Abstract
Adolescents' peer culture plays a key role in the development and maintenance of risk-taking behavior. Despite recent advances in developmental neuroscience suggesting that peers may increase neural sensitivity to rewards, we know relatively little about how the quality of peer relations impact adolescent risk taking. In the current 2-year three-wave longitudinal study, we examined how chronic levels of peer conflict relate to risk taking behaviorally and neurally, and whether this is modified by high-quality peer relationships. Forty-six adolescents completed daily diaries assessing peer conflict across 2 years as well as a measure of peer support. During a functional brain scan, adolescents completed a risk-taking task. Behaviorally, peer conflict was associated with greater risk-taking behavior, especially for adolescents reporting low peer support. High levels of peer support buffered this association. At the neural level, peer conflict was associated with greater activation in the striatum and insula, especially among adolescents reporting low peer support, whereas this association was buffered for adolescents reporting high peer support. Results are consistent with the stress-buffering model of social relationships and underscore the importance of the quality of adolescents’ peer relationships for their risk taking.
Keywords: peers, risk taking, adolescence, friendship, fMRI
Adolescence is a developmental period characterized by increased risk-taking behavior including smoking, drug and alcohol use and risky sexual behaviors such as unprotected sex (Dahl, 2004; Steinberg, 2008). Adolescents' peer culture is thought to play a key role in the development and maintenance of these health-risk behaviors (Prinstein et al., 2001; Steinberg and Monahan, 2007). During adolescence, there is an increase in the amount of time spent with peers, a greater orientation toward peer acceptance and conformity, an increased importance of close friendships and the emergence of romantic relationships (La Greca and Harrison, 2005; Steinberg and Monohan, 2007). As time spent with peers increases, so does the likelihood of experiencing negative peer interactions. Difficulties in establishing and maintaining positive peer relationships are associated with multiple negative developmental outcomes including delinquency, risky sexual behavior and substance use (Parker and Asher, 1987; Kupersmidt et al., 1995; Woodward and Fergusson, 1999; Hussong, 2000).
NEUROBIOLOGY OF PEER INFLUENCE
Research has increasingly focused on biological factors to explain why peers are so influential during adolescence. Recent models from developmental neuroscience suggest that increased risk taking arises during adolescence because of the developmentally early maturation of brain systems involved in reward sensitivity and incentive processing compared with relatively protracted development of brain systems involved in cognitive control (Steinberg, 2008; Somerville et al., 2010). This dual systems perspective posits that a neural imbalance between affective and cognitive control brain regions underlies adolescent risk taking. For example, functional neuroimaging studies have consistently found that adolescents demonstrate hyperactivation in the striatum and insula during reward processing and risk taking, and these activations are associated with increased risk-taking behaviors (Ernst et al., 2005; Durston et al., 2006; Galvan et al., 2006, 2007; van Leijenhorst et al., 2010).
According to Romer and Hennessy’s (2007) biosocial–affect model, peers serve to increase the affective nature of decision making, leading to an even greater attraction to risk taking (Romer and Hennessy, 2007). Thus, peers may heighten vulnerability to risk taking by increasing reward sensitivity. Indeed, Chein et al. (2010) found that the mere presence of peers increased ventral striatum activation during risk taking among adolescents but not adults, highlighting the affective nature of peers during adolescence. Therefore, peers may increase risk taking by heightening the neural imbalance during adolescence.
QUALITY OF PEER RELATIONSHIPS
Despite these recent advances, we know relatively little about how the quality of peer relations impact adolescent risk taking. Two primary dimensions characterizing adolescent friendships include support, which reflects positive quality relationships, and conflict, which reflects negative quality relationships (Berndt and Perry, 1986). Although the presence of peers may relate to heightened risk taking and reward sensitivity generally, there are likely individual differences, such that the quality of peer relationships increases or decreases susceptibility to risk taking. Indeed, the quality of adolescents’ friendships (e.g. intimacy and support), rather than the quantity of friends, is the strongest predictor of adolescent adjustment, including engagement in substance use (Hansell, 1985)
Peer conflict
Peers dominate adolescents’ social worlds, and negative peer experiences are particularly stressful. Indeed, difficulties in maintaining positive peer relationships are associated with a host of negative outcomes, including aggression, delinquency and substance use (Parker and Asher, 1987; Kupersmidt et al., 1995; Woodward and Fergusson, 1999; Hussong, 2000). When examining multiple social risk factors (e.g. group acceptance, group rejection, social support, peer conflict), conflict with one’s best friend is the strongest predictor of adolescent delinquency (Kupersmidt et al., 1995). Adolescent risk taking may be partly based on the impetus to overcome or eliminate the negative feelings resulting from poor relationships (Brady et al., 2009). Therefore, peer conflict may result in greater risk taking because adolescents are more oriented toward the rewards attained from engaging in risky behavior.
Peer support
As adolescents place greater emphasis on peer relationships, close friendships become adolescents’ primary source of social support (Furman and Buhrmester, 1992). Adolescents who do not have close positive peer relationships are less likely to receive emotional support in times of stress (Hussong, 2000). Without such support, adolescents may be more susceptible to the negative effects of conflict. According to the stress-buffering model of social relationships, social connection and support provide individuals with the psychological resources necessary to cope with stress, thereby decreasing the likelihood of engaging in risky behavior as a means of coping (Cohen et al., 2001). Thus, high levels of peer support may buffer adolescents from engaging in risk-taking behavior as a response to high levels of peer conflict. Indeed, prior research has found that adolescents who report low levels of peer support experience greater risk-taking behavior following stressful events, whereas those who report high peer support are buffered from this effect (Brady et al., 2009).
Alternatively, it is possible that high levels of peer support may increase risky behavior. According to the peer socialization of risk model, adolescents with high levels of support from peers engage in greater levels of risk-taking behavior, perhaps because of modeling, greater opportunities or social norms in the peer group (Jessor, 1993; Brady et al., 2009). Indeed, high levels of peer support have been associated with substance use (McCubbin et al., 1985; Wills et al., 2004) and delinquency (Windle, 1992). Perhaps this is due to the nature of peer relationships, such that peers tend to focus on social activities with positive hedonic qualities and to engage in spontaneous and impulsive behaviors (Wills et al., 2004).
CURRENT STUDY
In the current 2-year three-wave longitudinal study, we examined how chronic levels of peer conflict relate to risk taking at the behavioral and neural level, and whether this is modified by high-quality peer relationships. During the fall of their 9th or 10th grade, and again 1 year later, 46 adolescents completed daily diary checklists for 2 weeks. Each night adolescents indicated whether they argued with a close friend, boyfriend or girlfriend. By asking adolescents to indicate whether they argued with friends each day for 2 weeks across 2 years, we were able to measure the chronicity of peer conflict (i.e. occurring at high levels across both years). Daily diary reports are ideal measurements for capturing the frequency with which events occur (Telzer and Fuligni, 2013) as they ‘capture life as it is lived’ and are less susceptible to recall biases (Bolger et al., 2003). Moreover, prior research has shown the value of correlating daily events to neural processes in order to connect key adolescent experiences to differential activation patterns (e.g. Forbes et al., 2010; Telzer et al., 2010). At each wave of daily diaries, adolescents also completed a measure of peer support. A few months after completing the second wave of daily diaries, adolescents underwent a functional brain scan, during which they completed a risk-taking task. We hypothesized that more chronic peer conflict would be associated with increased risk taking behaviorally and heightened affective neural response (e.g. ventral striatum and insula) during the risk-taking task. Moreover, we tested whether this association was modified by adolescents’ reports of supportive friendships. That is, do high levels of peer support buffer or exacerbate the association between peer conflict and neural sensitivity to risk taking?
METHODS
Participants
Participants were recruited through one high school in the Los Angeles metropolitan area to participate in the daily diary waves (Wave 1 and Wave 2) of data collection occurring across 2 years of high school. Those who completed both Waves 1 and 2 (93 adolescents) were recruited for the neuroimaging session (Wave 3). Forty-six adolescents (20 males, 26 females) completed all three waves of data collection. At the first wave of data collection, adolescents completed daily diary checklists and a questionnaire in the fall of their 9th or 10th grade (Mage = 14.8 years; range = 14–16 years). One year later in the fall of their 10th or 11th grade, adolescents completed the same daily diary checklists and questionnaire (Mage = 15.9 years; range = 15–17 years). A few months after completing the second wave of daily diaries and questionnaires, adolescents completed a functional magnetic resonance imaging (fMRI) scan as well as self-report measures of risk-taking behavior (Mage = 16.3 years). In addition, the primary caregiver of each participant (mothers = 36, fathers = 10) completed self-report measures of their child’s conduct problems at the first wave of daily diaries. Participants were from relatively lower socioeconomic backgrounds, with an average yearly family income of $28 365, with 19.6% of primary caregivers (usually the mother) unemployed and 29.3% of secondary caregivers (usually the father) unemployed. Participants completed written consent and assent in accordance with the University of California, Los Angeles (UCLA’s) Institutional Review Board.
Peer relationship measures
Peer conflict
At Waves 1 and 2, adolescents completed a daily diary for 14 consecutive days. Each night before going to bed, they indicated whether they had experienced peer conflict by checking off if two events had occurred (‘argued with a close friend’ and ‘argued with a boyfriend or girlfriend’). To create a measure that represents the chronicity of peer conflict, we coded whether adolescents experienced any peer conflict on any day at each wave (0 = no peer conflict experienced at either wave, 1 = peer conflict experienced on at least 1 day at one wave only, 2 = peer conflict experienced on at least 1 day at both waves). In addition, we took into consideration the level of peer conflict by coding whether adolescents experienced peer conflict on at least 25% of the days (1 = experienced peer conflict on 25% of days or more at one wave only, 2 = experienced peer conflict on 25% of days or more at both waves). By taking the sum of whether they experienced peer conflict at each wave and whether they experienced high peer conflict (i.e. >25% of days), we created a scale that ranged from 0 to 4 (0 = no conflict experienced at either wave; 1 = low conflict experienced at one wave only; 2 = low conflict experienced at both waves or high conflict experienced at one wave; 3 = low conflict experienced at one wave and high conflict experienced at the other wave; 4 = high conflict experienced at both waves). See Table 1 for frequencies of peer conflict by gender. Although females tended to report slightly higher levels of peer conflict (M = 1.8, s.d. = 1.4) than males (M = 1.2, s.d. = 1.6), males and females did not differ significantly from each other t(44) = 1.3, ns.
Table 1.
Frequencies of peer conflict by gender
| Peer conflict | N female | N male | Total |
|---|---|---|---|
| 0 | 7 | 10 | 17 |
| 1 | 2 | 5 | 7 |
| 2 | 9 | 0 | 9 |
| 3 | 5 | 1 | 6 |
| 4 | 3 | 4 | 7 |
Note: Males and females did not differ significantly on peer conflict. 0 = no conflict experienced at either wave; 1 = low conflict experienced at one wave only; 2 = low conflict experienced at both waves or high conflict experienced at one wave; 3 = low conflict experienced at one wave and high conflict experienced at the other wave; 4 = high conflict experienced at both waves.
Peer support
At Waves 1 and 2, adolescents completed the Inventory of Peer Attachment (Armsden and Greenberg, 1987). The scale measures how much adolescents feel they can trust, communicate with and are supported by their peers. Using a five-point scale (1 = almost never to 5 = almost always), adolescents answered nine questions about their friends in the past month (e.g. ‘I trusted my friends’, ‘I could count on my friends when I needed to talk’, ‘My friends showed that they understand me’). At each wave, the nine items were averaged. A measure of peer support was created by taking the sum of the two waves (range = 1–10), such that higher scores indicated greater peer support. The scale had good internal consistency at each wave (Wave 1: α = 0.92; Wave 2: α = 0.94). Males and females differed significantly on their reports of peer support, such that females (M = 8.01, s.d. = 1.00) reported higher levels than males (M = 6.38, s.d. = 1.76), t(44) = 3.97, P < 0.001.
Control measures
To examine the role of peer relationships on adolescent risk taking, it is necessary to take into account potential confounding factors that may be correlated with both the quality of peer relationships and risk-taking behavior. Relatively greater adolescent risk taking could be explained simply by the fact that adolescents spend more time with friends than do adults, and adolescents who spend more time with their peers have greater opportunities to experience negative peer relationships. Indeed, research has found that adolescents who spend more time with their friends after school report higher levels of delinquency, substance use and susceptibility to peer pressure (Flannery et al., 2010). Therefore, in the current study, we control for the amount of time adolescents spend with their friends. In addition, higher incidences of peer conflict and risky behavior have been associated with earlier conduct problems (Woodward and Fergusson, 1999), suggesting that conduct problems could account for the association between negative peer relations and risky behavior. In order to control for this possibility and to more directly test how negative peer relationships impact adolescent risk taking, we control for adolescents’ conduct problems.
Time with friends
To control for the amount of time adolescents spent with their friends, participants completed the daily diary checklist each night at Waves 1 and 2. They indicated each night whether they had spent time with their friends, boyfriend or girlfriend outside of school each day. We created an index for each wave that represented the proportion of days they spent with their friends.
Conduct problems
During the first wave of data collection, the Child Behavior Checklist (Achenbach, 1991) was completed by parents and their child to assess problem behaviors. The externalizing subscale includes 32 items tapping a range of conduct problem behaviors (e.g. argues a lot, destroys things, gets in fights, lacks guilt, lies and cheats, threatens others). The parent and youth report of adolescents’ externalizing was used as a control variable to index conduct problems. The scale had good internal consistency (parent report: α = 0.83; adolescent report: α = 0.85).
Self-report risk taking
Risk-taking behavior
At the time of the brain scan (Wave 3), adolescents completed a modified version of the Adolescent Risk-Taking Scale (Alexander et al., 1990) to measure how often they engaged in risky behaviors. Adolescents responded to nine items using a four-point scale (0 = never, 1 = once or twice, 2 = several times and 3 = many times) to indicate the frequency with which they have engaged in the following behaviors: raced on a bike or boat, did something risky or dangerous on a dare, broke a rule that their parents set just for the thrill of seeing if they could get away with it, stole or shoplifted, slipped out at night while their parents thought they were asleep, willingly rode in a car with someone who was a dangerous driver, tagged or defaced publicproperty, drove in a car without wearing a seatbelt and had sex without using protection. The scale had decent internal consistency (α = 0.75).
fMRI paradigm
To examine neural sensitivity to risk, participants completed the Balloon Analogue Risk Task (BART; Lejuez et al., 2002). Importantly, behavioral performance on the BART correlates with real-life risk behaviors such as adolescent smoking, sexual promiscuity, addiction and drug use (Lejuez et al., 2003; Bornovalova et al., 2009), suggesting that this task provides a scanner-compatible proxy for measuring real-world behaviors.
On each trial of the task, participants were shown a virtual red-colored balloon and were given the option to inflate the balloon, which can either grow larger or explode. The larger the balloon is inflated, the greater the monetary reward but the higher the probability of explosion. Participants press one of two buttons to either inflate (pump) the balloon or to ‘cash out’. Each trial begins with the presentation of a balloon and ends when the balloon either explodes or the participant cashes out (Figure 1). The participant receives a monetary payoff (25 cents) for each pump on which the balloon is successfully inflated and can stop inflating the balloon at any point and keep the accumulated payoff. The greater the balloon is inflated, the higher the monetary reward. However, if the balloon explodes before cashing out, the participant receives no payoff for that trial, but earnings from the previous trials are not affected. Participants are instructed that the greater they pump the balloon, the more money they can earn. The number of inflations before explosion varied probabilistically according to a Poisson distribution. This pattern models the unpredictable rewards and punishments that characterize real-world risky behaviors. As pumping progresses during a trial, explosion probability increases exponentially. The explosion point of each balloon was drawn from a uniform distribution from 1 to 12 pumps. After each pump, the balloon image disappeared (1–3 s, variable duration) until the outcome was displayed: a larger balloon or an exploded one. At the end of each trial, the screen was blank for a varying duration (1–12 s, average 4 s). In addition to the red balloons, participants were also presented with white balloons that had no cash value associated with pumps. The white balloons were not included in analyses in the current study. The task was self-paced and was performed during one 9 min run. Because the task was self-paced but time limited, participants completed varying number of total balloons. Participants received their total earnings in cash at the end of the task.
Fig. 1.
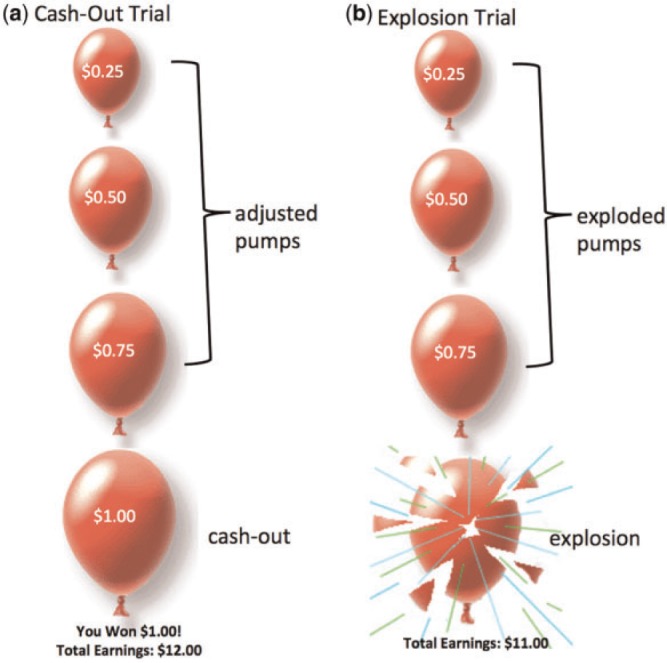
Examples of trials on the BART. (a) risk-taking trial that results in a cash-out outcome (b) risk-taking trial that results in an explosion.
Behavioral measures on the BART include mean response time to pumps and mean response time to cash outs. Response times were calculated for each individual pump and for each cashed balloon. The mean response times were derived by taking the average response times across all individual pumps that were eventually cashed (i.e. adjusted pumps) and the average response times on each cashed-out trial. For the pumps, we analyzed the adjusted pumps, which represent pumps on balloons that did not explode. This is preferable to examining pumps on balloons that did explode because the number of pumps is necessarily constrained on balloons that explode (Lejuez et al., 2002; Cavalca et al., 2012). Response times for balloons that eventually exploded were therefore not calculated for the behavioral response time data. Faster response times are generally indicative of more impulsive decision making. In addition, percent of balloons successfully cashed is an index of safer behavior, whereas percent of balloons that resulted in an explosion represents more risky and impulsive behavior. Greater average pumps on balloons that were cashed represent a greater orientation toward risk. Finally, total earnings on the task represent the adaptive nature of participants’ decisions. Less money earned overall suggests less adaptive decisions either because they are being overly safe (i.e. cashing out early) or overly risky (exploding more balloons).
fMRI data acquisition and analysis
fMRI data acquisition
Imaging data were collected using a 3 Tesla Siemens Trio MRI scanner. The tasks were presented on a computer screen, which were projected through scanner-compatible goggles. The BART task consisted of 270 functional T2*-weighted echoplanar images (EPIs) (slice thickness, 4 mm; 34 slices; TR = 2 s; TE = 30 m/s; flip angle = 90°; matrix = 64 × 64; FOV = 200 mm; voxel size 3 × 3 × 4 mm3). A T2*-weighted, matched-bandwidth (MBW), high-resolution anatomical scan and magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scan were acquired for registration purposes (TR: 2.3; TE: 2.1; FOV: 256; matrix: 192 × 192; sagittal plane; slice thickness: 1 mm; 160 slices). The orientation for the MBW and EPI scans was oblique axial to maximize brain coverage.
fMRI data preprocessing and analysis
Neuroimaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Preprocessing for each participant’s images included slice timing to adjust for temporal differences in slice acquisition within each volume and spatial realignment to correct for head motion (no participant exceeded 2 mm). The realigned and slice-timing-corrected functional data were coregistered to the high-resolution MPRAGE, which were then segmented into cerebrospinal fluid, gray matter and white matter. The normalization transformation matrix from the segmentation step was then applied to the functional and structural images, thus transforming them into standard stereotactic space as defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping. The normalized functional data were smoothed using an 8 mm Gaussian kernel, full width at half maximum, to increase the signal-to-noise ratio.
Whole brain statistical analyses were performed using the general linear model (GLM) in SPM8. Each trial was convolved with the canonical hemodynamic response function (HRF). High-pass temporal filtering with a cutoff of 128 s was applied to remove low-frequency drift in the time series. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm with an autoregressive model order of 1. One GLM was defined for the BART, which included multiple regressors for each event type: pumps, cash outs and explosions. For the pumps, we analyzed the adjusted pumps, which represent pumps on balloons that did not explode. Pumps on balloons that exploded were therefore included in a separate regressor that was not used as a condition of interest. Convolution with the HRF was applied to each individual pump. Pumps, cash outs and explosions were modeled with a parametric regressor that tested for the linear relationship between brain activation and the magnitude of pumps, reward or loss. We used pump number as a parametric modulator, with each pump in a trial assigned a weight that increased linearly across pumps within a trial. On cash-out trials and explosions, this number represented how many pumps occurred before the cash out or explosion. The number of pumps was demeaned by subtracting the mean number of pumps from each pump number within the trial. Because the task was self-paced, the duration of each trial lasted from the onset of each balloon to the point at which the participant made a button response to pump or cash out that balloon. This value was calculated for each individual pump and not across the entire balloon from presentation to either explosion or cash out. Null events, consisting of the jittered intertrial intervals, were not explicitly modeled and therefore constituted an implicit baseline.
The following analyses were run at each voxel across the entire brain volume: (i) regression analyses examining how neural activation during risky decisions (i.e. increasing pumps) relates to chronic peer conflict, and (ii) moderation regression analyses examining whether peer support moderates the association between peer conflict and neural sensitivity to risk taking. To correct for multiple comparisons, we conducted a Monte Carlo simulation implemented using 3dClustSim in the software package AFNI (Ward, 2000). Results of 3dClustSim indicated a voxel-wise threshold of P < 0.005 combined with a minimum cluster size of 28 voxels for the whole brain, corresponding to P < 0.05, family-wise error (FWE) corrected. Note that results from this sample of adolescents have been published previously (Telzer et al., 2013a,b, 2014).
RESULTS
Behavioral results
Performance on the BART
On average, participants completed 20.6 red balloons (range 13–34), successfully cashed out 64.8% of red balloons (range 43–95%) and exploded 33.6% of red balloons (range 10–56%). Participants inflated red balloons (adjusted pumps) 3.7 pumps on average (range 1.9–6.2) with an average maximum of 6.5 pumps (range 3–11). The average total number of adjusted pumps across all cashed balloons (i.e. number of trials included in fMRI analyses) was 49 (range 19–86). Participants earned $15.70 on average (range $8.25–26.75) and were significantly slower when making decisions to cash out (M = 0.91, s.d. = 0.32) than to inflate balloons (M = 0.77, s.d. = 0.26), t(45) = 3.36, P < 0.005. Providing ecological validity for the BART, participants who reported greater real-life risk taking earned less money on the BART (r = −0.31, P < 0.05) and exploded marginally more balloons (r = 0.27, P = 0.07).
Peer conflict and behavioral links to risk taking
In regression analyses in SPSS, we regressed peer conflict on behavioral performance on the BART and self-report risk-taking behavior. Greater peer conflict was associated with faster reaction times when pumping the balloons during the BART (β = −0.29, P < 0.05). However, after controlling for all confounds (gender, conduct problems, time spent with friends), this association was no longer significant, although the effect size remained the same (β = −0.29, P = 0.15). Peer conflict was not associated with average number of pumps or with percent of balloons cashed out or exploded. Peer conflict was related to greater self-reported risk-taking behavior (β = 0.56, P < 0.001), but this effect became non-significant when all control variables were entered into the model (β = 0.24, P = 0.12).
Peer support and behavioral links to risk taking
Peer support and peer conflict were not significantly correlated. We regressed behavioral performance on the BART and self-reported risk-taking behavior on peer support, controlling for peer conflict and all confounds. Peer support was not associated with behavioral performance on the BART. Peer support was associated with lower self-reported risk-taking behavior (β = −0.37, P < 0.05).
Peer support modifies the association between peer conflict and risk taking
Moderation analyses were estimated by computing interaction terms by first centering the moderator variable (peer support) and multiplying it by the centered version of peer conflict. The interaction term, the centered moderator and centered peer conflict were entered into regression analyses to predict risk-taking behavior on the BART as well as self-reported risk taking. All variables were entered as continuous measures. Gender, conduct problems and time spent with friends were entered as covariates. Peer support significantly moderated the association between peer conflict and the percent of balloons that were cashed (β = 0.42, P < 0.05), the percent of balloons that exploded (β = −0.37, P < 0.05) and self-reported risk taking (β = −0.27, P < 0.05). In this analysis with the interactions in the model, peer support was associated with more balloons that were cashed and fewer balloons that exploded (β = 0.37, P < 0.05 and β = −0.36, P < 0.05, respectively).
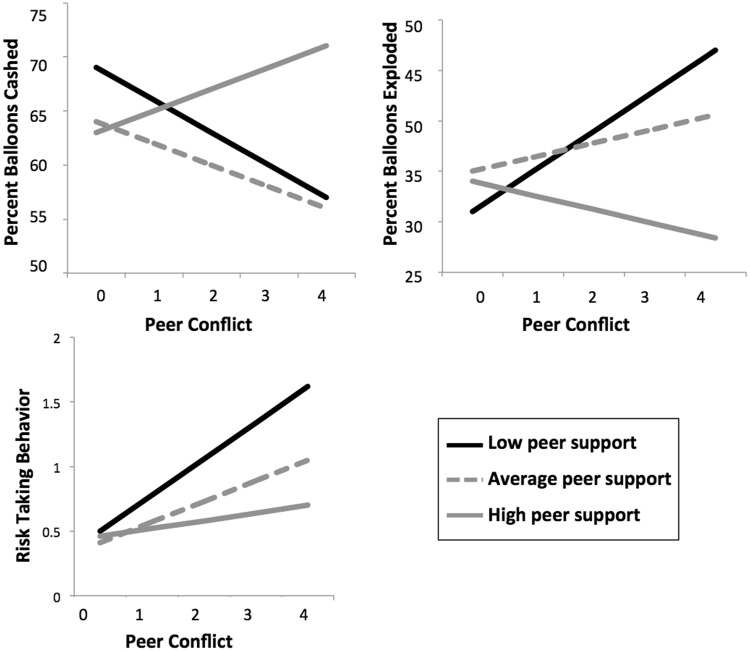
For descriptive purposes to explore the significant interactions, we divided the sample into thirds, representing those who reported low levels of peer support (N = 17), medium levels of peer support (N = 13) and high levels of peer support (N = 18). As shown in Figure 2, higher levels of peer conflict were associated with fewer balloons successfully cashed and greater balloons that resulted in an explosion when adolescents reported low levels of peer support. High levels of peer support buffered this association. In terms of self-reported risk taking, higher levels of peer conflict were related to greater levels of risk taking, especially for adolescents reporting low levels of peer support. High levels of peer support buffered this association.
Fig. 2.
Peer support modulates the effect of peer conflict on risk-taking behavior during the BART and self-reported risk taking. Peer conflict is coded as: 0 = no conflict either year, 1 = low conflict 1 year only, 2 = low conflict both years, 3 = low conflict 1 year, high conflict 1 year, 4 = high conflict both years.
Neuroimaging Results
Peer conflict and neural correlates of risk taking
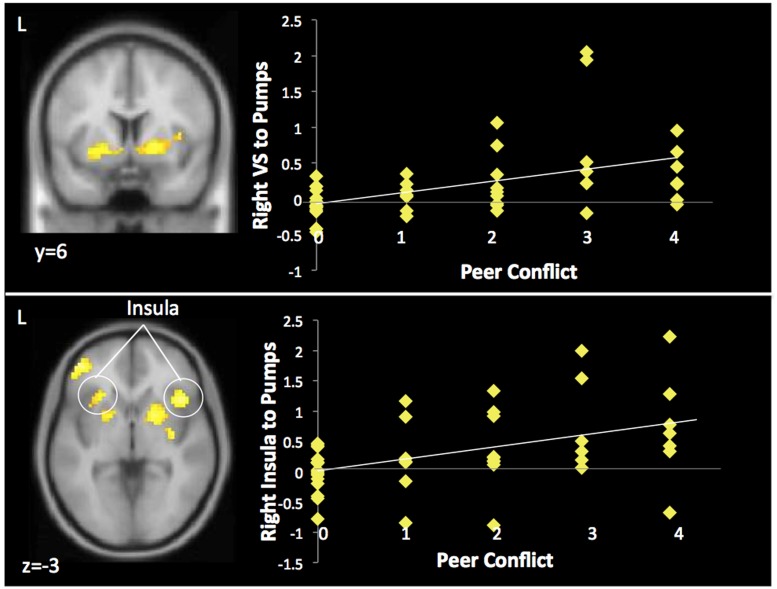
In whole-brain regression analyses, we correlated peer conflict with neural activation to pumps, explosions and cash outs. During pumps, greater peer conflict was associated with greater activation in the bilateral insula, the bilateral ventral striatum (see Figure 3), the right ventrolateral prefrontal cortex (VLPFC), the supplementary motor area (SMA), and the bilateral dorsolateral PFC (DLPFC) (see Table 2). We added the potential confounds as covariates (gender, conduct problems and time spent with friends), and these effects remained significant. Next, in order to control for potential differences in task performance, we controlled for the number of total balloons that participants successfully cashed, the average number of adjusted pumps per balloon and the maximum number of pumps across the task. All the imaging effects remained significant, suggesting that the neural differences are not due to differences in the task context (i.e. having a smaller number of pumps per trial) and are due to differences in peer conflict. Peer conflict was not associated with neural activation during cash outs or explosions.
Fig. 3.
Neural activation in the striatum and insula correlated with greater peer conflict during pumps. Peer conflict is coded as: 0 = no conflict either year, 1 = low conflict 1 year only, 2 = low conflict both years, 3 = low conflict 1 year, high conflict 1 year, 4 = high conflict both years.
Table 2.
Peer conflict and neural correlates of risk taking
| Convert | Anatomical region | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Pumps | ||||||
| R VS | 12 | 5 | −10 | 3.53 | 87 | |
| L VS | −10 | 5 | −10 | 3.54 | 71a | |
| L insula | −24 | 23 | −5 | 4.05 | a | |
| R insula | 38 | 20 | 0 | 3.86 | 85 | |
| R DLPFC | 45 | 41 | 28 | 3.89 | 45 | |
| L DLPFC | −45 | 17 | 13 | 4.22 | 185b | |
| R VLPFC | −47 | 47 | 1 | 3.69 | b | |
| SMA | −9 | 11 | 49 | 3.89 | 83 |
Note: L and R refer to left and right hemispheres; x, y and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. All regions are listed at cluster-forming threshold of P < 0.005 with 28 contiguous voxels, corresponding to P < 0.05 FWE corrected. The following abbreviations were used for the specific brain regions: VS, ventral striatum; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; SMA, supplementary motor area. a,bRegions marked with the same superscript letter are part of the same cluster.
Peer support and neural correlates of risk taking
In whole brain regression analyses controlling for peer conflict, we correlated peer support with neural activation during pumps, cash outs and explosions. Peer support was associated with increased medial PFC activation during cash outs [t(44) = 5.41, P < 0.005 corrected, k = 462 contiguous voxels, x, y, z = −18, 56, 1] and pumps [t(44) = 4.07, P < 0.005 corrected, k = 187 contiguous voxels, x, y, z = 9, 50, 10] and to greater activation in the temporal parietal junction [t(44) = 3.96 P < 0.005 corrected, k = 35 contiguous voxels, x, y, z = 60, −55, 28] and inferior frontal gyrus [t(44) = 3.04, P < 0.005 corrected, k = 30 contiguous voxels, x, y, z = −33, 23, −17] during pumps. Peer support was not associated with neural activation during explosions.
Peer support modifies the association between peer conflict and neural correlates of risk taking
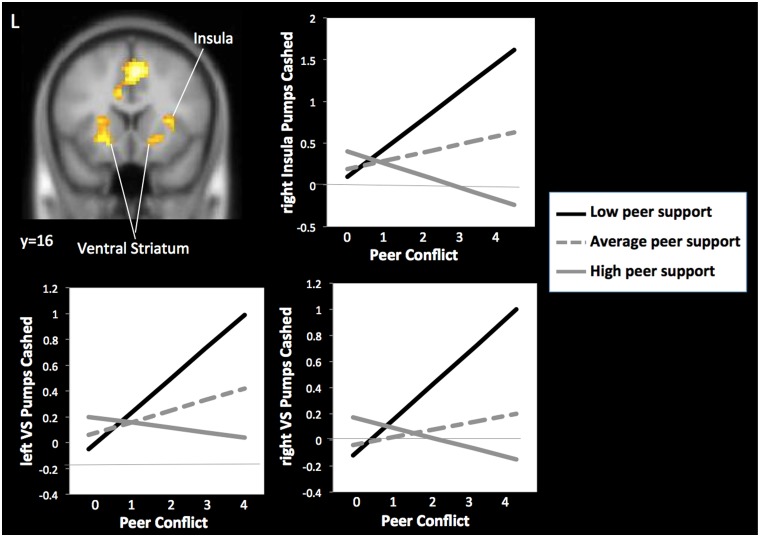
We ran moderation analyses using the same interaction terms described in the behavioral analyses. This interaction term was used as a regressor in whole brain regression analyses during pumps, cash outs and explosions. The centered moderator and centered peer conflict were also entered as regressors. Gender, conduct problems and time spent with friends were entered as covariates. As shown in Table 3, we found a significant interaction in the right insula and the bilateral ventral striatum during increasing pumps (for other significant clusters, see Table 3). For descriptive purposes, we extracted the parameter estimates from the striatum and insula. We divided the sample into thirds representing those reporting low, average, and high levels of peer support. We then graphed the association between peer conflict and neural activation for adolescents in these three groups. As shown in Figure 4, adolescents who reported low peer support and higher levels of peer conflict showed an exaggerated neural response in the insula (B = 0.24, SE = 0.11, P < 0.005), the right ventral striatum (B = 0.21, SE = 0.06, P = 0.007) and the left ventral striatum (B = 0.19, SE = 0.04, P < 0.005) during pumps. In contrast, high levels of peer support buffered this effect, such that peer conflict was not associated with neural activation in the right ventral striatum (B = −0.06, SE = 0.06, ns) and was actually associated with attenuated neural response in the insula (B = −0.22, SE = 0.05, P < 0.001) and left ventral striatum (B = −0.08, SE = 0.02, P < 0.05).
Table 3.
Peer support moderates peer conflict and neural correlates of risk taking
| Convert | Anatomical region | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Pumps | ||||||
| R VS | 16 | 18 | −6 | −3.62 | 28 | |
| L VS | −18 | 18 | −5 | −4.10 | 39 | |
| R insula | 34 | 20 | 4 | −3.83 | 30 | |
| L Fusiform | −33 | −67 | −17 | −3.98 | 147 | |
| L DLPFC | −42 | 50 | 16 | −4.06 | 154 | |
| R DLPFC | 33 | 53 | 31 | −4.18 | 93 | |
| Cuneus | 0 | −82 | 16 | −3.39 | 55 | |
| Cuneus | 0 | −79 | 37 | −3.88 | 82 | |
| SMA | 6 | 14 | 49 | −5.13 | 382 | |
| L Post-central gyrus | −30 | −40 | 67 | −4.48 | 974 | |
| R Pre-central gyrus | 33 | −10 | 61 | −3.62 | 56 | |
| Supramarginal gyrus | −57 | −19 | 43 | −3.7 0 | 46 | |
| R cerebellum | 12 | −70 | 17 | −4.20 | 47 | |
| L cerebellum | −33 | −67 | −17 | −3.78 | 46 | |
| Cash outs | Pre-central gyrus | −36 | −28 | 61 | 3.27 | 51 |
| Precuneus | 15 | −46 | 61 | 3.59 | 59 | |
| SMA | 6 | −10 | 64 | 3.38 | 43 |
Note: L and R refer to left and right hemispheres; x, y and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. All regions are listed at cluster-forming threshold of P < 0.05 FWE corrected. The following abbreviations were used for the specific brain regions: VS, ventral striatum; SMA, supplementary motor area
Fig. 4.
Peer support modulates the effect of peer conflict on ventral striatum and insula activation during risk taking. Peer conflict is coded as: 0 = no conflict either year, 1 = low conflict 1 year only, 2 = low conflict both years, 3 = low conflict 1 year, high conflict 1 year, 4 = high conflict both years.
During cash outs, we found a significant interaction in the precuneus, SMA and the left pre-central gyrus (Table 3). There were no significant interactions with peer support during explosions.
Linking neural response to behavioral measures of risk taking
Our final set of analyses examined whether neural activation in the insula and ventral striatum were correlated with behavioral performance on the BART and self-reported risk taking. We created functional regions of interest (ROI) from the clusters of activation that were significant in the contrast that correlated peer conflict on neural activation during pumps. We extracted parameter estimates of signal intensity from the entire functional ROIs and correlated neural activation with behavioral and self-report measures in SPSS. This analysis therefore tests whether activation in the specific functional regions that were correlated with greater peer conflict were also correlated with greater risk taking. Results indicate that greater activation in the insula and ventral striatum were associated with faster response times during pumps on the BART (rs = −0.34 and −0.29, Ps < 0.05) and cashing out on fewer balloons (rs = −0.36 and −0.39, Ps < 0.05). Greater activation in the insula and ventral striatum were also associated with greater self-reported risk taking (rs = 0.35 and 0.33, Ps < 0.05).
Discussion
Adolescents engage in high rates of health-compromising risky behavior. Adolescents’ peer culture plays an important role in the development and maintenance of risk-taking behavior. Despite recent advances in developmental neuroscience suggesting that peers may increase neural sensitivity to rewards (Chein et al., 2010), we know relatively little about how the quality of peer relations impact adolescent risk taking. In the current study, we took a comprehensive approach to better understand the role that peers play in facilitating risk taking. Our results are consistent with the stress-buffering model of social relationships (Cohen et al., 2001). Importantly, although peer conflict was associated with greater risk-taking behavior and heightened sensitivity in neural regions involved in affect and reward processing during risk taking, high levels of peer support served to buffer these associations. Our findings highlight the important role that peers play in the development of risky behavior and underscore the importance of the quality of adolescents’ peer relationships.
Behaviorally, our findings indicate that peer conflict is associated with greater risk-taking behavior. These findings are consistent with other research demonstrating the negative role that poor peer relationships play in youths’ lives (Parker and Asher, 1987; Kupersmidt et al., 1995; Woodward and Fergusson, 1999; Hussong, 2000; Peake et al., 2013). In contrast, peer support was associated with less risky behavior. These findings are consistent with theories of social support that suggest that adolescents with more supportive friendships are less susceptible to externalizing symptoms (Hussong, 2000).
Importantly, the relationship between peer conflict and risky behavior was moderated by peer support. Although adolescents experiencing more chronic levels of peer conflict reported greater risk taking, those reporting high levels of peer support were buffered from engaging in greater risk taking on the BART and in their real-life self-reported risk taking. Only adolescents with low peer support demonstrated heightened risk-taking behavior following more chronic levels of peer conflict. These findings are consistent with the stress-buffering model of social relationships (Cohen et al., 2001) and suggest that adolescents who have more supportive and close friendships are less susceptible to the negative effects of conflict. Perhaps these adolescents are more likely to receive emotional support from their friends (Sandler et al., 1989), which provides a means of coping with stress. Thus, positive and supportive peer relations play an important protective role in youths’ lives, buffering them from risky behavior.
Each of our behavioral effects was paralleled by greater activation in neural regions involved in reward sensitivity during risk taking. Specifically, more chronic levels of peer conflict were associated with increased activation in the striatum and the insula during increasing pumps on the BART. Given that we performed a parametric analysis examining how the brain responds to increasing levels of risk (i.e. increasing pumps), the insula and ventral striatum appear to be sensitive to the amount of risk, with greater risks eliciting more activation in these brain regions. Indeed, the insula is involved in tracking risk in the environment (Singer et al., 2009), and the striatum has been consistently linked with sensitivity to rewards, with greater rewards eliciting greater striatal activity (e.g. Galvan et al., 2006; Telzer et al., 2013b). These findings suggest that chronic peer conflict sensitizes neural systems involved in reward sensitivity. Therefore, peer conflict may relate to higher levels of risk taking because risks become comparatively more rewarding and salient. Indeed, ventral striatum and insula activation were associated with behavioral indexes of risk taking. In accordance with the biosocial-affect model (Romer and Hennessy, 2007), negative peer relationships serve to increase the affective nature of risk taking, leading to an even greater attraction to risks. Thus, greater peer conflict may lead to heightened risk taking because adolescents are more oriented toward the rewards attained from engaging in risky behavior, and so the affective nature of risk taking increases.
Importantly, this peer effect is not uniform, as we found that the quality of adolescents’ peer relationships modulates neural sensitivity to risk taking. We found significant interactions at the neural level, consistent with the behavioral effects. More chronic levels of peer conflict were associated with greater activation in the ventral striatum and the insula for adolescents reporting low levels of peer support. This effect was buffered for adolescents reporting high levels of peer support. Therefore, high risk-taking behavior among adolescents experiencing more chronic peer conflict and low peer support is driven by this underlying neural process, whereby increased levels of risk-taking behavior are paralleled by heightened activation in the striatum and the insula.
Our behavioral and neural findings support the stress-buffering model of social relationships (Cohen et al., 2001) and are contrary to the peer socialization of risk model, which would suggest that peer support would amplify rather than attenuate the effects of peer conflict (Jessor, 1993; Brady et al., 2009). Prior research may have found an association between peer support and adolescent risk taking because the effect was being driven by the fact that adolescents with more supportive friends were also spending more time with their friends, and this extra time accounted for their greater risk taking due to peer socialization. Indeed, higher levels of risk taking occur in the presence of friends (Chein et al., 2010). By controlling for time spent with peers in the current study, we can be more confident that our effects are due to the relational quality of adolescents’ friendships. Although our measure of peer support captures high-quality friendships across 2 years, our measure does not capture the types of close peer relationships that may support the peer socialization of risk model. For instance, deviant peer association is a strong predictor of risk-taking behavior, and so strong peer bonds may be a negative influence depending on the peers’ level of deviance; high relationship quality may increase the influence of a deviant peer (Poulin et al., 1999). Thus, although we have carefully mapped the quality of adolescents’ peer friendships, future studies should carefully measure the types of peers adolescents have as close friends.
Our findings are especially striking given our stringent controls. By controlling for the amount of time adolescents spent with their friends across 4 weeks over a 2 year period, we can be confident that our findings are not explained simply by the fact that adolescents who spend more time with their friends have both greater opportunities to experience conflict with their friends and to engage in more risk-taking behavior. Moreover, by controlling for youths’ conduct problems, reported by both the teen and the parent, we can also be confident that our findings are not due to an underlying behavioral problem of the adolescent.
In addition to neural regions involved in reward processing, we also found significant activations in brain regions involved in regulation and motor responses. In particular, we found that adolescents who reported more chronic peer conflict demonstrated heightened activation in the VLPFC, DLPFC and SMA. In addition, we found similar interactions with peer support in these brain regions, such that higher peer support modulated the peer conflict effect. Adolescence is marked by maturation of motor inhibition and impulse control (Casey et al., 2008). The heightened activation in regulatory and motor regions may suggest impaired impulse control in both motor and cognitive domains, which may result in riskier behavior. The sensorimotor cortex has been implicated in an ‘urge to move’ (Weiland et al., 2012). Thus, SMA activation during increasing pumps may represent an impulsive response in preparation for making a motor response during risk taking. Although we interpret that greater activation in brain regions involved in regulation and motor responses are suggestive of impaired and inefficient neural processing among adolescents experiencing more chronic peer conflict, heightened activation in these regions has also been interpreted as reflecting efficient neural processing (see Poldrack, 2010).
A few limitations should be acknowledged. First, our sample size of 46 is potentially small for analyses examining moderation, such that only a few individuals will be included in each level of peer conflict by peer support. These effects should therefore be interpreted with relative caution. Nonetheless, the consistent nature of the moderation effects across multiple measures and across both brain and behavioral indexes gives us confidence in these findings. Second, behavioral performance on the BART ranged widely across adolescents, with the maximum number of pumps ranging from 3 to 11. Thus, while some adolescents experienced relatively low levels of risk taking (i.e. three pumps as a maximum level of risk), others experienced a relatively high risk-taking context (i.e. 11 pumps as a maximum level of risk), and so the task was experienced differently across participants. To account for this potential confound, we controlled for maximum number of pumps in our analyses, and the results remained significant. Moreover, maximum number of pumps was not associated with peer conflict or peer stress. Therefore, we have relative confidence that our effects are not due to differences in the task environment across participants. However, future research should replicate these effects in a more controlled experimental task.
In conclusion, we took a comprehensive approach to understand how chronic levels of peer conflict over a 2 year period relate to adolescent risk taking. By examining daily conflict across 14 days on 2 subsequent years, we were able to carefully quantify chronic levels of peer conflict in youths’ lives. We found that more chronic peer conflict was associated with greater risk-taking behavior, which was paralleled by heightened activation in brain regions involved in affect and reward processing. Importantly, these associations were buffered when adolescents had supportive peer relationships. Together, our findings underscore how important positive supportive peer relationships are for adolescents.
Acknowledgments
Support for this study was provided by the NIH (R01HD057164-S and R01HD057164: Fuligni) an NSF Doctoral Dissertation Improvement Grant (Telzer) and a University of California Institute of Mexico and the United States Dissertation Research Grant (Telzer).
REFERENCES
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Alexander CS, Kim YJ, Ensminger M, Johnson KE, Smith BJ, Dolan LJ. A measure of risk taking for young adolescents: reliability and validity assessments. Journal of Youth and Adolescence. 1990;19:559–69. doi: 10.1007/BF01537176. [DOI] [PubMed] [Google Scholar]
- Armsden GC, Greenberg MT. The inventory of parent and peer attachment: individual differences and their relationship to psychological well-being in adolescence. Journal of Youth and Adolescence. 1987;16:427–45. doi: 10.1007/BF02202939. [DOI] [PubMed] [Google Scholar]
- Berndt TJ, Perry TB. Children’s perceptions of friendships as supportive relationships. Developmental Psychology. 1986;22:640–8. [Google Scholar]
- Bolger N, Davis A, Rafeli E. Diary methods: capturing life as it is lived. Annual Review of Psychology. 2003;54:579–616. doi: 10.1146/annurev.psych.54.101601.145030. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Cashman-Rolls A, O'Donnell JM, et al. Risk taking differences on a behavioral task as a function of potential reward/loss magnitude and individual differences in impulsivity and sensation seeking. Pharmacology Biochemistry and Behavior. 2009;34:685–92. doi: 10.1016/j.pbb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Brady SS, Dolcini MM, Harper GW, Pollack LM. Supportive friendships moderate the association between stressful life events and sexual risk taking among African American adolescents. Health Psychology. 2009;28(2):238–48. doi: 10.1037/a0013240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalca E, Kong G, Liss T, Reynolds EK, et al. A preliminary experimental investigation of peer influence on risk-taking among adolescent smokers and non-smokers. Drug and Alcohol Dependence. 2012;129:163–6. doi: 10.1016/j.drugalcdep.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albertm D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2010;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Gottlieb BH, Underwood LG. Social relationships and health: challenges for measurement and intervention. Advances in Mind-Body Medicine. 2001;17:129–41. [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Flannery DJ, Williams LL, Vazsonyi AT. Who are they with and what are they doing? Delinquent behavior, substance use, and early adolescents’ after-school time. American Journal of Orthopsychiatry. 1999;69:247–53. doi: 10.1037/h0080426. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, et al. Healthy adolescents' neural response to reward: associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child Adolescent Psychiatry. 2010;49:162–72. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman W, Buhrmester D. Age and sex differences in perceptions of networks of personal relationships. Child Development. 1992;63:103–15. doi: 10.1111/j.1467-8624.1992.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Developmental Science. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Hansell S. Adolescent friendship networks and distress in school. Social Forces. 1985;63:698–714. [Google Scholar]
- Hussong AM. Perceived peer context and adolescent adjustment. Journal of Research on Adolescence. 2000;10(4):391–415. [Google Scholar]
- Jessor R. Successful adolescent development among youth in high-risk settings. American Psychologist. 1993;48:117–26. doi: 10.1037//0003-066x.48.2.117. [DOI] [PubMed] [Google Scholar]
- Kupersmidt JB, Burchinal M, Patterson CJ. Developmental patterns of childhood peer relations as predictors of externalizing behavior problems. Development and Psychopathology. 1995;7:825–43. [Google Scholar]
- La Greca AM, Harrison HW. Adolescent peer relations, Friendships and romantic relationships: do they predict social anxiety and depression? Journal of Clinical Child and Adolescent Psychology. 2005;34:49–61. doi: 10.1207/s15374424jccp3401_5. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavior measure of risk taking: the Balloon Analogue Risk Task BART. Journal of Experimental Psychology Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence. 2003;26:320–45. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- McCubbin HI, Needle RH, Wilson M. Adolescent health risk behaviors: family stress and adolescent coping as critical factors. Family Relations. 1985;34:51–62. [Google Scholar]
- Parker JG, Asher SR. Peer relations and later personal adjustment: are low-accepted children at risk? Psychological Bulletin. 1987;102:357–89. doi: 10.1037//0033-2909.102.3.357. [DOI] [PubMed] [Google Scholar]
- Peake S, Dishion TJ, Stormshak EA, Moore WE, Pfeifer JH. Risk-taking and social exclusion in adolescence: neural mechanisms underlying peer influences on decision-making. Neuroimage. 2013;82:23–4. doi: 10.1016/j.neuroimage.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Interpreting developmental changes in neuroimaging signals. Human Brain Mapping. 2010;31:872–8. doi: 10.1002/hbm.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein MJ, Boergers J, Spirito A. Adolescents’ and their friends’ health-risk behavior: factors that alter or add to peer influence. Journal of Pediatric Psychology. 2001;26(5):287–98. doi: 10.1093/jpepsy/26.5.287. [DOI] [PubMed] [Google Scholar]
- Poulin F, Dishion TJ, Haas E. The peer influence paradox: friendship quality and deviancy training within male adolescent friendships. Merrill-Palmer Quarterly. 1999;45:42–61. [Google Scholar]
- Romer D, Hennessy M. A biosocial-affect model of adolescent sensation seeking: the role of affect evaluation and peer-group influence in adolescent drug use. Prevention Science. 2007;8(2):89–101. doi: 10.1007/s11121-007-0064-7. [DOI] [PubMed] [Google Scholar]
- Sandler IN, Miller P, Short JL, Wolchik SA. Social support as a protective for children in stress. In: Elle D, editor. Children’s Social Networks and Social and Supports. New York: Wiley; 1989. pp. 277–307. [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Science. 2009;13(8):334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–33. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A soial neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Monahan K. Age difference in resistance to peer influence. Developmental Psychology. 2007;43(6):1531–43. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ. Positive daily family interactions eliminate gender differences in internalizing symptoms during adolescence. Journal of Youth and Adolescence. 2013;42(10):1498–511. doi: 10.1007/s10964-013-9964-y. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, Fuligni AJ. Gaining while giving: an fMRI study of the rewards of family assistance among White and Latino youth. Social Neuroscience. 2010;5:508–18. doi: 10.1080/17470911003687913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Gálvan A. The effects of poor quality sleep on brain function during risk taking in adolescence. Neuroimage. 2013a;71:275–83. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Gálvan A. Meaningful family relationships: neurocognitive buffers of adolescent risk taking. Journal of Cognitive Neuroscience. 2013b;25:374–87. doi: 10.1162/jocn_a_00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Gálvan A. Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proceedings of the National Academy of Sciences USA. 2014;111:6600–5. doi: 10.1073/pnas.1323014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity Across adolescence. Cerebral Cortex. 2010;20(1):61–9. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Ward BD. 2000. Simultaneous inference for fMRI data. Available: http://afni.nimh.nih.gov/pub/dist/doc/manuals/AlphaSim.pdf. [Google Scholar]
- Weiland BJ, Welsh RC, Yau WYW, Zucker RA, Zubeita JK, Heitzeg MM. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug and Alcohol Dependence. 2013;128:130–9. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Resko JA, Ainette MG, Mendoza D. Role of parent support and peer support in adolescent substance use: a test of mediated effects. Psychology of Addictive Behaviors. 2004;18:122–34. doi: 10.1037/0893-164X.18.2.122. [DOI] [PubMed] [Google Scholar]
- Windle M. A longitudinal study of stress buffering for adolescent problem behaviors. Developmental Psychology. 1992;28:522–30. [Google Scholar]
- Woodward LJ, Fergusson DM. Childhood peer relationship problems and psychosocial adjustment in late adolescence. Journal of Abnormal Child Psychology. 1999;27(1):87–104. doi: 10.1023/a:1022618608802. [DOI] [PubMed] [Google Scholar]