Abstract
People expect to be treated equivalently as others in like circumstances. The present study investigated that whether and how equal or unequal treatments of others in like circumstances affected individuals’ responses to unfairness through justifying their reference points for fairness considerations. Twenty-five participants were scanned while they were playing an adapted version of the Ultimatum Game as responders. During the experiment, the participant was not only informed of the offer given by her/his proposer but also informed of the division scheme of another proposer–responder pair. It turned out that participants were more likely to accept unequal offers and reported higher fairness ratings when other responders received unequal offers compared with equal offers. Stronger bilateral anterior insula and dorsal anterior cingulate gyrus activities were observed when only participants (but not other responders) received equal offers, whereas greater right dorsolateral prefrontal cortex activity was found when both of them received unequal offers, especially when participants accepted the unequal offers. Taken together, the results demonstrated that whether others in like circumstances were offered equally also plays an important role in responders’ fairness-related social decision making.
Keywords: unfairness, reference point, Ultimatum Game, AI, dACC, DLPFC
INTRODUCTION
In social interactions, humans are motivated by fairness considerations. To eliminate inequality they consider unfair, people may reject an unequal distribution even at a cost to themselves. Relevant evidence is provided by studies using the Ultimatum Game (UG) (Sanfey et al., 2003; Tabibnia et al., 2008; Dulebohn et al., 2009; Güroğlu et al., 2010, 2011). During the UG, one player (proposer) proposes how to divide a sum of money. If the other player (responder) accepts the division scheme, both of them get the suggested division of money, and if the responder rejects the division scheme, neither of them receives any. Results showed that the responder accepted all equal offers but was likely to reject unequal offers, especially for offers <20% of the total (Güth et al., 1982; Camerer and Thaler, 1995), indicating the important role of perceived fairness in decision making. Furthermore, responders’ judgments of fairness in the UG are not only affected by the proposed income disparity but also by contextual factors, such as the intentions of the proposer (Falk et al., 2003; Sutter, 2007; Güroğlu et al., 2010, 2011), the social distance between the proposer and the responder (Bohnet and Frey, 1999; Wu et al., 2011a) and the loss or gain context (Buchan et al., 2005; Zhou and Wu, 2011; Guo et al., 2013).
Among contextual factors influencing judgments of fairness, people care about others in similar circumstances as themselves (i.e. their peers) (Bewley, 1999; Wu et al., 2011b) and expect to be treated equivalently as them (Bohnet and Zeckhauser, 2004). For example, employees usually do not compare their salary with the income of the employer, but rather with the wages of employees in similar positions (Babcock et al., 1996). The question of how people’s fairness considerations depend on the treatment of peers is of fundamental importance, with far-reaching implications ranging from the design of social welfare systems to the optimal provision of incentives in firms. Thus, using functional magnetic resonance imaging (fMRI), the present study aimed to elucidate how individuals’ fairness considerations are modulated by equal or unequal treatments of their peers and the underlying neural mechanisms by adopting a modified version of the UG.
Several brain regions involved in fairness considerations have been identified in previous neuroimaging studies using the UG paradigm, including anterior insula (AI), dorsal anterior cingulate cortex (dACC) and dorsolateral prefrontal cortex (DLPFC) (Sanfey et al., 2003; Dulebohn et al., 2009; Güroğlu et al., 2010, 2011; Corradi-Dell’Acqua et al., 2013). Empirical evidences recently suggested that AI and/or dACC were engaged in detecting and responding to norm violations and thus activities of AI and dACC during unfair offers in UG might be associated with behaviors violating social norms (Montague and Lohrenz, 2007; Spitzer et al., 2007; King-Casas et al., 2008; Güroğlu et al., 2010, 2011; Strobel et al., 2011; Civai et al., 2012; Chang and Sanfey, 2013). The engagement of DLPFC in UG has also been related to fairness-related decision making (Sanfey et al., 2003; Güroğlu et al., 2010, 2011). Notably, it has been consistently demonstrated that right DLPFC function was negatively related to acceptance rates of unequal divisions in UG, indicating that right DLPFC may play a key role in fairness-related acceptance behaviors (van't Wout et al., 2005; Knoch et al., 2006, 2008).
To test whether and how equal or unequal treatments given to others in like circumstances affected responders’ fairness considerations and its underlying neural basis, we designed an adapted version of the UG. In the adapted UG, two pairs played the UG, while the participants played as responders in one of the pairs (Figure 1A). In the Unequal-single condition, the offers to the participants were unequal (i.e. Ұ5:Ұ45 or Ұ10:Ұ40), while the offers to the responders in the other pair were equal (i.e. Ұ25:Ұ25). In the Unequal-both condition, the offers to the responders in both pairs were the same unequal offers. In the Equal condition, the offers in both pairs were equal.
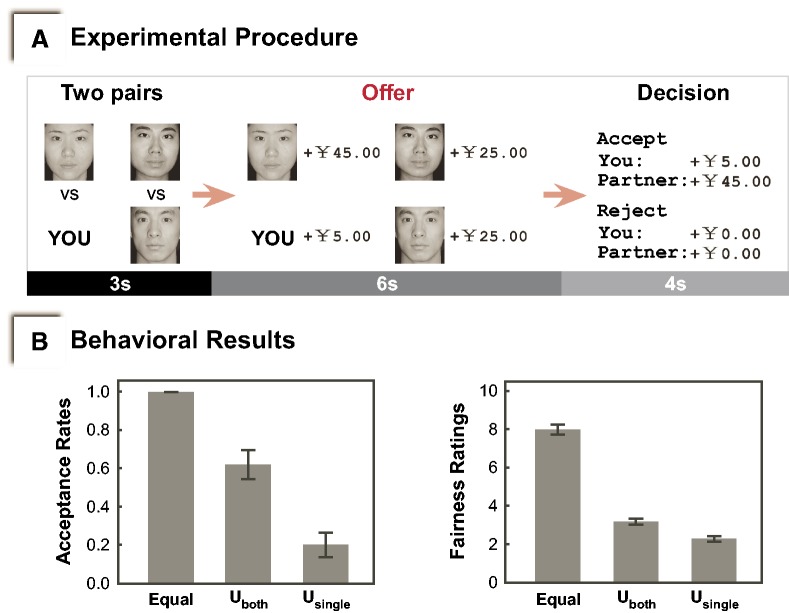
Fig. 1.
(A) Experimental Procedure. Participants were scanned while playing the game for 24 trials, 8 in each condition. Equal offers were given to the participants and the responders in the other pair in the Equal condition. Participants and the other responders received the same unequal offers in the Unequal-both condition, whereas in the Unequal-single condition, only participants were offered unequally. (B) Behavioral Results. Lower acceptance rates and fairness ratings were revealed for unequal trials than equal trials. Furthermore, acceptance rates and fairness ratings of Unequal-both trials were significantly higher than that of Unequal-single trials. Uboth = Unequal-both. Usingle = Unequal-single. Error bars indicate s.e.m.
We were interested in responders’ behavioral and neural responses to unequal offers in the Unequal-single and Unequal-both conditions. Equal split is a default social norm during fairness considerations when there is no salient contextual cue to indicate otherwise (Messick and Schell, 1992; Messick, 1995; Civai et al., 2012). However, when a salient contextual cue has been provided, the reference point for fairness considerations can deviate from an equal split. A recent study showed that past experience could lead people to accept more unequal divisions by shifting the reference point for fairness judgments (Herz and Taubinsky, 2013). Chang and Sanfey (2013) further argued that individuals’ context-specific expectations could be used as fairness reference points, and higher expectation violations were associated with increased activity in dACC. In the present study, we directly set an equally treated or unequally treated reference group of responders, which provided a direct contextual cue for the shift of a fairness reference point. When other responders were offered equal splits, the equality norm in fairness judgments would be confirmed. Proposers’ norm-violating behaviors in the Unequal-single condition should be more obvious than in the Unequal-both condition, and their unequal offers should trigger stronger perception of unfairness. Thus, we expected greater activations in AI/dACC associated with norm violations in the Unequal-single relative to Unequal-both condition. When other responders received offers below an equal split, the reference points for fairness might be shifted below equality. Unequal offers should be perceived less unfair, leading to lower acceptance thresholds. Thus, right DLPFC activity related to acceptance behaviors in UG should be observed in the Unequal-both condition relative to the Unequal-single condition.
METHODS
Participants
Twenty-five right-handed volunteers from the university community with normal or corrected-to-normal vision [18 women, mean age = 21.44 ± 3.38 (s.d.) years] participated in this experiment. None of the participants reported a significant abnormal neurological history. All the participants gave informed consent before scanning.
Materials
Seventy-two face pictures were selected from Chinese Facial Affective Picture System (Gong et al., 2011) as materials, which were randomly allocated to three conditions (Unequal-single, Unequal-both and Equal), 24 face pictures in each condition. For each condition, eight faces were selected as the proposers in the participant’s pair, eight as the proposers and eight as the responders in the other pair, half of which were women faces. The pictures were counterbalanced across different conditions so as to equalize the emotional valence, arousal and attractiveness.
Procedure
Before scanning, participants were told the rules of the game and that they would play as responders with 24 different proposers, respectively. They were also told that another 24 students also played the same game as responders with another 24 different proposers, and all the offers about dividing money were collected before the experiment from real people. During each trial, participants would not only view the offer from their proposers but also the offer from one of the other 24 pairs, without receiving feedback on the responder behavior in the other pair. Participants were told that they would judge the fairness of present offer in a context where the responder in the other pair was treated differently, and they should make final decisions according to their own perceived fairness. Participants were also informed that, for both pairs in each trial, responders’ decisions would be revealed only to their own proposers and thus their decision would not affect the offer of proposers in the other pair; all the offers and decisions among trials were mutual independent. In addition, participants were told that they would be paid according to their decision (after some kind of transformation to reduce the amount of money involved). They would be paid with the amount of money obtained from a random selection of 10% trials in the game (if the final payment was below 50RMB, they would be paid 50RMB).
Then they completed 24 trials (Figure 1A) in the scanner, 8 in each condition. All the trials were presented randomly and functional images were acquired simultaneously. Each trial began with a 4 s presentation of the faces of two pairs, followed by a 6 s presentation of the offers from two proposers. Equal offers (Ұ25:Ұ25) were given to the participants and the responders in the other pair in the Equal condition. In the Unequal-both condition, the offers to the participants were the same as the offers to the responders in the other pair (four trials of Ұ5:Ұ45 and four trials of Ұ10:Ұ40). In the Unequal-single condition, the offers to the participants were unequal (four trials of Ұ5:Ұ45 and four trials of Ұ10:Ұ40), while the offers to the responders in the other pair were always equal (Ұ25:Ұ25). After that, a 3 s decision cue appeared and participants were required to decide (accept or reject) within 3 s. Each trial was jittered with interstimulus intervals from 1 to 3 s, during which a black fixation cross was presented.
After scanning, the participants were presented with the same stimuli as inside the scanner and asked to rate how fair they felt for each offer using a nine-point Likert-type scale where 1 indicated extremely unfair and 9 indicated extremely fair.
fMRI image acquisition and analysis
Scanning was carried out on a 3 T Siemens scanner at the fMRI Lab (East China Normal University, Shanghai). Functional images were acquired using a gradient echo echo-planar imaging (EPI) sequence (TR = 2200 ms, TE = 30 ms, FOV = 220 mm, matrix size = 64 * 64). Thirty five slices paralleled to the AC–PC line (slice thickness = 3 mm, gap = 0.3 mm) were acquired and covered the whole brain. The first five TRs acquired were discarded to allow for T1 equilibration. Before the functional run, a high-resolution structural image was acquired using a T1-weighted, multiplanar reconstruction sequence (TR = 1900 ms, TE = 3.42 ms, 192 slices, slice thickness = 1 mm, FOV = 256 mm, matrix size = 256 * 256).
Data preprocessing and statistical analyses were performed with Statistical Parametric Mapping (SPM5, Wellcome Department of Cognitive Neurology, London). During data preprocessing, the first five functional images were discarded to allow scanner equilibrium effects. Then all data were realigned spatially to the first image of the first time series. The mean EPI image of each participant was computed and spatially normalized to the Montreal Neurological Institute (MNI) single participant template by using the ‘unified segmentation’ function in SPM5. The resulting parameters were subsequently applied to the functional images, and all images were thus transformed into standard MNI space (resampled to 2 * 2 * 2 mm3 voxel size). At last, the data were smoothed with an 8 mm full-width half-maximum isotropic Gaussian kernel.
Statistical analyses were performed using the general linear model implemented in SPM5. An event-related design was used at the first-level analysis with three types of events (Equal, Unequal-both and Unequal-single). Events were convolved with a canonical hemodynamic response function and its time derivatives. All the encoding trials were modeled with null duration from the onset time of the offers. Additional regressors of no interest were created for face presentation and decision. Six regressors modeling movement-related variance and one modeling the overall mean were also employed in the design matrix. High pass temporal filtering with a cutoff of 128 s was also applied in the models. For each subject at the first-level analysis, simple main effects for three types of events (Equal, Unequal-both and Unequal-single) were computed by applying the ‘1 0’ contrasts. The three first-level individual contrast images were then analyzed at the second group level using a random-effects model (flexible factorial design in SPM5).
The (Unequal-both + Unequal-single)/2 − Equal contrast was computed to search for brain regions related to unfairness. The reverse contrasts were also computed. Distinct activations between different unequal conditions were calculated by the Unequal-single − Unequal-both and reverse contrasts. To further test how equal or unequal offers to others in similar circumstances affect participants’ brain activations associated with unfairness, specific activations identified in the Unequal-single − Unequal-both and reverse contrasts were used to compute regions of interest (ROIs). All the significant voxels in the activated clusters within 6 mm spherical regions centered on the peak or local maximum coordinates were included in each ROI. ROIs were defined in the same way throughout the article.
To identify brain regions associated with acceptance behaviors in the Unequal-both condition, a new model containing only accepted trials in the Unequal-both condition and Equal condition (all trials in the Equal condition were accepted; see Behavioral results) was conducted. Three participants were excluded from this analysis because of lack of accepted trials in the Unequal-both condition. We further searched for brain regions whose BOLD signal change detected from the above contrast varied with the corresponding acceptance rate through a correlation analysis. A voxel-level threshold of P < 0.001 (uncorrected) and a spatial extent threshold of k > 15 were consistently used throughout the article.
RESULTS
Behavioral results
Paired t-tests revealed lower acceptance rates and fairness ratings for Unequal-both trials than Equal trials (ts > 5.05, Ps < 0.01) (Figure 1B). Furthermore, acceptance rates and fairness ratings of Unequal-both trials were significantly higher than that of Unequal-single trials (ts > 5.10, Ps < 0.01, significant at 5% with sequential Bonferroni correction) (Figure 1B).
fMRI results
Overall brain activities during unequal trials compared with equal trials
Data analyses revealed that bilateral AI (MNI -32 26 2 and 40 20 0) and DLPFC (MNI -40 56 4 and 34 6 56) survived by contrasting unequal trials with equal trials [(Unequal-both + Unequal-single)/2 − Equal], whereas no region was activated in the reverse contrast (Table 1). When Unequal-single trials were compared with Equal trials, greater activities in bilateral AI (MNI -32 26 2 and 32 28 -2), dACC (MNI 14 24 28) and left DLPFC (MNI -44 52 6) were observed. The Unequal-both vs Equal contrast revealed activations in left AI (MNI -30 26 2) and bilateral DLPFC (MNI -40 56 4 and 46 22 44). We further compared the accepted Unequal-both trials with Equal trials. Similar activations in left AI (MNI -28 26 2) and bilateral DLPFC (MNI -42 54 4 and 40 16 52) were found. Additionally, right AI (MNI 42 22 0) was also activated. The reverse contrasts showed no activated ROI.
Table 1.
Overall brain activities during Unequal trials compared with Equal trials
| Side | Region | Peak activation |
t value | Voxels | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Unequal–Equal | ||||||
| L | Supplementary motor area | −4 | 18 | 48 | 11.36 | 51 733 |
| L | AI | −32 | 26 | 2 | 8.38 | |
| R | 40 | 20 | 0 | 4.54 | ||
| L | DLPFC | −40 | 56 | 4 | 5.07 | |
| R | 34 | 6 | 56 | 4.25 | ||
| L | Middle temporal gyrus | −52 | −18 | −10 | 5.81 | 433 |
| L | −52 | 0 | −20 | 3.91 | 26 | |
| R | Superior frontal gyrus | 30 | 66 | 2 | 4.73 | 55 |
| L | Medial temporal pole | −48 | 10 | −30 | 4.71 | 76 |
| R | Hippocampus | 24 | −38 | −2 | 4.41 | 93 |
| R | Fusiform gyrus | 42 | −14 | −32 | 4.21 | 58 |
| R | Superior temporal gyrus | 50 | −10 | −8 | 4.16 | 93 |
| R | 58 | −36 | 10 | 3.84 | 112 | |
| L | Superior medial gyrus | −6 | 56 | 38 | 3.71 | 26 |
| L | Posterior insula | −32 | −30 | 20 | 3.65 | 19 |
| Equal–Unequal | ||||||
| No regions | ||||||
Note: Coordinates (mm) are in MNI space. L = left hemisphere; R = right hemisphere. P < 0.001(uncorrected), k > 15.
Distinct brain activities between different unequal conditions
The Unequal-single–Unequal-both contrast revealed stronger activities in bilateral AI (MNI -34 26 2 and 26 18 -18) and dACC (MNI 8 22 28), whereas a cluster located in right DLPFC (MNI 44 22 44) was observed in the reverse contrast (Table 2, Figure 2). Further ROI analyses based on activations in bilateral AI, dACC and right DLPFC were conducted. Results revealed that bilateral AI and dACC showed greater activities during Unequal-single trials than Unequal-both trials, whereas right DLPFC activity was greater during Unequal-both trials than Unequal-both trials (ts > 4.22, Ps < 0.01) (Figure 2).
Table 2.
Distinct brain activities between different Unequal conditions
| Side | Region | Peak activation |
t value | Voxels | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Unequal-single–Unequal-both | ||||||
| R | Rolandic operculum | 44 | −4 | 14 | 8.33 | 57 552 |
| L | AI | −34 | 26 | 2 | 6.21 | |
| R | 26 | 18 | −18 | 5.27 | ||
| R | Anterior cingulate cortex | 8 | 22 | 28 | 5.34 | |
| R | Linual gyrus | 40 | −82 | −18 | 3.66 | 16 |
| Unequal-both–Unequal-single | ||||||
| R | DLPFC | 44 | 22 | 44 | 4.21 | 108 |
Note: Coordinates (mm) are in MNI space. L = left hemisphere; R = right hemisphere. P < 0.001(uncorrected), k > 15.
Fig. 2.
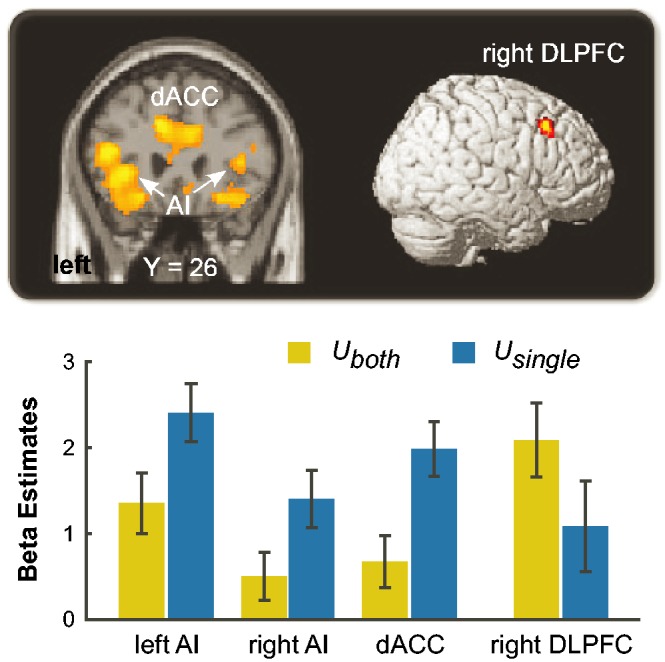
Distinct brain activities between different unequal conditions. Activities in bilateral AI (MNI -34 26 2 and 26 18 -18) and dACC (MNI 8 22 28) were greater for Unequal-single condition relative to Unequal-both trials, whereas right DLPFC (MNI 44 22 44) activity was stronger during Unequal-both trials than Unequal-single trials. Uboth = Unequal-both. Usingle = Unequal-single. Error bars indicate s.e.m. P < 0.001(uncorrected), k > 15.
Correlation analyses on acceptance rate
Correlation analyses were performed to determine brain regions whose BOLD signal change detected from the accepted Unequal-both vs Equal contrast varied with the corresponding acceptance rate. Significant negative correlations were found in right DLPFC (MNI 30 18 56). We then overlapped activations from correlation analyses with activations from the Unequal-both–Equal contrast. Clusters in right DLPFC overlapped (Figure 3).
Fig. 3.
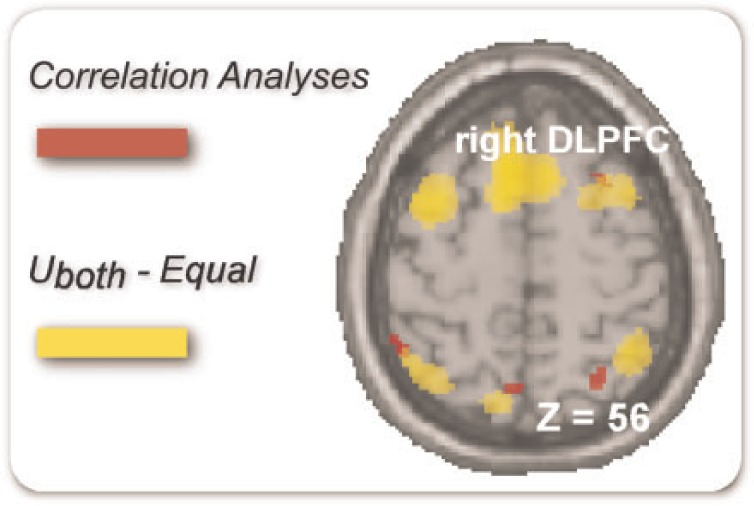
Overlap. Clusters in right DLPFC overlapped between correlation analyses and the Unequal-both–Equal contrast. Uboth = Unequal-both. P < 0.001(uncorrected), k > 15.
Parametric analyses on fairness ratings
Parametric analyses on fairness ratings were also conducted. Bilateral AI (MNI -34 24 4 and 26 22 -14) and DLPFC (MNI -28 14 56 and 32 10 60) showed negative correlations with fairness ratings, indicating higher brain activities in these regions when participants reported lower fairness ratings. No regions showed significant positive correlations with fairness ratings.
DISCUSSION
This study sought to investigate whether and how equal or unequal treatments given to others in like circumstances affected responders’ fairness considerations in the UG. At the behavioral level, decreased fairness ratings and acceptance rates were found in both unequal conditions relative to the equal condition. More importantly, responders were more likely to accept unequal offers and reported higher fairness ratings in Unequal-both condition relative to Unequal-single condition, indicating that unequal offers were perceived less unfair when their peers received unequal offers compared with when their peers received equal offers. At the neural level, greater right DLPFC activity and lower AI/dACC activities were observed during Unequal-both trials relative to Unequal-single trials. These findings demonstrate that equal or unequal offers given by proposers are not the only determinant of responders’ judgments of fairness in UG. Whether their peers are offered equally also plays an important role in their behavioral and neural responses to unfairness.
Unequal offers in UG have been considered as violations of fairness norm (de Quervain et al., 2004; Spitzer et al., 2007; King-Casas et al., 2008; Güroğlu et al., 2010, 2011). Consistent with prior arguments that AI was associated with norm violations (Montague and Lohrenz, 2007; Spitzer et al., 2007; King-Casas et al., 2008; Strobel et al., 2011; Chang and Sanfey, 2013), data analyses showed that greater AI activity was observed during unequal offers relative to equal offers. The role of AI in reacting to norm violations was further supported by the result that more AI activity was found in the Unequal-single condition relative to the Unequal-both condition. Participants reported higher fairness ratings and accepted more often in the Unequal-both condition compared with Unequal-single condition, suggesting that the equal split serving as the social norm during fairness considerations in the standard UG should not be taken for granted as the reference point for fairness judgments when other responders are treated unequally. On the other hand, the equal split is confirmed as the reference point for fairness considerations when other responders are offered equally. Thus, proposers’ norm-violating behaviors in the Unequal-single condition would be more obvious, leading to greater AI activities associated with norm violations in the Unequal-single trials relative to the Unequal-both trials. Accompanied by AI activity, dACC also showed significant activation in the Unequal-single condition rather than the Unequal-both condition, indicating the involvement of AI/dACC network in norm violations (Güroğlu et al., 2010, 2011).
Another important brain region involved in fairness considerations is DLPFC. The engagement of DLPFC in decision making is related to top-down executive control of impulses to reject unfair offers (Sanfey et al., 2003) or accept unfair offers (Güroğlu et al., 2010, 2011). In the present study, right DLPFC was activated during the Unequal-both trials relative to the Unequal-single trials. When restricted to accepted trials, the Unequal-both vs Equal contrast also activated right DLPFC, indicating that right DLPFC may relate to acceptance behaviors in the Unequal-both condition. Correlation analyses further revealed that the activations of right DLPFC during the accepted Unequal-both trials showed negative correlation with acceptance rates. The right DLPFC overlapped in the correlation analyses and the Unequal-both–Equal contrast, suggesting individuals with lower acceptance rates had higher right DLPFC activity when their peers were also treated unequally like themselves. This is consistent with converging evidence using repetitive transcranial magnetic stimulation (Knoch et al., 2006) and cathodal transcranial direct current stimulation (Knoch et al., 2008), which revealed that disrupted function of the right DLPFC, but not left DLPFC, increased acceptance rates of unequal offers in UG. Together, our results suggested that right DLPFC might be engaged in executive control of acceptance impulses (Güroğlu et al., 2010, 2011).
Our conclusions come from a design in which the comparison between the participant and another person was made salient. The explicit provision of the comparison may have encouraged participants, consciously or unconsciously, to incorporate the comparison into decision making more fully. Life also makes comparisons explicit, so the relevance of our conclusions still stands. Nonetheless, future research should investigate the salience of the comparison as a potential moderating variable.
We consider three further interpretations of our results. First, AI and dACC may be more responsive to the Unequal-single rather than the Unequal-both condition because the former is a mismatch condition and the latter a match condition; the mismatch condition may just make the unfairness more salient and hence easier to detect. On this interpretation, individuals should respond to unfairness more quickly in the Unequal-single than Unequal-both condition. Using the same materials and procedure as the fMRI experiment, we asked 23 new participants to press different buttons to indicate whether they were offered an equal amount in each trial. There was no significant difference in reaction time (or accuracy) between the Unequal-single and Unequal-both conditions (reaction time, Unequal-single: 1248 ± s.d. = 426 ms vs Unequal-both: 1239 ± 485 ms, t(22) = 0.22, P = 0.83). To interpret the non-significant difference, note that Ilic et al. (2013) found, for different materials, a match-mismatch difference in reaction time of ∼10% of total reaction time, i.e. for reaction time of around 1240 ms, if there was a match-mismatch difference, a difference of around 124 ms would be reasonable to expect. We found a difference of 9 ms in the wrong direction (standard error = 40 ms, corrected to 42 ms, as per Dienes, 2011). A Bayes factor was calculated, with the predictions of H1 modeled as a half-normal with an s.d. of 124 ms (as per Dienes, 2011, forthcoming), giving B = 0.28. The B indicates the data provide substantial support for the null. That is, the unfairness is not easier to detect per se in the Unequal-single vs the Unequal-both condition.
A second interpretation of our results is that the sharing of unfairness is seen as less aversive generally, and our result is a specific case of this more general phenomenon. For example, it may be that if an equal split were given to the participant and an unequal split to the other pair (a condition we did not use), AI and dACC would also be more active than in the Unequal-both condition. This conjecture deserves testing but if found false our hypothesis would still stand. The participants were asked to make decisions for themselves and not for the responder in the other pair; whether the responder in the other pair was treated equally or unequally only provided a contextual cue for the participant’s fairness considerations. Participants did not receive any feedback about the responder’s behavior in the other pair. Plausibly, participants focused on their own fairness. It has been argued that people do not share others’ pain automatically when they are involved in an additional task distracting attention from the pain of others (Gu and Han, 2007). Thus, participants may not share the pain of the responder in the other pair explicitly or automatically when the unfairness occurs only to the other (and not to oneself).
A third interpretation is based on AI being sensitive to others' unfairness when participants are asked to accept or reject unequal divisions for themselves or on the behalf of another person (Civai et al., 2012; Corradi-Dell'Acqua et al., 2013). Thus, one might predict the total amount of AI activation to increase according to the sum of the unfairness for self and other. This theory is falsified by the data; it predicts greater AI activation in the Unequal-both rather than Unequal-single condition. In our paradigm, participants were only asked to play the UG as responders and make decisions for themselves with the co-occurent information on the offer to the responder in the other pair, completely changing the perspective from the task in Civai et al. (2012) and Corradi-Dell'Acqua et al. (2013).
Recent neuroimaging studies have focused on how to distinguish confounds in the UG to further isolate the variables involved in fairness-related decision-making process. For example, Tabibnia et al. (2008) separated the preference for fairness from the desire for material gain. Tricomi et al. (2010) further distinguished the aversion to inequality from social image or reciprocity. Our study contributes to this research program. During the present study, two conditions were manipulated in which participants received the same unequal offers but were provided different information on whether others like them were treated equally, allowing us to probe directly how people’s fairness considerations were affected by the comparisons between the referent others and themselves during the same amount of inequality. Different patterns of behavioral and neural responses between the Unequal-single and Unequal-both conditions highlights that people's fairness considerations are dynamic and there is no one-to-one correspondence between unequal and unfair. What people seek may be not absolute equality but relative fairness.
CONCLUSIONS
The present study investigated that whether and how responders’ behavioral and neural responses to unfairness in UG were affected by equal or unequal offers to their peers through justifying responders’ reference points for fairness considerations. Specifically, participants were more likely to accept unequal offers and reported higher fairness ratings when their peers received unequal offers compared with when their peers received equal offers. More AI/dACC activities were observed when only participants (but not their peers) received equal offers, whereas greater right DLPFC activity was found when both of them received unequal offers, especially when participants accepted the unequal offers. Evidence from correlation analyses further revealed greater DLPFC activity for individuals with lower acceptance rates, indicating right DLPFC might be related to executive control of acceptance impulses. Taken together, the results demonstrated that equal or unequal offers given by proposers are not the only determinants of responders’ judgments of fairness in UG, whether others in like circumstances were offered equally also plays an important role in responders’ fairness-related social decision-making processes.
Conflict of Interest
None declared.
Acknowledgments
This research was supported by National Natural Science Foundation of China (31271090), Innovation Program of Shanghai Municipal Education Commission (12ZS046).
REFERENCES
- Babcock L, Wang X, Loewenstein G. Choosing the wrong pond: social comparisons in negotiations that reflect a self-serving bias. Quarterly Journal of Economics. 1996;111:1–19. [Google Scholar]
- Bewley TF. Why Wages Don’t Fall During a Recession. Cambridge, MA: Harvard University Press; 1999. [Google Scholar]
- Bohnet I, Frey BS. Social distance and other-regarding behavior in dictator games: comment. The American Economic Review. 1999;89:335–9. [Google Scholar]
- Bohnet I, Zeckhauser R. Social comparisons in ultimatum bargaining. The Scandinavian Journal of Economics. 2004;106:495–510. [Google Scholar]
- Buchan NR, Croson RTA, Johnson EJ, Wu G. Gain and loss ultimatums. In: Morgan J, editor. Advances in Applied Microeconomics. Greenwich, CT: JAI Press; 2005. [Google Scholar]
- Camerer C, Thaler RH. Anomalies: ultimatums, dictators and manners. Journal of Economic Perspectives. 1995;9:209–19. [Google Scholar]
- Chang LJ, Sanfey AG. Great expectations: neural computations underlying the use of social norms in decision-making. Social Cognitive and Affective Neuroscience. 2013;8:277–84. doi: 10.1093/scan/nsr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civai C, Crescentini C, Rustichini A, Rumiati RI. Equality versus self-interest in the brain: Differential roles of anterior insula and medial prefrontal cortex. Neuroimage. 2012;62:102–12. doi: 10.1016/j.neuroimage.2012.04.037. [DOI] [PubMed] [Google Scholar]
- Corradi-Dell’Acqua C, Civai C, Rumiati RI, Fink GR. Disentangling self- and fairness-related neural mechanisms involved in the Ultimatum Game: an fMRI study. Social Cognitive and Affective Neuroscience. 2013;8:424–31. doi: 10.1093/scan/nss014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ-F, Fischbacher U, Treyer V, et al. The neural basis of altruistic punishment. Science. 2004;305:1254–8. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- Dienes Z. Bayesian versus Orthodox statistics: which side are you on? Perspectives on Psychological Sciences. 2011;6:274–90. doi: 10.1177/1745691611406920. [DOI] [PubMed] [Google Scholar]
- Dienes Z. How Bayesian statistics are needed to determine whether mental states are unconscious. In: Overgaard M, editor. Behavioural Methods in Consciousness Research. Oxford: Oxford University Press; forthcoming. [Google Scholar]
- Dulebohn JH, Conlon DE, Sarinopoulos I, Davison RB, McNamara G. The biological bases of unfairness: Neuroimaging evidence for the distinctiveness of procedural and distributive justice. Organizational Behavior and Human Decision. 2009;110:140–51. [Google Scholar]
- Falk A, Fehr E, Fischbacher U. On the nature of fair behavior. Economic Inquiry. 2003;41:20–6. [Google Scholar]
- Gong X, Huang Y, Wang Y, Luo Y. Revision of the Chinese facial affective picture system. Chinese Mental Health Journal. 2011;25:40–6. [Google Scholar]
- Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. NeuroImage. 2007;36:256–67. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Guo X, Zheng L, Zhu L, et al. Increased neural responses to unfairness in a loss context. Neuroimage. 2013;77:246–53. doi: 10.1016/j.neuroimage.2013.03.048. [DOI] [PubMed] [Google Scholar]
- Güroğlu B, van den Bos W, Rombouts SA, Crone EA. Unfair? It depends: neural correlates of fairness in social context. Social Cognitive and Affective Neuroscience. 2010;5:414–23. doi: 10.1093/scan/nsq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güroğlu B, van den Bos W, van Dijk E, Rombouts SA, Crone EA. Dissociable brain networks involved in development of fairness considerations: understanding intentionality behind unfairness. NeuroImage. 2011;57:634–41. doi: 10.1016/j.neuroimage.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Güth W, Schmittberger R, Schwarze B. An experimental analysis of ultimatum bargaining. Journal of Economic Behavior and Organization. 1982;3:367–88. [Google Scholar]
- Herz H, Taubinsky D. Market Experience is a Reference Point in Judgments of Fairness. Available: http://ssrn.com/abstract=2297773 [Accessed July 29, 2013] [Google Scholar]
- Ilic O, Kovic V, Styles SJ. In the absence of animacy: superordinate category structure affects subordinate label verification. PLoS One. 2013;8:e83282. doi: 10.1371/journal.pone.0083282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321:806–10. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Nitsche MA, Fischbacher U, Eisenegger C, Pascual-Leone A, Fehr E. Studying the neurobiology of social interaction with transcranial direct current stimulation-the example of punishing unfairness. Cerebral Cortex. 2008;18:1987–90. doi: 10.1093/cercor/bhm237. [DOI] [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314:829–32. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Messick DM. Equality, fairness, and social conflict. Social Justice Research. 1995;8:153–73. [Google Scholar]
- Messick DM, Schell T. Evidence for an equality heuristic in social decision making. Acta Psychologica. 1992;80:311–23. [Google Scholar]
- Montague PR, Lohrenz T. To detect and correct: norm violations and their enforcement. Neuron. 2007;56:14–8. doi: 10.1016/j.neuron.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–8. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Fischbacher U, Herrnberger B, Gron G, Fehr E. The neural signature of social norm compliance. Neuron. 2007;56:185–96. doi: 10.1016/j.neuron.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Strobel A, Zimmermann J, Schmitz A, et al. Beyond revenge: neural and genetic bases of altruistic punishment. Neuroimage. 2011;54:671–80. doi: 10.1016/j.neuroimage.2010.07.051. [DOI] [PubMed] [Google Scholar]
- Sutter M. Outcomes versus intentions: on the nature of fair behavior and its development with age. Journal of Economic Psychology. 2007;28:69. [Google Scholar]
- Tabibnia G, Satpute AB, Lieberman MD. The sunny side of fairness preference for fairness activates reward circuitry (and disregarding unfairness activates self-control circuitry) Psychological Science. 2008;19:339–47. doi: 10.1111/j.1467-9280.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Rangel A, Camerer CF, O'Doherty JP. Neural evidence for inequality-averse social preferences. Nature. 2010;463:1089–91. doi: 10.1038/nature08785. [DOI] [PubMed] [Google Scholar]
- van’t Wout M, Kahn RS, Sanfey AG, Aleman A. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex affects strategic decision-making. Neuroreport. 2005;16:1849–52. doi: 10.1097/01.wnr.0000183907.08149.14. [DOI] [PubMed] [Google Scholar]
- Wu Y, Leliveld MC, Zhou X. Social distance modulates recipient’s fairness consideration in the dictator game: an ERP study. Biological Psychology. 2011a;88:253–62. doi: 10.1016/j.biopsycho.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou Y, van Dijk E, Leliveld MC, Zhou X. Social comparison affects brain responses to fairness in asset division: an ERP study with the Ultimatum Game. Frontiers in Human Neuroscience. 2011b;5:131. doi: 10.3389/fnhum.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wu Y. Sharing losses and sharing gains: increased demand for fairness under adversity. Journal of Experimental Social Psychology. 2011;47:582–8. [Google Scholar]