Abstract
Talking about emotion and sharing emotional experiences is a key component of human interaction. Specifically, individuals often consider the reactions of other people when evaluating the meaning and impact of an emotional stimulus. It has not yet been investigated, however, how emotional arousal ratings and physiological responses elicited by affective stimuli are influenced by the rating of an interaction partner. In the present study, pairs of participants were asked to rate and communicate the degree of their emotional arousal while viewing affective pictures. Strikingly, participants adjusted their arousal ratings to match up with their interaction partner. In anticipation of the affective picture, the interaction partner’s arousal ratings correlated positively with activity in anterior insula and prefrontal cortex. During picture presentation, social influence was reflected in the ventral striatum, that is, activity in the ventral striatum correlated negatively with the interaction partner’s ratings. Results of the study show that emotional alignment through the influence of another person’s communicated experience has to be considered as a complex phenomenon integrating different components including emotion anticipation and conformity.
Keywords: social influence, conformity, emotion regulation, IAPS, fMRI
INTRODUCTION
Communicating emotional evaluations constitutes an essential component of human social interaction and serves important functions such as strengthening social relationships, providing collective knowledge about how to deal with emotional events and stimulating the cognitive processing of emotional experiences (Seehausen et al., 2012; Matejka et al., 2013; for a review see Rimé, 2009). Acknowledging the fact that individuals often consider the reactions of others when evaluating the meaning and impact of a given situation, Manstead and Fischer (2001) introduced the concept of ‘social appraisal’ into emotion research and stated that the way in which individuals evaluate an emotional event is affected by the way in which others (apparently) evaluate that same event. The rationale for this comes from social comparison theory (Festinger, 1954), which holds that individuals have a need to evaluate their own opinions and beliefs by comparison with others. Schachter and Singer (1962) applied this idea to the emotion domain by showing that individuals actively seek out information concerning how others evaluate and react to an emotional situation. Although a number of studies supported the idea that emotional experiences are influenced by another person’s appraisal (e.g. Evers et al., 2005; Mumenthaler and Sander, 2012), the neural mechanisms of such an emotional alignment in social interaction are still unclear.
By communicating about previous emotional experiences, people allow their interaction partner to anticipate an emotional stimulus and to regulate their emotion in advance. In studies on the neural correlates of emotion anticipation, participants were presented with threat and no-threat cues before affective and neutral pictures (Waugh et al., 2008; Simmons et al., 2011). These studies have shown that the anterior insula is likely to be implicated in emotion anticipation. Anticipating emotional stimuli might help to prepare cognitive control processes and might facilitate the use of emotion regulation strategies once the emotional stimulus occurs (e.g. Herwig et al., 2007; Vanderhasselt et al., 2013). One emotion regulation strategy that has received particular interest in emotion regulation research is cognitive reappraisal. Cognitive reappraisal involves reinterpreting the meaning of an affective stimulus in a way that alters the emotional response (Gross and Thompson, 2007; Gross, 2008). Reappraisal results in changes of self-reported emotional experience accompanied by increased activity in dorsal anterior cingulate and prefrontal cortex, as well as a decrease or increase—depending on whether the person is down- or upregulating—of activity in brain regions involved in emotion processing, such as the amygdala (Kanske et al., 2011; Ochsner et al., 2012). Specifically highlighting a relationship between emotion anticipation and regulation, Carlson and Mujica-Parodi (2010) report a correlation between anticipatory insula activity and participants’ disposition to downregulate their emotions using cognitive reappraisal.
Previous studies investigating expectancy effects and anticipatory emotion regulation used only non-social cues (yet nonetheless involving human assessment and emotional evaluation to some degree) before an affective stimulus and did not investigate how emotion is modulated by another person’s appraisal in an interactive setting. Hearing about an interaction partner’s current experience, however, might also involve empathic responses. Empathy can be defined as the ability to understand and share the feelings of another person (whereas sympathy and theory of mind both involve an understanding but no sharing of another’s state; Saxe et al., 2006; de Vignemont and Singer, 2006) and has been related to a network of brain regions including the bilateral insula, the anterior cingulate and mid-cingulate cortex (e.g. Wicker et al., 2003; for a quantitative meta-analysis of functional neuroimaging studies on empathy see Fan et al., 2011).
In addition to this informative social influence (including the aforementioned phenomena: emotion anticipation, anticipatory emotion regulation and empathic responses) people tend to conform to the opinion of others because they want to behave correctly, obtain social approval or maintain a favorable self-concept (normative social influence; Cialdini and Goldstein, 2004). Recent studies have shown that effects of group opinion on likeability and attractiveness ratings were related to activity in the ventral striatum (Berns et al., 2005; Klucharev et al. 2009, 2011; Campbell-Meiklejohn et al., 2010; Zaki et al., 2011).
In the present study, we investigated how emotional arousal ratings and physiological responses elicited by affective pictures are influenced by another person’s communicated arousal rating in a one-to-one social interaction. To this end, we invited two participants at a time and asked them to report their emotional arousal when confronted with affective pictures. That is, participants rated how emotionally agitated they felt and then the rating was shown to the interaction partner. Participants took turns in being the first or second rater. In the position of the second rater (on which we specifically focused with regard to our research question), participants were influenced by the interaction partner’s rating, which was shown before the picture. Notably, our task distinguished the different phenomena involved (e.g. emotion anticipation and empathic responses, and conformity) on the basis of the temporal sequence. That is, emotion anticipation and empathy could be measured when participants were presented with the interaction partner’s rating, whereas conformity played a role during picture rating.
We hypothesized that participants would align their arousal ratings to conform to the interaction partner’s ratings. This ‘emotional conformity’ or rather alignment should be accompanied by increased anticipatory activity in anterior insula when the interaction partner reports increased emotional arousal in response to an upcoming picture. Specifically, we expected activity in anterior insula to correlate with the interaction partner’s arousal ratings. On the basis of the recent reports on the neural correlates of conformity, we hypothesized that social influence on an individual’s emotional experience would be reflected in altered neural activity in the ventral striatum and in emotion processing regions such as the amygdala.
METHODS
Participants
We invited 20 pairs of participants to take part in our study and to complete the experiment together. Participants in a pair were of the same sex and did not know each other before the experiment (i.e. they had no relationship of any kind). Neuroimaging data were obtained for n = 20 participants (8 men), that is, one participant of each pair rated emotional arousal inside and the other one performed the task outside the magnetic resonance imaging (MRI) scanner. Data are only reported for the subjects who rated the pictures inside the scanner. Because perceived similarity is important for making social comparisons (Festinger, 1954), all participants were students and of similar age. Participants were on average 23.95 years old (s.d. = 3.91) and right-handed as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971).
To explore whether the dependent measures of our task correlate with individual differences in emotion processing, emotion regulation, as well as the tendency for making social comparisons, participants completed a test battery including the Emotion Regulation Questionnaire (ERQ; Gross and John, 2003), the Trait Emotional Intelligence Questionnaire (TEIQue-SF; Freudenthaler et al., 2008) and the Iowa-Netherlands Comparison Orientation Measure (Jonas and Huguet, 2008). The ERQ, specifically, measures a person’s tendency for cognitive reappraisal.
The study was approved by a local ethics committee and conducted in accordance with the Declaration of Helsinki. Subjects were paid for their participation and gave written informed consent.
Interactive rating task and stimulus material
To investigate how emotional arousal is influenced by another person’s arousal ratings, we used an interactive rating task. In this task, participants rated their emotional arousal elicited by affective pictures together with an interaction partner. Both participants—one inside and one outside the MRI scanner—took turns in being the first or second rater. In the position of the second rater (‘second-rater’ condition), they first saw the rating of the interaction partner and then the picture. Importantly, participants could anticipate how emotionally aroused they would feel on the basis of the interaction partner’s rating. Emotional arousal was measured because it is easier to compare emotional arousal with an interaction partner than comparing emotional valence or the quality of a specific emotion (such as fear and disgust).
Pictures were taken from the International Affective Picture System (IAPS, Lang et al., 2005) based on their mean normative ratings for valence and arousal, which are given on a nine-point scale in the technical manual. We selected 90 negative/unpleasant and emotionally arousing pictures (valence: M = 2.69, s.d. = 0.90; arousal: M = 6.04, s.d. = 0.69) and 54 neutral pictures (valence: M = 5.25, s.d. = 0.55; arousal: M = 3.10, s.d. = 0.55). Negative pictures displayed threatening scenes, objects, animals or wounded people (44.44% of the negative pictures were threat-related, 11.11% represented sad and 44.44% represented disgust pictures)1. Neutral stimuli consisted of pictures of household objects, landscapes, buildings, animals and social gatherings.
To reliably generate three different conditions for the second rater, the rating of the interaction partner (i.e. the ‘other’ person) was pre-determined: (i) ratings could be two points lower than the original IAPS norm rating of the respective picture (underestimation = ‘negative under’ condition), (ii) equal to the original IAPS norm rating ( = ‘negative equal’ condition) or (iii) two points higher than the original IAPS norm rating (overestimation = ‘negative over’ condition). Importantly, pictures in the different conditions were in fact matched on the basis of the IAPS norm ratings. In addition to these three ‘second rater’ conditions with negative pictures, our task also included a neutral second-rater condition (‘neutral’). Because participants took turns during the experiment in being the first or second rater, we also had a negative and a neutral ‘first-rater’ condition. Negative and neutral pictures were also presented in an ‘alone’ condition. In the alone conditions, participants rated their emotional arousal on their own without having the opportunity to communicate. In the present report, we focus on the question how people are influenced by another person’s emotional experience. Therefore, only data from the four second-rater conditions are reported.
In total, we had four conditions of interest: negative under, negative equal, negative over and neutral. For each participant, a set of 18 (negative or neutral) pictures was randomly assigned to each condition. Each set consisted of the same number of eight threatening, two sad and eight disgusting pictures. In addition, half of the pictures in each negative as well as neutral set depicted people or had a social content, and half of them depicted non-social scenes. The five sets of negative pictures were matched with respect to valence [F(4,85) = 0.29, P = 0.87], arousal [F(4,85) = 0.09, P = 0.99] and picture luminance [F(4,85) = 0.32, P = 0.87]. The same was true for the three sets of neutral pictures [valence: F(2,51) = 0.09, P = 0.92; arousal: F(4,85) = 0.11, P = 0.90; luminance: F(4,85) = 0.09, P = 0.92].
Experimental procedure
When a pair of participants arrived at the laboratory, one of them was randomly assigned to rate the pictures inside the MRI scanner. Participants were instructed together and completed a practice session under supervision to become familiar with the interactive rating task.
The experiment in the scanner consisted of three runs. Each run lasted ∼20 min and consisted of four blocks: one block in which participants rated pictures alone, one first-rater block and two second-rater blocks. Participants believed that when being in the first-rater position, their interaction partner was in the second-rater position and vice versa. In fact, however, both participants were presented with the identical task. The order of blocks was randomized. Each block contained 12 trials and was preceded by an instruction cue for 5 s (which stated ‘you rate alone’, ‘you rate first’ or ‘interaction partner rates first’). Within the blocks, trials were presented in a pseudo-randomized order in a mixed blocked/event-related design.
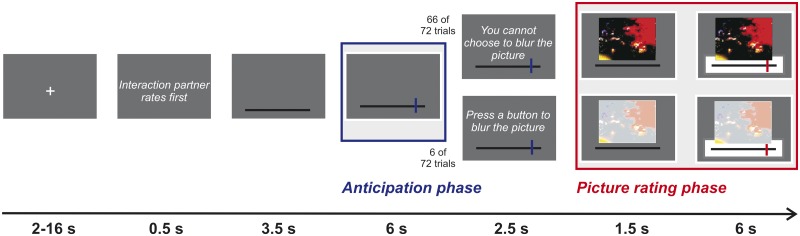
Trials were presented with jittered interstimulus intervals (minimum = 2 s, maximum = 16 s, M = 6.72 s), which were optimized using OptSeq2 (www.surfer.nmr.mgh.harvard.edu). A trial started with a fixation cross followed by a short reminder of the current condition. For a schematic description of the experimental trial in the second-rater conditions see Figure 1. First, a rating scale was presented. After 3.5 s the rating of the interaction partner was displayed on this rating scale for 6 s, allowing the participant to anticipate the upcoming picture ( = picture anticipation phase). IAPS ratings were transformed from a nine-point scale to a visual analogue scale ranging from −135 to +135. Then, the picture was presented (=picture rating phase). After 1.5 s of picture presentation, a rating scale below the picture appeared and participants were given 6 s to enter their arousal rating. Participants could enter their rating 1.5 s after the picture was presented. Participants were given 6 s for their response. To enter their arousal rating, participants indicated a position (from −135 to +135) along a continuous line between two end points (‘no emotional arousal’ and ‘high emotional arousal’) by pressing three buttons (labeled ‘left’, ‘right’ and ‘confirm’) on a response device. Visual analog scale data were converted back to the 1–9 scale common for IAPS pictures.
Fig. 1.
Schematic description of an experimental trial in the second-rater conditions. During the anticipation phase, the blue bar depicted the rating of the interaction partner. During the picture rating phase, the participant saw the picture and rated the picture (red bar). The possibility to blur the picture occurred only in 6 of the 72 second-rater trials.
To make the ratings of the interaction partner more relevant, participants were told that communicating their emotional experience (arousal) in the first-rater condition had the purpose of warning the interaction partner about the upcoming stimulus. Therefore, in a few trials the second rater had the opportunity to blur the picture (i.e. to make the details of the pictures less discernible, see Figure 1). In these cases the picture was depicted with a white veil. This possibility, however, occurred only six times during the whole experiment (two times per run) and affected all conditions equally (i.e. this possibility occurred twice in the neutral condition, twice in the negative over condition, once in the negative under condition and once in the negative equal condition). On average, subjects chose the option to blur the image on 0.9 (s.d. = 1.59) trials of six trials in which this option was available. Fourteen subjects did not choose the option to blur the image at all. In all analyses of the picture rating phase, trials in which participants had the opportunity to blur the image were excluded.
The experiment was conducted using Presentation (Neurobehavioral Systems Inc., Albany, CA) running on a Microsoft Windows operating system. Pictures were presented via a pair of stereoscopic MRI compatible goggles (VisuaStim, Resonance Technology, Los Angeles, CA). As additional psychophysiological measures, we recorded participants’ skin conductance response (SCR) and heart rate. For technical reasons, heart rate data were available only for 16 subjects. After the experiment, participants were debriefed. Self-reports revealed that no participant was suspicious of the experimental manipulation.
Functional magnetic resonance imaging data acquisition and analysis
Blood oxygen level-dependent (BOLD) signal changes (i.e. neural activity) during the experiment were recorded using a 3T scanner (Trio, Siemens, Erlangen, Germany) using a 12-channel head coil. Functional imaging data were acquired with a gradient echo T2*-weighted echo-planar sequence (repetition time = 2 s, echo time =30 ms, flip angle = 70°, 64 × 64 matrix, field of view = 192 mm, voxel size = 3 × 3 × 3 mm). A total of 37 axial slices (3 mm thick, no gap) were sampled for whole-brain coverage. Imaging data were acquired in three separate runs of 590 volumes each. A high-resolution T1-weighted anatomical scan of the whole brain was acquired (256 × 256 matrix, voxel size = 1 × 1 × 1 mm).
Image analysis was performed using SPM8 (www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 7.11.1 (Mathworks Inc., Sherborn, MA). Echo-planar images were realigned, unwarped, coregistered to the respective participant’s T1 scan that was normalized to a standard T1 template based on the Montreal Neurological Institute (MNI) reference brain, resampled to 3 mm isotropic voxels, and spatially smoothed with an isotropic 8 mm full width at half maximum Gaussian kernel.
After preprocessing, subject-specific first-level analyses were conducted with regressors for each of the experimental conditions. We specifically modeled the picture anticipation phases (6 s; Figure 1) and the picture rating phases (7.5 s) of the second-rater conditions (including the second under, second equal and second over conditions). Separate regressors were included for negative and neutral pictures. The regressor for the negative picture anticipation phases was parametrically modulated on a trial-by-trial basis by the ratings of the interaction partner (which corresponded to the position of the rating bar displayed on the screen). The regressor for the negative picture rating phases was parametrically modulated on a trial-by-trial basis by the participant’s own ratings and by the ratings of the interaction partner. These two parametric modulators were entered independently into the design matrix, that is, without using the serial orthogonalization that is used as default in SPM (for a similar approach see Gläscher et al., 2010; Wunderlich et al., 2011). This ensured that only the additional variance that could not be explained by any other regressor was assigned to the respective effect and thus prevented spurious confounds between regressors.
Regressors of no interest included two regressors modeling the time periods when participants waited for their interaction partner’s rating (3.5 s) as well as the periods when participants could chose to blur the picture (2.5 s). Further regressors modeled the phases of the alone and first-rater conditions (separately for negative and neutral pictures) as well as the cue phases (0.5 s) and the motor responses defined as time periods from the first button press to the press of the confirm button. The six motion-correction parameters estimated from the realignment procedure were entered as covariates of no interest.
At the group level, estimated beta weights were entered into random effects analyses. All reported activations survived a threshold of P < 0.05 after clusterwise familywise error correction for multiple comparisons over the entire brain at a cluster-defining threshold of P < 0.001, uncorrected. For follow-up analyses and illustration purposes, we calculated an additional model in which picture anticipation and picture rating phases were split up into separate onset regressors for the three second-rater conditions. Parameter estimates in the functional regions of interest (ROIs) were extracted using the MarsBaR toolbox for SPM (marsbar.sourceforge.net).
Acquisition and analysis of psychophysiological data
In addition to functional magnetic resonance imaging (fMRI) and rating data, we measured heart rate and SCR. Heart rate was measured with a pulse plethysmograph on the left thumb. Skin conductance was recorded using a pair of Ag/AgCl electrodes placed on the intermediate phalanges of the left index and middle fingers and an MRI compatible sampling device (Brain Products, Gilching, Germany).
Heart rate and SCRs were analyzed in the anticipation phases (6 s) and in the picture rating phases (7.5 s). Both measures were analyzed using MATLAB. For SCR data analyses we additionally used the MATLAB-based software LedaLab V3.3.1 (www.ledalab.de). In LedaLab, a continuous decomposition analysis was applied to extract the phasic information underlying the SCR, which aims at retrieving the signal characteristics of the underlying sudomotor nerve activity (Benedek and Kaernbach, 2010).
Statistical analyses of behavioral and psychophysiological data
Statistical analyses were performed using SPSS (SPSS Inc., Chicago, IL). The significance level for all tests was P < 0.05. Parameters were analyzed using repeated measures analyses of variance (ANOVAs) and post hoc t-test. For significant main effects we report  (partial eta squared) and for post hoc t-tests Cohen’s d as a measure of effect size. All within-subjects effects were Greenhouse-Geisser corrected whenever the assumption of sphericity was violated (ε < 1.0). In those cases, we also report corrected degrees of freedom.
(partial eta squared) and for post hoc t-tests Cohen’s d as a measure of effect size. All within-subjects effects were Greenhouse-Geisser corrected whenever the assumption of sphericity was violated (ε < 1.0). In those cases, we also report corrected degrees of freedom.
To investigate the influence of the interaction partner’s ratings, we compared arousal ratings, skin conductance and heart rate data between the three negative second-rater conditions (under, equal, over) in 1 × 3 repeated measures ANOVAs. SCR and heart rate data were analyzed during both the picture anticipation and the picture rating phases. As a validity check and to test for an effect of emotion (i.e. whether negative IAPS pictures lead to increased arousal ratings, skin conductance and heart rate), we additionally computed paired t-test comparing ratings and physiological data in the second rater negative equal and neutral conditions.
RESULTS
Rating data
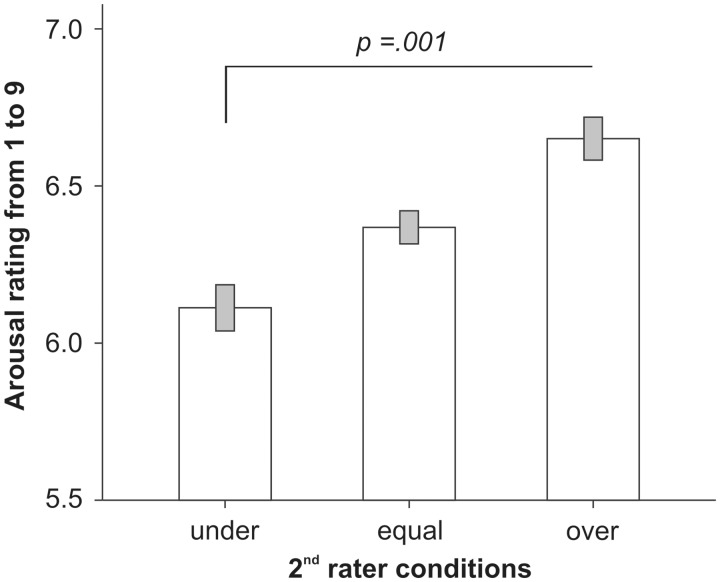
As a validity check, we first compared the negative equal with the neutral condition. As expected, participants reported significant more emotional arousal when presented with negative compared with neutral pictures [t(19) = 19.87, P < 0.001, d = 4.44; Table 1]. Importantly, the 1 × 3 repeated measures ANOVA on the ratings of the negative pictures in the under, equal and over conditions revealed an effect of condition [F(2,38) = 11.34, P < 0.001, 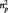 = 0.374]. Paired t-tests showed that participants’ ratings were higher after seeing an overestimation than after seeing an underestimation of arousal by their interaction partner [negative over vs negative under: t(19) = 4.09, P = 0.001, d = 0.915; Figure 2]. We also compared the negative equal condition with the first and alone negative picture conditions (in which no social influence was exerted). As expected, paired t-tests showed that the second rater negative equal condition, in which the rating of the interaction partner was equal to the original IAPS norm rating, did not differ from alone and first-rater conditions (Ps ≥ 0.394).
= 0.374]. Paired t-tests showed that participants’ ratings were higher after seeing an overestimation than after seeing an underestimation of arousal by their interaction partner [negative over vs negative under: t(19) = 4.09, P = 0.001, d = 0.915; Figure 2]. We also compared the negative equal condition with the first and alone negative picture conditions (in which no social influence was exerted). As expected, paired t-tests showed that the second rater negative equal condition, in which the rating of the interaction partner was equal to the original IAPS norm rating, did not differ from alone and first-rater conditions (Ps ≥ 0.394).
Table 1.
Arousal ratings [means and standard error of the mean (SEM), ranging from 1 to 9], heart rates (in beats/min) and SCRs (in µS) for the four conditions: negative under, negative equal, negative over and neutral
| Arousal rating | Heart rate (beats/min) | SCR (µS) | |
|---|---|---|---|
| M (SEM) | M (SEM) | M (SEM) | |
| N = 20 | N = 16 | N = 20 | |
| Picture rating phase | |||
| Negative under | 6.11 (0.17) | 68.475 (2.588) | 0.380 (0.064) |
| Negative equal | 6.37 (0.17) | 68.025 (2.668) | 0.355 (0.057) |
| Negative over | 6.65 (0.16) | 68.401 (2.722) | 0.408 (0.069) |
| Neutral | 1.70 (0.17) | 67.972 (2.670) | 0.250 (0.053) |
| Picture anticipation phase | |||
| Negative under | — | 68.397 (2.750) | 0.341 (0.058) |
| Negative equal | — | 68.367 (2.745) | 0.313 (0.051) |
| Negative over | — | 68.132 (2.703) | 0.410 (0.065) |
| Neutral | — | 68.093 (2.710) | 0.239 (0.051) |
Fig. 2.
Arousal ratings for negative pictures in the three second-rater conditions, in which the previous rating of the interaction partner was lower than (under), equal to (equal) or higher than (over) the original IAPS norm rating.
Psychophysiological data
In line with the literature, we found increased SCRs when subjects were viewing the negative pictures when comparing skin conductance during the picture rating phase in the negative equal and neutral conditions [t(19) = 2.40, P = 0.027, d = 0.54; Table 1; Bradley et al., 2008]. The 1 × 3 repeated measures ANOVA on SCR data in the three negative second-rater conditions revealed no effect of condition during the picture rating phase [F(2,38) = 0.957, P = 0.393]. In the picture anticipation phase, the 1 × 3 repeated measures ANOVA showed a trend for an effect of condition [F(2,38) = 2.861, P = 0.070, 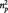 = 0.131] with greatest SCRs in the second over condition.
= 0.131] with greatest SCRs in the second over condition.
Heart rate did not differ during the viewing of negative equal compared with neutral pictures [t(15) = −0.273, P = 0.788; Table 1]. The 1 × 3 repeated measures ANOVA on heart rate data in the three negative second-rater conditions revealed no effect of condition [F(2,30) = 0.357, P = 0.211]. Heart rate also did not differ between the three second-rater conditions in the picture anticipation phase [F(2,30) = 1.641, P = 0.703].
fMRI data
Neural correlates during picture anticipation
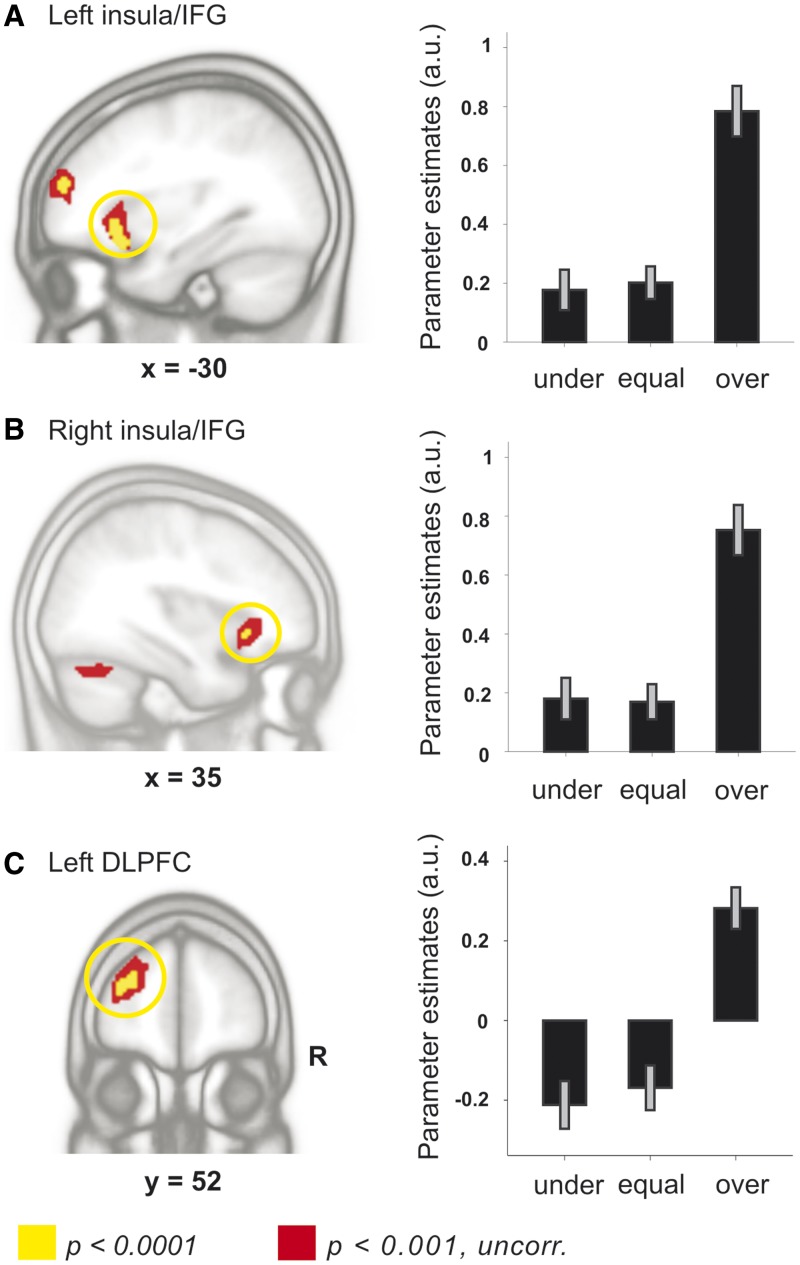
In the picture anticipation phase, BOLD signal changes correlated positively—on a trial-by-trial basis—with arousal ratings of the interaction partner in bilateral anterior insula extending into inferior frontal gyrus (IFG) as well as left dorsolateral prefrontal cortex (DLPFC) among other regions (Table 2 and Figure 3). That is, neural activity in these regions was enhanced when participants saw a higher arousal rating by the interaction partner compared with when they saw a lower rating. In the reverse contrast, which tested for negative correlations with the ratings of the interaction partner, we did not find any significant activations.
Table 2.
Neural activity in the picture anticipation phase in correlation with arousal ratings of the interaction partner
| Anatomical region | L/R | Number of voxels in cluster | Z score of local maximum | MNI peak voxel coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Positive trial-by-trial correlation | ||||||
| Occipital lobe | R | 694 | 4.78 | 9 | − 70 | −5 |
| Anterior insula/IFG | L | 171 | 4.75 | −30 | 20 | −14 |
| DLPFC | L | 173 | 4.16 | −24 | 59 | 22 |
| Anterior insula/IFG | R | 114 | 4.1 | 33 | 26 | −5 |
| Parietal lobe/precuneus | L | 117 | 3.93 | −3 | −76 | 40 |
| Negative trial-by-trial correlation | ||||||
| Occipital lobe | R | 78 | 3.97 | 36 | −94 | 4 |
L, left hemisphere; R, right hemisphere.
Note: All reported activations survived a threshold of P < 0.05 after clusterwise familywise error correction for multiple comparisons over the entire brain at a cluster-defining threshold of P < 0.001, uncorrected.
Fig. 3.
Changes in neural activity in the picture anticipation phase. Left panel: BOLD signal changes correlated positively with arousal ratings of the interaction partner in left (A) and right anterior insula extending into IFG (B) as well as left DLPFC (C). Right panel: For illustration purposes, we calculated an additional model that included separate onset regressors for the three second-rater conditions, in which the previous rating of the interaction partner was lower than (under), equal to (equal) or higher than (over) the original IAPS norm rating. We plotted parameter estimates (mean and standard error of the mean in arbitrary units) within the functional ROIs in the three second-rater conditions.
We tested whether insula activity is influenced by self-reported habitual use of cognitive reappraisal (Carlson and Mujica-Parodi, 2010) and found that activity in bilateral anterior insula/IFG correlated positively with reappraisal scores measured with the ERQ [left anterior insula/IFG: r = 0.52, P < 0.019, 95% confidence interval (0.101; 0.783); right anterior insula/IFG: r = 0.505, P < 0.023, 95% confidence interval (0.081; 0.775)].
Neural correlates during picture viewing
In the picture rating phase, BOLD signal changes in bilateral amygdala correlated positively with participants’ own ratings in all conditions with negative pictures on a trial-by-trial basis (Table 3A and Figure 4A). That is, the more arousal was reported by the participant, the more neural activation occurred in the amygdala. In the reverse contrast, which tested for negative correlations with participants’ own arousal ratings, we found activity in right superior frontal gyrus, bilateral inferior parietal lobe and left dorsolateral prefrontal gyrus.
Table 3.
Neural activity in the picture rating phase in correlation with own arousal ratings (A) and in correlation with arousal ratings of the interaction partner (B)
| Anatomical region | L/R | Number of voxels in cluster | Z score of local maximum | MNI peak voxel coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| (A) Positive trial-by-trial correlation | ||||||
| No ROI at peak voxel | R | 444 | 5.02 | 6 | −31 | −8 |
| Voxels within anatomical amygdala mask | R | 20 | 4.29 | 24 | −7 | −14 |
| Voxels within anatomical amygdala mask | L | 1 | 3.24 | −18 | −7 | −17 |
| Negative trial-by-trial correlation | ||||||
| Inferior parietal lobe | R | 504 | 4.36 | 54 | −31 | 43 |
| Inferior parietal lobe | L | 585 | 4.35 | −48 | −61 | 49 |
| Superior frontal gyrus | R | 622 | 4.32 | 9 | 35 | 58 |
| Dorsolateral prefrontal gyrus | L | 288 | 4.17 | −48 | 23 | 40 |
| (B) Positive trial-by-trial correlation | ||||||
| No suprathreshold clusters | ||||||
| Negative trial-by-trial correlation | ||||||
| Ventral striatum (caudate nucleus) | L | 87 | 4.25 | −6 | 11 | 4 |
L, left hemisphere; R, right hemisphere.
Note: All reported activations survived a threshold of P < 0.05 after clusterwise familywise error correction for multiple comparisons over the entire brain at a cluster-defining threshold of P < 0.001, uncorrected.
Fig. 4.
Changes in neural activity in the picture rating phase. (A) BOLD signal changes in bilateral amygdala correlated positively with own arousal ratings in all conditions. (B) Left panel: BOLD signal changes correlated negatively with the interaction partner’s ratings (displayed in the anticipation phase) in bilateral ventral striatum. Right panel: We plotted parameter estimates (mean and standard error of the mean in arbitrary units) within the functional ROI in the three second-rater conditions.
To test our hypothesis with regard to the influence of the interaction partner’s ratings on neural activity during picture viewing, we correlated the interaction partner’s ratings with BOLD signal changes during this phase on a trial-by-trial basis. We found that the interaction partner’s ratings correlated negatively with activity in left ventral striatum (Table 3B and Figure 4B). That is, the more arousal was reported by the interaction partner, the less neural activation occurred in the striatum. No region showed a positive correlation with the ratings of the interaction partner.
We additionally tested whether activity differed in the three second-rater conditions within anatomical ROIs of the left and right amygdala (defined by the Automated Anatomical Labelling software; Tzourio-Mazoyer et al., 2002). The 2 (hemisphere) × 3 (condition) repeated measures ANOVA revealed an effect of condition [F(2,38) = 4.513, P = 0.017, 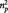 = 0.192], but neither an effect of hemisphere [F(1,19) = 0.029, P = 0.866] nor an interaction between condition and hemisphere [F(2,38) = 0.350, P = 0.707].
= 0.192], but neither an effect of hemisphere [F(1,19) = 0.029, P = 0.866] nor an interaction between condition and hemisphere [F(2,38) = 0.350, P = 0.707].
To investigate the relationship between activity related to participants’ own ratings and activity related to the interaction partner’s ratings, we performed an additional covariate analysis. To this end, we extracted the parameter estimates within a functional ROI of the ventral striatum from the contrast defined above (negative trial-by-trial correlation with interaction partner’s ratings; Table 3B and Figure 4B). We entered these parameter estimates as an across-subjects covariate in the contrast testing for trial-by-trial correlations with own arousal ratings (Table 3A and Figure 4A). This analysis did not reveal significant correlations. For completeness we report that we found a negative correlation between ventral striatum and left amygdala in an exploratory analysis at a lenient threshold (P = 0.08, small volume corrected by a mask of the bilateral anatomical amygdala after clusterwise familywise error correction for multiple comparisons over the entire brain at a cluster-defining threshold of P < 0.005, uncorrected). We do not draw conclusions from this result, but future studies might want to investigate a possible relationship between activity in striatum and amygdala.
DISCUSSION
To investigate how another person’s communicated experience (in the case of the present study, the exposure to affective pictures) influences emotional arousal, we used an interactive rating task. Our study yielded three main results. First, participants showed an alignment of their emotional reactions with their interaction partner’s ratings (i.e. ‘emotional conformity’). Second, when participants anticipated the affective pictures, changes in neural activity in bilateral anterior insula/IFG, and left DLPFC correlated with the interaction partner’s ratings. Third, when participants saw the pictures, their own ratings correlated positively with activity in the amygdala while interaction partner’s ratings correlated negatively with activity in the left ventral striatum.
Emotional alignment in the interactive rating task
During the interactive rating task, we successfully induced emotion: participants rated their emotional arousal as higher in response to negative pictures compared with neutral pictures. We also replicated previous findings that negative pictures elicit physiological responses by showing increased SCRs during the viewing of negative compared with neutral pictures (Lang et al., 1993).
Our study is the first showing emotional alignment processes in a social interaction by demonstrating that participants’ arousal ratings were influenced by ratings of their interaction partner. That is, participants rated their arousal in response to the pictures as higher when the interaction partner supposedly had also indicated high emotional arousal. It is important to note that the pictures in the different conditions were matched for emotional arousal based on the IAPS norm ratings.
This ‘emotional conformity’ effect is in line with and extends recent evidence that individuals tend to respond in a similar way or to seek common ground with other people when performing cognitive tasks (Berns et al., 2005), rating the attractiveness of faces (Klucharev et al., 2009, 2011; Zaki et al., 2011) or the likability of music (Berns et al., 2010; Campbell-Meiklejohn et al., 2010), when recollecting episodic memory (Edelson et al., 2011), or when rating personality traits (Korn et al., 2012).
The design of our study, however, differed from other studies investigating social conformity in three important ways. First, in previous studies investigating conformity, there were typically three phases (e.g. Klucharev et al., 2009; Zaki et al., 2011): in a first phase, subjects rated a stimulus. Then, they learned how other people (i.e. a group of people) have rated that stimulus. This rating is manipulated and greater, less than or equal to the participant’s initial rating. In the third phase, participants rerate the stimulus. The difference in the rating between third and first phase determines how much the social influence has changed the participant’s evaluation. Because emotional arousal in response to an affective stimulus, however, is likely to attenuate due to a repeated exposure (unlike other kinds of ratings such as attractiveness ratings), we used a different approach to investigate effects of social influence. In our study, participants were influenced by an interaction partner’s rating that already preceded the first (and only) exposure of that stimulus, and an effect of social influence was determined by the difference between the ratings following an interaction partner’s overestimations compared with ratings following underestimations. Second, we investigated how social influence affects negative emotion rather than positive affective phenomena such as likability and attractiveness, which were investigated in most previous conformity studies. Third, in our study, participants were only influenced by one person (the interaction partner) and not by a group of people or an expert (i.e. conditions that could further increase the pressure for social conformity; see Meshi et al., 2012; Schilbach et al., 2013a). Participants were influenced by the interaction partner’s appraisals, although they might have been aware that there are great individual differences in emotional reactivity between people. That is, people usually respond differently to affective pictures, and one can never be sure for a given picture whether the interaction partner would respond as ‘most people would’ to that specific image (although participants in our study were all students, of same sex and similar age and, thus, might have perceived each other as similar). In contrast, subjects in our study experienced that the interaction partner reported a higher or lower emotional arousal than most other people would in two-thirds of the cases. In sum, our study investigating the modulation of emotional arousal using the interactive rating task should have a high ecological validity and allowed us to also investigate effects of emotion anticipation in addition to the examination of conformity effects.
Anticipatory responses to a reported emotional experience
During the picture anticipation phase, the interaction partner’s ratings correlated positively with activity in a network of brain regions comprising the bilateral anterior insula extending into IFG and DLPFC. That is, the more negative the rating of the interaction partner was, the more activity occurred in this network. Activation of this network has been observed when participants were presented with a cue indicating that another person receives a painful stimulus (Singer et al., 2004). In our experimental paradigm, the interaction partner’s rating not only indicated the other person’s emotional response but also served as a social cue that helped to anticipate the upcoming affective stimulus. A number of studies showed that the anterior insula is involved in anticipation of aversive stimuli elicited by non-social cues (Herwig et al., 2007; Carlson et al., 2011; Simmons et al., 2011; Denny et al., 2014). Anticipation of high arousing pictures in our study was also accompanied by increased skin conductance.
Investigating effects of conformity with regard to likability ratings of music songs, Berns et al. (2010) found that activity in the anterior insula and the anterior cingulate cortex was positively correlated with participants’ tendency to change their evaluations of a song. In our study, a high or low arousal rating given by an interaction partner before the stimulus presentation might have triggered the use of emotion regulation strategies that help to modulate (increase or decrease) the response to the stimulus, for instance, by reinterpreting the upcoming picture. In line with this notion, we found a positive correlation of activity in bilateral anterior insula/IFG with participants’ self-reported habitual use of cognitive reappraisal measured with the ERQ. This relation has also been reported by Carlson and Mujica-Parodi (2010), who found a correlation between anticipatory insula activity and participants’ disposition to reappraise. Hence, in our study, seeing a high arousal rating given by the interaction partner upregulated the emotional response, whereas a low arousal rating downregulated emotion. However, it is also possible that participants engaged in (anticipatory) emotion downregulation when seeing a high arousal rating and consecutively gave a lower arousal rating. Nevertheless, our data show that emotion regulation seems to be rather a contributing factor than a factor that counters the behavioral emotion alignment effect. Notably, in our task, participants received no explicit instruction to reappraise or downregulate their emotion. Instead, our task mirrored a real-life communication about emotional events.
Interplay between emotion processing and conformity
When participants saw the affective picture, participants’ own ratings correlated positively with activity in the bilateral amygdala. That is, the more arousing the pictures were rated by the participant the more activity in the amygdala occurred, which is in line with the well-established role of the amygdala in emotion processing (for a review see Phan et al., 2004).
In contrast to participants’ own ratings, interaction partners’ ratings were negatively correlated with activity in the ventral striatum. That is, the less arousing the pictures were rated by the interaction partner the more activity was found in ventral striatum.
Activity in ventral striatum has been consistently found in almost all studies investigating the neural correlates of conformity with a group opinion (e.g. Berns et al., 2010; Campbell-Meiklejohn et al., 2010; Klucharev et al., 2011; Zaki et al., 2011). Campbell-Meiklejohn et al. (2010), for instance, argue that striatal activity is related to reward when participants agree with the opinion of others. Activity in ventral striatum has also been associated with the processing of prediction errors, that is, discrepancies between expectations and outcomes (Schultz, 2006; with regard to aversive emotional events see Delgado et al., 2008). Therefore, previous studies on social conformity have interpreted striatal activity signaling the need for ‘going along’ with a group opinion (Klucharev et al., 2009).
In our study, we found a negative correlation between activity in ventral striatum and the interaction partner’s ratings. Although participants agreed with their interaction partners’ ratings in all conditions (i.e. in both over- and underestimations), the need for going along with the other person’s opinion was greater the (unexpectedly) lower the rating of the interaction partner was (e.g. in trials of the negative under condition that led to emotional downregulation). This result has to be interpreted in the context of our interactive rating task. As mentioned earlier, our interactive rating task differed from previous conformity tasks in which participants received social information about a stimulus ‘after’ forming their own opinion of it. Here, participants received social information ‘before’ seeing an affective picture, which might have enabled the aforementioned anticipatory processes. Therefore, we do not only interpret activity in the ventral striatum with respect to social reward elicited by the agreement with others but also with respect to emotional downregulation. Although amygdala and ventral striatum activity cannot be interpreted as pure markers of negative and less-negative (or positive) emotion (e.g. Paton et al., 2006), recent meta-analyses suggest that activity in amygdala and activity in the ventral striatum are biased toward negative and positive affective experience, respectively (e.g. Wager et al., 2008). In sum, our results indicate that emotional alignment as found in our study differs from other types of conformity.
Our study makes predictions for generalizations from the current design that should be tested in future studies. First, because we focused on negative emotion (in contrast to the conformity studies that investigated positive affective phenomena such as likability and attractiveness), it would be interesting to also investigate the social modulation of positive emotion. Second, it should be noted that participants in our study interacted with each other by communicating their emotional arousal on a verbally labeled scale. In real-life social interactions, however, emotional alignment may often include non-verbal communication (e.g. gesture, emotional facial expressions, prosody). Thus, future studies should clarify whether verbal and non-verbal types of social alignment differ from each other. Third, emotional alignment could be investigated from the perspective of cue integration (Zaki, 2013), which suggests that Bayesian inference provides a benchmark for understanding social information processing as it does for physical perception. For example, if people receive ratings about affective pictures from two different persons, they should update their estimates of the other persons’ reliability—a process that has been associated with the ventral striatum (Klucharev et al., 2009; Meshi et al., 2012). The ratings from the two persons should then be integrated according to their reliability.
CONCLUSION
Our study is one of the first that could show emotional alignment in a social setting, that is, an effect of social influence on emotional arousal exerted by a single interaction partner. The tendency to emotionally align was related to activity in bilateral anterior insula/IFG, and DLPFC in anticipation of the pictures. This anticipatory neural activity was positively correlated with individual differences in reappraisal use. During picture viewing, social influence was reflected in altered neural activity in ventral striatum in response to the interaction partner’s ratings. Our approach is in concordance with the recent proposal of an interaction-based ‘second person’ approach to social neuroscience (Schilbach et al., 2013b). This approach stresses that social cognition is fundamentally different when people are in interaction with others rather than merely observing a social interaction. For example, reciprocally engaging in eye contact with another person differs conceptually and neurally from passively perceiving the eye gaze of another person. Our task mirrored a social interaction, in which participants exchanged their opinion about an affective picture via ratings. Taken together, by showing that people integrate information from others into their own emotional appraisals and by investigating the neural correlates of such an emotion alignment in a social interaction, our study is in line with that ‘second person’ approach to and provides a step toward investigating real-time social encounters in a truly interactive manner. Because social influenceability, emotion regulation and the tendency to communicate about emotion differ between cultures (Bond and Smith, 1996; De Leersnyder et al., 2013), it would be interesting to use our interactive rating task to also investigate cultural differences.
Acknowledgments
This study was financially supported by the Cluster of Excellence ‘Languages of Emotion’ at Freie Universität Berlin, which is funded by the DFG (German Research Foundation). We thank Evelyn Schnapka for analyzing heart rate and skin conductance data.
Footnotes
1We used the following neutral and negative IAPS pictures (Lang et al., 2005):
Neutral: 1602, 2038, 2191, 2272, 2358, 2383, 2385, 2393, 2396, 2397, 2435, 2480, 2485, 2513, 2514, 2516, 2560, 2570, 2580, 2595, 2630, 2745, 2749, 2791, 2840, 5410, 5500, 5510, 5520, 5530, 5531, 5635, 5740, 7000, 7004, 7009, 7010, 7025, 7035, 7041, 7050, 7057, 7060, 7100, 7130, 7150, 7175, 7190, 7217, 7233, 7235, 7500, 7710, 9210.
Negative: 1019, 1022, 1040, 1050, 1051, 1052, 1070, 1080, 1101, 1110, 1111, 1114, 1120, 1200, 1201, 1205, 1220, 1270, 1274, 1300, 1301, 1302, 1321, 1525, 1930, 1931, 1932, 2717, 2800, 2900, 3000, 3005, 3010, 3015, 3030, 3051, 3053, 3060, 3062, 3063, 3064, 3068, 3069, 3080, 3100, 3102, 3110, 3140, 3170, 3181, 3261, 3266, 6021, 6022, 6212, 6510, 6550, 7380, 8230, 8480, 8485, 9008, 9040, 9181, 9182, 9252, 9253, 9265, 9290, 9300, 9301, 9320, 9410, 9420, 9433, 9471, 9480, 9490, 9560, 9561, 9570, 9571, 9611, 9620, 9622, 9630, 9635, 9903, 9911, 9921.
REFERENCES
- Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods. 2010;190(1):80–91. doi: 10.1016/j.jneumeth.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Capra CM, Moore S, Noussair C. Neural mechanisms of the influence of popularity on adolescent ratings of music. NeuroImage. 2010;49(3):2687–96. doi: 10.1016/j.neuroimage.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Chappelow J, Zink CF, Pagnoni G, Martin-Skurski ME, Richards J. Neurobiological correlates of social conformity and independence during mental rotation. Biological Psychiatry. 2005;58(3):245–53. doi: 10.1016/j.biopsych.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Bond R, Smith PB. Culture and conformity: a meta-analysis of studies using Asch’s (1952b, 1956) line judgment task. Psychological Bulletin. 1996;119(1):111–37. [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45(4):602–7. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Meiklejohn DK, Bach DR, Roepstorff A, Dolan RJ, Frith CD. How the opinion of others affects our valuation of objects. Current Biology. 2010;20(13):1165–70. doi: 10.1016/j.cub.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Social Cognitive and Affective Neuroscience. 2011;6(1):74–81. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Mujica-Parodi LR. A disposition to reappraise decreases anterior insula reactivity during anxious anticipation. Biological Psychology. 2010;85(3):383–5. doi: 10.1016/j.biopsycho.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Cialdini RB, Goldstein NJ. Social influence: compliance and conformity. Annual Review of Psychology. 2004;55(1974):591–621. doi: 10.1146/annurev.psych.55.090902.142015. [DOI] [PubMed] [Google Scholar]
- De Leersnyder J, Boiger M, Mesquita B. Cultural regulation of emotion: Individual, relational, and structural sources. Frontiers in Psychology. 2013;4:55. doi: 10.3389/fpsyg.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2008;363(1511):3787–800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Ochsner KN, Weber J, Wager TD. Anticipatory brain activity predicts the success or failure of subsequent emotion regulation. Social Cognitive and Affective Neuroscience. 2014;9(4):403–11. doi: 10.1093/scan/nss148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? Trends in Cognitive Sciences. 2006;10(10):435–41. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Edelson M, Sharot T, Dolan RJ, Dudai Y. Following the crowd: brain substrates of long-term memory conformity. Science. 2011;333(6038):108–11. doi: 10.1126/science.1203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers C, Fischer AH, Mosquera PMR, Manstead ASR. Anger and social appraisal: a “spicy” sex difference? Emotion. 2005;5(3):258–66. doi: 10.1037/1528-3542.5.3.258. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35(3):903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Festinger L. A theory of social comparison processes. Human Relations. 1954;7(2):117–40. [Google Scholar]
- Freudenthaler HH, Neubauer AC, Gabler P, Scherl WG, Rindermann H. Testing and validating the trait emotional intelligence questionnaire (TEIQue) in a German-speaking sample. Personality and Individual Differences. 2008;45(7):673–8. [Google Scholar]
- Gläscher J, Daw N, Dayan P, O’Doherty JP. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron. 2010;66(4):585–95. doi: 10.1016/j.neuron.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Review of General Psychology. 1998;2(3):271–99. [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- Herwig U, Baumgartner T, Kaffenberger T, et al. Modulation of anticipatory emotion and perception processing by cognitive control. NeuroImage. 2007;37(2):652–62. doi: 10.1016/j.neuroimage.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Jonas KJ, Huguet P. What day is today? A social-psychological investigation into the process of time orientation. Personality and Social Psychology Bulletin. 2008;34(3):353–65. doi: 10.1177/0146167207311202. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex. 2011;21(6):1379–88. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Klucharev V, Munneke MAM, Smidts A, Fernandez G. Downregulation of the posterior medial frontal cortex prevents social conformity. Journal of Neuroscience. 2011;31(33):11934–40. doi: 10.1523/JNEUROSCI.1869-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucharev V, Hytönen K, Rijpkema M, Smidts A, Fernández G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61(1):140–51. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Korn CW, Prehn K, Park SQ, Walter H, Heekeren HR. Positively biased processing of self-relevant social feedback. Journal of Neuroscience. 2012;32(47):16832–44. doi: 10.1523/JNEUROSCI.3016-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8. 2008 University of Florida, Gainsville, FL. [Google Scholar]
- Manstead ASR, Fischer AH. Social appraisal: the social world as object of and influence on appraisal processes. In: Scherer KR, Schorr A, Johnstone T, editors. Appraisal Processes in Emotion: Theory, Methods, Research. New York: Oxford University Press; 2001. pp. 221–32. [Google Scholar]
- Matejka M, Kazzer P, Seehausen M, et al. Talking about emotion: prosody and skin conductance indicate emotion regulation. Frontiers in Psychology. 2013;4:260. doi: 10.3389/fpsyg.2013.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Biele G, Korn CW, Heekeren HR. How expert advice influences decision making. PloS One. 2012;7(11):e49748. doi: 10.1371/journal.pone.0049748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumenthaler C, Sander D. Social appraisal influences recognition of emotions. Journal of Personality and Social Psychology. 2012;102(6):1118–35. doi: 10.1037/a0026885. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251(1):E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439(7078):865–70. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectrums. 2004;9(4):258–66. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Rimé B. Emotion elicits the social sharing of emotion: theory and empirical review. Emotion Review. 2009;1(1):60–85. [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16(2):235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Schachter S, Singer JE. Cognitive, social, physiological determinants of emotional state. Psychological Review. 1962;69(5):379–99. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Schultze T, Mojzisch A, Vogeley K. To you I am listening: perceived competence of advisors influences judgment and decision-making via recruitment of the amygdala. Social Neuroscience. 2013a;8(3):189–202. doi: 10.1080/17470919.2013.775967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, et al. Toward a second-person neuroscience. The Behavioral and Brain Sciences. 2013b;36(4):393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Seehausen M, Kazzer P, Bajbouj M, Prehn K. Efects of empathic paraphrasing—extrinsic emotion regulation in social conflict. Frontiers in Psychology. 2012;3:482. doi: 10.3389/fpsyg.2012.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Human Brain Mapping. 2011;32(11):1836–46. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, Kühn S, De Raedt R. “Put on your poker face”: neural systems supporting the anticipation for expressive suppression and cognitive reappraisal. Social Cognitive and Affective Neuroscience. 2013;8(8):903–10. doi: 10.1093/scan/nss090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, et al. The neuroimaging of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotion. New York: Guilford Press; 2008. pp. 249–71. [Google Scholar]
- Waugh CE, Wager TD, Fredrickson BL, Noll DC, Taylor SF. The neural correlates of trait resilience when anticipating and recovering from threat. Social Cognitive and Affective Neuroscience. 2008;3(4):322–32. doi: 10.1093/scan/nsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Symmonds M, Bossaerts P, Dolan RJ. Hedging your bets by learning reward correlations in the human brain. Neuron. 2011;71(6):1141–52. doi: 10.1016/j.neuron.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J. Cue integration: a common framework for social cognition and physical perception. Perspectives on Psychological Science. 2013;8(3):296–312. doi: 10.1177/1745691613475454. [DOI] [PubMed] [Google Scholar]
- Zaki J, Schirmer J, Mitchell JP. Social influence modulates the neural computation of value. Psychological Science. 2011;22(7):894–900. doi: 10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]