Abstract
Functional neuroimaging studies have implicated the default mode network (DMN) in autobiographical memory (AM). Convergent evidence from a lesion approach would help clarify the role of the DMN in AM. In this study, we used a voxelwise lesion-deficit approach to test the hypothesis that regions of the DMN are necessary for AM. We also explored whether the neural correlates of semantic AM (SAM) and episodic AM (EAM) were overlapping or distinct. Using the Iowa Autobiographical Memory Questionnaire, we tested AM retrieval in 92 patients with focal, stable brain lesions. In support of our hypothesis, damage to regions within the DMN (medial prefrontal cortex, mPFC; posterior cingulate cortex, PCC; inferior parietal lobule, IPL; medial temporal lobe, MTL) was associated with AM impairments. Within areas of effective lesion coverage, the neural correlates of SAM and EAM were largely distinct, with limited areas of overlap in right IPL. Whereas SAM deficits were associated with left mPFC and MTL damage, EAM deficits were associated with right mPFC and MTL damage. These results provide novel neuropsychological evidence for the necessary role of parts of the DMN in AM. More broadly, the findings shed new light on how the DMN participates in self-referential processing.
Keywords: default mode network, autobiographical memory retrieval, semantic autobiographical memory, episodic autobiographical memory, self-referential processing
INTRODUCTION
Autobiographical memory and the default mode network
The ability to recollect and re-experience autobiographical events is essential for the development, maintenance and awareness of our unique personal narrative and identity. Autobiographical memory (AM) is often characterized as ‘personal episodic memory’, or the process of mentally traveling back in time to relive an event from our past with temporal and spatial specificity (e.g. remembering your college graduation, including where you were and how you felt) (Tulving, 1983).
Neuroimaging and lesion studies have implicated multiple brain regions in AM, including prefrontal (medial prefrontal cortex, mPFC; ventromedial prefrontal cortex, vmPFC; dorsomedial prefrontal cortex, dmPFC), temporal (lateral temporal cortex, LTC; medial temporal lobe, MTL) and parietal (posterior cingulate cortex/retrosplenial, PCC/Rsp; precuneus, pC; inferior parietal lobule, IPL) regions (Scoville and Milner, 1957; Kroll et al., 1997; Svoboda et al., 2006; Tranel and Jones, 2006; Berryhill et al., 2007; Thaiss and Petrides, 2008). Interestingly, there is substantial overlap between these structures and the brain regions that are typically included in the ‘default mode network’ (DMN) (Andreasen et al., 1995; Buckner et al., 2008; Spreng et al., 2009; Buckner, 2012). The DMN, which includes the mPFC, PCC/Rsp, IPL, and LTC and MTL, derives its name from the consistent finding that these structures are more active during ‘rest’ conditions (periods of unconstrained thought) than during externally focused, cognitively demanding tasks (Shulman et al., 1997; Mazoyer et al., 2001; Raichle et al., 2001; Buckner et al., 2008). Although the functional significance of the DMN is not entirely understood, converging evidence suggests that the DMN may be involved in various types of self-referential processing, including spontaneous thought or mind wandering, and AM retrieval (Andreasen et al., 1995; Raichle et al., 2001; Mckiernan et al., 2003; D'Argembeau et al., 2005; Mason et al., 2007; Buckner et al., 2008; Qin and Northoff, 2011; Whitfield-Gabrieli et al., 2011). Other neuroimaging studies have pointed to the role of the DMN (in particular MTL and LTC) in mental simulation, scene construction, future thinking and theory of mind (Buckner and Carroll, 2007; Buckner et al., 2008; Hassabis and Maguire, 2007; Spreng et al., 2009; Andrews-Hanna et al., 2010). However, while neuroimaging studies have implicated the DMN in AM, no large-scale lesion study has yet examined the necessary role of DMN regions in AM retrieval. The primary goal of the present study was to establish whether certain parts of the DMN play a ‘critical’ role in AM.
Semantic and episodic AM
A secondary goal of the current study was to explore the neural correlates of ‘semantic’ AM (SAM) and ‘episodic’ AM (EAM) retrieval. Cognitively, SAM refers to context-free general knowledge from AM (e.g. your address, your grandfather’s name, the name of your high school), whereas EAM refers to context-specific aspects of our autobiography, memories that are tied to specific times and places (e.g. a specific vacation the week after you graduated from high school). In terms of the neural circuitry, cognitive neuroscience research has documented both differences and similarities between SAM and EAM. Dissociations between SAM and EAM have been highlighted in neuropsychological research, with the MTL consistently implicated in episodic memory and EAM, but not in semantic memory or SAM. Functional neuroimaging studies in healthy populations have also shown both associations and dissociations between SAM and EAM.
Early neuropsychological studies in patients with temporal lobectomies, most notably studies with patient H.M., provided the first compelling evidence for the critical role of the MTL in episodic memory (Scoville and Milner, 1957; Squire, 1992; Spiers et al., 2001). Additionally, these findings along with other lesion and neuroimaging studies provided support for a neural dissociation between episodic and semantic memory (Kapur, 1999; Burianova and Grady, 2007 for review; Moscovitch et al., 2005 for review; Tulving, 1972; Tulving et al., 1988; Vargha-Khadem et al., 1997; Wheeler and Mcmillan, 2001; Svoboda and Levine, 2009; But see Squire and Zola-Morgan, 1991; Squire, 1992). For example, patients with retrograde amnesia following MTL damage tended to have significant impairments in episodic memory retrieval whereas semantic memory retrieval remained relatively intact (Kapur, 1999 for review; Vargha-Khadem et al., 1997). Building on these neurological cases, subsequent neuropsychological research provided similar support for dissociations between SAM and EAM (Tulving, 1972, 2002; Cermak and O'Connor, 1983; Damasio et al., 1985; Tulving et al., 1988; Klein and Gangi, 2010). Reports of individuals with profound retrograde amnesia after brain injury, including the MTL, have shown that aspects of SAM can remain intact despite severe deficits in EAM (e.g. Cermak and O'Connor, 1983; Damasio et al., 1985; Klein and Gangi, 2010 for review; Tulving et al., 1988; Rathbone et al., 2009). For example, Damasio et al. (1985) studied an amnesic patient who was able to recall semantic facts about his life (e.g. jobs he held, names of family members and friends), but was unable to situate these facts in a temporal context (p. 254). In contrast, patients with extensive left temporal lobe lesions or semantic dementia exhibited the opposite pattern, with SAM severely deficient, but EAM relatively intact (e.g. Eslinger, 1998; Hodges and Graham, 2001; Piolino et al., 2003; Hodges and Patterson, 2007). In sum, these studies demonstrated the crucial role of the MTL in EAM. By comparison, these findings did not reveal consistent neural correlates of SAM.
Functional neuroimaging studies in neurologically healthy populations have indicated that the brain regions underlying SAM and EAM are both overlapping and distinct (Addis et al., 2004a,b; Holland et al., 2011; Levine et al., 2004; Martinelli et al., 2013). For instance, a recent meta-analysis of 38 functional magnetic resonance imaging (fMRI) studies found that both SAM and EAM were associated with activity in the left mPFC, PCC, parahippocampal gyrus (PHG) and inferior parietal regions (Martinelli et al., 2013). However, EAM tends to elicit greater activity than SAM in brain regions including the hippocampus, PHG, pC, PCC and the temporal parietal junction (e.g. Addis et al., 2004b; Holland et al., 2011; Martinelli et al., 2013). In contrast, SAM has been associated with greater activity in frontal and lateral temporal regions, such as the mPFC, middle and inferior temporal regions (e.g. fusiform gyrus) (e.g. Addis et al., 2004b; Levine et al., 2004; Martinelli et al., 2013). These neuroimaging findings suggest that SAM and EAM may have both overlapping and distinct neural correlates.
Taken together, neuropsychological research indicates that there are primarily differences between the brain regions critical for SAM and EAM, whereas neuroimaging studies show that there are also similarities. However, because of the potential limitations in previous lesion studies (e.g. small sample size), the precise differences and/or similarities between the neural circuitry associated with SAM as compared with EAM remain underspecified. Group-level voxelwise lesion-deficit methods can provide strong approaches to explore such brain–behavior relationships, taking advantage of the power afforded by large sample sizes and more distributed lesion coverage. For example, voxelwise lesion-deficit approaches make it possible (in principle) to identify how damage in certain brain regions may be consistently and specifically associated with deficits in a certain behavior (e.g. Rorden et al., 2007; Rudrauf et al., 2008). However, to date, no large-scale lesion study has directly compared the neural correlates of SAM and EAM.
In the present study, we performed a group-level voxelwise lesion analysis to test the hypothesis that regions within the DMN are critical for AM. Specifically, we predicted that damage to brain regions within the DMN (mPFC, PCC/Rsp, IPL, LTC and MTL) should be associated with impaired AM retrieval. We also explored whether the neural correlates of SAM and EAM are overlapping or distinct.
METHODS
Participants
The sample consisted of two groups: a brain damaged (BD) group (N = 92), and a neurologically normal, healthy comparison group (N = 34) (Table 1). For the BD group, 92 subjects (mean age 53.7 ± 13.5) with stable focal brain lesions were selected from the Cognitive Neuroscience Patient Registry of the University of Iowa’s Department of Neurology. Patients with different lesion etiologies and locations (right hemisphere, R; left hemisphere, L; bilateral, B) were included (hemorrhage [8L/3R/4B], infarct [18L/17R/4B], surgical resection of focal lesions (benign tumor [3L/6R/8B]), herpes simplex encephalitis [3R/1B] and surgical treatment for epilepsy [5L/6R/4B] or trauma [1L/1B]) (Figure 1).
Table 1.
Participant group demographics
| Group | Age (years) | Education (years) | Sex | Handedness | Chronicity (years) | Laterality |
|---|---|---|---|---|---|---|
| BD (N = 92) | 53.7 (13.5) | 13.6 (2.8) | 51M/41F | 74R/5L/13M | 9.1 (6.3) | 35L/35R/22B |
| NC (N = 34) | 43.2 (13.4) | N/A | N/A | N/A | N/A | N/A |
Notes: Demographic information, lesion laterality and chronicity are reported for brain-damaged subjects. Age is reported for the normal comparison group. Additional demographic information was not available for the comparison group, as the data from these subjects were collected as part of a previous study (Tranel and Jones, 2006)—however, the normal participants were drawn from a Registry of healthy persons that we have used extensively in our research, and this cohort has demographic features that are very similar to those of our brain-damaged population.
BD, brain damaged; NC, normal comparison; age and education are reported in group means with s.d. in parentheses; M, male; F, female; Handedness: R, right handed; L, left handed; M, mixed handedness; Chronicity is the time between lesion onset and experimental testing; Laterality indicates the hemispheric location of the lesion: L, left hemisphere; R, right hemisphere; B, bilateral.
Fig. 1.
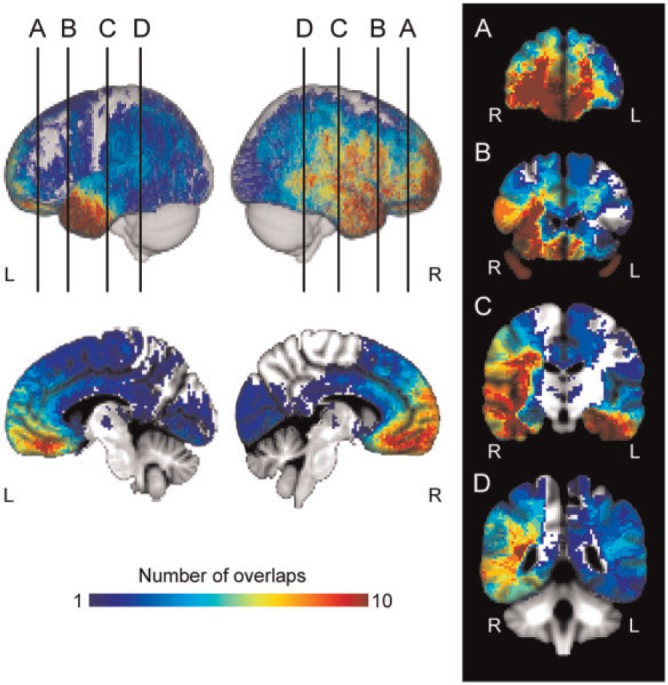
Lesion overlap map (NMap) for all brain-damaged subjects. Overlap maps are displayed in MNI-152 space. Lateral left, lateral right, midsagittal (right, left) are displayed. (A-D) Coronal slices are represented from anterior to posterior regions. Coronal images are displayed in radiologic convention. Color code indicates total number of overlapping lesions at each voxel.
All patients had stable (non-progressive) and circumscribed brain lesions, and all were characterized neuropsychologically and neuroanatomically in the chronic epoch (>3 months after lesion onset), according to standard protocols of the Benton Neuropsychology Laboratory (Tranel, 2009) and the Laboratory of Human Neuroanatomy and Neuroimaging (Damasio and Damasio, 1989; Frank et al., 1997). Additionally, all subjects with any psychiatric disorders or other neurological illnesses were excluded.
For the normal comparison group, data from 34 neurologically healthy subjects (mean age = 43 ± 13.5) were used; 33 of the subjects participated in an earlier study (Tranel and Jones, 2006), 1 additional subject was tested for this study.
All participants gave informed consent according to a protocol approved by the Institutional Review Board of the University of Iowa.
Lesion mapping procedures
All subjects underwent structural scanning procedures. Magnetic resonance (MR) images were acquired in a 1.5-T GE Sigma scanner with a 3D SPGR sequence (1.5 mm contiguous T1 weighted coronal cuts). If subjects were unable to undergo MR scanning, computerized axial tomography (CT) data were collected. Lesion maps were generated using the MAP-3 method, a technique used to generate a 3-dimensional lesion overlap map in template space, (Frank et al., 1997; Fiez et al., 2000), in which boundaries of the lesions for a given subject are visually identified on MR/CT scans and manually transferred onto a normal reference brain (P.C. local standard space; resolution, 0.94 × 0.94 × 1.6 mm) based on the delineation of homologous anatomical landmarks. This procedure requires anatomical expertise but circumvents the problems of interindividual registration encountered with lesion data and the problems of combining subjects scanned with different imaging modalities. Lesion delineation and transfer were done using Brainvox (Frank et al., 1997). One advantage of this time-consuming approach is that it preserves anatomical boundaries and tissue compartments in the mapping of the lesions onto the reference brain, enabling group-level analysis.
All subject lesion masks were warped and resampled to Montreal Neurological Institute (MNI-152) template space (resolution, 2 mm3) using AFNI (Cox, 1996). Lesion overlap maps (NMaps) were created by summing the three-dimensional MAP-3 binary lesion mask for all subjects (Figure 1).
AM task and scoring procedure
All subjects completed the Iowa Autobiographical Memory Questionnaire (IAMQ) (Jones et al., 1998). The IAMQ is a comprehensive self-report inventory that assesses AM across the life span. In particular there are five IAMQ time periods including: early childhood and adolescence (birth–18 years), young adulthood (19–39 years), middle adulthood (40–59 years), late adulthood (60+ years) and recent life (past year). We focused on retrieval of AM related to information acquired before lesion onset (retrograde memory). Consistent with prior studies using the IAMQ (Tranel and Jones, 2006), we also verified answers for each participant against a collateral—usually a family member or spouse who knew the participant well and who could confirm the accuracy of each memory. Although we did not assess confabulation directly, our verification method prevented the inclusion of confabulated or inaccurate memories. Moreover, there were very few instances where a collateral was unable to verify the accuracy of an answer. Importantly, any memory that could not be verified was not included in the AM score for that time period.
The composite AM score for the IAMQ was calculated as the sum of the total number of correct (and verified) responses divided by the total number of possible responses for each time period (total correct/total possible). The total number of possible responses for composite AM did not include questions that were left blank or not verified by the collateral. Thus, participants were not penalized for missing or unverified answers.
Memory components
We also decomposed the IAMQ into SAM (e.g. What was your street address in high school?) and EAM (e.g. Recall an incident when you were in high school) components based on the type of AM required for each question. Although the IAMQ was not originally designed to calculate separate SAM and EAM components, the questionnaire was created to assess both SAM and EAM, similar to the well-validated Autobiographical Memory Interview (Kopelman et al., 1989). In the IAMQ, the SAM questions asked about various personal semantic memories such as names of family members, pets and former employers. In contrast, the EAM questions asked participants to recall a specific autobiographical event that happened during a particular time period (e.g. Recall an incident when you were in high school). Whereas SAM questions were scored as 0 or 1 (correct/incorrect), each EAM question was scored from 0–3 depending on the level of episodic detail and vividness of the memory. Lastly, total SAM and EAM scores were calculated for each time period similar to composite AM—where the total SAM and EAM scores for all correct responses were divided by the total number possible for each component.
Behavioral data analysis
To perform the lesion analysis (Rudrauf et al., 2008), subject performance was dichotomized as impaired or unimpaired. First, we selected a unique retrograde retrieval epoch (out of the scores for the four retrograde time periods) for each subject based on the time before lesion onset (see ‘Retrieval Epoch’ below). Next, we divided the composite AM scores into SAM and EAM components (see ‘Memory Components’ above). All scores were then z-transformed for normalization. Subjects were defined as impaired based on comparison to the normal healthy comparison group (1.65 s.d. below the mean; <5th percentile).
Retrieval epoch
To have a retrieval epoch that would be generally calibrated across participants, the retrograde retrieval epoch was calculated by first subtracting 5 years from age of lesion onset to find the ‘reference time period.’ For example, if age at lesion onset was 55, after subtracting five, the reference time period would be middle adulthood. We then used the time period ‘before’ the reference time period to define the retrograde retrieval epoch (e.g. reference time period = middle adulthood; retrograde retrieval epoch = young adulthood). Next, to dichotomize each patient into ‘impaired’ and ‘unimpaired’ groups for the lesion analysis, we compared the z-score for the selected retrograde time period (e.g. young adulthood) for each patient with the average z-score across all healthy participants for that exact same time period. For example, if the selected retrograde time period for a patient was ‘young adulthood’, then the patient’s z-scores for all memory types (composite AM, SAM and EAM) for the young adulthood epoch was compared with the average z-scores for all memory types across all healthy comparison participants for the young adulthood time period. If the AM scores were 1.65 s.d. below the mean of the healthy participants, the patient was classified as ‘impaired’.
Neuropsychological variables
All patients were tested on various neuropsychological measures including verbal memory (Rey Auditory-Verbal Learning Test, AVLT: scores for Trial 5; 30 min delayed recall reported), visuospatial memory (Complex Figure Test, CFT: 30 min delayed recall score reported), language (Token Test score from the Multilingual Aphasia Examination reported), word identification and reading (Wide Range Achievement Test—Revised, WRAT-R: Reading Standard Score reported) and general mood (Beck Depression Inventory-II, BDI-II, raw score reported). These neuropsychological variables were included to investigate potential group differences in general memory, language, reading ability or mood (Table 2), so that any such differences could be taken into account in interpreting differences in AM retrieval scores.
Table 2.
Brain damaged participant group characteristics for composite memory
| Variable | Unimpaired composite AM (N = 62) | Impaired composite AM (N = 30) |
|---|---|---|
| Age (years) | 51 (13.7) | 59.2 (11.1)* |
| Education (years) | 14.3 (2.8) | 12.2 (2.5)* |
| Sex | 35M/27F | 16M/14F |
| Handedness | 53R/2L/7M | 21R/3L/6M |
| Chronicity (years) | 10.1 (6.1) | 7.3 (6.5) |
| Laterality | 16B/17L/29R | 6B/18L/6R |
| Rey AVLT: Trial 5/30 min recall | 11.6(2.6)/9.3(3.9) (N = 61) | 10.1(3.3)/8.0(3.8) (N = 29) |
| CFT: 30 min recall | 19.1 (6.4) | 14.9 (7.6)* (N = 28) |
| Token test | 42.7 (4.3) (N = 54) | 42.1 (2.3)* (N = 25) |
| WRAT-R: Reading Standard Score | 101.6 (13.8) (N = 55) | 92.8 (10.4) (N = 23) |
| BDI-II | 6.2 (5.4) (N = 58) | 6.6 (5.1) (N = 28) |
Notes: Mann–Whitney U test, *significant at P < 0.05, two-tailed, Bonferroni-corrected for multiple comparisons, α = 0.006. The overall group N’s are indicated (62 for unimpaired composite AM, 30 for impaired composite AM), although it should be noted that there are some instances of missing values (N’s are indicated for those measures with missing values).
Voxelwise proportional MAP3 lesion-deficit analysis
Lesion-deficit relationships and statistical power were estimated using voxelwise proportional difference maps (PM3) (Rudrauf et al., 2008). PM3 expresses, for every voxel, the proportion of subjects whose lesion includes the voxel and who have a deficit (NLD) relative to the total number of subjects with a deficit (ND), minus the proportion of subjects with a lesion at the voxel and no deficit (NLnD) relative to the total number of subjects with no deficit (NnD). The formula can be expressed with the equation Prob (L | D) − Prob (L | nD), the conditional probability of a lesion (L) given a deficit (D) minus the conditional probability of a lesion given no deficit (nD). For example, at a given voxel, if all patients with a lesion have a deficit, the PM3 = 1, whereas PM3 = 0 when half the patients have a lesion and a deficit, and the remainder have a lesion and no deficit. The PM3 maps were thresholded using exact statistics from permutation tests (Rudrauf et al., 2008). The statistical thresholds for the PM3 analysis were determined based on preliminary power analyses, i.e. ‘effective coverage maps’ (ECMs). Effective coverage is a voxelwise measure of the maximum lesion-deficit relationship permitted by the sample, at a given statistical threshold (e.g. P < 0.05) (Rudrauf et al., 2008). In other words, effective coverage defines the brain regions where effects can/cannot be detected at a given significance threshold. To create ECMs (Figures 2A and 3A), we first calculated at each voxel the maximum lesion-deficit relationship permitted which took into account: (i) the number of subjects with a lesion and a deficit at a given voxel (NLD), (ii) the number of subjects with a deficit in the full sample (ND), (iii) the number of subjects with a lesion and no deficit at a given voxel (NLnD), and (iv) the number of subjects with no deficit in the full sample (NnD). For example, in a full sample of 92 subjects, including 6 subjects with a deficit and 86 without a deficit, if 8 subjects had a lesion at a given voxel of interest, the maximum number of subjects permitted by the sample that could have both a deficit and a lesion at that voxel would be 6, and the value of effective coverage would be calculated as follows: [6 NLD/6 ND] − [2 NLnD/86 NnD] = .97. Next, the ECMs were thresholded at P < 0.05 using exact statistics (Rudrauf et al., 2008).
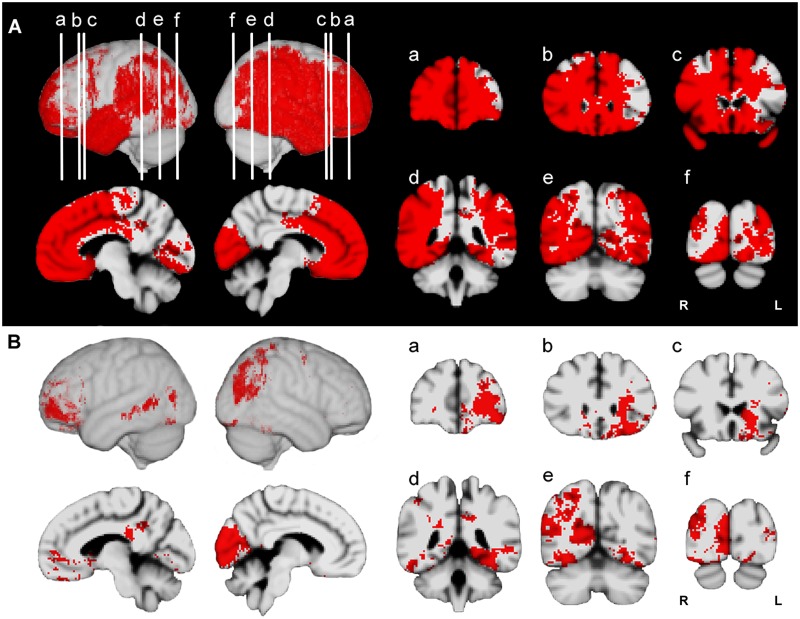
Fig. 2.
Lesion proportion difference maps (PM3) and ECM for composite AM. (A) Effective coverage map (red indicates effective coverage at P < 0.05). (B) Thresholded PM3 results for composite AM (red indicates lesion-deficit effects significant at P < 0.05). PM3 maps are displayed on in MNI-152 space. Lateral left, lateral right, midsagittal (right, left) are displayed. Coronal slices a–f: anterior to posterior regions. Coronal images are displayed in radiologic convention.
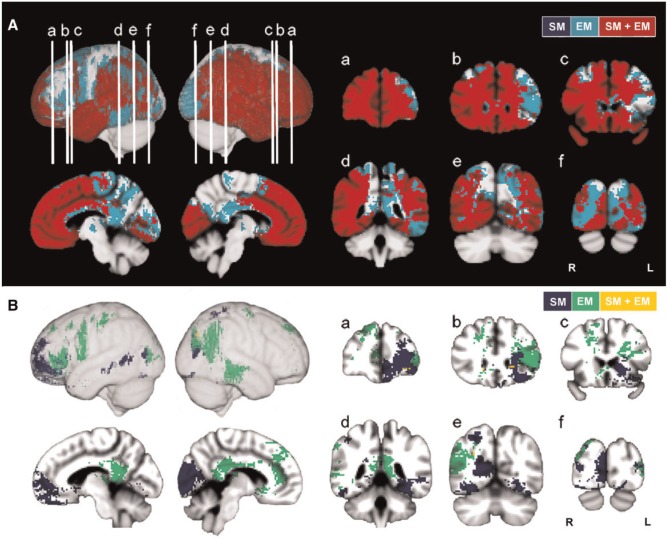
Fig. 3.
Lesion proportion difference maps (PM3) and ECM for episodic and semantic AM. (A) Effective coverage map (color codes: blue = effective coverage based on semantic AM; cyan = effective coverage based on episodic AM; red = effective coverage for episodic and semantic; ECM maps are significant at P < 0.05). (B) Thresholded PM3 results for semantic and episodic AM are represented on the same brain (color codes: blue = semantic AM (SM), green = episodic AM (EM), yellow = both (SM + EM); effects represented for both memory types are significant at P < 0.05). PM3 maps are displayed in MNI-152 space. Lateral left, lateral right, midsagittal (right, left) are displayed. Coronal slices a–f: anterior to posterior regions. Coronal images are displayed in radiologic convention.
We selected the one-tailed, uncorrected threshold of P < 0.05 as the main threshold for the PM3 analysis. The rationale for using an uncorrected threshold was that we implemented a hypothesis-driven approach with specific predictions of lesion-deficit relationships for particular brain regions (the DMN sectors named above—mPFC, IPL, LTC, MTL and PCC/Rsp). In addition, the availability of well-characterized neurological patients with focal lesions to these brain regions is obviously a major limiting factor in work like this, and we sought to maximize the signal-to-noise in our experimental data. We also performed preliminary power analyses (as described above), and limited our analyses to regions where effective coverage was sufficient at P < 0.05, given our lesion sample.
AM impairment in patients with lesions to the PCC/Rsp
We acknowledge the limitations in the interpretation of group-level voxelwise lesion analyses for participants with brain injury to the PCC/Rsp (a component of the DMN), as lesions to this region are rare. While we report group-level lesion-deficit results including three participants with PCC/Rsp damage, we also present a statistical ranking of these individuals as case studies to better characterize their performance in relation to other brain-injured participants within the sample. For these ranked statistical results, we reasoned that if the PCC/Rsp is critical for AM, all of these three patients should be relatively impaired on AM retrieval. We ranked all subjects based on their scores for each of the AM components, and computed their individual and quartile ranks. We report the rankings of AM scores for the three PCC/Rsp cases below.
RESULTS
Behavioral results
Subjects were dichotomized for lesion-deficit analysis into impaired and unimpaired groups for each of the AM components (‘composite’, ‘semantic’ and ‘episodic’). For each memory type, ‘impaired’ groups had mean AM scores that were significantly lower than ‘unimpaired’ groups (Mann–Whitney U test, each P < 0.001).
There were no significant differences between ‘episodic’ AM groups for any demographic or neuropsychological variables (Mann–Whitney U Test, each P > 0.05, Bonferroni corrected P = 0.006). However, there were significant differences between ‘composite’ AM groups for age, education, CFT 30 min recall and the Token test (Mann–Whitney U Test, P < 0.05, Bonferroni corrected  = 0.006), and between ‘semantic’ AM groups for the CFT 30 min recall (Mann–Whitney U Test, P < 0.05, Bonferroni corrected
= 0.006), and between ‘semantic’ AM groups for the CFT 30 min recall (Mann–Whitney U Test, P < 0.05, Bonferroni corrected  = 0.006). Although there were no significant group differences, the impaired ‘composite’ AM group also had lower means for chronicity (Table 2). Importantly, after controlling for all of these variables (age, education, chronicity, CFT 30 min recall and the Token test) in follow-up analyses, significant differences between ‘impaired’ and ‘unimpaired’ groups remained for both ‘composite’ AM (F5,73 = 5.61, P < 0.001, partial
= 0.006). Although there were no significant group differences, the impaired ‘composite’ AM group also had lower means for chronicity (Table 2). Importantly, after controlling for all of these variables (age, education, chronicity, CFT 30 min recall and the Token test) in follow-up analyses, significant differences between ‘impaired’ and ‘unimpaired’ groups remained for both ‘composite’ AM (F5,73 = 5.61, P < 0.001, partial  2 = 0.28) and ‘semantic’ AM (F5,73 = 4.17, P < 0.01, partial
2 = 0.28) and ‘semantic’ AM (F5,73 = 4.17, P < 0.01, partial  2 = 0.22).
2 = 0.22).
Voxelwise lesion-deficit analysis results
Composite AM
In support of the main hypothesis, deficits in ‘composite’ retrograde AM were significantly associated with damage to regions within the DMN (Figure 2). Effects were present in portions of the left mPFC, including the vmPFC and extending to frontopolar regions. While prefrontal effects were primarily localized in the left hemisphere, a few significant voxels were found in the right posterior orbitofrontal cortices. Although our sample size of patients with PCC/Rsp lesions was limited, significant effects were found in the left PCC. The Rsp and pC were not implicated in either hemisphere. However, we were limited by statistical power in these regions (Figure 2A). The IPL was implicated in the right hemisphere only. Effects were also found in left LTC (within Brodmann area 21) and bilateral MTL components (posterior PHG) of the DMN.
Besides the core components of the DMN, significant effects were present in right superior lateral parietal and lateral occipital cortices. Effects were also found bilaterally in ventral temporal and occipital regions including the fusiform gyrus and portions of the cuneus and lingual gyrus (more extensive in the right hemisphere).
Semantic and episodic AM
When breaking down the IAMQ into SAM and EAM components, overlapping and distinct regions of the DMN were implicated (Figure 3). Both SAM and EAM deficits were associated with limited areas of overlapping damage to the right IPL, with more extensive parietal effects for EAM. While damage to the mPFC and MTL was associated with SAM and EAM deficits, the effects were not overlapping. More specifically, effects for the mPFC and MTL appeared to show distinct lateralization for each AM component. For SAM, mPFC and MTL effects were left lateralized and similar to those for ‘composite’ AM, including left vmPFC, frontopolar and posterior parahippocampal regions. In contrast, significant effects for EAM were right lateralized and found primarily in the right dorsal mPFC, anterior cingulate cortex (ACC) (encompassing Brodmann areas 8, 9, and 32) and parahippocampal regions. In the PCC extending to Rsp, significant effects were found bilaterally only for EAM. However, statistical power was more limited in the PCC/Rsp for SAM and ‘composite’ AM as compared with EAM.
SAM deficits were also associated with damage to temporal (ventral and lateral portions), parietal and occipital regions. In fact, the effects for SAM were nearly identical to those regions implicated for ‘composite’ AM. Analyses for EAM implicated a more distinct pattern of effects on the lateral surface including the left inferior frontal gyrus, primary motor and premotor cortex, and the right superior parietal cortices and inferior temporal gyrus.
Patients with PCC/Rsp lesions have low IAMQ scores
To examine the relative impact of PCC/Rsp lesions on AM retrieval in more detail, we ranked all subjects according to their AM scores. We focused on three rare cases with focal PCC/Rsp lesions. We found that all patients with PCC/Rsp lesions were ranked in the lowest quartile for ‘composite’ AM score (mean = 0.71 ± 0.10), and in the lowest half of all subjects (cases all individually ranked < 35 out of 92; with ranking of 1 = lowest AM score, 92 = highest AM score) for SAM and EAM scores (mean SAM score = 0.77 ± 0.15; mean EAM score = 0.46 ± 0.12). Thus, PCC/Rsp lesions were associated with relatively impaired AM retrieval across all AM components.
DISCUSSION
In this study, we performed a voxelwise lesion-deficit analysis to test the hypothesis that regions of the DMN are necessary for AM retrieval. Our findings fully support this hypothesis. We found that brain damage to regions within the DMN was associated with impairments in AM retrieval, including both SAM and EAM. We also found that all three patients with PCC/Rsp lesions were relatively impaired in AM retrieval when ranked within the entire sample of brain-damaged participants. Significant impairments in AM retrieval were also observed after damage to the occipital cortex.
Our results are convergent with recent functional imaging literature on the DMN (Raichle et al., 2001; Greicius et al., 2003) and its implication in AM (Andreasen et al., 1995; Buckner et al., 2008; Spreng et al., 2009). We found that AM retrieval was impaired after damage to each of the main components of the DMN, including mPFC, PCC/Rsp, LTC, MTL and IPL regions. The brain regions identified in the present study also demonstrate a remarkable overlap with the core AM network identified in a meta-analysis of functional imaging studies of AM (Svoboda et al., 2006). The findings indicate that SAM and EAM rely on distinct and partially overlapping brain regions, in a manner that is consistent with neuroimaging (e.g. Addis et al., 2004b; Levine et al., 2004; Martinelli et al., 2013) and neuropsychological (e.g. Tulving, 1972; Cermak and O'Connor, 1983; Damasio et al., 1985; Tulving et al., 1988; Klein and Gangi, 2010; Irish et al., 2012) findings. Additionally, the SAM results are compatible with a recent meta-analysis associating DMN regions with semantic memory (Binder et al., 2009).
More broadly, these results could have important implications for research on other types of self-related processes associated with the DMN, including future thinking, spontaneous thought and self-referential cognition (Buckner and Carroll, 2007; Mason et al., 2007; Buckner et al., 2008; Andrews-Hanna et al., 2010; Spreng and Grady, 2010; Qin and Northoff, 2011). For example, several neuroimaging studies indicate that tasks requiring past AM retrieval and future thinking recruit DMN regions (Okuda et al., 2003; Schacter et al., 2012 for review; Szpunar et al., 2007; Spreng and Grady, 2010). Moreover, neuropsychological studies suggest that both episodic and semantic memory deficits can contribute to impaired future thinking (Hassabis et al., 2007; Race et al., 2011; Irish et al., 2012). For instance, comparable deficits in future thinking were found both in patients with semantic dementia and patients with Alzheimer’s disease (Irish et al., 2012). Although lesion studies of future thinking have primarily focused on the MTL, no lesion study has yet investigated whether lesions to other DMN regions are associated with deficits in future thinking and AM retrieval. More generally, our findings also raise theoretical questions about the functional significance of the DMN. For example, is the DMN critical for a domain general process such as internal mentation, self-related processes (i.e. AM retrieval) or mental simulation (Buckner and Carroll, 2007; Buckner et al., 2008)? Or are separate self-related processes associated with separate subsystems of the DMN (Andrews-Hanna et al., 2010, 2014)? Neuroimaging studies to date have found evidence to support both the domain general function of the DMN in simulation (e.g. Spreng et al., 2009; Spreng and Grady, 2010), and the division of the DMN into separate MTL and dorsomedial PFC subsystems involved in different types of self-related processes (e.g. future thinking, self-referential processing/mentalizing) (e.g. Andrews-Hanna et al., 2010, 2014). Thus, future large-scale lesion studies are warranted to examine whether overlapping or distinct DMN regions are critical for future thinking, spontaneous thought, self-referential cognition and AM retrieval (including SAM and EAM).
We also observed that damage to the occipital cortex, including portions of the cuneus in the right hemisphere and lingual gyrus in both hemispheres, was associated with significant impairments in composite AM and SAM retrieval. It is interesting to note that regions within the occipital cortex have been implicated in several functional imaging studies of AM (see Svoboda et al., 2006 for meta-analysis), as well as in previous neuropsychological studies (Ogden, 1993; Brown and Chobor, 1995; Hunkin et al., 1995; Rubin and Greenberg, 1998; Greenberg et al., 2005 for review). Based on such evidence, researchers have proposed a construct of ‘visual amnesia’, highlighting the integral role of long-term visual memory and the occipital cortex in AM retrieval (Rubin and Greenberg, 1998). However, some of the aforementioned studies included patients with damage to both occipital cortex and MTL regions (Rubin and Greenberg, 1998; Greenberg et al., 2005). Thus, one potential explanation for the present findings is that patients with damage to the occipital cortex also had damage extending to MTL regions. To address this possibility, we examined the lesion extent of all patients with occipital damage and AM impairments in our sample. We found that all patients (3/3) with right occipital cortex damage and AM deficits had lesions extending to the MTL (e.g. parahippocampal regions). In contrast, only one out of three patients with damage to the left occipital cortex had a lesion that encompassed MTL regions. Together, these results suggest that the association between the occipital cortex and AM deficits found in the present study cannot be fully explained by damage to MTL regions. More research is warranted to further characterize behavioral as well as neurobiological alterations during AM retrieval in patients with lesions restricted to the occipital cortex.
There are some limitations to our study that should be noted. The IAMQ was not originally designed to measure SAM and EAM separately. Therefore, the total number of EAM and SAM questions was not equivalent, as there were more SAM questions. This was perhaps reflected in the PM3 results (see Figures 2 and 3), as the lesion-deficit effects for the composite and SAM were similar. Although questions were categorized as either SAM or EAM, it is also possible that some questions contained or elicited both components of AM. For example, some SAM questions (e.g. What was the date of the birth of your first child?) could have elicited a specific EAM (e.g. the memory of what happened when your first child was born). More generally, the issue of overlap in SAM and EAM is not unique to the IAMQ, and appears to be common across studies using retrospective AM stimuli (Levine et al., 2002; Gilboa, 2004; Moscovitch et al., 2005). In fact, several neuroimaging studies have used prospectively collected AM stimuli to circumvent these methodological concerns (e.g. Levine et al., 2004; Svoboda and Levine, 2009). Future lesion studies could explore neuroanatomical dissociations between SAM and EAM (e.g. differences in laterality) by using tasks such as the Autobiographical Interview (Levine et al., 2002) or other prospectively collected AM stimuli, designed to assess SAM and EAM independently. Additionally, more recent theoretical work has highlighted behavioral and neuroanatomical distinctions between different types of SAM (i.e. personal facts as compared with repeated personal events), and general semantic and EAM (Irish et al., 2011; Renoult et al., 2012). For example, Renoult et al. (2012) suggest that SAM including personal facts aligns more closely with the neural correlates of general semantic memory as compared with EAM. Moreover, different types of EAM deficits (e.g. lack of emotion detail, loss of spatial context) may be related to damage in disparate brain regions. For instance, another study demonstrated that different types of dementia were associated with distinct EAM retrieval deficits (Irish et al., 2011). Thus, future neuropsychological work could also compare the neural correlates for different types of SAM, general semantic memory and EAM including a detailed analysis of the content of AM.
We would also note that the lesion-deficit effects found in the PCC/Rsp should be interpreted with caution. Although we found in the follow-up analysis that all subjects with lesions including PCC/Rsp regions were relatively impaired across all AM components, there were only three subjects in this study with lesions in this region. While the PCC/Rsp is a region that is uncommonly damaged in stroke (being located in a watershed region with tributaries from the middle cerebral artery and posterior cerebral artery), such lesions may be more frequently caused by tumor resections (e.g. Oszvald et al., 2012). Thus, it might be possible to further test the critical role of the PCC/Rsp in AM and other self-related processes by recruiting individuals with tumor resections to this region. Finally, we acknowledge the use of uncorrected statistical thresholds in reporting the main results, but note that our analyses were hypothesis-driven and focused on specific brain sectors (DMN components).
CONCLUSION
Our findings provide novel neuropsychological evidence for the necessary role of DMN regions in AM retrieval. To our knowledge, this is the first large-scale lesion study to demonstrate that core components of the DMN are critical for AM retrieval. More generally, the DMN has been hypothesized to play a central role in self-referential processing. Our study brings support to this hypothesis by showing that regions of the DMN play a causal role in normal AM retrieval, a prototypical form of self-referential processing. The findings further suggest that the regions of the DMN play an overlapping and distinct role in SAM and EAM, though further lesion studies are necessary.
Acknowledgments
The authors thank Dr Robert Jones for providing data for healthy comparison participants. We also thank R. Henson for her efforts in scheduling the patients for this study.
This work was supported by a grant from the National Institutes of Health (NS19632).
REFERENCES
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage. 2004a;23(4):1460–71. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004b;14(6):752–62. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, et al. Remembering the past: two facets of episodic memory explored with positron emission tomography. The American Journal of Psychiatry. 1995;152(11):1576–85. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65(4):550–62. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Saxe R, Yarkoni T. Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. Neuroimage. 2014;91(0):324–35. doi: 10.1016/j.neuroimage.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. The Journal of Neuroscience. 2007;27(52):14415–23. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Chobor KL. Severe retrograde amnesia. Aphasiology. 1995;9(2):163–70. [Google Scholar]
- Buckner RL. The serendipitous discovery of the brain's default network. NeuroImage. 2012;62(2):1137–1145. doi: 10.1016/j.neuroimage.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna J, Schacter D. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Burianova H, Grady CL. Common and unique neural activations in autobiographical, episodic, and semantic retrieval. Journal of Cognitive Neuroscience. 2007;19(9):1520–34. doi: 10.1162/jocn.2007.19.9.1520. [DOI] [PubMed] [Google Scholar]
- Cermak LS, O'Connor M. The anterograde and retrograde retrieval ability of a patient with amnesia due to encephalitis. Neuropsychologia. 1983;21(3):213–34. doi: 10.1016/0028-3932(83)90039-8. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25(2):616–24. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Damasio A, Eslinger PJ, Damasio H, Van Hoesen GW, Cornell S. Multimodal amnesic syndrome following bilateral temporal and basal forebrain damage. Archives of Neurology. 1985;42(3):252. doi: 10.1001/archneur.1985.04060030070012. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. Lesion Analysis in Neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- Eslinger PJ. Autobiographical memory after temporal lobe lesions. Neurocase. 1998;4(6):481–95. [Google Scholar]
- Fiez JA, Damasio H, Grabowski TJ. Lesion segmentation and manual warping to a reference brain: intra- and interobserver reliability. Human Brain Mapping. 2000;9(4):192–211. doi: 10.1002/(SICI)1097-0193(200004)9:4<192::AID-HBM2>3.0.CO;2-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R, Damasio H, Grabowski T. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5(1):13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory—one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42(10):1336–49. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Eacott MJ, Brechin D, Rubin DC. Visual memory loss and autobiographical amnesia: a case study. Neuropsychologia. 2005;43(10):1493–502. doi: 10.1016/j.neuropsychologia.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences. 2007;104(5):1726–31. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends in Cognitive Sciences. 2007;11(7):299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham KS. Episodic memory: insights from semantic dementia. Philosophical Transaction Royal Social London B Biological Sciences. 2001;356:1423–34. doi: 10.1098/rstb.2001.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. The Lancet Neurology. 2007;6(11):1004–014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- Holland AC, Addis DR, Kensinger EA. The neural correlates of specific versus general autobiographical memory construction and elaboration. Neuropsychologia. 2011;49(12):3164–77. doi: 10.1016/j.neuropsychologia.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkin NM, Parkin AJ, Bradley VA, et al. Focal retrograde amnesia following closed head injury: a case study and theoretical account. Neuropsychologia. 1995;33(4):509–23. doi: 10.1016/0028-3932(94)00136-d. [DOI] [PubMed] [Google Scholar]
- Irish M, Addis DR, Hodges JR, Piguet O. Considering the role of semantic memory in episodic future thinking: evidence from semantic dementia. Brain. 2012;135(7):2178–91. doi: 10.1093/brain/aws119. [DOI] [PubMed] [Google Scholar]
- Irish M, Hornberger M, Lah S, Miller L, Pengas G, Nestor PJ. Profiles of recent autobiographical memory retrieval in semantic dementia, behavioural-variant frontotemporal dementia, and Alzheimer's disease. Neuropsychologia. 2011;49(9):2694–702. doi: 10.1016/j.neuropsychologia.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Jones R, Grabowski T, Tranel D. The neural basis of retrograde memory: evidence from positron emission tomography for the role of non-mesial temporal lobe structures. Neurocase. 1998;4(6):471–9. [Google Scholar]
- Kapur N. Syndromes of retrograde amnesia: a conceptual and empirical synthesis. Psychological Bulletin. 1999;125(6):800. doi: 10.1037/0033-2909.125.6.800. [DOI] [PubMed] [Google Scholar]
- Klein SB, Gangi CE. The multiplicity of self: neuropsychological evidence and its implications for the self as a construct in psychological research. Annals of the New York Academy of Sciences. 2010;1191:1–15. doi: 10.1111/j.1749-6632.2010.05441.x. [DOI] [PubMed] [Google Scholar]
- Kopelman M, Wilson B, Baddeley A. The autobiographical memory interview: a new assessment of autobiographical and personal semantic memory in amnesic patients. Journal of Clinical and Experimental Neuropsychology. 1989;11(5):724–44. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Kroll NE, Markowitsch HJ, Knight RT, von Cramon DY. Retrieval of old memories: the temporofrontal hypothesis. Brain. 1997;120(8):1377–99. doi: 10.1093/brain/120.8.1377. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17(4):677. [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: a prospective functional MRI study. Journal of Cognitive Neuroscience. 2004;16(9):1633–46. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Martinelli P, Sperduti M, Piolino P. Neural substrates of the self-memory system: new insights from a meta-analysis. Human Brain Mapping. 2013;34(7):1515–29. doi: 10.1002/hbm.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Research Bulletin. 2001;54(3):287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Mckiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, et al. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. Journal of Anatomy. 2005;207(1):35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden JA. Visual object agnosia, prosopagnosia, achromatopsia, loss of visual imagery, and autobiographical amnesia following recovery from cortical blindness: case MH. Neuropsychologia. 1993;31(6):571–89. doi: 10.1016/0028-3932(93)90053-3. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, et al. Thinking of the future and past: the roles of the frontal pole and the medial temporal lobes. NeuroImage. 2003;19(4):1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Oszvald Á, Quick J, Franz K. Resection of gliomas in the cingulate gyrus: functional outcome and survival. Journal of Neurooncology. 2012;109(2):341–8. doi: 10.1007/s11060-012-0898-0. [DOI] [PubMed] [Google Scholar]
- Piolino P, Belliard S, Desgranges B, Perron M, Eustache F. Autobiographical memory and autoneotic consciousness in a case of semantic dementia. Cognitive Neuropsychology. 2003;20(7):619–39. doi: 10.1080/02643290242000899. [DOI] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57(3):1221–33. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. The Journal of Neuroscience. 2011;31(28):10262–9. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone CJ, Moulin CJ, Conway MA. Autobiographical memory and amnesia: using conceptual knowledge to ground the self. Neurocase. 2009;15(5):405–18. doi: 10.1080/13554790902849164. [DOI] [PubMed] [Google Scholar]
- Renoult L, Davidson PS, Palombo DJ, Moscovitch M, Levine B. Personal semantics: at the crossroads of semantic and episodic memory. Trends in Cognitive Sciences. 2012;16(11):550–8. doi: 10.1016/j.tics.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, Bonilha L. Improving lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19(7):1081–8. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Greenberg DL. Visual memory-deficit amnesia: a distinct amnesic presentation and etiology. Proceedings of the National Academy of Sciences. 1998;95(9):5413–16. doi: 10.1073/pnas.95.9.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrauf D, Mehta S, Bruss J, Tranel D, Damasio H, Grabowski TJ. Thresholding lesion overlap difference maps: application to category-related naming and recognition deficits. Neuroimage. 2008;41(3):970–84. doi: 10.1016/j.neuroimage.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76(4):677–94. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9(5):648–63. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA, Burgess N. Hippocampal amnesia. Neurocase. 2001;7(5):357–82. doi: 10.1076/neur.7.5.357.16245. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience. 2010;22(6):1112–23. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99(2):195. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–6. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Svoboda E, Levine B. The effects of rehearsal on the functional neuroanatomy of episodic autobiographical and semantic remembering: a functional magnetic resonance imaging study. The Journal of Neuroscience. 2009;29(10):3073–82. doi: 10.1523/JNEUROSCI.3452-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy ofautobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proceedings of the National Academy of Sciences. 2007;104(2):642–7. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss L, Petrides M. Autobiographical memory of the recent past following frontal cortex or temporal lobe excisions. European Journal of Neuroscience. 2008;28(4):829–40. doi: 10.1111/j.1460-9568.2008.06381.x. [DOI] [PubMed] [Google Scholar]
- Tranel D. The Iowa-Benton school of neuropsychological assessment. In: Grant T, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric Disorders. 3rd edn. New York: Oxford University Press; 2009. pp. 66–83. [Google Scholar]
- Tranel D, Jones RD. Knowing ‘what’ and knowing ‘when’. Journal of Clinical and Experimental Neuropsychology. 2006;28(1):43–66. doi: 10.1080/13803390490919344. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. New York, NY: Academic Press; 1972. pp. 381–402. [Google Scholar]
- Tulving E. Elements of Episodic Memory. Vol. 2. New York: Oxford University Press; 1983. [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annual Review of Psychology. 2002;53(1):1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tulving E, Schacter DL, McLachlan DR, Moscovitch M. Priming of semantic autobiographical knowledge: a case study of retrograde amnesia. Brain and Cognition. 1988;8(1):3–20. doi: 10.1016/0278-2626(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(5324):376–80. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Mcmillan CT. Focal retrograde amnesia and the episodic—semantic distinction. Cognitive, Affective, and Behavioral Neuroscience. 2001;1(1):22–36. doi: 10.3758/cabn.1.1.22. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castañón A, et al. Associations and dissociations between default and self-reference networks in the human brain. NeuroImage. 2011;55(1):225–32. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]