaction term (i.e. the PPI regressor). Parameter estimates from the PPI regressor were calculated by comparing functional connectivity for each condition relative to baseline. We used bilateral ventral striatum regions of interest (ROIs) within each subject (identical to the DTI ventral striatum ROIs, anatomically defined using FIRST subcortical segmentation) to extract connectivity measures to the MPFC seed region. Average parameter estimates from the PPI regressor were calculated within these ROIs reflecting functional connectivity between these regions within each subject. These values were then used for functional connectivity correlational analyses with the self-esteem measures across subjects.
Post hoc analyses
Both probabilistic tractography and PPI are hypothesis-driven analysis techniques requiring a priori ROI selection, and we had specific predictions regarding the regions underlying in self-esteem representation. However, it is possible that regions other than the hypothesized areas contribute to self-esteem as well. To account for this possibility and increase the specificity of the results, we conducted an additional series of post hoc tests. Each of the post hoc tests followed the same analytical procedures per connectivity modality as the hypothesized regions with the exception of the area being examined.
For the fMRI analyses, 10 additional cortical ROIs were taken from peak coordinates in three recent studies on evaluative self-referential processing by Beer and Hughes (2010), D'Argembeau et al. (2012) and Eisenberger et al. (2011). These ROIs included areas within the medial OFC, lateral OFC, posterior cingulate cortex (PCC), vMPFC, dMPFC, vACC, dACC, pSTS and anterior insula. We used these ROIs for additional PPI analyses, seeding in both left and right accumbens, and tested their correlation with both state and trait self-esteem measures.
For the DTI analyses, we investigated eight additional bilateral white matter pathways linking areas involved in self-referential processing and evaluative cognition. These include the uncinate fasciculus (amygdala–MPFC pathway), cingulum bundle (PCC–MPFC pathway), orbitostriatal tracts (accumbens–OFC pathway) and ventral cingulostriatal tracts (accumbens–vACC pathway). White matter integrity measures were extracted for each of these tracts and correlated with both state and trait self-esteem measures.
RESULTS
Consistent with the psychometric literature on self-esteem, trait and state self-esteem measures were highly related within the current sample (R46 = 0.447, P = 0.0006). Furthermore, during the fMRI portion of the experiment, both trait and state self-esteem were correlated with the endorsement of positive words (trait: R36 = 0.417, P = 0.009; state: R = 0.354, P = 0.0295), inversely correlated with the endorsement of negative words (trait self-esteem: R36 = −0.487, P = 0.002; state self-esteem: R36 = −0.556, P < 0.001) and correlated with the differences between positive and negative trait words (trait self-esteem: R36 = 0.515, P < 0.001; state self-esteem: R36 = 0.525, P < 0.001).
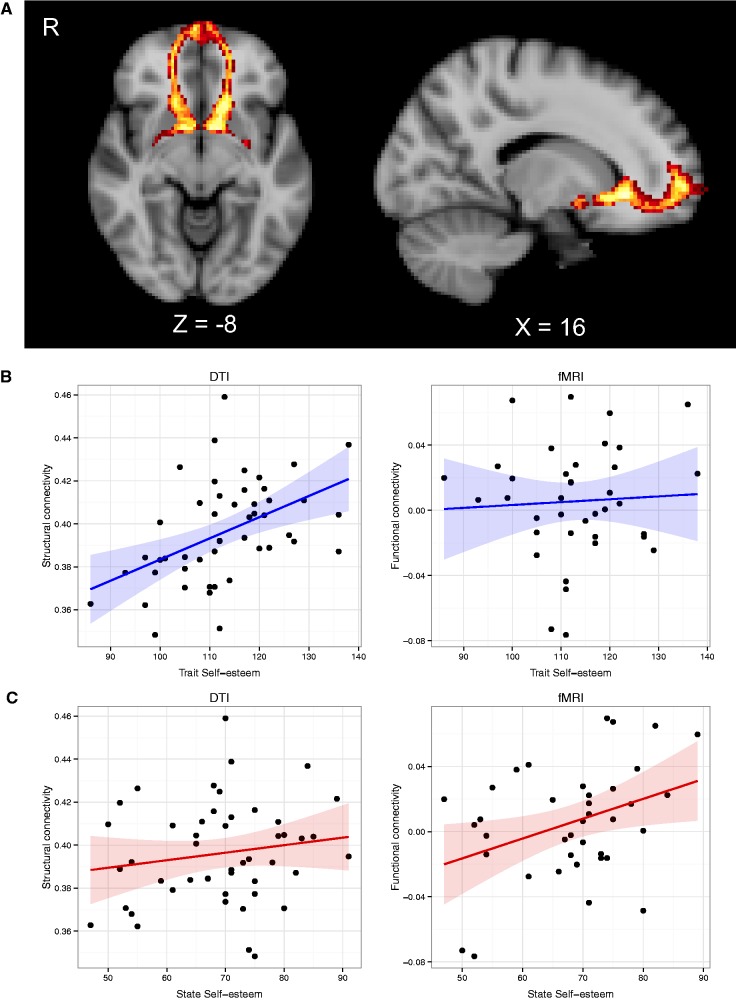
Cross-subject DTI probabilistic tractography analyses revealed a robust white matter pathway linking the MPFC and bilateral ventral striatum, indicating a direct anatomical connection between these regions (Figure 1A). Along these tracts, average white matter integrity showed a significant correlation to trait self-esteem in both the left (R46 = 0.46, P = 0.001) and right hemispheres (R46 = 0.47, P = 0.0007). State self-esteem was not correlated with white matter integrity within these tracts (left hemisphere: R46 = 0.095, P = 0.519; right hemisphere: R46 = 0.16, P = 0.278). Results of the trait self-esteem correlations for both imaging modalities are plotted in Figure 1B.
Fig. 1.
White matter tractography map and correlations of self-esteem measures to structural and functional frontostriatal connectivity. (A) Cross-subject probabilistic tractography results displaying bilateral MPFC to ventral striatum white matter tracts reveal a robust anatomical connection between these regions. Slices are marked with MNI coordinates. (B) Scatterplots displaying the correlations of ‘trait’ self-esteem to both functional and structural connectivity measures of the right ventral striatum to MPFC. Structural connectivity showed a significant relationship to trait self-esteem, whereas functional connectivity did not (N = 48). (C) Scatterplots displaying the correlations of ‘state’ self-esteem to each connectivity measure of the right ventral striatum to MPFC. Conversely to the trait self-esteem findings, state self-esteem showed a significant relationship with functional connectivity between these regions, whereas structural connectivity did not (N = 38). The same patterns were mirrored in the left hemisphere. Shaded areas represent 95% confidence interval.
From the fMRI analyses, state self-esteem was significantly correlated with bilateral functional connectivity of MPFC and ventral striatum when subjects were viewing positively valenced words high in self-relevance in both the left (R36 = 0.33, P = 0.046) and right (R36 = 0.37, P = 0.024) hemispheres. Neither negatively valenced words nor words low in self-relevance showed a significant correlation to either measures of self-esteem. In contrast to the DTI findings, trait self-esteem was not significantly correlated with functional connectivity between these regions (left hemisphere: R36 = 0.26, P = 0.112; right hemisphere: R36 = 0.06, P = 0.738). Results of the state self-esteem correlations for both imaging modalities are plotted in Figure 1C.
From the post hoc analyses, of the 40 additional correlational tests generated by the fMRI procedure, there was only one significant finding; trait self-esteem was negatively correlated to functional connectivity of the right accumbens and lateral OFC coordinate (R36 = −0.31, P = 0.049) from Beer and Hughes (2010). The results from all of the post hoc fMRI analyses are summarized in Supplementary Table S1. From the post hoc DTI analyses, there were two significant findings: bilateral ventral cingulostriatal (connecting the ventral striatum to the vACC) tracts were positively correlated to trait self-esteem (left hemisphere: R46 = 0.34, P = 0.016; right hemisphere: R46 = 0.37, P = 0.009), mirroring the DTI results from our original analysis in frontostriatal connections from ventral striatum to MPFC. However, because of the probabilistic nature of the DTI tractography routine, adjacent ventral striatal–MPFC frontostriatal tracts and ventral striatal–vACC cingulostriatal tracts share partially overlapping tractography results, which the method cannot completely delineate. To account for this spatial overlap and increase the specificity of our results, two additional multiple regression analyses were conducted. Within each hemisphere, we used white matter integrity measures from both the frontostriatal tracts from our original analysis and the cingulostriatal tracts from the post hoc analysis as predictors of trait self-esteem. This allows us to test for the unique variance accounted for by each of the tracts while removing the variance that can be explained by both tracts. From these analyses, frontostriatal tracts remain significant (left hemisphere: β = 195.57, P = 0.027; right hemisphere: β = 235.032, P = 0.03), while cingulostriatal tracts become non-significant (left hemisphere: β = 11.59, P = 0.866; right hemisphere: β = −8.283, P = 0.929). Thus, these results show that when both tracts are included in a model predicting trait self-esteem, only the frontostriatal tracts connecting ventral striatum to the MPFC remain significant predictors of trait self-esteem. The results from all of the post hoc DTI analyses are summarized in Supplementary Table S2.
DISCUSSION
Despite the fact that empirical work has associated high self-esteem with decreased risk for a host of affective and psychiatric disorders and greater responsiveness to their treatment, the brain systems that support this phenomenon have remained largely unelaborated. Higher-order cognitive functions and personality dispositions like self-esteem are complex, and the brain systems that support these phenomena may be the result of interactions among brain regions rather than reflecting the function of any single area working in isolation. To this end, our findings provide convergent evidence that self-esteem is related to frontostriatal connectivity linking areas involved in self-referential processing to those underlying positive evaluation and reward. We demonstrate that stable aspects of self-esteem maintenance are reflected in frontostriatal structural white matter anatomy, whereas momentary feelings of self-esteem are reflected in transient frontostriatal functional coupling of these regions.
An important point to highlight from these results is how the each connectivity modality congruently reflected the relative stability of each self-esteem measure. Namely, stable ‘trait’ self-esteem was related to stable white matter anatomy, and transient ‘state’ self-esteem was related to transient functional coupling. Not only do these results provide convergent evidence that self-esteem is reflected in these frontostriatal circuits but they also suggest that this system can represent self-esteem variability operating on both short-term and long-term timescales. This distinction may be particularly useful when evaluating the efficacy of treatments designed to target changes in self-concept or attitudes about one’s self.
There are several types of cognitive and behavioral approaches aimed at increasing feelings of self-worth and boosting self-esteem (Young, 1994; Newns et al., 2003), but some researchers have questioned their value and efficacy (Baumeister et al., 2003). Because the different brain imaging modalities reflect unique information about an individual’s self-esteem, they may be useful indicators in objectively evaluating the efficacy of these approaches across both the short term and long term. The current study suggests that elevated short-term feelings of state self-esteem increase frontostriatal functional connectivity, but this momentary coupling does not reflect long-term trait self-esteem, which is better reflected in the underlying white matter anatomy. However, this dissociation may not mean that both connectivity modalities are unrelated to one another. Though neuroanatomy is relatively static when compared with the dynamic nature of brain function, increases in white matter integrity have been demonstrated in healthy adults following long-term training regiments (Scholz et al., 2009). To the extent that an individual regularly experiences feelings of high state self-esteem, it is possible that the repeated recruitment of these frontostriatal circuits may increase the structural integrity of white matter tracts within this system over time. This, in turn, may then lead to an increase in trait self-esteem, reflecting the results from our DTI analysis. Future longitudinal studies using self-esteem enhancement therapies will be necessary to test this possibility.
One popular account of self-esteem in the psychological literature is the sociometer theory of self-esteem (Leary et al., 1995; Leary and Baumeister, 2000). This theory postulates that state self-esteem evolved as a monitor of others’ actions and evaluations toward the self and alerts a person to the possibility of social rejection within their environment. However, it is unclear how this theory of self-esteem fits with our current findings. Our task is devoid of any social manipulation and was designed to avoid explicit social comparisons. Moreover, our post hoc analyses did not reveal any significant correlations of self-esteem and brain structure or function to areas previously implicated in the neural underpinnings of the sociometer self-esteem effect (Eisenberger et al., 2011). One possibility for the absence of results related to self-esteem function in these areas could simply be due to the lack of a social manipulation in the task. However, another possibility is that self-esteem is less anchored in social evaluation than previously assumed, and our results reflect a more endogenously generated self-evaluation process. Additional work will be needed to tease apart these possibilities and begin to build brain-based theories of both trait and state self-esteem.
Though the findings in the current study support the role of structural and functional frontostriatal connections underlying multiple aspects of self-esteem, there were three other significant findings from our post hoc analyses. From the DTI post hoc tests, bilateral cingulostriatal tracts connecting the ventral striatum to the vACC were both correlated with trait self-esteem. In addition to the MPFC and ventral striatum, the vACC is another important node in these frontostriatal networks previously implicated in studies of evaluative self-referential processing (Moran et al., 2006; Somerville et al., 2010). However, because of their proximity and the probabilistic nature of the tractography routine, these tracts share a partially overlapping spatial extent with the frontostriatal tracts from the original analysis. When controlling for the shared variance explained both of the tracts in a multiple regression model, only the original frontostriatal tracts remain a significant predictor of trait self-esteem. This suggests that the relationship of cingulostriatal tract integrity to trait self-esteem can be completely accounted for by its overlap with the frontostriatal tracts from the original analysis, adding specificity to the results. From the fMRI post hoc tests, we found that trait self-esteem was negatively correlated with functional connectivity of the right ventral striatum and lateral OFC. This result fits with previous work showing that the activation in the lateral OFC is associated with a reduction in positively biased self-evaluations (Beer and Hughes, 2010). However, it is important to note that this is the only significant correlation across all of our fMRI analyses to be related to trait self-esteem, the only one to not be bilateral and the only one to be negatively correlated with either self-esteem measure. Because of these issues and given the number of statistical tests in our post hoc analyses, caution should be taken when interpreting this finding. Nonetheless, this result warrants further investigation into the role of the lateral OFC in regulating evaluative attitudes about the self.
In conclusion, we provide evidence that individual differences in self-esteem are reflected in both structural and functional frontostriatal circuits linking areas underlying self-referential cognition to ones involved in positive evaluation. Taken together, the current findings suggest that these frontostriatal circuits may give rise to feelings of self-esteem by integrating information about the self with positive affect and reward. Given the evidence that high self-esteem may buffer people against the possibility of acquiring conditions such as depression and anxiety, these frontostriatal connectivity measures may be useful in both objectively measuring an individual’s risk for these disorders as well as evaluating the efficacy of treatments targeting them. These results also add to a growing body of literature on evaluative self-referential cognition and suggest that self-esteem may be better characterized by distributed brain network properties rather than the function of any individual region alone.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
The authors thank Courtney Rogers for help with data collection and Dylan Wagner for help with data analysis. R.S.C. is a National Science Foundation graduate research fellow.
This work was supported by a grant from the National Institute of Mental Health (R01MH059282) to T.F.H.
REFERENCES
- Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: a theory-based subtype of depression. Psychological Review. 1989;96:368. [Google Scholar]
- Anderson NH. Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology. 1968;9(3):272–9. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Bagozzi RP, Heatherton TF. A general approach to representing multifaceted personality constructs: application to state self-esteem. Structural Equation Modeling: A Multidisciplinary Journal. 1994;1(1):35–67. [Google Scholar]
- Baumeister RF, Campbell JD, Krueger JI, Vohs KD. Does high self-esteem cause better performance, interpersonal success, happiness, or healthier lifestyles? Psychological Science in the Public Interest. 2003;4(1):1–44. doi: 10.1111/1529-1006.01431. [DOI] [PubMed] [Google Scholar]
- Beer JS, Hughes BL. Neural systems of social comparison and the “above-average” effect. Neuroimage. 2010;49(3):2671–9. doi: 10.1016/j.neuroimage.2009.10.075. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine. 2003;50(5):1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Blascovich J, Tomaka J. Measures of self-esteem. In: Robinson JP, Shaver PR, Wrightsman LS, editors. Measures of Personality and Psychological Attitudes. San Diego: Academic Press; 1991. pp. 115–60. [Google Scholar]
- Butler AC, Hokanson JE, Flynn HA. A comparison of self-esteem lability and low trait self-esteem as vulnerability factors for depression. Journal of Personality and Social Psychology. 1994;6(1):166. doi: 10.1037//0022-3514.66.1.166. [DOI] [PubMed] [Google Scholar]
- Carmines EG, Zeller RA. On establishing the empirical dimensionality of theoretical terms: an analytical example. Political Methodology. 1974:75–96. [Google Scholar]
- Ciesla JA, Roberts JE. Self-Directed thought and response to treatment for depression: a preliminary investigation. Journal of Cognitive Psychotherapy. 2002;16(4):435–453. [Google Scholar]
- D’Argembeau A, Jedidi H, Balteau E, Bahri M, Phillips C, Salmon E. Valuing one’s self: medial prefrontal involvement in epistemic and emotive investments in self-views. Cerebral Cortex. 2012;22(3):659–67. doi: 10.1093/cercor/bhr144. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24(8):1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Muscatell KA, Byrne Haltom KE, Leary MR. The neural sociometer: brain mechanisms underlying state self-esteem. Journal of Cognitive Neuroscience. 2011;23(11):3448–55. doi: 10.1162/jocn_a_00027. [DOI] [PubMed] [Google Scholar]
- Fleming JS, Courtney BE. The dimensionality of self-esteem: II. Hierarchical facet model for revised measurement scales. Journal of Personality and Social Psychology. 1984;46(2):404–21. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Greenberg J, Solomon S, Pyszczynski T, et al. Why do people need self-esteem? Converging evidence that self-esteem serves an anxiety-buffering function. Journal of Personality and Social Psychology. 1992;63(6):913–22. doi: 10.1037//0022-3514.63.6.913. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Baumeister RF. Binge eating as escape from self-awareness. Psychological Bulletin. 1991;110(1):86–108. doi: 10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Polivy J. Development and validation of a scale for measuring state self-esteem. Journal of Personality and Social Psychology. 1991;60(6):895–910. [Google Scholar]
- Heatherton TF, Wyland CL. Assessing self-esteem. In: Lopez SJ, Snyder CR, editors. Assessing Positive Psychology. Washington, DC: APA; 2003. pp. 219–33. [Google Scholar]
- Heller AS, Johnstone T, Light SN, et al. Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. The American Journal of Psychiatry. 2013;170(2):197–206. doi: 10.1176/appi.ajp.2012.12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes BL, Beer JS. Protecting the self: the effect of social-evaluative threat on neural representations of self. Journal of Cognitive Neuroscience. 2013;25(4):613–22. doi: 10.1162/jocn_a_00343. [DOI] [PubMed] [Google Scholar]
- Jbabdi S, Behrens TEJ, Smith SM. Crossing fibres in tract-based spatial statistics. Neuroimage. 2010;49(1):249–56. doi: 10.1016/j.neuroimage.2009.08.039. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Leary MR, Baumeister RF. The nature and function of self-esteem: sociometer theory. Advances in Experimental Social Psychology. 2000;32:1–62. [Google Scholar]
- Leary MR, Tambor ES, Terdal SK, Downs DL. Self-esteem as an interpersonal monitor: the sociometer hypothesis. Journal of Personality and Social Psychology. 1995;68(3):518. [Google Scholar]
- Lewinsohn PM, Mischel W, Chaplin W, Barton R. Social competence and depression: the role of illusory self-perceptions. Journal of Abnormal Psychology. 1980;89(2):203–12. doi: 10.1037//0021-843x.89.2.203. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Social psychology as a natural kind. Trends in Cognitive Sciences. 2009;13(6):246–51. doi: 10.1016/j.tics.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18(9):1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Newns K, Bell L, Thomas S. The impact of a self-esteem group for people with eating disorders: an uncontrolled study. Clinical Psychology and Psychotherapy. 2003;10(1):64–8. [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience. 2012;7(5):604–9. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Shapiro AM, Gamble SA. Level and perceived stability of self-esteem prospectively predict depressive symptoms during psychoeducational group treatment. The British Journal of Clinical Psychology. 1999;38:425–9. doi: 10.1348/014466599162917. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University; 1965. [Google Scholar]
- Schoenemann PT, Sheehan MJ, Glotzer LD. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nature Neuroscience. 2005;8(2):242–52. doi: 10.1038/nn1394. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neuroscience. 2009;12(11):1370–1. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF. Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cerebral Cortex. 2010;20(12):3005–13. doi: 10.1093/cercor/bhq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs KD, Voelz ZR, Pettit JW, et al. Perfectionism, body dissatisfaction, and self-esteem: an interactive model of bulimic symptom development. Journal of Social and Clinical Psychology. 2001;20(4):476–97. [Google Scholar]
- Wagner DD, Haxby JV, Heatherton TF. The representation of self and person knowledge in the medial prefrontal cortex. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3(4):451–70. doi: 10.1002/wcs.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Uddin LQ, Takahashi H, et al. Superiority illusion arises from resting-state brain networks modulated by dopamine. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(11):4363–7. doi: 10.1073/pnas.1221681110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JE. Cognitive Therapy for Personality Disorders: A Schema-Focused Approach. Sarasota, FL: Professional Resource Press; 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.